Introduction
Growth hormone (GH) regulates sex-specific drug metabolizing enzyme (DME) expression (1) and altered clearance of multi-enzymatic substrates following GH replacement has been demonstrated. (2, 3) Baseline pharmacokinetic alterations have also been described suggesting GH deficiency itself alters DME activity. (3–5) However, effects on individual enzymes have not been characterized. We therefore assessed activities of DMEs likely to be affected in children with GH deficiency, a condition affecting 1 in 3,500 children, (6) and compared them to pediatric historical controls. (7)
Results
Evaluable molar ratios were available in 12 GH deficient (GHD) children (9 males) and 24 historical controls with (n=12) and without (n=12) cystic fibrosis (CF) (14 males). Given the lack of difference between controls with and without CF (8), ratios for these groups were combined. Demographics and probe substrate doses are presented in Table 1. All subjects were Caucasian and sex distribution was independent of study group (p = 0.47). GHD subjects were significantly older (11.5 vs. 6.8 years, p < 0.0001) than controls. CYP2D6 genotype was determined in all subjects and was concordant with the DM/DX ratio. Four subjects (n=1 GHD, n=3 control) were genotypic poor metabolizers (PMs) and excluded from CYP2D6 analysis. Five subjects (n=1 GHD, n=4 control) were phenotypic rapid acetylators and, given the limited number in each group, were excluded from NAT-2 analysis.
Table 1.
Subject demographics, pharmacologic probe substrate doses and urinary molar ratio values
| Parameter | GHD | Historical control | p |
|---|---|---|---|
| Age (years)* | 11.5 (10.0, 12.9) | 6.8 (6.2, 7.4) | < 0.0001 |
| Gender (% male) * | 75% | 58% | 0.47 |
| Weight (kg) * | 29.2 (24.2, 34.2) | 22.2 (20.7, 23.7) | 0.002 |
| Caffeine dose (mg/kg)* | 0.43 (0.34, 0.52) | 0.53 (0.49, 0.58) | 0.03 |
| DM dose (mg)* | 14.6 (12.1, 17.1) | 11.1 (10.3, 11.8) | 0.002 |
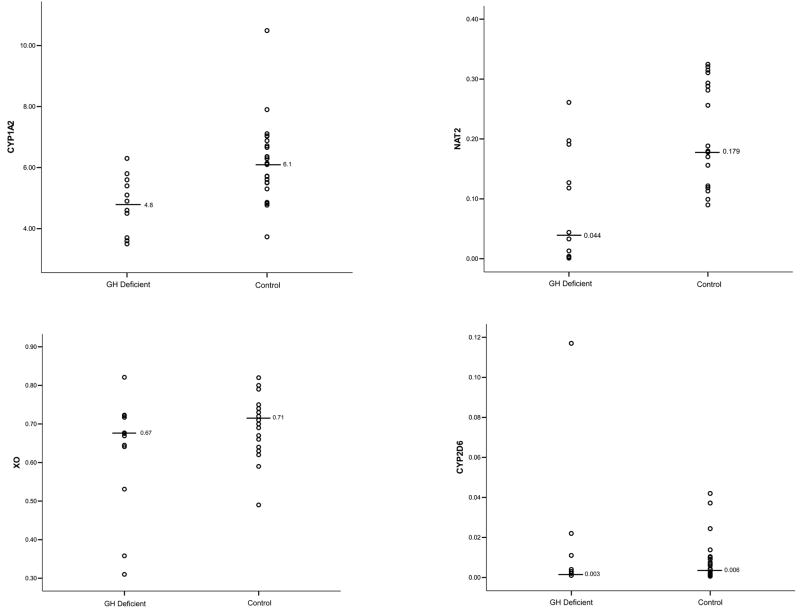
| CYP1A2 ratio++ | 4.8 [3.5, 6.3] (n=12) | 6.1 [3.7, 10.5] (n=24) | 0.001 |
| NAT-2 ratio++ | 0.044 [0.001, 0.261] (n=11) | 0.179 [0.090, 0.325] (n=20) | 0.007 |
| XO ratio++ | 0.67 [0.31, 0.82] (n=12) | 0.71 [0.49, 0.82] (n=24) | 0.23 |
| CYP2D6 ratio++ | 0.003 [0.001, 0.117] (n=11) | 0.006 [0.001, 0.042] (n=21) | 0.63 |
Values presented as mean (95% CI)
Values presented as median [min,max]
Molar ratios are summarized in Table and Figure 1. Lower ratio values indicate reduced activity for CYP1A2, NAT-2 and XO and increased activity for CYP2D6. Unadjusted CYP1A2 and NAT-2 ratios were significantly lower in GHD children. In contrast, no apparent differences in activities of CYP2D6 and XO were noted. Observed mean percent differences were 29%, 122%, 11%, and 38% for CYP1A2, NAT-2, XO and CYP2D6. Post hoc analysis revealed the power to determine a 50% difference in CYP1A2, NAT-2, XO and CYP2D6 activities was 0.99, 0.83, 0.99 and 0.07. Minimum detectable differences (β = 0.2, α = 0.05) for CYP1A2, NAT-2, XO and CYP2D6 were 23%, 111%, 24% and 188%.
Figure 1.
Caffeine and DM urinary molar ratios in children with GH deficiency and healthy controls
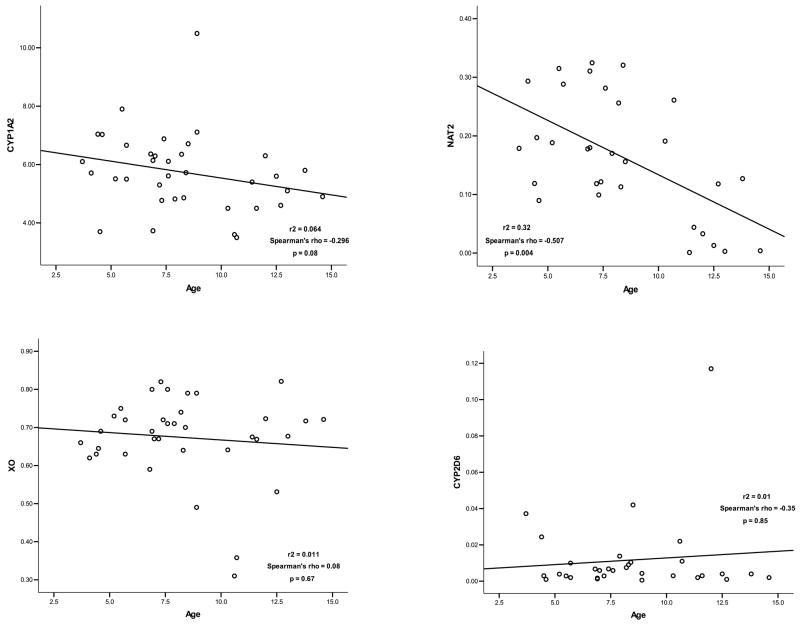
There were no apparent correlations between age and enzyme activity for CYP1A2 (Spearman’s ρ = −0.296, p=0.08), XO (Spearman’s ρ = 0.08, p=0.67) or CYP2D6 (Spearman’s ρ = −0.35, p=0.85) (Figure 2). In contrast, NAT-2 activity was significantly correlated with age (Spearman’s ρ = −0.507, p=0.004). Adjustment of ratio comparisons confirmed values for NAT-2 were confounded by age and revealed no significant differences (p = 0.44). The difference in XO activity also achieved statistical significance following age adjustment (p = 0.03). All other relationships were stable with respect to age correction and age-adjusted CYP1A2 and XO ratios were significantly lower in GHD children.
Figure 2.
Caffeine and DM molar ratios as a function of age
Discussion
Hormones are important in regulating expression and activity of DMEs and other enzymes (e.g., 11 beta-hydroxysteroid dehydrogenase 1) and alterations in clearances of multi-enzymatic drug substrates and endogenous compounds (e.g., cortisol) and have been observed in patients with endocrinopathies (9, 10), conditions associated with hormonal dysregulation (11) and cell culture models. (12) Pharmacokinetic alterations have also been noted in GHD adults (2) and children (3–5). Given the role of GH in regulating sex-specific CYP450 expression and activity (1), it is possible these changes occur consequent to dysregulation of GH and/or its downstream mediators. However, the impact of GH deficiency on individual DMEs has not been investigated. We therefore evaluated activities of specific DMEs in children with idiopathic GH deficiency and compared them to those previously obtained in children with presumably normal GH levels.
Age-adjusted CYP1A2 and XO ratios were significantly lower in GHD children suggesting activities of these isoforms may be reduced by GH dysregulation. CYP2D6 ratios were also lower in GHD children although the difference was not statistically significant. Post-hoc analysis revealed sufficient power to detect the estimated effect size for all enzymes except CYP2D6 (power = 0.07). In addition to lacking statistical significance, the observed difference in CYP2D6 is likely of minimal clinical relevance since it is within the range of normal intra-individual variability previously reported in adults and of a magnitude less than expected to produce significant alterations in clearance of CYP2D6 substrates. (13) Conversely, the difference in CYP1A2 is comparable to that noted in a previous interaction study between caffeine and omeprazole; (14) a finding which suggests GH deficiency may significantly alter the clearance of concomitantly administered CYP1A2 substrates despite falling within the range of reported normal intra-individual variability (4.5 – 49.3%). (15)
Observation of reduced CYP1A2 activity is consistent with prior pharmacokinetic data (3) which demonstrated a theophylline elimination half-life in GHD children (7.2 hours) substantially greater (~54%) than that previously reported in children with asthma (3.7 hours) (16). The magnitude of effect noted in our study (29%), however, was less than expected from these pharmacokinetic data. Since enzymes other than CYP1A2 contribute to theophylline elimination, it is possible that their activities were similarly reduced by GH dysregulation and consequently, represent a collective effect of GH deficiency on multiple enzymes/isoforms. Differences in body mass between children with GH deficiency and asthma may have also contributed to previous results.
Although maturation of NAT-2 appears to occur over the first 4 years of life, apparent oral clearance values for isoniazid, a prototypical NAT-2 substrate, decrease by approximately 45% from age 6 to 16 years. (17) We noted a similar decrease in NAT-2 activity with increasing age and our ability to identify GH-mediated effects on NAT-2 was therefore confounded by potential developmental influences. Given that the onset of puberty is significantly delayed in GHD children (18), however, it is likely that the “developmental” ages of our populations were more similar than suggested by chronologic age. Although the observed difference (122%) in NAT-2 activity was greater than expected solely from differences in developmental stage, age-adjustment of values revealed no significant differences.
We conclude that activities of CYP1A2 and XO may be significantly reduced in GHD children. Observed alterations in enzyme activity may have important clinical implications since clearance of concomitantly administered pharmacologic substrates may be altered by GH deficiency in children and adults. Adjustment of dosing regimens for drugs that are substrates for these isoforms may therefore be necessary. Additional prospective studies utilizing age-matched controls are required to confirm these preliminary results. GH may also alter activities of other phase I (CYP2C19, CYP2C9, CYP3A4) and II (glucuronyl transferases, sulfotransferases) DMEs and investigation of these isoforms is warranted.
Methods
Study sites
Four institutions within the Pediatric Pharmacology Research Unit (PPRU) network participated: University of Louisville (Louisville, KY); Children’s Mercy Hospitals and Clinics (Kansas City, MO), Case Western Reserve University (Cleveland, OH) and University of California at San Diego (San Diego, CA). The protocol received approval from each Institutional Review Board and the PPRU Network Steering Committee. Informed consent was obtained from each subject’s parent and assent was obtained from children ≥ 7 years. Procedures were performed in accordance with ethical standards of each Institutional Review Board and Good Clinical Practice guidelines. The study was also registered with the clinical trials registry sponsored by the United States National Library of Medicine (www.clinicaltrials.gov, study identifier NCT00458991).
Study subjects
Pre- or early pubertal (Tanner stage 1 or 2) children (4–14 years) with short stature and idiopathic GH deficiency were eligible. Short stature was defined as presence of the following: height less than 5th percentile for age and sex or deceleration across two major percentiles on standard pediatric growth curves and growth velocity less than 5 cm/year. GH deficiency was defined as failure to raise serum GH concentrations greater than 10 μg/L following provocative testing with at least two secretagogues. (19) Historical controls included children ages 3–8 years with and without CF. (7)
Children were excluded if they had an acute viral illness, fever or were receiving thyroid hormone, corticosteroids or any medications and/or foods known to induce or inhibit CYP450, NAT-2 or XO. (20–22) History of prior rhGH treatment, illicit drug/tobacco use or exposure to second hand smoke > 8 hours/day was also exclusionary. Children with a non-idiopathic etiology of GH deficiency or history of hepatic, renal, cardiac or thyroid disorders were ineligible. Presence of organ dysfunction was based on clinical history and recent laboratory tests, if available.
Phenotyping procedure
Subjects abstained from caffeine or methylxanthine containing foods, charbroiled foods, cruciferous vegetables and grapefruit/grapefruit juice for 48 hours prior to study. After an overnight fast, urine was collected and each subject was given four-ounces of Coca-Cola® (~11.5 mg caffeine) and 0.5 mg/kg (maximum 30 mg) of dextromethorphan (DM) (Robitussin® Pediatric Cough, Wyeth, Madison, NJ). Urine was collected for 8 hours in containers containing 2 grams of ascorbic acid to maintain pH less than 4. Start and stop times of urine collection, total volume collected and urine pH were recorded. Each sample was mixed and a 50mL aliquot frozen at −70°C until analysis.
CYP2D6 genotyping
Blood samples (5 mL) were collected and genomic DNA isolated using a Qiagen® blood DNA kit (Qiagen, Inc., Valencia, CA). CYP2D6 genotype was determined as described previously (23) and alleles designated according to standard nomenclature (http://www.cypalleles.ki.se). The following allelic variants were included in the analysis: *2, *3, *4, *5, *6, *7, *8, *9, *10, *12, *14, *17, *29, *40, *41[2988G], *41[2988A], *42, *45/46 and *1×2, *2×2, *4×2 gene duplications. Subjects with two non-functional alleles were classified as PMs.
Analytical procedure
Urinary 5-acetylamino-6-formylamino-3-methyluracil (AFMU), 1-methylxanthine (1X), 1-methyluric-acid (1U) and 1,7-dimethyluric acid (17U) concentrations were determined by reverse-phase HPLC as described previously (24) using a modification of the methods of Grant et al.. (25) Retention times and collision-induced dissociation provided specific identification and quantification of the analytes via comparison of peak areas with those of analytical standards in drug-free urine specimens. Response was linear from 1μM to 333μM for all analytes. Intra- and inter-day coefficients of variation were less than 5%, with a lower limit of quantitation assigned as the low standard (1μM).
Urinary concentrations of DM and its metabolite dextrorphan (DX) were determined via HPLC with fluorescence detection as previously described. (26) Data were normalized to an internal standard (levallorphan tartrate) and molar amounts determined using a seven-point standard curve prepared daily in drug-free urine using peak height measurements. The analytical method demonstrated linearity over the range of standard concentrations (0–10 nM for DM; 0–1,000 nM for DX) evaluated (r2 > 0.99). Intra- and inter-day coefficients of variation were less than 10%, with a lower limit of quantitation of 0.1μM for all analytes.
Phenotype assignment
The molar ratio of caffeine 7-demethylation products (AFMU + 1X + 1U) to the 8-hydroxylation product (17U) was used as a marker of CYP1A2 activity. (27) The hydroxylation molar ratio, 1U/(1U + 1X), was used as a measure of XO activity. (28) NAT-2 activity was assessed using the molar ratio of AFMU/(AFMU + 1X + 1U), with a ratio of ≥ 0.34 indicative of a rapid acetylator phenotype. (29) The molar ratio of DM to DX was used to estimate CYP2D6 activity. Subjects with a DM/DX ratio of > 0.3 were classified as PMs and those with a ratio of < 0.3 were classified as extensive metabolizers (EM). (30)
Statistical analysis
Residuals were calculated for each measure and their distributions examined. Demographics were compared using the Student’s t test assuming equal variances. Sex distribution was evaluated using the Fisher’s Exact test. Molar ratios were compared using the Mann Whitney test. Given the lack of difference between controls with and without CF (8), ratios for these groups were combined. The relation between age and enzyme activity was explored graphically and statistically (Spearman correlation coefficient). Comparisons of enzyme activity were also age adjusted via fitting of linear models using study group as a categorical factor and age as a continuous covariate. Post hoc power calculations were performed to determine minimum detectable differences and actual power to detect a 50% difference in enzyme activity. An effect size of 50% was selected based on prior pharmacokinetic data demonstrating theophylline clearance values in GHD children approximately 54% lower than those in children with asthma. (3) Statistical analyses were performed using SPSS for Windows®, version 13.0 (SPSS Inc., Chicago, IL) and R®, version 2.5.1. (R Foundation for Statistical Computing, Vienna, Austria, www.r-project.org) with α =0.05.
Acknowledgments
We gratefully acknowledge the principal investigators (PIs) and research personnel at the following collaborating PPRU network sites: Case Western Reserve University, Cleveland, OH (PI: Jeffrey Blumer, M.D.); University of California San Diego, San Diego, CA (PI: Edmund Capparelli, Pharm.D.). We also thank Elizabeth McDowell, RN, CCRC, Misty Cummins, RN, Pam Clark, M.D., Mike Foster, M.D., Janice Sullivan, M.D., Mike Venneman, BSN, RN, CCRC, Alex Karmazin, M.D. and Campbell Howard, M.D. for their assistance with subject identification and recruitment; Angela Griffin, BS, Tao Lin, MS, Bill Pierce, Ph.D. and Hilary Yokley, for their analytical assistance; and Doug Lorenz. MA, MSPH for his assistance with statistical analysis.
Supported in part by the National Institute of Child Health and Human Development (NICHD) Pediatric Pharmacology Research Unit (PPRU) Network (grant numbers 1 U10 HD31313-15 and 1 U10 HD 0495934-04) and by grants from the Katharine B. Richardson Foundation and Philip Astrowe Trust.
Footnotes
Disclosures
Dr. Davis currently works for and holds stock in Genentech, Inc. At the time of this study, he had no such affiliation.
References
- 1.Waxman DJ, O’Connor C. Growth hormone regulation of sex-dependent liver gene expression. Mol Endocrinol. 2006;20:2613–29. doi: 10.1210/me.2006-0007. [DOI] [PubMed] [Google Scholar]
- 2.Cheung NW, Liddle C, Coverdale S, Lou JC, Boyages SC. Growth hormone treatment increases cytochrome P450-mediated antipyrine clearance in man. J Clin Endocrinol Metab. 1996;81:1999–2001. doi: 10.1210/jcem.81.5.8626872. [DOI] [PubMed] [Google Scholar]
- 3.Redmond GP, Bell JJ, Nichola PS, Perel JM. Effect of growth hormone on human drug metabolism: time course and substrate specificity. Pediatr Pharmacol (New York) 1980;1:63–70. [PubMed] [Google Scholar]
- 4.Redmond GP, Bell JJ, Perel JM. Effect of human growth hormone on amobarbital metabolism in children. Clin Pharmacol Ther. 1978;24:213–8. doi: 10.1002/cpt1978242213. [DOI] [PubMed] [Google Scholar]
- 5.Rifkind AB, Saenger P, Levine LS, Pareira J, New MI. Effects of growth hormone on antipyrine kinetics in children. Clin Pharmacol Ther. 1981;30:127–32. doi: 10.1038/clpt.1981.137. [DOI] [PubMed] [Google Scholar]
- 6.Lindsay R, Feldkamp M, Harris D, Robertson J, Rallison M. Utah Growth Study: growth standards and the prevalence of growth hormone deficiency. J Pediatr. 1994;125:29–35. doi: 10.1016/s0022-3476(94)70117-2. [DOI] [PubMed] [Google Scholar]
- 7.Kennedy MJ, Abdel-Rahman SM, Kashuba AD, Leeder JS. Comparison of various urine collection intervals for caffeine and dextromethorphan phenotyping in children. J Clin Pharmacol. 2004;44:708–14. doi: 10.1177/0091270004266624. [DOI] [PubMed] [Google Scholar]
- 8.Kennedy MJ, Scripture CD, Kashuba AD, Scott CS, Gaedigk A, Kearns GL. Activities of cytochrome P450 1A2, N-acetyltransferase 2, xanthine oxidase, and cytochrome P450 2D6 are unaltered in children with cystic fibrosis. Clin Pharmacol Ther. 2004;75:163–71. doi: 10.1016/j.clpt.2003.10.005. [DOI] [PubMed] [Google Scholar]
- 9.Matzke GR, Frye RF, Early JJ, Straka RJ, Carson SW. Evaluation of the influence of diabetes mellitus on antipyrine metabolism and CYP1A2 and CYP2D6 activity. Pharmacotherapy. 2000;20:182–90. doi: 10.1592/phco.20.3.182.34775. [DOI] [PubMed] [Google Scholar]
- 10.Saenger P, Rifkind AB, New MI. Changes in drug metabolism in children with thyroid disorders. J Clin Endocrinol Metab. 1976;42:155–9. doi: 10.1210/jcem-42-1-155. [DOI] [PubMed] [Google Scholar]
- 11.Kotlyar M, Carson SW. Effects of obesity on the cytochrome P450 enzyme system. Int J Clin Pharmacol Ther. 1999;37:8–19. [PubMed] [Google Scholar]
- 12.Li RS, Nakagawa Y, Liu YJ, Fujisawa Y, Sai S, Nakanishi T, et al. Growth hormone inhibits the 11 beta-Hydroxysteroid dehydrogenase type 1 gene promoter activity via insulin-like growth factor I in HepG2 cells. Horm Metab Res. 2008;40:286–8. doi: 10.1055/s-2008-1058076. [DOI] [PubMed] [Google Scholar]
- 13.Kashuba AD, Nafziger AN, Kearns GL, Leeder JS, Shirey CS, Gotschall R, et al. Quantification of intraindividual variability and the influence of menstrual cycle phase on CYP2D6 activity as measured by dextromethorphan phenotyping. Pharmacogenetics. 1998;8:403–10. doi: 10.1097/00008571-199810000-00005. [DOI] [PubMed] [Google Scholar]
- 14.Rost KL, Roots I. Accelerated caffeine metabolism after omeprazole treatment is indicated by urinary metabolite ratios: coincidence with plasma clearance and breath test. Clin Pharmacol Ther. 1994;55:402–11. doi: 10.1038/clpt.1994.49. [DOI] [PubMed] [Google Scholar]
- 15.Kashuba AD, Bertino JS, Jr, Kearns GL, Leeder JS, James AW, Gotschall R, et al. Quantitation of three-month intraindividual variability and influence of sex and menstrual cycle phase on CYP1A2, N-acetyltransferase-2, and xanthine oxidase activity determined with caffeine phenotyping. Clin Pharmacol Ther. 1998;63:540–51. doi: 10.1016/S0009-9236(98)90105-9. [DOI] [PubMed] [Google Scholar]
- 16.Ellis EF, Koysooko R, Levy G. Pharmacokinetics of theophylline in children with asthma. Pediatrics. 1976;58:542–7. [PubMed] [Google Scholar]
- 17.Rey E, Gendrel D, Treluyer JM, Tran A, Pariente-Khayat A, d’Athis P, et al. Isoniazid pharmacokinetics in children according to acetylator phenotype. Fundam Clin Pharmacol. 2001;15:355–9. doi: 10.1046/j.1472-8206.2001.00044.x. [DOI] [PubMed] [Google Scholar]
- 18.Darendeliler F, Hindmarsh PC, Preece MA, Cox L, Brook CG. Growth hormone increases rate of pubertal maturation. Acta Endocrinol (Copenh) 1990;122:414–6. doi: 10.1530/acta.0.1220414. [DOI] [PubMed] [Google Scholar]
- 19.Wilson TA, Rose SR, Cohen P, Rogol AD, Backeljauw P, Brown R, et al. Update of guidelines for the use of growth hormone in children: the Lawson Wilkins Pediatric Endocrinology Society Drug and Therapeutics Committee. J Pediatr. 2003;143:415–21. doi: 10.1067/s0022-3476(03)00246-4. [DOI] [PubMed] [Google Scholar]
- 20.Balis FM, Holcenberg JS, Zimm S, Tubergen D, Collins JM, Murphy RF, et al. The effect of methotrexate on the bioavailability of oral 6-mercaptopurine. Clin Pharmacol Ther. 1987;41:384–7. doi: 10.1038/clpt.1987.45. [DOI] [PubMed] [Google Scholar]
- 21.Michalets EL. Update: clinically significant cytochrome P-450 drug interactions. Pharmacotherapy. 1998;18:84–112. [PubMed] [Google Scholar]
- 22.Pacher P, Nivorozhkin A, Szabo C. Therapeutic effects of xanthine oxidase inhibitors: renaissance half a century after the discovery of allopurinol. Pharmacol Rev. 2006;58:87–114. doi: 10.1124/pr.58.1.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gaedigk A, Simon SD, Pearce RE, Bradford LD, Kennedy MJ, Leeder JS. The CYP2D6 activity score: translating genotype information into a qualitative measure of phenotype. Clin Pharmacol Ther. 2008;83:234–42. doi: 10.1038/sj.clpt.6100406. [DOI] [PubMed] [Google Scholar]
- 24.Kennedy MJ, Davis DA, Smith NB, Gaedigk A, Pearce RE, Kearns GL. Six-Month, Prospective, Longitudinal, Open-Label Caffeine and Dextromethorphan Phenotyping Study in Children with Growth Hormone Deficiency Receiving Recombinant Human Growth Hormone (rhGH) Replacement. Clin Ther. 2008 doi: 10.1016/j.clinthera.2008.09.012. In Press. [DOI] [PubMed] [Google Scholar]
- 25.Grant DM, Tang BK, Kalow W. A simple test for acetylator phenotype using caffeine. Br J Clin Pharmacol. 1984;17:459–64. doi: 10.1111/j.1365-2125.1984.tb02372.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Blake MJ, Gaedigk A, Pearce RE, Bomgaars LR, Christensen ML, Stowe C, et al. Ontogeny of dextromethorphan O- and N-demethylation in the first year of life. Clin Pharmacol Ther. 2007;81:510–6. doi: 10.1038/sj.clpt.6100101. [DOI] [PubMed] [Google Scholar]
- 27.Campbell ME, Spielberg SP, Kalow W. A urinary metabolite ratio that reflects systemic caffeine clearance. Clin Pharmacol Ther. 1987;42:157–65. doi: 10.1038/clpt.1987.126. [DOI] [PubMed] [Google Scholar]
- 28.Grant DM, Tang BK, Campbell ME, Kalow W. Effect of allopurinol on caffeine disposition in man. Br J Clin Pharmacol. 1986;21:454–8. doi: 10.1111/j.1365-2125.1986.tb05222.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tang BK, Kadar D, Qian L, Iriah J, Yip J, Kalow W. Caffeine as a metabolic probe: validation of its use for acetylator phenotyping. Clin Pharmacol Ther. 1991;49:648–57. doi: 10.1038/clpt.1991.82. [DOI] [PubMed] [Google Scholar]
- 30.Schmid B, Bircher J, Preisig R, Kupfer A. Polymorphic dextromethorphan metabolism: co-segregation of oxidative O-demethylation with debrisoquin hydroxylation. Clin Pharmacol Ther. 1985;38:618–24. doi: 10.1038/clpt.1985.235. [DOI] [PubMed] [Google Scholar]