Abstract
Exploring the development of nonviral nucleic acid delivery vectors with progressive, specific, and novel designs in molecular architecture is a fundamental way to investigate how aspects of chemical and physical structure impact the transfection process. In this study, macromolecules comprised of a four-arm star poly(ethylene glycol) and termini modified with one of five different heparin binding peptides have been investigated for their ability to bind, compact, and deliver DNA to mammalian cells in vitro. These new delivery vectors combine a PEG-derived stabilizing moiety with peptides that exhibit unique cell-surface binding ability in a molecular architecture that permits multivalent presentation of the cationic peptides. Five peptide sequences of varying heparin binding affinity were studied; each was found to sufficiently bind heparin for biological application. Additionally, the macromolecules were able to bind and compact DNA into particles of proper size for endocytosis. In biological studies, the PEG-star peptides displayed a range of toxicity and transfection efficiency dependent on the peptide identity. The vectors equipped with peptides of highest heparin binding affinity were found to bind DNA tightly, increase levels of cellular internalization, and display the most promising transfection qualities. Our results suggest heparin binding peptides with specific sequences hold more potential than nonspecific cationic polymers to optimize transfection efficiency while maintaining cell viability. Furthermore, the built-in multivalency of these macromolecules may allow simultaneous binding of both DNA at the core of the polyplex and heparan sulfate on the surface of the cell. This scheme may facilitate a bridging transport mechanism, tethering DNA to the surface of the cell and subsequently ushering therapeutic nucleic acids into the cell. This multivalent star shape is therefore a promising architectural feature that may be exploited in the design of future polycationic gene delivery vectors.
INTRODUCTION
The delivery of nucleic acids by nonviral vectors is an intense area of research due to the promise of synthetic materials to be tailored for specific drug targets (1, 2). It has been found that even moderate chemical modifications of the vector architecture may significantly affect the ability of the molecule to transfect cells efficiently (3–8). Under many conditions of polycation–DNA complex (polyplex) formation, the surface charge on positively charged polyplexes is neutralized, which allows polyplex–polyplex aggregation to occur, and even in the absence of complete charge neutralization, cationic polyplexes can bind and flocculate with oppositely charged serum proteins. By preventing this aggregation, polyplexes can remain in circulation for extended time periods, avoid clearance by macrophages, and maintain a small, uniform size for vascular penetration and cellular endocytosis under in vivo conditions (9–12). The addition of poly(ethylene glycol) chains to polycationic vectors has previously been shown to favorably stabilize polycation/DNA complexes, known as polyplexes, against flocculation in conditions containing salt and serum, which has improved their potential for in vivo gene delivery (13).
Nitrogen-dense polycations such as Poly(ethyleneimine) (PEI) have been shown to have high transfection efficiency; however, the cytotoxicity of this and many similar vectors are high in many cell lines (14). To retain high transfection efficiency and minimize toxicity, modified polycationic systems have been created. For instance, the modification of PEI with PEG has been developed either as a copolymer or with a grafted architecture (15–17). In addition, PEGylated poly(L-lysine) vectors have recently been shown to have very low cytotoxicity as well as notable stability in vivo, although increasing the transfection efficiency of these vectors is still a current research goal (18–20). To understand the architectural impact of these vectors, a wide variety of novel methods for the incorporation of hydrophilic moieties are being examined. For example, supramolecular vectors utilizing a PEG moiety grafted onto preformed DNA/polycationic vectors (21) or even rotaxane-type vectors utilizing cyclodextrin molecules threaded around hydrophilic polymers (22–24) have been developed. Additionally, low molecular weight branched PEI conjugated onto the end of star-shaped PEG molecules has been found to have promising transfection potential (25). In our work reported here, we have incorporated cationic peptide chains derived from the binding portion of heparin binding proteins (HBPs) onto the termini of star-shaped PEG macromolecules to create multivalent DNA vectors that can both efficiently complex DNA and target heparan sulfate-containing endocytic receptors.
There have been numerous reports implicating the role of proteoglycans in the uptake of polyplexes into cells (26, 27). We hypothesize that polyplexes containing heparin binding peptides may increase cell-surface adhesion and subsequent uptake, more so than classical nonspecific polycationic vectors. This may occur through specific interaction with ubiquitous heparan sulfate moieties on cell surfaces, which are similar to heparin in structure. Specific HBP–proteoglycan binding may enhance cellular uptake via sequence-specific association. This could eliminate the need to design highly charge-dense vectors that nonspecifically bind heparan sulfate (5, 28), and negatively impact cell viability (14). Such approaches employing PEG-HBPs for complexation of nucleic acids therefore offer unique opportunities for controlling and improving DNA delivery.
The specificity and affinity of protein interactions with heparin are governed by the specific placement of cationic amino acid residues relative to the sulfation pattern of heparin. This pattern may provide a strategy for manipulating heparin binding affinity and DNA delivery. Literature reports suggest that heparin-interacting protein (HIP) and antithrombin III (ATIII) bind to similar pentasaccharide sequences found in heparin (29). Four tetrasaccharides are thought to be required for efficient heparin binding of human platelet factor 4 (PF4) (30), thus providing higher affinity binding for PF4 over that of HIP and ATIII. Furthermore, peptides that contain multiple arginine residues have been suggested to have high heparin binding affinity, since similar structures are found in heparin binding proteins (31, 32).
The reported PEG-star peptide bioconjugates have been previously exploited to assemble noncovalently cross-linked hydrogels (33–37) via the interactions of the heparin binding termini (peptide mimics of the heparin binding domains of ATIII (38), HIP (39), PF4 (30, 40)) with PEG-star molecules modified with heparin. The mechanical and biological properties of such hydrogels can be engineered on the basis of the heparin–HBP interactions and applied in the controlled delivery of proteins (35, 40). Electrostatic interactions are a key component in HBP–heparin associations (41); therefore, it is likely that these PEG star HBP conjugates will also be capable of mediating the binding, condensation, and delivery of other polyanions such as DNA. Furthermore, the multivalent nature of these molecular stars may permit the simultaneous interaction of the bioconjugate with both DNA at the core of the polyplex and glycosaminoglycan at the cell surface through the interaction of both DNA-bound peptide arms and unbound arms on the surface of the nanoparticle. This simultaneous interaction may tether the DNA complexes to the cell surface, which may subsequently act to usher nucleic acid-based therapeutics into cells.
To this end, we have measured the heparin binding affinities and kinetics for the HBPs, examined their complexation with DNA, and correlated this information with the transfection efficiencies of the resulting polyplexes. Polyplexes formed with two of the structures described here, PEG-star PF4ZIP and PEG-star RRP, inhibit serum-mediated aggregation and exhibit promising transfection in the presence of serum. In addition, physical characterization of polyplexes created with these novel vectors as well in vitro biological activity profiles are presented. Figure 1 and Table 1 show details of the PEG-star peptide structure, the proposed transfection scheme, and the amino acid sequences used to create these unique bioconjugates.
Figure 1.
(a) Chemical structure of PEG-star peptides depicting their molecular architecture. “PEPTIDE” refers to one of 5 different peptides listed in Table 1. n = 60. (b) Proposed scheme for the transfection of mammalian cells with pDNA using PEG-star HBP vectors. The star polymers are multivalent and peptides functionalized on the termini of the PEG-star should be competent to bind both DNA in the core of the polyplex and heparan sulfate on the surface of cells simultaneously.
Table 1.
Peptides Incorporated into the PEG-Star Peptide Vectors
EXPERIMENTAL PROCEDURES
I. Materials
For the synthesis of the PEG-HBPs, low molecular weight heparin sodium salt (LMWH) from porcine intestinal mucosa (average molecular weight 3000 g/mol) was obtained from Sigma (St. Louis, MO), and heparin from porcine intestinal mucosa (average molecular weight 6000 g/mol) was purchased from Celsus Lab., Inc. (Cincinnati, OH). Hydroxyterminated four-arm PEG (Mn = 10 300; Mw = 11 300 g/mol; Mw/Mn = 1.1) and thiol-terminated, four-arm PEGs (Mn = 10 000; Mw = 10 800 g/mol; Mw/Mn = 1.1) were purchased from Polymer Source (Dorval, Quebec, Canada). Deionized water (18 ΩM) was applied in all the experiments (Barnstead NANOpure Diamond Ultrapure water Systems, Barnstead Inc, Dubuque, IA). Amino acids, rink amide MBHA (4-methylbenzhydrylamine) resin, and HBTU were purchased from Novabiochem Corp. (San Diego, CA). DMF and acetonitrile (HPLC grade) were obtained from Fisher Scientific (Fairlawn, NJ). General chemicals were all purchased from Sigma-Aldrich unless otherwise specified. Triethylamine (TEA) was distilledfrom CaH2. All other reagents were used as received.
Branched polyethylenimine (PEI), a positive control used in some experiments, was obtained from Aldrich. For the execution of biological studies, pCMVβ plasmid DNA and gWiz-Luc were acquired from PlasmidFactory (Bielefeld, Germany) and Aldevron (Fargo, ND), respectively. Nuclease-free water, Opti-MEM, and DMEM (supplemented with 10% fetal bovine serum (FBS), 100 units/mL penicillin, 100 µg/mL streptomycin, 0.25 µg/mL amphortericin), and PBS were all purchased from Invitrogen (Carlsbad, CA). For the luciferase assay, cell culture lysis buffer and the luciferase substrate assay reagent were purchased from Promega (Madison, WI). For the viability assay, a Bio-RAD DC protein assay kit was purchased from Bio-Rad (Hercules, CA), and bovine albumin (98%) was purchased from Sigma (St. Louis, MO).
II. Synthesis and Characterization of the PEG-HBPs
The synthesis of the PEG-star HBP conjugates was conducted via addition reactions between vinyl sulfone-terminated four-arm star-PEG and cysteine-terminated HBPs. The synthetic protocols for preparing the peptides and bioconjugates for the PEG-ATIII, PEG-HIP, and PEG-PF4ZIP have been described previously (35, 36, 40).
Synthesis of RRP and sHIP
The RRP peptide RRRRRRRGGG and the sHIP peptide CKAAKRPKAAKDKQTK were prepared on Rink Amide MBHA resin via solid-phase peptide synthesis with Fmoc protection using a PTI PS3 peptide synthesizer (Protein Technologies, Inc., AZ). A preparative-scale Delta 600 HPLC (Waters) equipped with a Waters Symmetry 300 C18 column (5 µm particle size, 19 × 150 mm) was applied for the crude RRP peptide purification. Solvent A (water/0.1% TFA) and solvent B (acetonitrile/0.1% TFA) were employed as HPLC solvents in programmed gradients. Peptides isolated as single fractions via HPLC were characterized via ESI mass spectrometry. The mass of the purified RRP was confirmed via ESI mass spectrometry, m/z = 641.9 [(M+2H)2+, calcd 642.2] and m/z = 428.0 [(M+3H)3+, calcd 428.5]. The mass of the purified sHIP was similarly confirmed, m/z = 886.2 [(M+2H)2+, calcd 887.5], m/z = 591.3 [(M+3H)3+, calcd 591.7], and m/z = 443.8 [(M+-4H)3+, calcd 444.0] (see Supporting Information). These peptides were used in heparin affinity measurements and circular dichroic spectroscopy, while peptides with an N-terminal cysteine were used in the synthesis of the PEG conjugates. Because of the virtually identical synthesis and purification, it is assumed that this slight modification in sequence does not significantly alter the peptide for our purposes.
Synthesis of PEG-RRP and PEG-sHIP Conjugates
The synthesis of PEG-RRP and PEG-sHIP conjugates was accomplished using a similar method applied previously for the synthesis of other PEG-HBP conjugates (36). Characterization data for these macromolecules via size exclusion chromatography and NMR are given in the Supporting Information.
III. Heparin Affinity Measurements
Affinity Liquid Chromatography
The heparin affinities of the HBPs and PEG-HBPs were first determined via heparin-sepharose affinity liquid chromatography on an ÄKTA Explorer FPLC equipped with a HiTrap Heparin HP column (5 mL, ~10 mg heparin/mL gel, Amersham Biosciences Corp, Piscataway, NJ), using previously described procedures in which peptides bound to a heparin-modified affinity column are eluted with buffer of varying concentrations of sodium chloride (35).
Surface Plasmon Resonance (SPR)
SPR characterization of the binding of HBPs to LMWH was performed on a Biacore 3000 instrument (Biacore Inc., Piscataway, NJ), employing a high-capacity LMWH surface created on a commercially available streptavidin-coated SA chip via treatment with biotinylated LMWH, as previously described (35). Equilibrium dissociation constants (KD) were calculated from experimentally determined association and dissociation rate constants, via standard fitting protocols previously described (35) and described in the Supporting Information.
IV. DNA Binding Assay
To determine whether these materials bind and complex pDNA, a series of gel shift assays were performed with polyplexes formulated at a range of charge ratios. This charge ratio represents the number of positive charges on the peptide side chain to the number of phosphate groups along the DNA backbone. A more thorough treatment of this calculation for the PEG-star peptides can be found in the Supporting Information.
Polyplexes were formed by adding a solution of the PEG-HBP (150 µL) dissolved in nuclease-free water to an equal volume of solution of plasmid DNA (15 µg) at various charge ratios. After a 30 min incubation period, 1 µL of running buffer (Blue Juice, Invitrogen, Carlsbad, CA) was added to each sample. Ten microliters of each polyplex sample was then loaded into the wells of a 0.6% agarose gel prepared in TAE buffer containing 0.06 µg/mL ethidium bromide. The samples were subsequently electrophoresed in an electric field of 60 V.
V. Circular Dichroic Spectroscopy (CD)
Circular dichroic spectroscopy was used to characterize the structures of select peptides while bound to pDNA. Characterization was conducted on PF4ZIP and HIP peptides in solution with pDNA (rather than on PEG-HBPs) in order to preserve the optical clarity of the solutions for CD measurements. CD spectra were acquired at 25 °C on a Jasco J-810 spectropolarimeter (Jasco Inc, Easton, MD) equipped with a Jasco PTC-424S temperature controller. Polyplexes consisting of heparin binding peptides and pDNA were formulated at a charge ratio of 5, and the final DNA concentration of the polyplex formulation was chosen to be 1 µg/100 µL in PBS to match that used in DNA transfection experiments. To form the polyplexes, either PF4ZIP or HIP was added to the pDNA solution to reach a final peptide concentration of 15.2 µM or 21.6 µM, respectively. Polyplexes at a charge ratio of 1 were formed at the same concentration (21.6 µM) of HIP, but with 1 µg/20 µL pDNA.
Samples were equilibrated at 25 °C for 30 min prior to data collection. The equilibration of the samples was indicated by the absence of further changes in the CD signal at longer equilibration times. All CD spectra were taken in a 1 mm path length quartz cuvette, at wavelengths from 200 to 250 nm. Data points were recorded at every nanometer with a 4.0 s response time. (Refer to Supporting Information for spectra.)
VI. Polyplex Size and Zeta Potential Measurements
In order to characterize polyplex stability, the size and zeta potential of polyplexes formulated by complexing pDNA with the PEG-HBPs were measured via dynamic light scattering using a Zetasizer Nano ZS [Malvern Instruments (Malvern, UK) equipped with a 633 nm laser]. Ultrapure water, Opti-MEM, and DMEM were filtered with a 0.2 µm filter before any polyplexes were formed to remove particulates and dust from the initial solutions. The solutions were not filtered after polyplex formation. Polyplexes were formed in a similar manner as described above for the gel shift assays, except 3 µg pDNA was used and the polyplexes were created at a charge ratio of 5. Measurements were performed at 25 °C. After initial incubation for 1 h, the polyplex solutions were diluted to 900 µL with Millipore water, Opti-MEM (serum-free media), or DMEM containing 10% fetal bovine serum. Solutions were then immediately transferred to a cuvette and measured. Polyplex size measurements were taken consecutively at 0, 20, 40, 60, and 120 min. Zeta potential measurements were taken consecutively at 0 and 20 min.
VII. Cell Viability and Transfection Efficiency
BHK-21 cells were cultured under the conditions recommended by the ATCC. Twenty-four hours before transfection, cells were seeded in 24 well plates at 50 000 cells per well and incubated in supplemented DMEM at 37 °C and 5% CO2. Immediately before transfection, the polyplexes were created with pDNA (gWiz-Luc) as described above for the previous experiments (gel-shift and particle size assays). For the delivery experiments, the polyplexes were created at a range of charge ratios as well as a charge ratio of 0, which represents the delivery of naked pDNA. One hour after polyplex formation, 600 µL of Opti-MEM or DMEM was added to each polyplex solution. Prior to the transfections, the media in each well was aspirated off, the cells were washed with PBS, and 300 µL of the polyplex solution was added. This volume was used to normalize the delivery of DNA to each well to 1 µg. Controls of both untransfected cells and cells transfected with pDNA only were also performed. Transfections at all charge ratios and the controls were performed in triplicate. Four hours after transfection, 800 µL of DMEM supplemented with 10% FBS was added to each well. Twenty-four hours after transfection, the media was removed from each well, and 1000 µL of fresh DMEM was added. Forty-eight hours after transfection, the media was removed from each well, the cells were washed with PBS, and 100 µL of lysis buffer was added to each well. The cell lysates were then assayed for luciferase expression using the protocol provided by Promega and protein content using the protocol provided with the Bio-Rad DC protein assay kit. The results of the protein assay were analyzed using a standard curve of bovine serum albumin and normalized against the untransfected control to obtain the fraction of cell survival.
VIII. Cellular Internalization
BHK-21 cells were cultured under conditions recommended by the ATCC. Twenty-four hours before transfection, cells were seeded in 6 well plates at 250 000 cells/well and incubated in DMEM supplemented with 10% FBS at 37 °C and 5% CO2. Formulation of polyplexes proceeded in the manner described above except 250 µL of each PEG-Star HBP was added to 5 µg Cy5-labeled DNA (250 µL total DNA solution volume) to formulate polyplexes at an N/P ratio of 5. After incubation for 1 h, 1 mL of Opti-MEM was added to each polyplex solution. The cells were washed with PBS and each well was transfected with a corresponding polyplex solution in Opti-MEM and subsequently incubated for 4 h. Afterwards, the transfection media was aspirated and each well was washed twice, with 2 mL and then 1 mL PBS, to remove bound polyplexes from the cell surface. The cells were subsequently incubated for two more hours in DMEM with 10% FBS to allow further trafficking of DNA. After this final incubation period, the media was removed and cells were again washed with 1 mL PBS and subsequently trypsinized at 37 °C for 10–15 min. Next, 1 mL DMEM was added to each well and the cells were transferred to Falcon tubes and spun at 1000 rpm for 10 min. The supernatant was then removed, 1 mL PBS was used to wash the pellet, and the cells were spun again in the same conditions. Again, the supernatant was removed and the pellet was resuspended in PBS supplemented with 2% FBS. The cell suspensions were analyzed with a FACS Calibur flow cytometer, using appropriate forward and side scatter gates, and the Cy5 fluorescence intensity of 50 000 cells in each sample was measured. A control of untransfected cells was used to create a gate less than 1% of cells exhibiting Cy5 fluorescence. This gate was used for subsequent samples transfected with the PEG-HBPs to determine the percent of cells transfected.
RESULTS AND DISCUSSION
I. PEG-HBP Synthesis and Characterization
Polysaccharides, such as heparan sulfate, are widely distributed on a broad range of cell types. Many cell-surface receptors involve the binding of an external molecule to heparan sulfate to initiate internalization via receptor-mediated endocytosis. By improving the polyplex-cell surface interaction involving these anionic polysaccharides, the cellular internalization, and thus transfection efficiency, may also be improved. For example, adeno and adeno-associated viral vectors with heparan sulfate and heparin binding domains have been previously constructed as gene delivery vectors (42, 43), to enhance cellular internalization via heparan-sulfate-associated receptors. Additionally, adenoviral vectors with modified fiber coat proteins containing cationic poly(lysine) have been shown to increase the transduction of macrophages, as well as endothelial, smooth muscle, glioblastoma, and T cells. (44) However, to the best of our knowledge, polymeric DNA delivery agents comprising heparin binding domains attached to star-shaped PEG molecules have not yet been applied for pDNA delivery and may offer advantages in both reduced aggregation and improved transfection efficiency.
In this study, we chose to examine the PEG-HBP macromolecules for their ability to complex pDNA and deliver it into a mammalian cell line. As displayed in Figure 1, five different heparin binding peptides (HIP, sHIP, ATIII, PF4ZIP, and RRP) were selected for attachment to the PEG-star macromolecules. All five peptides are positively charged at physiological pH and should therefore be capable of electrostatically complexing DNA. The peptide sequences were each selected for their high affinity for heparin. The HIP and ATIII peptides are derived from the heparin binding domains of heparin-interacting protein and antithrombin III, respectively (38, 39). The peptide denoted sHIP represents a motif generated by scrambling the HIP sequence, which was synthesized to confirm the role of sequence specificity in heparin binding (45) and to test the hypothesis that heparin binding alone, outside of changes in peptide composition and charge density, plays a role in controlling DNA delivery. The PF4ZIP sequence is a dimeric coiled-coil, heparin binding peptide modeled after the heparin binding domain of human platelet factor 4 (40). The RRP sequence (Table 1) was chosen on the basis of the fact that arginine is a commonly identified residue in randomly synthesized 7-mer peptides that have been shown to efficiently bind to and immobilize heparin (31, 32). Additionally, polyarginine peptides have been shown to have promising transfection properties (46). The glycine residues were included in the RRP sequence to provide flexible linkers between the short oligo-arginine sequence and the PEG backbone.
The previously unreported PEG-RRP and PEG-sHIP conjugates have been successfully synthesized via Michael addition reactions between vinyl sulfone-terminated four-arm PEG (47) and cysteine-terminated HBPs, using a similar method applied previously for the synthesis of other PEG-HBP conjugates (36). Functionalization of the PEG-star termini with the HBPs has yielded values of 77% (35), 63% (36), and 59% (37) for the PEG-HBP modified with the heparin binding proteins HIP, ATIII, and PF4ZIP, respectively. Similarly, in these investigations, the compositions of the PEG-RRP and the PEG-sHIP were determined from their 1H NMR spectrum (see Supporting Information). Functionalization of the PEG-RRP was found to be 119%. This may result from association of the RRP peptides during synthesis and purification. Significant aggregation is not suggested to occur between the PEG-RRP conjugates, as solutions of the PEG-RRP remain clear and nonviscous. Functionalization of the PEG-sHIP was found to be 100%. It has been previously demonstrated that the PEG-HBPs readily associate with multivalent heparin moieties to form noncovalently associated hydrogels (35–37). Therefore, although the precise number of termini modified with RRP cannot be determined, we anticipated that a sufficient number of PEG termini were modified with the RRP to support network, and therefore polyplex, formation (functionalization ≥2). The functionalization values of 77% (PEG-HIP), 100% (PEG-sHIP), 63% (PEG-ATIII), 59% (PEG-PF4ZIP), and 119% (PEG-RRP) were used in all charge ratio calculations for PEG-HBP/pDNA polyplexes.
II. Heparin Binding Affinity of HBPs
Previous reports have demonstrated that targeting ligands, such as monoclonal antibodies, peptides, and sugars, can be conjugated to DNA carriers to promote receptor-mediated endocytosis (48, 49). Additionally, a limited number of reports suggest that modification of viral vectors via the use of heparin and heparan-binding peptides enhance binding with heparan sulfate-associated endocytic receptors on a broad range of cell types, thus increasing internalization (42, 43). Heparin-sepharose affinity chromatography and surface plasmon resonance (SPR) experiments were therefore performed to compare the heparin binding affinity of different HBPs to confirm the potential difference in cellular uptake between PEG-HBP/pDNA complexes. Results of these experiments are summarized in Table 2. For heparin affinity chromatography experiments, the concentration of NaCl required for HBP elution from a heparin-sepharose column is used as an indication of the affinity of the peptide for heparin. Elution from the heparin-sepharose column required a salt concentration of 1160 ± 10 mM for RRP and 929 ± 4 mM for sHIP (Table 2). Comparison of these values to those obtained with the previously synthesized peptides (HIP: 695 ± 14 mM, ATIII: 594 ± 2 mM, PF4ZIP: 882 ± 3 mM) indicates that RRP has the highest heparin binding affinity. The enhanced heparin binding of the nonspecific RRP sequence is likely due to the high cationic charge density of this peptide and suggests the potential for the PEG-RRP to elicit the highest cellular uptake of the PEG-HBPs.
Table 2.
Heparin Binding Affinity Data (at 25 °C) for Heparin Binding Peptides Determined via Affinity Chromatography and SPR at pH = 7.4
| HBP | salt required for elution from heparin column (mM) |
ka (M−1 s−1) | kd (s−1) | KD (M) | PEG-HBP | salt required for elution from heparin column (mM) |
|---|---|---|---|---|---|---|
| PF4ZIPa | 882 ± 3 | 2.24 ± 0.05 × 105 | 2.56 ± 0.10 × 10−3 | 1.14 ± 0.03 × 10−8 | PEG-PF4ZIP | 798 ± 13 |
| RRP | 1160 ± 10 | 2.64 ± 0.08 × 105 | 1.18 ± 0.12 × 10−3 | 4.47 ± 0.34 × 10−9 | PEG-RRP | 1271 ± 1 |
| HIPb | 695 ± 14 | 1.10 ± 0.08 × 103 | 4.64 ± 0.02 × 10−3 | 4.21 ± 0.3 × 10−6 | PEG-HIP | 628 ± 7 |
| sHIP | 929 ± 4 | 9.29 ± 0.17 × 104 | 5.62 ± 0.13 × 10−3 | 6.05 ± 0.03 × 10−8 | PEG-sHIP | 763 ± 4 |
| ATIIIb | 594 ± 2 | 1.56 ± 0.06 × 102 | 2.00 ± 0.3 × 10−3 | 1.28 ± 0.13 × 10−5 | PEG-ATIII | 558 ± 1 |
The sHIP peptide, which is composed of a scrambled HIP sequence, was actually found to have higher binding affinity for heparin than the natural HIP sequence. Although this result was not anticipated, the sHIP sequence serves as an excellent comparison for assessing the role of heparin binding affinity in DNA transfection efficiency because it removes the complications of differences in composition and charge density. In order to support this initial finding, further SPR binding experiments were conducted.
The interaction of the five HBPs with a low molecular weight heparin-modified SA chip surface was investigated and results are reported in Table 2. No mass transport limitations, linked reactions, or nonspecific binding of HBP to SA chips were observed in any of the SPR measurements, as assessed via manufacturer-recommended standard control experiments (see Supporting Information). Analysis of the association and dissociation regions of the binding curves provided relative association and dissociation rates for comparison to previous studies conducted on the same chip surface (35, 37). The measured association of the RRP with LMWH proceeds with a rate constant of 2.64 ± 0.08 × 105 M−1 s−1 based on the analysis of the association regions of the binding curves, while the dissociation proceeds with a rate constant of 1.18 ± 0.12 × 10−3 s−1, yielding a KD of 4.47 ± 0.34 × 10−9 M. Similar calculations resulted in a KD value of 6.05 ± 0.03 × 10−8 M for the sHIP peptide (Table 2). Comparison of these results to our previous studies indicates that faster association kinetics for RRP, sHIP, and PF4ZIP, relative to those reported for the ATIII and HIP (35), are the primary cause of the lower calculated KD values, and the trend of the dissociation constants is consistent with the chromatography results. Furthermore, relative to the reported value for HIP, the KD value of the sHIP peptide is lower and agrees with our chromatography results. This confirms the higher heparin affinity of the sHIP sequence versus the natural HIP sequence. Overall, the more rapid association rate, coupled with the similar dissociation rate of RRP, sHIP, and PF4ZIP versus the other HBPs, suggest the strong binding of anionic heparin by these peptides, which suggests their strong interaction with cell surfaces.
Heparin affinity of the PEG-HBP conjugates was also investigated using column chromatography. As shown in Table 2, the trend in heparin binding affinity of the PEG conjugates correlate well with the free peptides, with PEG-RRP, PF4ZIP, and sHIP displaying highest binding affinity. In general, the PEG-HBP conjugates required slightly lower salt concentration for elution from the heparin-modified column. This seemed surprising, since multivalent presentation of binding epitopes often results in binding enhancements due to statistical and multivalent effects. However, the PEG-HBP conjugates are of low molecular weight and low valency (3–4 peptides per conjugate), and such polymers often do not show significant multivalent enhancement (50). In any case, the focus of these studies was to determine the heparin binding affinity of the PEG-HBPs for correlation to DNA delivery efficiency, and our results demonstrate that the heparin binding affinities of the HBPs have similar trends regardless of their conjugation to PEG.
As a control, we performed chromatography experiments on PEI, which is a commonly used transfection reagent. It was found that PEI eluted at 1980 ± 0.002 mM NaCl, which indicates that this incredibly charge-dense polycation binds heparin with very high affinity. However, the PEI-heparin binding mechanism likely involves a great deal of cooperativity, which may cause the dissociation of pDNA at the cell surface and decreased cellular uptake. We propose that the multivalent cationic nature of the PEG-HBPs holds the most promise for simultaneous binding of DNA and cell-surface heparan via electrostatic interactions.
Cellular uptake and release of PEG-HBP/pDNA complexes via endocytosis may be directly affected by the progressive acidification from the physiological pH (7.4) of the extracellular medium to pH 6.5-6.0 in endosomes (51). The heparin binding kinetics between low molecular weight heparin and the peptides of highest heparin affinity (RRP, sHIP, and PF4ZIP) were monitored via SPR at pH values between 6 and 8, to determine potential changes in binding kinetics that might alter the process of cellular uptake or release of pDNA from the PEG-HBP/pDNA complex. An increase in the KD value signifies a decrease in the affinity of the peptide for heparin. As shown in Table 3, Table 4, and Table 5, KD values for PEG-RRP, sHIP, and PF4ZIP generally increase slightly as the pH is lowered below 7.4, which suggests that the PEG-HBP/pDNA bound to the cell-surface heparan sulfates will likely remain associated with these proteoglycans during internalization, but may begin to dissociate from heparan sulfate as the polyplex/heparan sulfate complexes begin to traffic through the progressively acidifying vesicles of the endocytic pathway. Such dissociation of the vectors from internalized heparan sulfate in the endosomes may enhance the intracellular motility of the complexes out of these vesicles.
Table 3.
Heparin Binding Affinity Data (at 25 °C) for PF4ZIP (peptide) at Various pH Values, as Determined via SPR
| pH | ka (M−1 s−1) | kd (s−1) | KD (M) |
|---|---|---|---|
| 6 | 1.11 ± 0.03 × 104 | 7.05 ± 0.03 × 10−4 | 6.35 ± 0.20 × 10−8 |
| 7.4 | 2.24 ± 0.05 × 105 | 2.56 ± 0.10 × 10−3 | 1.14 ± 0.03 × 10−8 |
| 8 | 1.97 ± 0.08 × 104 | 8.70 ± 0.49 × 10−3 | 4.42 ± 0.63 × 10−7 |
Table 4.
Heparin Binding Affinity Data (at 25 °C) for RRP (peptide) at Different pH Values, as Determined via SPR
| pH | ka (M−1 s−1) | kd (s−1) | KD (M) |
|---|---|---|---|
| 6 | 1.35 ± 0.13 × 104 | 1.00 ± 0.01 × 10−3 | 7.41 ± 0.82 × 10−8 |
| 7.4 | 2.64 ± 0.08 × 105 | 1.18 ± 0.12 × 10−3 | 4.47 ± 0.34 × 10−9 |
| 8 | 9.94 ± 0.26 × 103 | 5.33 ± 0.15 × 10−3 | 5.36 ± 0.01 × 10−7 |
Table 5.
Heparin Binding Affinity Data (at 25 °C) for sHIP (peptide) at Various pH Values, as Determined via SPR
| pH | ka (M−1 s−1) | kd (s−1) | KD (M) |
|---|---|---|---|
| 6 | 2.73 ± 0.08 × 104 | 1.36 ± 0.01 × 10−2 | 4.98 ± 0.12 × 10−7 |
| 7.4 | 9.29 ± 0.17 × 104 | 5.62 ± 0.13 × 10−3 | 6.05 ± 0.03 × 10−8 |
| 8 | 2.26 ± 0.22 × 104 | 1.82 ± 0.04 × 10−2 | 8.05 ± 0.86 × 10−7 |
III. DNA Binding
For use as potential nucleic acid delivery vectors, the conjugates must be able to package DNA into small particles capable of being endocytosed. The PEG-HBP/pDNA complexes are described by their charge ratio, which is a ratio that indicates the number of positively charged residues in the peptide sequence to the number of anionic phosphate residues in the DNA backbone. The amount of material needed to completely complex the DNA is established by this assay, with results shown in Figure 2. The lane in which pDNA no longer migrates through the gel is regarded as the charge ratio at which the PEG-HBPs completely complex pDNA (see Figure 2a, lanes 2–50). This assay indicates that the PEG-HBPs are able to complex pDNA at a charge ratio of 2 or lower. Similar values have been reported for PEGylated poly(L-lysine) vectors which have previously shown promising transfection characteristics (19, 20, 52).
Figure 2.
Results of the gel shift assays. (a) PEG-PF4ZIP. (b) PEG-RRP. (c) PEG-HIP. (d) PEG-sHIP. (e) PEG-ATIII. Polyplexes were created at the indicated charge ratios in water. Samples were electrophoresed in an agarose gel under a 60 V electric field.
At higher charge ratios, the bands produced by polyplexes created with PEG-RRP and PEG-ATIII appeared to fade and disappear (i.e., Figure 2b,e, lanes 5–50), which may indicate that these star polymers were able to bind pDNA sufficiently tightly to exclude the intercalating agent ethidium bromide used to visualize the bands. Moreover, the ATIII peptide has two aromatic residues that may be capable of intercalating pDNA; this may displace ethidium bromide as well and contribute to the low charge ratio needed to complex pDNA. It is worth noting that both PEG-RRP and PEG-ATIII demonstrate similar qualitative pDNA binding in these gel experiments, despite the significant differences in their heparin binding affinity, suggesting that the strength of heparin binding does not correlate to the ability of the polymer to complex pDNA, as might be expected since the associations involve different binding motifs.
CD characterization of peptide conformation at charge ratios of 5 (sufficient to completely complex DNA in all cases) and 1 (to increase the relative amount of bound peptide in the sample) indicated that the HBPs showed no significant change in conformation upon binding DNA (see Supporting Information), although changes in peptide conformation upon the binding of DNA have been previously observed for select peptides (53). Therefore, it is expected that observed differences in DNA binding and transfection efficiency result from the physicochemical properties of the peptide and not from additional conformational changes upon complexation.
IV. Polyplex Size
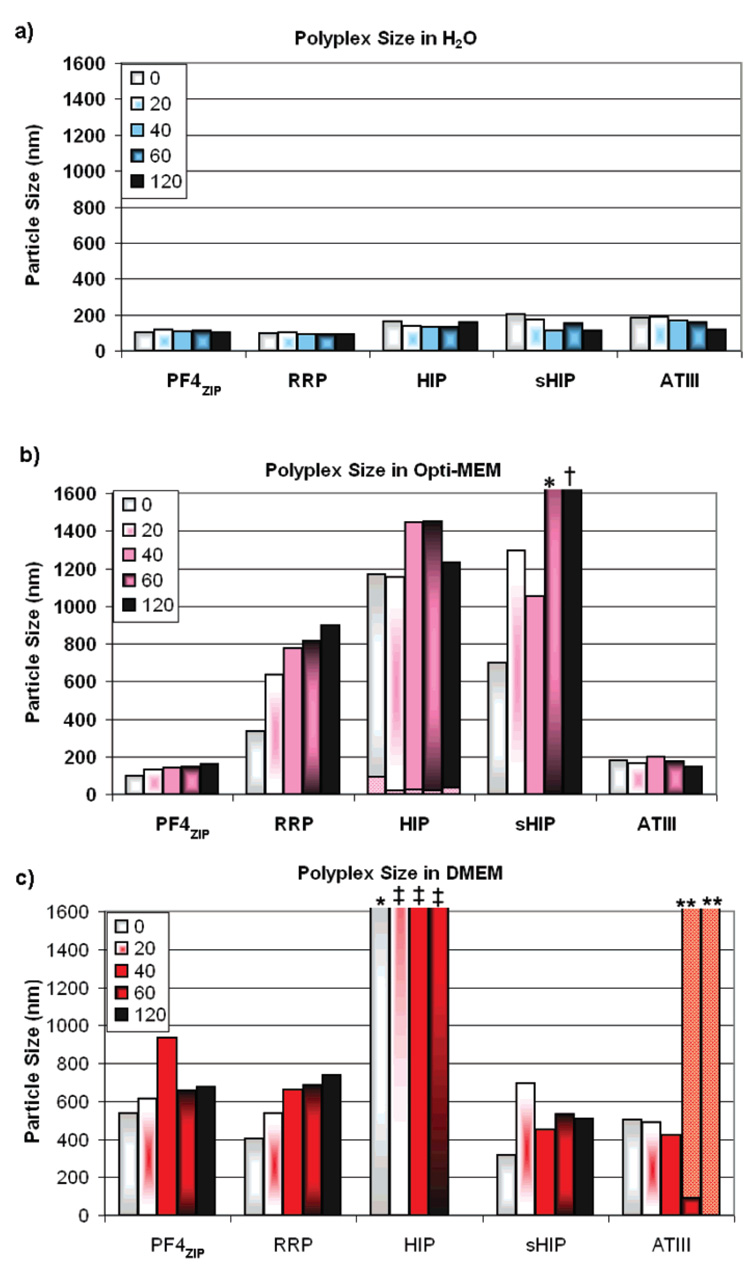
The salt and serum stability of polyplexes is critical for their application in vivo; if the particles are unstable at high ionic strength and in the presence of serum proteins, they may aggregate to form large particles which would be detrimental to their efficacy. Dynamic light scattering was therefore employed to measure the size of the PEG-HBP/pDNA polyplexes in various dispersants over time.
The size of the polyplexes formed between the star macromolecules and pDNA, shown in Figure 3, again displayed a dependence on the cationic peptide moiety of the vector. The polyplex solutions created with PEG-HIP in Opti-MEM and PEG-ATIII created in DMEM revealed bimodal peaks in size measurements. The large difference in size between the two populations likely indicates that the shapes of the polyplexes created with these vectors are too complex to fit to the simple algorithm supplied by the manufacturer, which assumes spherical particles. Furthermore, inspection of the size distribution fit depicts three slopes, which likely indicate more than one mode of motion over time and also suggest the lack of spherical shape of the particles. Previous work has shown that the morphology of DNA/polycation complexes varies with the chemical structure of the cationic vector (54). Particularly, AFM microscopy studies have shown that polyplexes created with PEGylated poly(amidoamines) exhibit both torodial and rodlike morphologies. (55) These studies also have reported the visualization of a dynamic equilibrium in the spatial conformations of the polyplexes (55). Our experiments are consistent with the potential existence of varied structures in polyplexes created with PEG-HIP in Opti-MEM and PEG-ATIII in DMEM, so an accurate assessment of particle size under these conditions cannot be made. No other polyplex/condition exhibited such bimodality. Further investigations of the size and morphology of these polyplexes via TEM studies are in progress.
Figure 3.
Particle sizes of PEG-HBP/pDNA polyplexes in various dispersants. Polyplexes were formed by adding an equal volume of PEG-HBP solution to pDNA at a charge ratio of 5 in water. Dynamic light scattering was used to measure the hydrodynamic diameter of the polyplexes at time 0 (immediately after addition of dispersant) and thereafter consecutively at 20, 40, 60, and 120 min. (a) Sizes of the polyplexes dispersed in water. (b) Sizes of the polyplexes dispersed in serum-free Opti-MEM. (c) Sizes of the polyplexes dispersed in DMEM containing 10% FBS. Overlaid bars are indicative of bimodal data. * denotes ~2 µm, ** denotes ~3 µm, † denotes ~4 µm, ‡ denotes 4.5–5 µm.
In water, the polyplex size varies between about 90 and 200 nm, which is consistent with the sizes of polyplexes created with un-PEGylated polycations (54, 56–58). Upon the addition of Opti-MEM, PEG-PF4ZIP and PEG-ATIII polyplexes did not aggregate and showed a small and uniform polyplex size over 120 min. In the presence of DMEM (containing 10% serum), all of the PEG-HBP polyplexes showed some increase in size. PEG-HIP polyplexes rapidly aggregated to almost 5 µm after 20 min. In contrast, PEG-PF4ZIP, PEG-sHIP, and PEG-RRP polyplexes yielded particles of roughly 500 nm upon initial DMEM addition that did not change significantly in size (relative to the other systems) over the course of 120 min. PEG-sHIP appears to be particularly stable in serum-containing media relative to the other PEG-HBP vectors. The utility of particles in this size range is suggested by previous studies. For example, particles as large as 200 nm can enter the endocytic pathway through clathrin- and caveolae-mediated endocytosis, and particles as large as 2 µm can enter the cell through macropinocytosis (59, 60). Additionally, it has been shown that polyplexes can enter the cell through multiple uptake pathways (61). The ability of the polyplexes to be trafficked in the nucleus may be less clear, as recent research has shown that the nuclear pore complex is capable of importing particles up to 39 nm in size (62). While this topic is still being actively investigated in in vivo systems utilizing slowly dividing cells, the scope of our current research focuses on in vitro procedures that likely allow polyplex diffusion into the nucleus during the rapid mitosis of cell lines in culture. Although the sizes of PEG-PF4ZIP, PEG-sHIP, and PEG-RRP polyplexes are somewhat larger than ideal, the size stability of these vectors over the course of 2 h evidences their promise for future studies, particularly given the low molecular weight of the PEG employed (~2700 Da per arm). The use of PEG star polymers of higher molecular weight may result in particles that are completely stable from aggregation in the presence of serum; previous studies have shown that PEG5000 is typically required to stabilize the size of polyplexes formed from PEG-PEI polymers (63).
V. Cell Viability Studies
To examine how well the PEG-HBPs perform under biological conditions, in vitro transfection studies were conducted with BHK-21 (golden Syrian hamster kidney) cells. A commonly used transfection polymer, PEI, was used as a positive control to compare the relative toxicity of the novel experimental vectors. The toxicity of polyplexes was measured via a protein assay of the cell lysates, normalized against a non-transfected control, to obtain the fraction of cell survival. This assay was performed on cells transfected both without serum (Opti-MEM) and in culture media containing 10% FBS (DMEM) to help draw conclusions about the serum stability of the complexes and their efficacy.
Figure 4a reveals the viability of BHK-21 cells transfected in Opti-MEM over a variety of charge ratios. The results show that the cell survival in the presence of PEG-PF4ZIP, PEG-HIP, and PEG-sHIP polyplexes remains high (>90%) up to a charge ratio of 15, and greater than 50% survival was found at a charge ratio of 30. PEG-sHIP shows particularly low toxicity, even at high charge ratio doses. In contrast, the polyplexes formed with PEI, PEG-RRP, and PEG-ATIII were found to be quite toxic. Notably, PEG-ATIII is extremely toxic—even more toxic than PEI, which is well-known to exhibit high cytotoxicity in many cell lines. PEG-RRP appears to have low cytotoxicity at a low charge ratio of 5, but the toxicity rapidly increases at higher doses. All three of these vectors, PEG-ATIII, PEG-RRP, and PEI, show less than 50% viability at an N/P ratio of 15 or above. Figure 4b depicts the viability of BHK-21 cells transfected in the presence of 10% serum; the data indicate the reduced toxicity of all of the vectors under these experimental conditions, which is likely to result from a simple reduction in cellular uptake of the polyplexes as a result of their larger size in serum, consistent with previous reports of decreasing cellular uptake of polycationic vectors in the presence of serum (6).
Figure 4.
Viability of BHK-21 cells after transfection with polyplexes created with PEG-HBPs in both (a) nonserum and (b) 10% serum conditions at the indicated charge ratios for 48 h. Cells were lysed after 48 h, and protein content was assayed and normalized against a non-transfected control to obtain the fraction of cell survival. Data are reported as a mean ± standard of deviation of three replicates.
The trend of toxicity among PEG-HBPs generally correlates with the cationic charge density of the peptide, with the PEG-RRP displaying greater cytotoxicity than the PEG-PF4ZIP or PEG-HIP. Other studies have also shown that the cytotoxicity of PEG-RRP is high even compared with other cationic peptides, such as antennapedia, TAT, and transportan (64), likely as a result of this high charge density (14). The RRP peptide employed in these studies has a charge density of 0.58 (7 arginines out of 12 total amino acids), while the ATIII, HIP, and PF4ZIP peptides have charge densities of 0.33, 0.31, and 0.18, respectively. The PEG-sHIP vector deviates slightly from this trend. However, the toxicity is very low and similar to PEG-HIP, which is expected given their identical composition. The unusually high toxicity of the PEG-ATIII polyplexes, despite the lower cationic charge density, most likely results from the higher hydrophobicity of the peptide (which contains two aromatic residues). This is consistent with the previously reported toxicity of positively charged and hydrophobic peptides of similar cationic charge (of about 5–7 positive charges per peptide) (65, 66). Importantly, the cell viability profiles of transfections performed with PEG-sHIP, PEG-PF4ZIP, and PEG-HIP are very promising in comparison to the unmodified PEI control and are also significantly improved relative to that reported for PEGylated PEI polymers (in which cells reveal less than 50% cell viability in transfection experiments performed above a charge ratio of 20) (17). PEG-PF4ZIP and PEG-sHIP, in particular, show extremely promising cell viability data, with at least 80% cell viability at a high charge ratio of 30, even under serum-free conditions.
VI. Transfection Efficiency Studies
To determine the overall transfection efficiency of the vectors, the PEG-HBPs were complexed with plasmids encoding the luciferase reporter gene for transfection of BHK-21 cells. A luciferase reporter gene assay was performed in both serum-free conditions (Opti-MEM) and conditions containing serum (DMEM with 10% FBS) to study the transfection efficiency of these vectors. Again, the widely used polymer, PEI, was included as a positive control, as this vector has shown high transfection efficiency despite its increased toxicity. Figure 5 depicts the results of these reporter gene assays.
Figure 5.
The transfection efficiency of PEG-HBP/pDNA polyplexes in (a) non-serum- and (b) serum-containing conditions. BHK-21 cells were transfected with polyplexes containing the reporter gene for luciferase and incubated for 48 h. A luciferase assay was performed on the cell lysates and data is displayed as RLU/mg protein. Data are reported as a mean ± standard of deviation of three replicates.
The results of transfection efficiency in Opti-MEM shows that PEG-PF4ZIP, PEG-sHIP, and PEG-RRP polyplexes demonstrated significantly higher transfection efficiencies (~1 × 108 RLU/mg protein) than PEG-HIP and PEG-ATIII polyplexes (<1 × 107 RLU/mg protein), as shown in Figure 5a. Although transfection efficiency for the PEI positive control is initially high, it was only measurable up to an N/P ratio of 10 in Opti-MEM due to the high toxicity of this vector at elevated doses, which detracts from its utility as a delivery vector. Similarly, delivery data for PEG-ATIII polyplexes in Opti-MEM and DMEM could only be obtained at maximum charge ratios of 5 and 25, respectively. Despite the apparent high transfection efficiency of PEG-ATIII, the toxicity profile is unsuitable for delivery applications, and this vector will not be discussed further.
The data in Figure 5a also indicate the similar transfection efficiencies of PEG-PF4ZIP, PEG-sHIP, and PEG-RRP polyplexes, despite the large difference in their aggregation behavior (Figure 3b), indicating that other steps in the transfection pathway such as endosomal escape and subsequent intracellular mobility play a large role in the overall transfection efficiency. It is well-documented that the intracellular travel of polyplexes likely involves acidic vesicles of the endosomal trafficking pathway (61). The KD of the HBPs with heparin measured at acidic pH values (Table 3–Table 5) suggests the potential for increased dissociation of the peptides from heparan sulfate (bound to the internal membrane of endosomal vesicles) during intracellular travel. A positive effect on the transfection efficiency pathway could result and may be due to the increase in the mobility of the pDNA once inside the cell.
In addition, these vectors showed maximum transfection efficiencies at a charge ratio of 5, suggesting the utility of these and similar vectors owing to the low toxicity (Figure 4a) and vector concentration needed to promote efficient delivery. The transfection efficiencies of PEG-RRP and PEG-PF4ZIP are nearly identical up to a charge ratio of 25, although the PEG-RRP vectors were highly toxic, and the PF4ZIP vectors showed little toxicity at the same transfection level. The PEG-sHIP exhibits similar transfection efficiency as that of PEG-PF4ZIP, with no toxicity, and a greater efficiency than PEG-HIP, consistent with the heparin binding data. Overall, the transfection assays in Opti-MEM strongly indicate the potential and enhanced utility of HBPs of specific sequences, relative to other nonspecific, high charge density polymers such as polyarginine and PEI, via the optimization of transfection efficiency per unit of cationic charge.
Comparison of the data in Figure 5a and b reveals that transfections with most of the PEG-HBPs in DMEM (Figure 5b) displayed a 10- to 100-fold reduction in efficiency when compared to transfections performed in Opti-MEM (Figure 5a). Such a decrease was not observed for PEI in these experiments. When coupled with the decrease in viability of cells transfected with PEI at increasing N/P ratios (80% at N/P 5, 40% at N/P 25), these results may suggest differences in endosomal escape, DNA protection from nucleases, and nuclear trafficking for the PEI relative to the PEG-HBPs. Engineering such responses in the PEG-HBPs could significantly improve their transfection performance in serum-containing media. In addition, the plateau in transfection efficiency of the polyplexes in DMEM did not occur until a charge ratio of approximately 20–25, which may reflect the increased amount of polymer required to sufficiently stabilize the pDNA polyplexes in medium containing serum. Indeed, the transfection efficiency of the PEG-sHIP vector, which appears to be more serum stable than the rest of the PEG-HBP vectors, plateaus at a lower charge ratio of 10, in agreement with this observation.
Taken altogether, the data from these investigations show a general correlation between the heparin binding affinity of the peptide and the transfection efficiency of the PEG-HBP conjugate. The heparin affinity chromatography and SPR experiments (Table 2) indicated heparin binding affinity in the order RRP > PF4ZIP ≈ sHIP > HIP > ATIII, which is very similar to the trend in transfection efficiency in Opti-MEM. That is, PEG-RRP, PEG-sHIP, and PEG-PF4ZIP show significantly increased transfection efficiencies over the PEG-HIP and PEG-ATIII vectors. This correlation suggests the importance of cell-surface association with glycosaminoglycans in the trafficking of these vectors, promoting enhanced cellular uptake by increasing polyplex–glycosaminoglycan affinity. To further examine specific events involving cellular binding and internalization, we investigated the cellular uptake of DNA via flow cytometry.
VII. Cellular Internalization Studies
To more specifically concentrate on the effect of cell-surface binding and internalization of the PEG-HBPs, flow cytometry was used to measure the amount of DNA internalized by transfected cells. BHK-21 cells were transfected with Cy5-labeled pDNA and subjected to analysis via flow cytometry. An N/P ratio of 5 was chosen, as it showed maximum transfection efficiency for the vectors in serum-free media. In all samples, greater than 80% of cells exhibited Cy5 fluorescence (data not shown). Figure 6 depicts the results of these internalization studies.
Figure 6.
The cellular uptake of Cy5-labeled DNA via polyplexes in non-serum-containing media. BHK-21 cells were transfected with polyplexes formulated with Cy5-labled DNA. Four hours after transfection, the cells were washed well with PBS and allowed to traffic for 2 more hours. The cells were then trypsinzed and analyzed via flow cytometry. Each sample represents the mean fluorescence in 50 000 cells.
The trend in cellular internalization of Cy5-labeled pDNA among PEG-HBPs (PEG-RRP > PEG-PF4ZIP > PEG-sHIP > PEG-HIP ≈ PEG-ATIII) is almost identical to the observed trend in heparin affinity (RRP > PF4ZIP ≈ sHIP > HIP > ATIII). Furthermore, the increased heparin binding affinity of sHIP versus HIP is reflected in the internalization experiments. Interestingly, PEG-ATIII, which has been shown to give stable polyplexes in non-serum-containing media (Figure 3b), shows very low cellular uptake. This result is likely due to the lowered affinity of this peptide for heparin (Table 2) and further illustrates the utility of cell-surface binding peptides for controlling internalization.
These cellular uptake results also corroborate the trend in luciferase gene expression and further substantiate our hypothesis that enhanced cell binding increases cellular delivery. Furthermore, the trend in the magnitude of internalization generally correlates with gene expression; for example, the internalization and transfection efficiency of PEG-RRP and PEG-PF4ZIP are about 1 order of magnitude higher than those of PEG-HIP. This may be due to the combined effect of the decreased polyplex stability (Figure 3b) and heparin binding affinity (Table 2) of the HIP peptide.
The two strongest binding PEG-HBPs, PF4ZIP and RRP, were internalized to a larger degree than the positive control, PEI, despite the higher heparin binding affinity of PEI. These data lend further confidence to our proposal that the multivalent star architecture of the PEG-star HBPs allows simultaneous binding of DNA at the core of the polyplex and heparan sulfate at the surface of the cell. Conversely, the PEI-DNA polyplex, dependent on cooperative interactions between the polymers to remain intact, may be more susceptible to competition with extracellular polyanions such as heparan sulfate. PEI from the surface of the polyplex may bind with heparan sulfate at the surface of the cell in a cooperative manner, leading to extracellular unpackaging of DNA. Clearly, the molecular architecture of the polymeric ligand, as well as the heparin binding affinity of the cationic moiety plays a large role in the cell-surface kinetics and internalization pathways of the polyplexes. It should be noted that although the cellular uptake of PEG-RRP and PEG-PF4ZIP was higher than that of PEI (Figure 6) their gene expression profiles were still lower than observed with PEI (Figure 5). This suggests that endosomal escape could be a barrier for the PEG-HBP vectors.
CONCLUSIONS
Five PEG-HBPs, capable of multivalent interactions with polyanionic biological molecules, have been shown to bind both heparin and plasmid DNA sufficiently well to make them useful for a variety of applications. Examination of dynamic light scattering data revealed that three polyplexes, produced with PEG-RRP, PEG-sHIP, and PEG-PF4ZIP, had notable stability against aggregation in cell culture media containing 10% serum. Transfection studies complemented these results and showed that these vectors also appear to have significant transfection efficiency in BHK-21 cells under both conditions. With the exception of the PEG-ATIII polyplex (which is very cytotoxic), increased heparin affinity correlated with increased transfection efficiency. This correlation was further indicated in the relationship between heparin affinity and cellular uptake. Most clearly, this is evidenced by the increased cellular uptake of PEG-sHIP polyplexes relative to those prepared with PEG-HIP; these are compositionally identical peptides which differ only in that the sHIP sequence demonstrated higher heparin affinity than the nonscrambled HIP sequence. These results suggest that cell-surface binding and uptake, mediated by heparan sulfate, play a large role in the overall transfection pathway of these vectors.
Comparison of polyplexes created with PEG-PF4ZIP and charge-dense polyplexes (PEG-RRP and PEI) demonstrates that an increase in heparin affinity alone can confer improved transfection efficiency without requiring an increase in the charge density and resulting toxicity. Furthermore, the PEG-HBPs which bind heparin mostly strongly, PEG-RRP and PEG-PF4ZIP, have enhanced cellular uptake compared to PEI, whose charge density is much higher than any of the PEG conjugates. These data corroborate our proposal that the multivalent presentation of peptides permit simultaneous interaction between DNA at the core of the polyplex and heparan sulfate at the surface of the cell without disruption of the polyplex, enhancing cellular uptake. Given the flexibility of synthetic strategies for producing PEG-HBPs with varying architectures and peptide identity, these results suggest that the star PEG-HBPs are promising and versatile candidates as gene delivery vectors.
ACKNOWLEDGMENTS
We gratefully acknowledge the National Institutes of Health (1-R01-EB003172-1) and the Arnold and Mabel Beckman Foundation for partial funding of the synthetic aspects of this project (KLK and LZ). TMR and KMF thank the Arnold and Mabel Beckman Foundation and the Department of Chemistry at the University of Cincinnati for partial funding of the biological studies performed herein. Additionally, we recognize and thank Nori Yamaguchi for assistance with the synthesis of some PEG–peptide conjugates and Yemin Liu for performing some of the gel-shift assays.
Footnotes
Supporting Information Available: Experimental details of the synthesis and characterization of PEG-RRP, heparin affinity studies, DNA binding assays, and CD spectra peptide–DNA complexes. This material is available free of charge via the Internet at http://pubs.acs.org.
LITERATURE CITED
- 1.Akhtar S. Non-viral cancer gene therapy: Beyond delivery. Gene Ther. 2006;13:739–740. doi: 10.1038/sj.gt.3302692. [DOI] [PubMed] [Google Scholar]
- 2.Reineke TM, Grinstaff MW. Designer materials for nucleic acid delivery. MRS Bull. 2005;30:635–638. [Google Scholar]
- 3.Deshpande MC, Davies MC, Garnett MC, Williams PM, Armitage D, Bailey L, Vamvakaki M, Armes SP, Stolnik S. The effect of poly(ethylene glycol molecular architecture on cellular interaction and uptake of DNA complexes. J. Controlled Release. 2004;97:143–156. doi: 10.1016/j.jconrel.2004.02.019. [DOI] [PubMed] [Google Scholar]
- 4.Liu Y, Wenning L, Lynch M, Reineke TM. New poly(D-glucaramidoamine)s induce DNA nanoparticle formation and efficient gene delivery into mammalian cells. J. Am. Chem. Soc. 2004;126:7422–7423. doi: 10.1021/ja049831l. [DOI] [PubMed] [Google Scholar]
- 5.Liu YM, Reineke TM. Hydroxyl stereochemistry and amine number within poly(glycoamidoamine)s affect intracellular DNA delivery. J. Am. Chem. Soc. 2005;127:3004–3015. doi: 10.1021/ja0436446. [DOI] [PubMed] [Google Scholar]
- 6.Liu Y, Reineke TM. Poly(glycoamidoamine)s for gene delivery. Stability of polyplexes and efficacy with cardiomyoblast cells. Bioconjugate Chem. 2006;17:101–108. doi: 10.1021/bc050275+. [DOI] [PubMed] [Google Scholar]
- 7.Liu Y, Wenning L, Lynch M, Reineke TM. Gene delivery with novel poly(L-tartaramidoamine)s. In: Svenson S, editor. Polymeric Drug Delivery Volume I-Particulate Drug Carriers. Washington, DC: American Chemical Society; 2006. [Google Scholar]
- 8.van de Wetering P, Moret EE, Schuurmans-Nieuwenbroek NME, van Steenbergen MJ, Hennink WE. Structure-activity relationships of water-soluble cationic methacrylate/methacrylamide polymers for nonviral gene delivery. Bioconjugate Chem. 1999;10:589–597. doi: 10.1021/bc980148w. [DOI] [PubMed] [Google Scholar]
- 9.Davis ME. Non-viral gene delivery systems. Curr. Opin. Biotechnol. 2002;13:128–131. doi: 10.1016/s0958-1669(02)00294-x. [DOI] [PubMed] [Google Scholar]
- 10.Pouton CW, Seymour LW. Key issues in non-viral gene delivery. Adv. Drug Delivery Rev. 1998;34:3–19. doi: 10.1016/s0169-409x(98)00048-9. [DOI] [PubMed] [Google Scholar]
- 11.Collard WT, Yang Y, Kwok KY, Park Y, Rice KG. Biodistribution, metabolism, and in vivo gene expression of low molecular weight glycopeptide polyethylene glycol peptide DNA co-condensates. J. Pharm. Sci. 2000;89:499–512. doi: 10.1002/(SICI)1520-6017(200004)89:4<499::AID-JPS7>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 12.Dash PR, Read ML, Barrett LB, Wolfert MA, Seymour LW. Factors affecting blood clearance and in vivo distribution of polyelectrolyte complexes for gene delivery. Gene Ther. 1999;6:643–650. doi: 10.1038/sj.gt.3300843. [DOI] [PubMed] [Google Scholar]
- 13.Verbaan FJ, Oussoren C, van Dam IM, Takakura Y, Hashida M, Crommelin DJ, Hennink WE, Storm G. The fate of poly (2-dimethyl amino ethyl)methacrylate-based polyplexes after intravenous administration. Int. J. Pharm. 2001;214:99–101. doi: 10.1016/s0378-5173(00)00642-6. [DOI] [PubMed] [Google Scholar]
- 14.Fischer D, Li Y, Ahlemeyer B, Krieglstein J, Kissel T. In vitro cytotoxicity testing of polycations: influence of polymer structure on cell viability and hemolysis. Biomaterials. 2003;24:1121–1131. doi: 10.1016/s0142-9612(02)00445-3. [DOI] [PubMed] [Google Scholar]
- 15.Kabanov AV, Vinogradov SV, Suzdaltseva YG, Alakhov VY. Water-soluble block polycations as carriers for oligonucleotide delivery. Bioconjugate Chem. 1995;6:639–643. doi: 10.1021/bc00036a001. [DOI] [PubMed] [Google Scholar]
- 16.Vinogradov SV, Bronich TK, Kabanov AV. Self-assembly of polyamine-poly(ethylene glycol) copolymers with phosphorothioate oligonucleotides. Bioconjugate Chem. 1998;9:805–812. doi: 10.1021/bc980048q. [DOI] [PubMed] [Google Scholar]
- 17.Sung S, Min SH, Cho KY, Lee S, Min Y, Yeom YI, Park J. Effect of polyethylene glycol on gene delivery of polyethylenimine. Biol. Pharm. Bull. 2003;26:492–500. doi: 10.1248/bpb.26.492. [DOI] [PubMed] [Google Scholar]
- 18.Ahn C, Chae SY, Bae YH, Kim SW. Synthesis of biodegradable multi-block copolymers of poly(L-lysine) and poly-(ethylene glycol) as non-viral gene carrier. J. Controlled Release. 2004;97:567–574. doi: 10.1016/j.jconrel.2004.04.002. [DOI] [PubMed] [Google Scholar]
- 19.Toncheva V, Wolfert MA, Dash PR, Oupicky D, Ulbrich K, Seymour LW, Schacht EH. Novel vectors for gene delivery formed by self-assembly of DNA with poly(L-lysine) grafted with hydrophilic polymers. Biochim. Biophys. Acta. 1998;1380:354–368. doi: 10.1016/s0304-4165(98)00004-x. [DOI] [PubMed] [Google Scholar]
- 20.Männistö M, Vanderkerken S, Toncheva V, Elomaa M, Ruponen M, Schacht E, Urtti A. Structure-activity relationships of poly(L-lysines): effects of pegylation and molecular shape on physicochemical and biological properties in gene delivery. J. Controlled Release. 2002;83:169–182. doi: 10.1016/s0168-3659(02)00178-5. [DOI] [PubMed] [Google Scholar]
- 21.Erbacher P, Bettinger T, Brion E, Coll J, Plank C, Behr J, Remy J. Genuine DNA/polyethylenimine (PEI) complexes improve transfection properties and cell survival. J. Drug Targeting. 2004;12:223–236. doi: 10.1080/10611860410001723487. [DOI] [PubMed] [Google Scholar]
- 22.Shuai X, Merdan T, Unger F, Kissel T. Supramolecular gene delivery vectors showing enhanced transgene expression and good biocompatibility. Bioconjugate Chem. 2005;16:322–329. doi: 10.1021/bc0498471. [DOI] [PubMed] [Google Scholar]
- 23.Wang L, Wang J, Dong C. Supramolecular inclusion complexes of star-shaped poly(ε-caprolactone) with α-cyclodextrin. J. Polym. Sci., Part A: Polym. Chem. 2005;43:4721–4730. [Google Scholar]
- 24.Ooya T, Choi HS, Yamashita A, Yui N, Sugaya Y, Kano A, Maruyama A, Akita H, Ito R, Kogure K, Harashima H. Biocleavable polyrotaxane-plasmid DNA polyplex for enhanced gene delivery. J. Am. Chem. Soc. 2006;128:3852–3853. doi: 10.1021/ja055868+. [DOI] [PubMed] [Google Scholar]
- 25.Petersen H, Kunath K, Martin AL, Stolnik S, Roberts CJ, Davies MC, Kissel T. Star-shaped poly(ethylene glycol)-block-polyethylene copolymers enhance DNA condensation of low moleular weight polyethylenimines. Biomacromolecules. 2002;3:926–936. doi: 10.1021/bm025539z. [DOI] [PubMed] [Google Scholar]
- 26.Mislick KA, Baldeschwieler JD. Evidence for the role of proteoglycans in cation-mediated gene transfer. Proc. Natl. Acad. Sci. U.S.A. 1996;93:12349–12354. doi: 10.1073/pnas.93.22.12349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kopatz I, Remy JS, Behr JP. A model for non-viral gene delivery: through syndecan adhesion molecules and powered by actin. J. Gene Med. 2004;6:769–776. doi: 10.1002/jgm.558. [DOI] [PubMed] [Google Scholar]
- 28.Koping-Hoggard M, Varum KM, Issa M, Danielsen S, Christensen BE, Stokke BT, Artursson P. Improved chitosan-mediated gene delivery based on easily dissociated chitosan polyplexes of highly defined chitosan oligomers. Gene Ther. 2004;11:1441–1452. doi: 10.1038/sj.gt.3302312. [DOI] [PubMed] [Google Scholar]
- 29.Liu S, Zhou F, Hook M, Carson DD. A heparin-binding synthetic peptide of heparin/heparan sulfate-interacting protein modulates blood coagulation activities. Proc. Natl. Acad. Sci. U.S.A. 1997;94:1739–1744. doi: 10.1073/pnas.94.5.1739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ibel K, Poland GA, Baldwin JP, Pepper DS, Luscombe M, Holbrook JJ. Low-resolution structure of the complex of human blood platelet factor 4 with heparin determined by small-angle neutron scattering. Biochim. Biophys. Acta. 1986;870:58–63. doi: 10.1016/0167-4838(86)90008-7. [DOI] [PubMed] [Google Scholar]
- 31.Fromm JR, Hileman RE, Caldwell EEO, Weiler JM, Linhardt RJ. Differences in the interaction of heparin with arginine and lysine and the importance of these basic amino acids in the binding of heparin to acidic fibroblast growth factor. Arch. Biochem. Biophys. 1995;323:279–287. doi: 10.1006/abbi.1995.9963. [DOI] [PubMed] [Google Scholar]
- 32.Caldwell EEO, Nadkarni VD, Fromm JR, Linhardt RJ, Weiler JM. Importance of specific amino acids in protein binding sites for heparin and heparan sulfate. Int. J. Biochem. Cell Biol. 1996;28:203–216. doi: 10.1016/1357-2725(95)00123-9. [DOI] [PubMed] [Google Scholar]
- 33.Seal BL, Panitch A. Viscoelastic behavior of environmentally sensitive biomimetic polymer matrices. Macromolecules. 2006;39:2268–2274. [Google Scholar]
- 34.Seal BL, Panitch A. Physical polymer matrices based on affinity interactions between peptides and polysaccharides. Biomacromolecules. 2003;4:1572–1582. doi: 10.1021/bm0342032. [DOI] [PubMed] [Google Scholar]
- 35.Yamaguchi N, Chae BS, Zhang L, Kiick KL, Furst EM. Rheological characterization of polysaccharide-poly-(ethylene glycol) star copolymer hydrogels. Biomacromolecules. 2005;6:1931–1940. doi: 10.1021/bm0500042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yamaguchi N, Kiick KL. Polysaccharide-poly-(ethylene glycol) star copolymer as a scaffold for the production of bioactive hydrogels. Biomacromolecules. 2005;6:1921–1930. doi: 10.1021/bm050003+. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang L, Furst EM, Kiick KL. Manipulation of hydrogel assembly and growth factor delivery via the use of peptide-polysaccharide interactions. J. Controlled Release. 2006;114:130–142. doi: 10.1016/j.jconrel.2006.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tyler-Cross R, Sobel M, McAdory LE, Harris RB. Structure-function relations of antithrombin III-heparin interactions as assessed by biophysical and biological assays and molecular modeling of peptide-pentasaccharide-docked complexes. Arch. Biochem. Biophys. 1996;334:206–213. doi: 10.1006/abbi.1996.0448. [DOI] [PubMed] [Google Scholar]
- 39.Liu S, Smith SE, Julian J, Rohde LH, Karin NJ, Carson DD. cDNA cloning and expression of HIP, a novel cell surface heparan sulfate/heparin-binding protein of human uterine epithelial cells and cell lines. J. Biol. Chem. 1996;271:11817–11823. doi: 10.1074/jbc.271.20.11817. [DOI] [PubMed] [Google Scholar]
- 40.Butcher DJ, Kowalska MA, Li S, Luo ZW, Shan SM, Lu ZX, Niewiarowski S, Huang ZW. A natural motif approach to protein design: A synthetic leucine zipper peptide mimics the biological function of the platelet factor 4 protein. FEBS Lett. 1997;409:183–187. doi: 10.1016/s0014-5793(97)00504-8. [DOI] [PubMed] [Google Scholar]
- 41.Spillmann D, Lindahl U. Glycosaminoglycan protein interactions-a question of specificity. Curr. Opin. Struct. Biol. 1994;4:677–682. doi: 10.1016/j.sbi.2017.12.011. [DOI] [PubMed] [Google Scholar]
- 42.Grifman M, Trepel M, Speece P, Gilbert LB, Arap W, Pasqualini R, Weitzman MD. Incorporation of tumor-targeting peptides into recombinant adeno-associated virus capsids. Mol. Ther. 2001;3:964–975. doi: 10.1006/mthe.2001.0345. [DOI] [PubMed] [Google Scholar]
- 43.Wickham TJ, Roelvink PW, Brough DE, Kovesdi I. Adenovirus targeted to heparan-containing receptors increases its gene delivery efficiency to multiple cell types. Nat. Biotechnol. 1996;14:1570–1573. doi: 10.1038/nbt1196-1570. [DOI] [PubMed] [Google Scholar]
- 44.Wickham TJ, Tzeng E, Shears LL, Roelvink PW, Li Y, Lee GM, Brough DE, Lizonova A, Kovesdi I. Increased in vitro and in vivo gene transfer by adenovirus vectors containing chimeric fiber proteins. J. Virol. 1997;71:8221–8229. doi: 10.1128/jvi.71.11.8221-8229.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Capila I, Linhardt RJ. Heparin-protein interactions. Angew. Chem., Int. Ed. 2002;41:390–412. doi: 10.1002/1521-3773(20020201)41:3<390::aid-anie390>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 46.Plank C, Tang MX, Wolfe AR, Szoka FC. Branched cationic peptides for gene delivery: Role of type and number of cationic residues in formation and in vitro activity of DNA polyplexes. Hum. Gene Ther. 1999;10:319–332. doi: 10.1089/10430349950019101. [DOI] [PubMed] [Google Scholar]
- 47.Lutolf MP, Tirelli N, Cerritelli S, Cavelli L, Hubbel JA. Systematic modulation of Michael-type reactivity of thiols through the use of charged amino acids. Bioconjugate Chem. 2001;12:1051–1056. doi: 10.1021/bc015519e. [DOI] [PubMed] [Google Scholar]
- 48.Bartsch M, Weeke-Klimp AH, Meijer DKF, Scherphof GL, Kamps J. Cell-specific targeting of lipid-based carriers for ODN and DNA. J. Liposome Res. 2005;15:59–92. doi: 10.1081/lpr-64961. [DOI] [PubMed] [Google Scholar]
- 49.Nishikawa M. Development of cell-specific targeting systems for drugs and genes. Biol. Pharm. Bull. 2005;28:195–200. doi: 10.1248/bpb.28.195. [DOI] [PubMed] [Google Scholar]
- 50.Lundquist JJ, Toone EJ. The cluster glycoside effect. Chem. Rev. 2002;102:555–578. doi: 10.1021/cr000418f. [DOI] [PubMed] [Google Scholar]
- 51.Mukherjee S, Ghosh RN, Maxfield FR. Endocytosis. Phys. Rev. 1997;77:759–803. doi: 10.1152/physrev.1997.77.3.759. [DOI] [PubMed] [Google Scholar]
- 52.Lee M, Han S, Ko KS, Koh JJ, Park JS, Yoon JW, Kim SW. Repression of GAD autoantigen expression in pancreas β-cells by delivery of antisense plasmid/PEG-g-PLL complex. Mol. Ther. 2001;4:339–346. doi: 10.1006/mthe.2001.0458. [DOI] [PubMed] [Google Scholar]
- 53.Gonçalves E, Kitas E, Seelig J. Structural and thermodynamic aspects of the interaction between heparan sulfate and analogues of melittin. Biochemistry. 2006;45:3086–3094. doi: 10.1021/bi052221t. [DOI] [PubMed] [Google Scholar]
- 54.Vijayanathan V, Thomas T, Antony T, Shirahata A, Thomas TJ. Formation of DNA nanoparticles in the presence of novel polyamine analogues: a laser light scattering and atomic force microscopic study. Nucleic Acids Res. 2004;32:127–134. doi: 10.1093/nar/gkg936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Martin AL, Davies MC, Rackstraw BJ, Roberts CJ, Stolnik S, Tendler SJB, Williams PM. Observation of DNA-polymer condensate formation in real time at a molecular level. FEBS Lett. 2000;480:106–112. doi: 10.1016/s0014-5793(00)01894-9. [DOI] [PubMed] [Google Scholar]
- 56.Bötcher C, Endisch C, Fuhrhop J, Catterall C, Eaton M. High yield preparation of oligomeric C-type DNA toroids and their characterization by cryoelectron microscopy. J. Am. Chem. Soc. 1998;120:12–17. [Google Scholar]
- 57.Arscott P, Li A, Bloomfield V. Condensation of DNA by trivalent cations. 1. effects of DNA length and topology on the size and shape of condensed particles. Biopolymers. 1990;30:619–630. doi: 10.1002/bip.360300514. [DOI] [PubMed] [Google Scholar]
- 58.Lin Z, Wang C, Feng X, Liu M, Li J, Bai C. The observation of the local ordering characteristics of spermidine-condensed DNA: atomic force microscopy and polarizing microscopy studies. Nucleic Acids Res. 1998;26:3228–3234. doi: 10.1093/nar/26.13.3228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Johannes L, Lamaze C. Clathrin-dependent or not: is it still the question? Traffic. 2002;3:443–451. doi: 10.1034/j.1600-0854.2002.30701.x. [DOI] [PubMed] [Google Scholar]
- 60.Rejman J, Oberle V, Zuhorn IS, Hoekstra D. Size dependent internalization of particles via the pathways of clathrin- and caveolae-mediated endocytosis. Biochem. J. 2004;377:159–169. doi: 10.1042/BJ20031253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Medina-Kauwe LK, Xie J, Hamm-Alvarez S. Intracellular trafficking of nonviral vectors. Gene Ther. 2005;12:1734–1751. doi: 10.1038/sj.gt.3302592. [DOI] [PubMed] [Google Scholar]
- 62.Pante N, Kann M. Nuclear pore complex is able to transport macromolecules with diameters of about 39mm. Mol. Biol. Cell. 2002;13:425–434. doi: 10.1091/mbc.01-06-0308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Glodde M, Sirsi SR, Lutz GJ. Physiochemical properties of low and high molecular weight poly(ethylene glycol)-grafted poly(ethylene imine) copolymers and their complexes with oligonucleotides. Biomacromolecules. 2006;7:347–356. doi: 10.1021/bm050726t. [DOI] [PubMed] [Google Scholar]
- 64.Jones SW, Christison R, Bundell K, Voyce CJ, Brockbank SMV, Newham P, Lindsay MA. Characterization of cell-penetrating peptide mediated peptide delivery. Br. J. Pharmacol. 2005;145:1093–1102. doi: 10.1038/sj.bjp.0706279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yang N, Strom MB, Mekonnen SM, Svendsen JS, Oystein R. The effects of shortening lactoferrin derived peptides against tumour cells, bacteria, and normal human cells. J. Pept. Sci. 2004;10:37–46. doi: 10.1002/psc.470. [DOI] [PubMed] [Google Scholar]
- 66.Zhang S, Xu Y, Wang B, Qiao W, Liu D, Li Z. Cationic compounds used in lipoplexes and polyplexes for gene delivery. J. Controlled Release. 2004;100:165–180. doi: 10.1016/j.jconrel.2004.08.019. [DOI] [PubMed] [Google Scholar]