Abstract
Background
Scant and equivocal research exists examining the effects of button-pressing on P300. Button-pressing may decrease P300 latency and amplitude. The melding of motor potentials and P300 may also confound studies of P300 topography, such as studies of temporal scalp-area asymmetries in schizophrenia.
Method
P300 was measured on button-press and silent-count tasks in control subjects. An estimate of motor activity was constructed from a simple reaction time task, with reaction times matched to the button-press task. The motor estimate was subtracted from the button-press P300 to assess Kok’s (1988) additive model. Lastly, lateral P300 from schizophrenia patients was compared with each condition’s P300.
Results
P300 was smaller and its topography different in the button-pressing task relative to silent-counting. The motor-correction procedure generated a P300 with normal topography. Comparison of the button-press P300 in controls to the silent-count P300 in schizophrenia patients reduced a significant lateral asymmetry to trend level. This asymmetry was significant after the correction procedure.
Conclusions
Button-pressing generates smaller P300 than silent-counting. Also, P300 topography in button-pressing tasks is confounded by motor potentials. The distortion can be corrected with a motor potential estimate. Motor potentials can occlude differences in P300 topography between groups.
Keywords: Button-press, Correction procedure, Event-related potential, Movement-related potential, P300, Topography
1. Introduction
The P300 event-related potential (ERP) is a positive potential that occurs approximately 300 ms after an infer-quently presented stimulus requiring detection, counting, or cognitive processing. One commonly used task is the auditory ‘oddball’ paradigm, which elicits P300 in response to rarely presented target pure tones interspersed among more frequent pure tone standards. The experimenter must decide whether to actively monitor the target-detection accuracy and reaction times (RTs) of the subjects through the use of a button-press, which provides thorough data regarding task performance, but may introduce movement-related artifacts to the ERP. Alternately, the experimenter may decide to rely on a verbal report of the covert silent-counting of the subject, which lacks the precision in behavioral monitoring of the button-press task.
Motoric response to a stimulus causes the occurrence of distinct movement-related potentials (MRPs). When a specific motor response is required, there will be response selection- and execution-related fields that arise. The temporal occurrence of these MRPs will have a lawful relation to the occurrence of the movement, and thus the MRPs can be thought of as being reverse time-locked to the button-press (Kornhuber and Deecke, 1965; Vaughan et al., 1968). Importantly, the P300 field is usually coincident with the movement or precedes it. Thus, these MRPs will distort the P300 field (see especially Kok, 1988). Since scalp potentials only reflect the sum of the electrical activity propagated to a given area, this motor negativity is not immediately distinguishable from the activity of P300 generators.
These MRPs include several components that precede the actual movement by several hundred milliseconds (Starr et al., 1995), and culminate with the actual cortico-spinal signals to move the finger (the motor-field). These components appear to arise in a progression from middle frontal gyrus working-memory areas, supplementary motor cortex, premotor cortex, and motor cortex (Ikeda et al., 1995; Kris-teva-Feige et al., 1997; Pedersen et al., 1998). The earliest MRPs are not necessarily lateralized, presumably arising in supplementary motor cortex and/or premotor cortex (e.g. Miller and Hackley, 1992). The lateralized readiness potential (LRP) or negative slope component, by contrast, is largest over the contralateral motor area, in close temporal proximity to the actual movement (Libet et al., 1982), beginning some 300 ms before and peaking approximately 100 ms or less before the movement (e.g. Miller and Ulrich, 1998; Hackley and Valle-Inclán, 1999). The later MRPs will present the greatest problem for P300 topography, because they arise simultaneously with P300. The LRP will be lateralized based on the responding hand, which is a severe confound for P300 topography if the same (e.g. dominant) hand is used to respond throughout the oddball task.
The precise effects of button-pressing on P300 elicited by pure tones are not well known. Results have been equivocal and contradictory. It is likely that the use of a button-pressing strategy results in an earlier and smaller P300 along the midline relative to a silent-counting strategy, as has been reported by Polich (1987) and Barret et al. (1987). However, Starr et al. (1995, 1997) reported no difference in P300 amplitude between button-pressing and silent-counting. To the degree that P300 amplitude reflects the amount of attentional resources devoted to task performance (e.g. Johnson, 1986), it is reasonable that P300 might be smaller on button-pressing tasks, since the subject does not need to maintain a representation of the current count. The alternative explanation that P300 reduction on a button-pressing task is related to an increase in task demands that exceeds attentional resources is untenable. The decrease of P300 latency on button-pressing tasks is not consistent with an increased cognitive load, since increased loads increase P300 latency (e.g. Salisbury et al., 1994). It is more likely that more cognitive resources are necessary when subjects silently count oddball tones, presumably related to the additional task demand of maintaining the accurate count. Although P300 amplitude becomes smaller as task difficulty or complexity exceeds attentional resources (e.g. Isreal et al., 1979), moderate increases in task demands well within the subject’s capabilities should increase P300 as the subject devotes more resources to the task.
In addition to a reduction in P300 amplitude on a button-press task due to resource allocation secondary to the motor act, the button-press motor response may directly affect the amplitude reduction through the melding of the MRPs described above with coincident P300 activity. These MRPs are known to be lateralized, and thus may be especially salient in studies of P300 topography. Although there is a general consensus that the presence of a motoric response in a go–nogo type paradigm reduces P300, presumably as a function of a general motor-related negativity (e.g. Pfefferbaum et al., 1985), to our knowledge a direct investigation of motor effects on widespread P300 topography elicited by pure tone stimuli has not been conducted. Barret et al. (1987) examined P300 topography from 4 lateral sites and reported greater amplitude reduction over the coronal midline site contralateral to the responding hand (C3). For the P300 elicited by complex stimuli (square waves perceived as fundamental frequencies and related harmonics), Kayser et al. (1998) showed that motor responses affected widespread P300 topography. Notably, the effects attributed to each hand were not the same. This point is of some importance for studies that attempt to cancel such motoric effects by counter-balancing response hands. From that same group, Tenke et al. (1998) showed that the use of motor responses generated a different set of sources and sinks as revealed by current-source density (CSD) transforms of the raw ERP data, and again that these sources and sinks were different for each hand.
This lack of research into the effects of button-pressing on widespread P300 scalp topography is more than trivial. As more dense recording arrays have been used to record the voltage field across the scalp, auditory P300 topography has been used to identify localized changes and regional abnormalities in P300 under different tasks or in different clinical populations. For example, in addition to a general overall amplitude reduction, P300 to pure tones has been reported to show left-lateralized reductions in schizophrenia (Faux et al., 1993; McCarley et al., 1993; Salisbury et al., 1998, 1999). This abnormality in P300 topography is associated with the size of a cortical area thought to contain one putative P300 generator, the left posterior superior temporal gyrus, and with the severity of thought disorder (Shenton et al., 1992; McCarley et al., 1993).
However, such an asymmetry in the P300 elicited by pure tones has not been found by other researchers (e.g. Pfefferbaum et al., 1989), leading to debate in the literature as to whether it is in fact a robust and consistent finding (see McCarley et al., 1991; Pfefferbaum et al., 1991). This discrepancy suggests that methodological factors, such as response mode, may affect the P300 topography, particularly after taking into account that most studies finding a left less than right P300 asymmetry in schizophrenia used silent-counts, whereas most that did not used button-presses (Bruder et al., 1996).
There have been suggestions for altering paradigms in order to remove the negativity contributed by the MRPs. An attempt to remove this artifact by counter-balancing response hand across subjects may not represent a workable solution. This method purports to cancel out the contralateral negativity concomitant with button-pressing by having half of the subjects respond with a left finger movement and half with a right finger movement. This approach presumes to treat the negativity associated with the MRPs from both sinistral and dextral button-presses as non-random, time-locked, and opposite polarity variation, which would not be the case if RTs differed between response hands. RT is generally longer for the non-dominant hand, so the MRPs for responses using different hands will occur at slightly different latencies. (A huge neuropsychological literature indicates such an RT difference between dominant and non-dominant hands, e.g. Purdue pegboard, finger-tapping.) Counter-balancing response hand thus introduces non-random time-locked variance with different latencies from each hand, since left- and right-hand MRPs are misaligned in time. In other words, MRPs from right-handed responses will be out of phase with the left-handed responses when they are averaged together. Rather than canceling out MRP-related variation, counter-balancing response hand across subjects introduces non-random variance due to the temporal misalignment of MRPs, which results in a wave-form containing even greater contamination. Also, the morphology of MRPs for each hand is not the same. Kutas and Donchin (1977) clearly demonstrated that the MRPs associated with dominant and non-dominant response hands showed different degrees of asymmetry, with the dominant hand MRP more lateralized. In addition, as indicated earlier, Kayser et al. (1998) using principal component analysis (PCA) and Tenke et al. (1998) using CSD transforms showed that the variance and source/sink activity, respectively, attributed to the responses with the left and right hand were not identical. PCA has been used to isolate a factor presumably reflecting P300 in several studies, but this approach has not gained universal acceptance, and if the response occurs only to targets it is unclear whether such a maneuver would isolate P300 without some contribution of motor-related variance.
Kok (1988) proposed an additive model to explain the effect of MRPs on P300. Using Kok’s terminology the model can be stated thus: true P300 = go P300 – MRP. If the actual activity related to P300 is equivalent to subtracting the MRP from the button-press P300, then a promising method for removing MRP contamination of P300 is to create a correction waveform of MRP activity that can be subtracted from the button-press P300 ERP waveform. Such a method has the advantage of being straightforward and easily comprehended. For example, Salisbury et al. (1994) formed a correction waveform by presenting a train of stimuli containing 100% target stimuli, identical to the target stimuli in a second oddball task, to which the subjects responded (simple reaction time (SRT) task). Thus, since the subject pressed a button to the tone on every trial, the correction waveform did not show a P300 and provided a relatively clean estimate of the MRP associated with the button-press (in addition to the early sensory potentials). By subtracting this correction waveform from the oddball target waveform, Salisbury et al. (1994) suggested that the MRP contamination would be eradicated. However, this particular method is limited. In cases where the subject is pressing a button to every tone, the RT steadily decreases, so that although the same hand is used to respond, the majority of MRPs on the correction task will occur with shorter latencies than those to the oddballs. Thus, the MRPs will have generally shorter latencies when averaged to form a corrective subtraction waveform. Once again, the misalignment of the MRPs in time will in fact distort the waveform further, rather than correct it. In addition, response selection processes may be significantly different for the oddball and the SRT tasks. For example, Miller and Ulrich (1998) and Hackley and Valle-Inclán (1999) showed that the intervals from the stimulus onset to LRP onset increased with the number of alternative responses in a forced-choice task, presumably reflecting a longer response selection stage of processing. In addition, Miller and Ulrich (1998) suggested that the interval from the LRP onset to movement was affected by the number of choices (from two to 6). It is unclear whether the response selection or response execution phases of the MRP would significantly differ in an SRT task (correction procedure) vs. an oddball task. As in Kok (1988), we assume that the motor processes on an SRT are roughly equivalent to a go trial. However, despite any differences in response selection processes and related MRPs, an SRT-derived correction waveform should provide a good estimate of the motor-field, the actual signal that arises in the motor cortex to move the finger, which is the primary culprit in distorting the P300 field and which should not differ much between tasks.
In this study, the effect of button-pressing on P300 to pure tones was assessed relative to a silent-counting task. The first aim of the study was to determine whether the act of button-pressing led to a general reduction of the P300 amplitude. The second aim of the study was to determine whether button-pressing significantly altered P300 scalp topography, and if so what the spatial extent of such alteration was. The third aim of this study was to assess whether an estimate of MRP activity that matched RTs between the button-pressing P300 task and the SRT task could obviate the effects of button-pressing. Lastly, the potential confounding effects of button-pressing in obscuring differences in lateral topography between schizophrenia patients and controls was assessed.
2. Method
2.1. Subjects
The 60 paid normal control participants in this study were recruited through newspaper advertisements for a ‘brain imaging study’. None of these subjects had a history of Axis I or II diagnosis, nor did they meet any SCID I or II diagnostic criteria (Spitzer et al., 1990a,b). In addition, no subject affirmed alcohol or drug abuse within the past year, or a history of dependence. Neurological illness and head trauma were absent among all subjects, and they each gave informed consent before participation. Of these 60 participants, one subject was dropped from analyses as the file containing the RTs was corrupted during the recording session. It was not possible to construct RT-matched correction waveforms for 13 subjects, who were thus dropped from analyses. The final 46 subjects averaged 28.9 years of age .SD = 8:4. with a range of 18–53, and included 36 men and 10 women. Age and gender did not affect the results below. All but one of the subjects was strongly right-handed, having a mean handedness of 0.77 .SD = 0:23. as determined by the Edinburgh Inventory (Oldfield, 1971).
2.2. ERP testing
Three distinct ERP paradigms were used in the study, presented in a counter-balanced fashion. One was an oddball paradigm wherein the subjects were asked to silently count binaurally presented oddball tones (97 dB sound pressure level (SPL), 1.5 kHz tones, 50 ms duration, 10 ms rise/fall, 15% of trials) among standard tones (97 dB SPL, 1 kHz). A second paradigm presented subjects with the same tones, but they were asked to press a button with their right thumb after every oddball tone heard. The third paradigm required the subjects to press a button after every binaurally presented target tone (97 dB SPL, 1.5 kHz, 100% of trials, SRT task). All tones were presented with a variable interstimulus interval of 1.3 ± 0.2 s. Each paradigm contained 200 stimuli and was presented once.
Electroencephalographic (EEG) activity was recorded from the scalp through 28 tin electrodes in preconfigured caps (ElectroCap International). Linked earlobes were used as the reference, the forehead as ground. Two electrodes located medially to the right eye, one above and one below, were used to monitor vertical eye movements and blinks. Electrodes placed at the outer canthi of the eyes were used to monitor horizontal eye movements. All electrode impedances were below 3 kΩ, and the ears were matched within 1 kΩ. The EEG amplifier bandpass was 0.15 (6 dB/octave rolloff) to 40 Hz (36 dB/octave rolloff). Single trial epochs were digitized at 3.5 ms/sample. Each epoch was of 900 ms duration, including a 100 ms prestimulus baseline.
Averaging and artifact rejection were done off-line. Epochs were digitally low-pass filtered at 8.5 Hz with a 24 dB/octave rolloff to remove ambient electrical noise, muscle artifact, and alpha contamination. Within each 200 trial block, epochs were baseline corrected by subtraction of the average prestimulus voltage. Vertical and horizontal eye movement artifacts were corrected with regression-based weighting coefficients (Semlitsch et al., 1986). Subsequently, epochs which contained voltage exceeding ±50 µV at F7, F8, Fp1, or Fp2 were rejected. Averages were computed for the brain responses to oddball tones in the button-press oddball paradigm and to tones in the SRT task after matching individual target RTs between the two tasks. Each oddball target trial RT was matched to an SRT trial RT within 5 ms. Using this trial-by-trial matching procedure, RT for each P300 trial was virtually simultaneous with an SRT trial. Only correct responses to oddball tones were matched for RT with responses from the SRT task. 70.3% of the oddball targets were matched to an SRT trial (range 33–100%). No matching was done for the silent-count paradigm. P300 was measured on all average wave-forms by designating peak P300 amplitude the most positive point from 250 to 450 ms at each recording site. In the case of double peaks, the later (P3b) peak was selected. Each site was manually inspected and adjusted if necessary, so that the same potential was measured from each site. Voltages were also normalized for overall amplitude differences by dividing by the condition’s Cz amplitude. (Note that dividing by any site, e.g. Pz, would be functionally equivalent.) Because of the selective RT matching in the button-press task, P300 latency was not assessed between conditions.
2.3. Analyses
Repeated measures analysis of variance (ANOVA) was used to test for effects between the different paradigms. Midline sites included Fz, Cz, and Pz. Frontal regions were defined as F3, C3, and FTC1, and their right-side homologues F4, C4, and FTC2. Temporal regions were defined as T3, T5, and TCP1, and their right-side homologues T4, T6, and TCP2. Results were considered significant at P ≤ 0:05, corrected where factors had more than two levels with the Huynh–Feldt epsilon.
3. Results
3.1. Target accuracy
For oddball counts in the button-press paradigm, subjects were 98.9% accurate. For the silent-count, subjects were 97.0% accurate. Button-press responses to tones in the SRT task were nearly 100% accurate (>99.9%).
3.2. RT
Mean RT to both the oddball target stimuli and the correction waveform SRT stimuli was 481.0 ± 93.0 ms. Thus, the LRP activity and P300 activity in the button-press oddball task would overlap to a great extent, and at least some of the LRP components on that task, including the motor-field, should be well estimated by the SRT wave-form.
3.3. P300 amplitude
P300 amplitudes were compared between the silent-counting and button-pressing conditions to assess differences caused by the response mode. Next, the corrected P300 waveform was compared to each response mode condition to assess the efficacy of the correction procedure. To obviate any apparent topographic differences likely caused by overall amplitude differences, comparisons were conducted on both the raw peak P300 measures and on normalized P300 measures. Raw and normalized P300 amplitudes for frontal, temporal, and midline sites are reported in Table 1. Fig. 1 presents the correction waveform for RT-matched trials on the SRT task. Fig. 2 presents the waveforms for the 3 P300 conditions: silent-count, button-press, and the corrected (subtracted) waveform.
Table 1.
Raw and normalized P300 amplitudes
| Raw data |
Normalized data |
|||||
|---|---|---|---|---|---|---|
| Silent-count | Button-press | Correction | Silent-count | Button-press | Correction | |
| Fz | 9.86 (4.82) | 5.93 (5.96) | 7.84 (6.42) | 0.67 (0.33) | 0.61 (0.61) | 0.73 (0.60) |
| Cz | 14.72 (5.67) | 9.74 (5.87) | 10.73 (6.54) | 1.00 (0.38) | 1.00 (0.60) | 1.00 (0.61) |
| Pz | 14.98 (6.07) | 11.82 (5.89) | 11.76 (6.65) | 1.02 (0.41) | 1.21 (0.60) | 1.10 (0.62) |
| F3 | 8.60 (4.22) | 4.89 (5.44) | 6.60 (5.44) | 0.58 (0.29) | 0.50 (0.56) | 0.61 (0.51) |
| FTC1 | 7.78 (3.02) | 5.02 (4.16) | 5.51 (4.25) | 0.53 (0.21) | 0.52 (0.43) | 0.51 (0.40) |
| C3 | 11.85 (4.65) | 8.15 (5.00) | 8.17 (5.32) | 0.80 (0.32) | 0.84 (0.51) | 0.76 (0.50) |
| F4 | 8.89 (4.28) | 5.89 (5.13) | 7.03 (5.60) | 0.60 (0.29) | 0.61 (0.53) | 0.66 (0.52) |
| FTC2 | 7.39 (2.96) | 5.74 (3.69) | 5.55 (4.08) | 0.50 (0.20) | 0.59 (0.38) | 0.52 (0.38) |
| C4 | 12.56 (4.71) | 9.71 (5.38) | 9.17 (5.89) | 0.85 (0.32) | 1.00 (0.55) | 0.85 (0.55) |
| T3 | 6.57 (2.01) | 4.88 (2.92) | 4.83 (3.36) | 0.45 (0.14) | 0.50 (0.30) | 0.45 (0.31) |
| TCP1 | 10.96 (4.39) | 8.61 (4.24) | 8.09 (4.59) | 0.74 (0.30) | 0.88 (0.44) | 0.75 (0.43) |
| T5 | 7.76 (3.75) | 5.93 (3.73) | 5.81 (3.96) | 0.53 (0.26) | 0.61 (0.38) | 0.54 (0.37) |
| T4 | 6.24 (2.10) | 4.36 (3.06) | 3.74 (3.82) | 0.42 (0.14) | 0.45 (0.31) | 0.35 (0.36) |
| TCP2 | 10.98 (4.27) | 8.19 (4.29) | 7.63 (4.86) | 0.75 (0.29) | 0.84 (0.44) | 0.71 (0.45) |
| T6 | 7.67 (3.76) | 5.22 (3.20) | 5.50 (4.09) | 0.52 (0.26) | 0.54 (0.33) | 0.51 (0.38) |
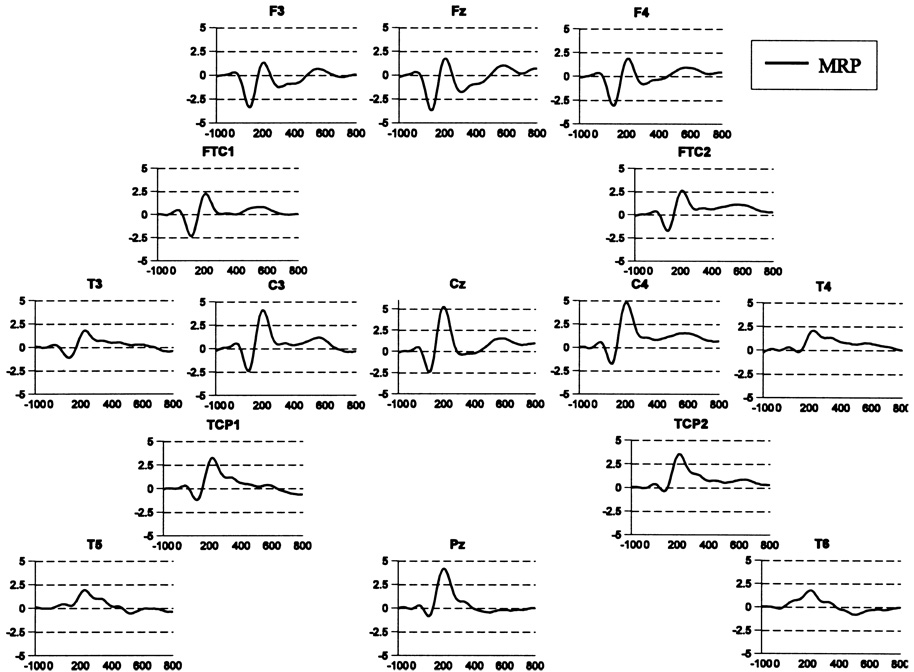
Fig. 1.
Grand averaged event-related brain potential waveform for the SRT task with a right hand button-press. N1 and P2 are evident, as well as a left-lateralized negativity over left motor areas between 300 and 400 ms (see C3 site vs. C4 site) that appears to show a corresponding positivity contralaterally more posteriorly (see T4 site).
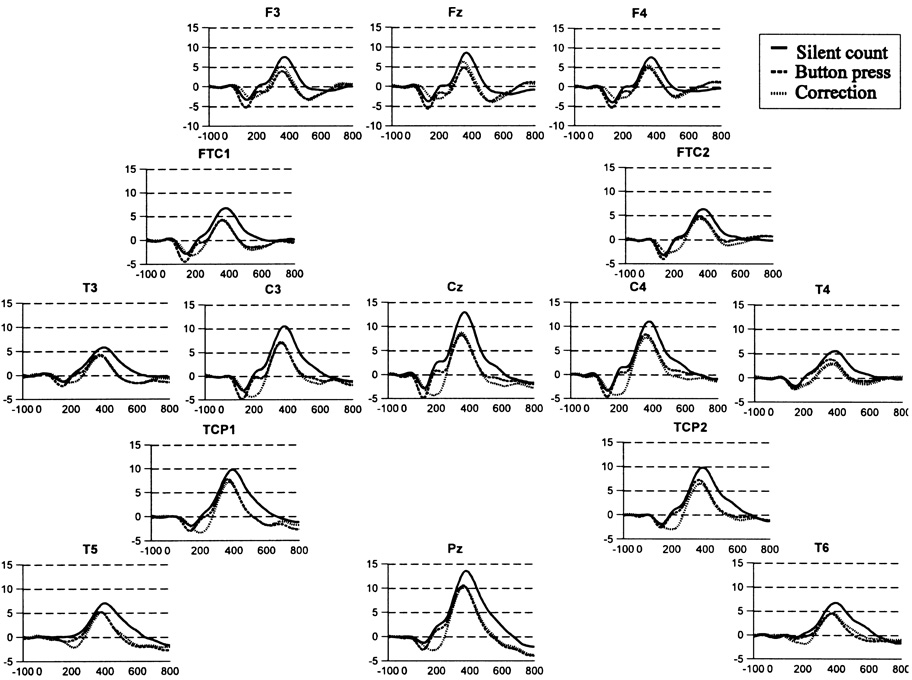
Fig. 2.
Grand averaged event-related brain potential waveforms to target tones on the oddball tasks. The correction waveform is the result of subtraction of the MRP waveform from the button-press waveform, thereby eradicating the movement potential activity (as well as the sensory potentials). Note the larger P300 for the silent-counting condition, and the general frontal reduction of P300 due to button-pressing.
3.4. Midline P300
To assess the differential effects on P300 amplitude of silent-counting and button-pressing, peak P300 amplitudes were analyzed along the traditional midline (Fz, Cz, and Pz). The act of button-pressing significantly reduced the amplitude of P300 compared to silent-counting (F(1,45) = 27:55, P < 0:001). For midline topography, both conditions showed the expected posteriorly larger P300 (F(2, 90) = 64.51, P < 0:001, ε= 0.72). However, the midline distribution differed between conditions (F(2,90) = 6.33, P < 0.004, ε = 0.93), with the silent-counting condition showing a more centro-parietal gradient and the button-pressing condition showing a steep poster-iorly maximal distribution. These midline topographic differences remained when the overall response mode P300 differences were erased by normalization (F(2,90) = 16.54, P < 0.001, ε = 0.87).
The correction procedure did not eradicate the overall amplitude difference when compared with the silent-counting condition (F(1,45) = 14.80, P < 0.001). In analysis of the raw data, the midline topographic difference between the silent-count and the button-press conditions remained after correction (F(2,90) = 3.94, P = 0.027, ε = 0.94). However, this may be confounded by the overall amplitude difference, as the correction procedure did abolish the midline topographic difference in the normalized data, as evinced by a lack of interaction between the correction procedure and the silent-count midline distributions (F(2,90) = 0.91, P < 0:39, ε = 0.92), and a significant interaction with the midline distribution of the button-pressing condition (F(2,90) = 15.51, P < 0.001, ε = 0.81). Furthermore, the correction procedure significantly increased midline P300 amplitude compared with the button-pressing condition (F(1,45) = 4.49, P < 0.04), largely by elevating the frontal and central amplitudes.
3.5. P300 lateral and regional topographies
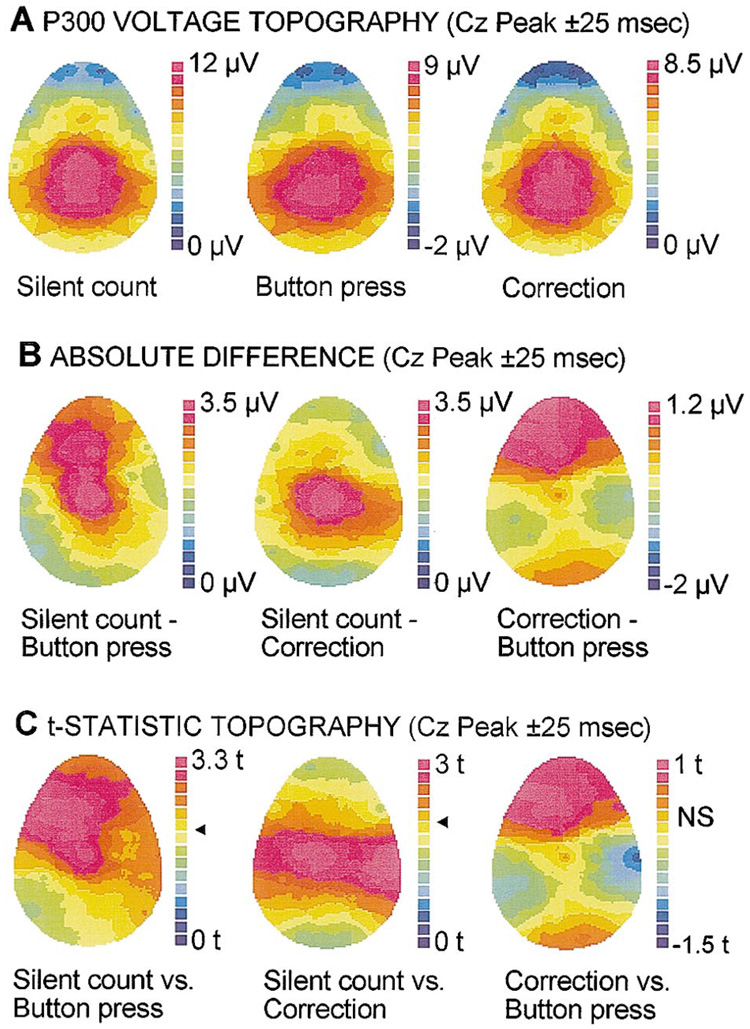
Topographic maps of the scalp distribution of voltage during a 50 ms window centered on Cz peak latency for the 3 tasks are presented in Fig. 3A. Note that there are lateral topographic differences between the tasks. The left-most map, representing P300 topography on the silent-count oddball task, is essentially symmetrical. By contrast, the middle map, representing P300 topography on a button-press task, is asymmetrical, with a slight decrease in voltage over the left central and anterior portions of the head, resulting in an ovoid shape. The right-most map, which represents the P300 topography on the motor-corrected button-press data, shows a symmetrical distribution. Fig. 3B presents the absolute voltage differences between the various tasks, obtained by subtraction of the relevant waveforms. The silent-count waveform shows clearly greater frontal and central P300 amplitude with an additional greater left frontal voltage relative to the button-press waveform (left-most map). The correction procedure erases the asymmetrical and midline frontal differences (center map). Comparison of the button-press and correction waveforms indicates a general frontal difference between the two conditions greatest on the left (right-most map). Fig. 3C indicates the significance of the differences in Fig. 3B. This method is less statistically rigorous than the analyses presented below, but indicates general patterns of significance between the groups, given that the traditional methods of analysis cannot sufficiently deal with large sets of electrodes. Button-pressing clearly causes a significant asymmetrical difference in the P300 topography that can be corrected via the correction procedure using this t-SPM analysis.
Fig. 3.
(A) Color-coded voltage maps displaying the scalp topography of P300 for the tasks. Note the distortion of the P300 field on the button-press task (ovoid shape) relative to the silent-count task (round shape). The correction technique restores the normal topography of P300. (B) Absolute differences between the tasks. Note the midline and left frontal differences between the silent-counting and button-pressing tasks. The correction procedure largely resolves the lateral frontal abnormality. (C) t-SPM maps indicate the significance of the absolute difference in (B). Note the highly significant differences over the left motor areas introduced by the act of pressing. The correction procedure compensates for these differences over contralateral motor areas, but P300 remains reduced.
Using more statistically rigorous repeated measures ANOVAs, left and right homologous sites were compared over motor and premotor regions (F3/4, C3/4, and FTC1/2), and over temporal regions (T3/4, T5/6, and TCP1/2). Analysis of P300 over the frontal regions between the silent-count and button-press tasks revealed a significant reduction in P300 amplitude for the button-pressing condition (F(1,45) = 21.98, P < 0.001), as was present for the midline amplitudes. Of primary importance was the presence of significantly different lateral voltage distributions between the tasks (F(1,45) = 6.17, P = 0.017). This asymmetry, with a left-sided P300 reduction on the right-handed button-press task, remained after P300 amplitude normalization (F(1,45) = 8.65, P = 0.005). The motor-correction procedure was successful in eradicating this MRP contamination, as evinced by a lack of a method by side interaction (F(1,45) = 0.67, P = 0.416) between the silent-count normalized data and the corrected waveform normalized data, and a significant method by side interaction (F(1,45) = 4.63, P = 0.037) when comparing the corrected waveform normalized data to the button-press normalized data.
These topographic differences did not reach significance over the temporal regions when analyzing all temporal sites. Although control subjects displayed larger mean P300 over the left temporal sites regardless of the specific response mode there was no significant temporal asymmetry in any condition (no main effect of side or interaction between side and method). However, inspection of Fig. 3 suggests that some of the contralateral MRP may be visible at the midtemporal areas (including T3). Because the lateral temporal asymmetry is typically assessed at T3 and T4, normalized P300 from those sites was analyzed. P300 was significantly larger on the left in these normal subjects at the midtemporal sites (F(1,45) = 4.05, P = 0.050). To assess whether the motor response mildly diminished the asymmetry, that is, if responding caused a slight but important change in the degree of lateral P300 asymmetry in controls, pairwise comparisons of each condition were conducted. The silent-count and corrected tasks showed a significant asymmetry when compared (F(1,45) = 4.85, P = 0.033). This effect was not apparent when the silent-count was compared to the button-press (F(1,45) = 2.70, P > 0.1), and reached only trend level when the button-press P300 was compared to the corrected waveform (F(1,45) = 3.18, P > 0.08). Inspection of the raw and normalized data (Table 1) suggests that the failure to detect a normal left-greater-than-right temporal P300 asymmetry in the button-press task is not due to a direct effect on P300 amplitude, but rather is related to an increase in the variance on the button-pressing task that decreases the effect size and which may mask the small magnitude asymmetry.
It is thus possible that the presence of MRPs, which occur likely up to several hundred milliseconds earlier in control subjects due to the long delays in responding in patients (e.g. Pfefferbaum et al., 1989; Salisbury et al., 1994), cause a reduction in the ability to detect the normal left-greater-than-right P300 asymmetry, which in turn may obscure the between group asymmetry. To assess this possibility, data from a previous P300 study of 35 male schizophrenia subjects (Salisbury et al., 1999) were utilized, strictly in a post hoc exploratory fashion. The patients performed the identical silent-count P300 task as normal subjects. The schizophrenic subjects’ mean normalized P300 was marginally smaller on the left than on the right for midtemporal sites (T3: 0.44; T4: 0.50, t(34) = −1.80, P = 0.08). When compared to 36 right-handed men from the current sample the normalized P300 evoked with silent-counting in both groups showed a significant group by side interaction (F(1,69) = 4.46, P < 0.038). Using the normalized P300 from the button-press task in controls, this interaction was reduced to trend-level significance (F(1,69) = 3.81, P = 0.055). By contrast, the normalized MRP-corrected P300 data did reveal a statistically significant group by side interaction (F(1,69) = 5.13, P = 0.027). Thus, it is apparent that button-pressing, although perhaps not the only factor, accounts for some of the failure to replicate a P300 temporal asymmetry in schizophrenia subjects.
4. Discussion
The results of this examination of response effects on P300 topography suggest that response mode is a critical factor in determining the overall amplitude and scalp distribution of P300. In normal subjects, there was a clear diminution of P300 amplitude in the case of a button-press. This finding must be interpreted as support for the contention that P300 is smaller when a button-press is required. This datum is compatible with a reduced need for resource allocation entailed by the cognitively simpler button-pressing task.
Additionally, button-pressing introduces MRP artifact that sums with P300 to produce a distortion of P300 topography. This melding of activity is greatest over anterior portions of the scalp. Studies that attempt to assess the distribution of P300 across the scalp are confounded by the use of a button-press response. This MRP contamination, however, can be at least partially eradicated by the use of a trial-by-trial RT matching procedure that creates a variance-matched and temporally aligned MRP-correction waveform, consistent with the additive model of Kok (1988). This distortion of the P300 field not only includes a reduction of the P300 recorded over the contralateral hemisphere likely caused by the motor-field of the responding finger, but also a more widespread reduction of P300 over frontal and central midline sites, likely the general negativity on ‘go’ trials described by Pfefferbaum et al. (1985).
This general reduction of P300 on a ‘go’ trial may be restricted to a ‘go–nogo’ paradigm. In a series of experiments using a forced-choice design, wherein subjects indicated the presence of a standard with one hand and a target with the other, Steinhauer and co-workers showed that P300 does not appear to be smaller than from a silent-count (Steinhauer and Hill, 1993; Hill et al., 1995). Comparisons of their target P300 to each condition showed no significant differences between normal post-pubescent subjects, although they found several other differences between tasks when also analyzing the local probabilities of standards. Further research of ‘go–nogo’ vs. forced-choice design effects on P300 amplitude and topography is necessary to indicate whether lateralized MRP effects on P300 are present in the latter case.
The MRP contamination of P300 is likely a cause of the debate over P300 topography in schizophrenia research. Post hoc comparison of the P300 on the button-pressing task with the P300 of schizophrenia patients suggested that a slight diminution of the effect size of the normal left-greater-than-right P300 asymmetry may be sufficiently large to interfere with the detection of a group reversal of P300 lateral temporal asymmetry. This is likely due to an increase of the variance at lateral sites in the button-press task relative to the silent-count task. This may be related to individual differences in the RT, which may cause variability in the degree of MRP and P300 overlap, and also to differences in the force of the response, which may alter the size and spread of the MRP, as activity in the motor cortex has been shown to vary with the force of the response (Dettmers et al., 1995). The likely role of MRPs in the lateral temporal asymmetry debate in schizophrenia is further indicated by the demonstration that an estimate of MRP activity from the SRT task both restored normal topography in the controls’ P300 and revealed a significant asymmetry in the schizophrenia group. For studies that search for group differences in the temporal scalp-area asymmetry, this small effect may be enough to obfuscate group asymmetry differences, and is likely to confound P300 topography more so in the controls than in the patients, since the patients will generally have average RTs greater than those of controls.
The present design was successful in indicating the presence of MRP activity in the P300 time range on tasks using a button-press, particularly over motor and premotor areas, in demonstrating an MRP-correction method to eradicate the offending MRPs from the ERP waveform based on Kok’s additive model (1988), and in demonstrating that button-pressing effects were likely to cause a failure in detecting the left temporal scalp-area reduction of P300 in schizophrenia. Motor potential contamination is a real and insidious confound which must be dealt with in any examination of P300 topography: studies that employ a button-press response to target stimuli must account for such motor-related factors before assessing P300 topography.
Acknowledgements
This work was supported in part by NIMH 40977, MERIT and Schizophrenia Center Awards from the Department of Veterans Affairs (R.W.M.); NIMH 01110 and 50747 (M.E.S.); and the National Alliance for Research in Schizophrenia and Depression (D.F.S.).
References
- Barret G, Neshige R, Shibasaki H. Human auditory and somatosensory event-related potentials: effects of response condition and age. Electro-enceph clin Neurophysiol. 1987;66:409–419. doi: 10.1016/0013-4694(87)90210-0. [DOI] [PubMed] [Google Scholar]
- Bruder GE, Tenke CE, Rabinowicz E, Towey JP, Malaspina D, Amador T, Kaufman CA, Gorman JM. Electrophysiological studies of brain activity in schizo-phrenia. In: Kaufman CA, Gorman JM, editors. Schizophrenia: new directions for clinical research and treatment. Lochmond, NY: Mary Ann Liebert, Inc; 1996. pp. 17–33. [Google Scholar]
- Dettmers C, Fink GR, Lemon RN, Stephan Km, Passingham RE, Silbers-weig D, Holmes A, Ridding MC, Brooks DJ, Frackowiak RS. Relation between cerebral activity and force in the motor areas of the human brain. J Neurophysiol. 1995;74:802–815. doi: 10.1152/jn.1995.74.2.802. [DOI] [PubMed] [Google Scholar]
- Faux S, McCarley RW, Nestor PG, Shenton ME, Pollak SD, Penhune V, Mondrow E, Marcy B, Peterson A, Horvath T, Davis KL. P300 topographic asymmetries are present in unmedicated schizophrenics. Electroenceph clin Neurophysiol. 1993;88:32–41. doi: 10.1016/0168-5597(93)90026-l. [DOI] [PubMed] [Google Scholar]
- Hackley SA, Valle-Inclán F. Accessory stimulus effects on response selection: does arousal speed decision making? J Cogn Neurosci. 1999;11:321–329. doi: 10.1162/089892999563427. [DOI] [PubMed] [Google Scholar]
- Hill S, Steinhauer S, Locke J. Event-related potentials in alcoholic men,their high-risk male relatives, and low-risk male controls. Alcohol Clin Exp Res. 1995;19:567–576. doi: 10.1111/j.1530-0277.1995.tb01550.x. [DOI] [PubMed] [Google Scholar]
- Ikeda A, Lüders HO, Shibasaki H, Collura TF, Burgess RC, Morris HM, Hamano T. Movement-related potentials associated with bilateral simultaneous and unilateral movements recorded from human supplementary motor area. Electroenceph clin Neurophysiol. 1995;95:323–334. doi: 10.1016/0013-4694(95)00086-e. [DOI] [PubMed] [Google Scholar]
- Isreal J, Wickens C, Donchin E. P300 amplitude changes during a tracking task as a function of continuous variations of tracking difficulty. Psychophysiology. 1979;16:175. [Google Scholar]
- Johnson R. A triarchic model of P300 amplitude. Psychophysiology. 1986;23:367–384. doi: 10.1111/j.1469-8986.1986.tb00649.x. [DOI] [PubMed] [Google Scholar]
- Kayser J, Tenke CE, Bruder GE. Dissociation of brain ERP topographies for tonal and phonetic oddball tasks. Psychophysiology. 1998;35:576–590. doi: 10.1017/s0048577298970214. [DOI] [PubMed] [Google Scholar]
- Kok A. Overlap between P300 and movement-related-potentials: a response to Verleger. Biol Psychol. 1988;27:51–58. doi: 10.1016/0301-0511(88)90005-1. [DOI] [PubMed] [Google Scholar]
- Kornhuber HH, Deecke L. Hirnpotentialaänderungen bei Wilkürbewegun-gen und passiven Bewegungen des Menschen: Bereitschaftspotential and reafferente Potentiale. Pflügers Archiv gesamte Physiol. 1965;248:1–17. [PubMed] [Google Scholar]
- Kristeva-Feige R, Rossi S, Feige B, Mergner T, Lücking CH, Rossini PM. The bereitschaftpotential paradigm in investigating voluntary movement organization in humans using magnetoencephalography. Brain Res Prot. 1997;1:13–22. doi: 10.1016/s1385-299x(97)80327-3. [DOI] [PubMed] [Google Scholar]
- Kutas M, Donchin M. The effect of handedness, of responding hand, and of response force on the contralateral dominance of the readiness potential. In: Desmedt JE, editor. Attention, voluntary contraction and event-related cerebral potentials, Prog Clin Neurophysiol. vol. 1. Basel: Karger; 1977. pp. 189–210. [Google Scholar]
- Libet B, Wright EW, Gleason CA. Readiness-potentials preceding unrestricted ‘spontaneous’ vs. pre-planned voluntary acts. Electroenceph clin Neurophysiol. 1982;54:322–335. doi: 10.1016/0013-4694(82)90181-x. [DOI] [PubMed] [Google Scholar]
- McCarley RW, Faux SF, Shenton ME, Nestor PG, Hollinger DP. Is there P300 asymmetry in schizophrenia? Arch Gen Psychiat. 1991;48:380–381. doi: 10.1001/archpsyc.1991.01810280096016. [DOI] [PubMed] [Google Scholar]
- McCarley RW, Shenton ME, O'Donnell BF, Faux SF, Kikinis R, Nestor PG, Jolesz FA. Auditory P300 abnormalities and left posterior superior temporal gyrus volume reduction in schizophrenia. Arch Gen Psychiat. 1993;50:190–197. doi: 10.1001/archpsyc.1993.01820150036003. [DOI] [PubMed] [Google Scholar]
- Miller J, Hackley SA. Electrophysiological evidence for temporal overlap among contingent mental processes. J Exp Psychol Gen. 1992;121:195–209. doi: 10.1037//0096-3445.121.2.195. [DOI] [PubMed] [Google Scholar]
- Miller J, Ulrich R. Locus of the effect of the number of alternative responses: evidence from the lateralized readiness potential. J Exp Psychol Hum Percept Perform. 1998;24:1215–1231. [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Pedersen JR, Johannsen P, Bak CK, Kofoed B, Saermark K, Gjedde A. Origin of human motor readiness field linked to left middle frontal gyrus by MEG and PET. NeuroImage. 1998;8:214–220. doi: 10.1006/nimg.1998.0362. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Ford JM, Weller BJ, Kopell B. ERPs to response production and inhibition. Electroenceph clin Neurophysiol. 1985;60:423–434. doi: 10.1016/0013-4694(85)91017-x. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Ford JM, White PM, Roth WT. P3 in schizophrenia isaffected by stimulus modality, response requirements, medication status, and negative symptoms. Arch Gen Psychiat. 1989;46:1035–1044. doi: 10.1001/archpsyc.1989.01810110077011. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Ford JM, White PM, Roth WT, Mathalon DH. Is there P300 asymmetry in schizophrenia? – Reply. Arch Gen Psychiat. 1991;48:381–383. [Google Scholar]
- Polich J. Response mode and P300 from auditory stimuli. Biol Psychol. 1987;25:61–71. doi: 10.1016/0301-0511(87)90067-6. [DOI] [PubMed] [Google Scholar]
- Salisbury DF, O’Donnell BF, McCarley RW, Nestor P, Faux S, Smith RS. Parametric manipulations of auditory stimuli differentially affect P3 amplitude in schizophrenics and controls. Psychophysiology. 1994;31:29–36. doi: 10.1111/j.1469-8986.1994.tb01022.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salisbury DF, Shenton ME, Sherwood AR, Fischer IA, Yurgelun-Todd DA, Tohen M, McCarley RW. First episode schizophrenic psychosis differs from first episode affective psychosis and controls in P300 amplitude over left temporal lobe. Arch Gen Psychiat. 1998;55:173–180. doi: 10.1001/archpsyc.55.2.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salisbury DF, Shenton ME, McCarley RW. P300 topography differs in schizophrenia and manic psychosis. Biol Psychiat. 1999;45:99–106. doi: 10.1016/s0006-3223(98)00208-x. [DOI] [PubMed] [Google Scholar]
- Semlitsch HV, Anderer P, Schuster P, Presslich O. A solution for reliable and valid reduction of ocular artifacts, applied to the P300 ERP. Psychophysiology. 1986;23:695–703. doi: 10.1111/j.1469-8986.1986.tb00696.x. [DOI] [PubMed] [Google Scholar]
- Shenton ME, Kikinis R, McCarley RW, Jolesz FA, Pollak SD, LeMay M, Wible CG, Hokama H, Martin J, Metcalf D, Coleman M. Left temporal lobe abnormalities in schizophrenia and thought disorder: a quantitative MRI study. New Engl J Med. 1992;327:604–612. doi: 10.1056/NEJM199208273270905. [DOI] [PubMed] [Google Scholar]
- Spitzer R, Williams J, Gibbon M, First M. The structured clinical interview for DSM-IIIR (SCID-II) – non-patient edition. Washington, DC: American Psychiatric Press; 1990a. [Google Scholar]
- Spitzer R, Williams J, Gibbon M, First M. The structured clinical interview for DSM-IIIR (SCID-II) – personality disorders. Washington, DC: American Psychiatric Press; 1990b. [Google Scholar]
- Starr A, Sandroni P, Michalewski HJ. Readiness to respond in a target detection task: pre- and post-stimulus event-related potentials in normal subjects. Electroenceph clin Neurophysiol. 1995;96:76–92. doi: 10.1016/0013-4694(94)00162-e. [DOI] [PubMed] [Google Scholar]
- Starr A, Aguinaldo T, Roe M, Michalewski HJ. Sequential changes of auditory processing during target detection: motor responding versus mental counting. Electroenceph clin Neurophysiol. 1997;105:201–212. doi: 10.1016/s0924-980x(97)00016-7. [DOI] [PubMed] [Google Scholar]
- Steinhauer S, Hill S. Auditory event-related potentials in children at high risk for alcoholism. J Stud Alcohol. 1993;54:408–421. doi: 10.15288/jsa.1993.54.408. [DOI] [PubMed] [Google Scholar]
- Tenke CE, Kayser J, Fong R, Leite P, Towey JP, Bruder GE. Response- and stimulus-related ERP asymmetries in a tonal oddball task: a Laplacian analysis. Brain Top. 1998;10:201–210. doi: 10.1023/a:1022261226370. [DOI] [PubMed] [Google Scholar]
- Vaughan HG, Jr, Costa LD, Ritter W. Topography of the human motor potential. Electroenceph clin Neurophysiol. 1968;25:1–10. doi: 10.1016/0013-4694(68)90080-1. [DOI] [PubMed] [Google Scholar]