Abstract
Objectives
It is controversial whether a semantic processing bias for strong associates is present in schizophrenia, and unknown whether the language abnormalities observed in schizophrenia can be attributed to dysfunctions early or late in cognitive processing. Combined behavioral and event-related potential (ERP) data can indicate the nature and timing of such abnormalities.
Methods
Sensibility judgements of dominant and subordinate homograph sentences were measured in 12 schizophrenia patients and 13 normal controls. ERPs were recorded to the disambiguating sentence-ending word.
Results
All subjects showed greatest misinterpretation of subordinate homograph sentences, but schizophrenia patients particularly misinterpreted these sentence types. For control subjects, subordinate homograph sentences that were classified as nonsensical showed greater N400 than those classified as sensible. By contrast, the N400 of patients was large, regardless of the sensibility judgement – patients’ brains initially responded to all subordinate sentences as if nonsensical. These data are consonant with a semantic bias. However, the patients’ N400 to dominant homograph sentence endings was also larger than that of controls, a finding not consonant with a semantic bias.
Conclusions
The behavioral results indicate a selective comprehension abnormality in schizophrenia dependent on the content of verbal memory. The ERP results suggest a pervasive contextual memory failure. A semantic activation decay model is proposed to explain these results.
Keywords: Associates, Homographs, Late positive complex, N400, Schizophrenia, Semantic memory
1. Introduction
Since Bleuler’s initial description of the fundamental psychological aspects of schizophrenia (Bleuler, 1911/1950) a disorder in the associations between concepts in the train of thought has been considered of central importance to schizophrenia, underlying the disordered thought reflected in the speech of patients. The precise cognitive mechanisms that underlie this schizophrenic thought disorder have yet to be established. Currently, two relatively distinct classes of theories have emerged, each with different notions of the cognitive processes involved. In one camp, schizophrenic thought disorder is hypothesized to reflect an abnormal activation of concepts in semantic conceptual space. This abnormality is thought to occur relatively early in the information processing stream, reflecting abnormal activation of semantic memory networks before active controlled and conscious attention processes have been engaged (e.g. Spitzer et al., 1993). This class of theories usually predicts a so-called semantic bias in schizophrenia, whereby dominant associates of words are preferentially favored, independent of contextual influences, because they are overly-activated and dominate or swamp higher-order cognitive mechanisms.
A variant of such early automatic semantic activation theories posits the fundamental abnormality as not one of overactivation at each node in the distributed network, but rather one of an unchecked, overly-broad spread of activation in the network. Rather than a generally greater amount of activation for nodes or associates, which also implies a greater spread of activation to associates normally only weakly activated, it may be that the abnormality is one purely related to a greater spread of activation such that weakly associated items are activated on par with more highly connected associates. Thus, in its strongest implementation, too many items crowd executive verbal mechanisms, with weak associates activated as greatly as strong associates. There is some evidence to suggest a greater spread of activation in schizophrenia. For example, Spitzer et al. (1993) showed that schizophrenia patients tended to activate antonyms of items more greatly than did controls, suggesting such a broader spread of activation, but only at very short presentation intervals. This effect can either suggest over-activation of the type discussed earlier, or simply a greater spread of activation without necessarily overly activating nodes. The latter effect may be modeled in a parallel distributed processing architecture by a lack of inhibitory control of spread of activation in local neural networks (Grunze et al., 1996). However, there is very little empirical evidence to suggest that schizophrenia patients generate weak associates more so than do controls in normal discourse or during testing when not under very rapid presentation rates. In fact the extant literature suggests the opposite; for example, Nestor et al. (1998) showed that schizophrenia subjects had an abnormal pattern of item recall for learned items in that they were unduly influenced by the strength of association, failing to recall items of low network connectivity. Hence it is unlikely that a pure broad spread of activation abnormality influences schizophrenic thought disorder, except at experimentally-induced rapid processing speeds.
The second influential class of theories posits that thought disorder in schizophrenia reflects an abnormality in later executive functions, for example a phasic disengagement of controlled, selective attention (e.g. Schwartz, 1982), or a failure to utilize verbal context to modulate the initial semantic activation appropriately (e.g. Cohen et al., 1999). As generally formulated currently, this class of theories does not support the presence of a semantic bias. For example, Cohen et al. (1999) argued that patients responded randomly to associates, choosing more dominant associates when subordinate associates were appropriate, and more subordinate associates when dominant associates were appropriate.
Thus, two matters of current debate can be illustrated. The first involves the stage of information processing affected in schizophrenia, whether relatively early in semantic activation, or later in executive functions [whether obligatory (contextual inhibition) or controlled (selective attention)]. The second issue for debate involves the presence of a semantic bias in schizophrenia. This issue is related to, yet independent from, the question of where in information processing an abnormality occurs. Although theories of overactive semantic facilitation generally predict a semantic bias, such automatic ‘hyperpriming’ is not essential for the presence of a semantic bias. The original formulation of a semantic bias (Chapman et al., 1964) suggested that it was caused by an abnormality in the utilization of context by attention processes. Some time later, Maher (1972) suggested that this bias might be explained by an abnormality in an active inhibitory process. The presence of a semantic bias is not necessarily reflective of an abnormality at a specific stage of information processing – it maybe early or late.
There is a fundamental difference in the behavior predicted by theories that posit a semantic bias and those that do not. Semantic bias theories suggest that comprehension abnormalities in schizophrenia will be entirely dependent on the content of semantic memory. By contrast, theories that do not suggest a bias predict that abnormalities in thinking will be independent of the content of the discourse. One tool for examination of the presence of a semantic bias is the use of homographs, words that are polysemous or have multiple unrelated meanings. For example, one may board the boat, nail the board, or receive room and board. The different meanings of these homographs can oft-times be arranged from strongest to weakest, or dominant to subordinate. Studies of homographs typically assess the utilization of biasing context and the path of associations in semantic conceptual space when a dominant or a subordinate meaning is semantically congruent.
Theories of late, controlled, executive dysfunction with-out a bias predict that schizophrenic patients would make more dominant meaning interpretations when context indicated that subordinate meanings were more appropriate, and, in addition, more subordinate interpretations when dominant meanings were indicated. Cohen et al. (1999) reported this pattern in a lexical disambiguation task. Patients presumably interpreted the word meaning randomly and independently from context due to a failure to use contextual cues. This result can also be modeled by a supraordinate selective attention abnormality. According to Schwartz’s model (1982), abnormal language in schizophrenia is the result of phasic lapses of attention whereby periods of normal performance alternate with periods of abnormal performance as selective attention becomes disengaged from performance. These phasic lapses are task independent, and thus, according to this theory, one would also predict no relation between the activation strength of semantic associates and performance.
By contrast, theories that suggest a semantic activation bias predict greater dominant meaning access for both dominant-biasing and subordinate-biasing context. Stronger but contextually inappropriate associates or meanings of homo-graphs mislead apperception, either because of too great activation in initial semantic excitation (e.g. Kwapil et al., 1990; Spitzer et al., 1994), a failure to maintain, modulate, or potentiate weak associates’ semantic strength in semantic memory (e.g. Nestor et al., 1998), a failure to properly use context to interpret meanings (e.g. Chapman et al., 1964; Maher, 1972; Truscott, 1970) or some combination of abnormal automatic and controlled operations.
Recently, Salisbury et al. (2000) reported on the use of event-related potentials (ERPs) to investigate semantic bias in schizophrenia. Two brain potentials bear particularly on this issue, being sensitive to language processing. The N400, a negative potential that occurs approximately 400 ms after a word, is inversely related to the facilitation, or priming, of a word by preceding material. The greater the priming of a word by preceding material, the smaller the N400 it evokes (e.g. Kutas and Hillyard, 1980; 1989; Polich, 1985). The N400 is also elicited by pictures of objects (Nigam et al., 1992; Pratarelli, 1994), and to color patches when the patches need to be named (Katayama and Yagi, 1992), but not to geometric figures that deviate from a predictable sequence, nor to musical notes that deviate from a known tune or scale (Besson and Macar, 1987). Thus, N400 appears to be uniquely associated with symbolic representations in semantic memory stores. The late positive complex (LPC) subsequent to N400 may reflect information extraction and analysis after individual word meanings are activated, the context-based inhibition of initial spread of activation, and the further analysis of global meaning (Halgren, 1990). Thus, the behavior of these potentials is influenced by priming, and can indicate whether a word has been primed or not. Salisbury et al. (2000) argued that if a semantic bias was present in schizophrenia, then subordinate associates should show disproportionately large N400 activity, as the patients would have selected the inappropriate dominant network of associates due to their bias. By contrast, a late memory- or attention-related failure would evoke large N400s to everything since all semantic facilitation would be absent. Subjects passively read sentences that affirmed the dominant or subordinate homograph meaning with little context. For example, a dominant sentence was ‘The bank was closed’ and a subordinate sentence was ‘The bank was steep’. The former refers to a financial institution, the latter to a river’s edge. Schizophrenia patients showed greater N400 to all sentence endings, suggesting a problem with maintenance of sentential context, a late executive abnormality. However, N400 was largest to the subordinate sentence-endings, and only that activity correlated with the degree of psychosis. Consequently, there was some evidence for each theory. In retrospect, that report was limited in that subjects simply passively read the sentences and did not indicate their eventual comprehension of the sentences. Hence, no behavioral evidence supporting or refuting the presence of a semantic processing bias was obtained.
The current study examined new samples of patients and controls on an expanded set of homograph stimuli. Subjects actively indicated whether the sentence was sensible or not to them, thus providing for analysis of comprehension patterns to the different sentence types and allowing for the sorting of ERPs based on interpretation. The study aims were to assess whether a semantic processing bias was observed for homograph comprehension in the absence of biasing context in schizophrenia, whether the electrical activity of the brain during semantic processing would be sensitive to such a semantic bias, and whether the pattern of ERP activity would indicate where in the processing stream an abnormality occurs. If a semantic bias was present, then schizophrenia patients should disproportionately judge more of the valid subordinate homograph meaning sentences as nonsensical. If this bias was due to a facilitation effect, occurring early in the processing stream, then they should show greater N400 to subordinate endings regardless of whether that sentence was judged sensible or nonsensical. If the bias was due to a later controlled abnormality, then they should show greater N400 to all sentence endings. If there was no semantic processing bias in schizophrenia subjects, they would make interpretation errors independently from homograph meaning strength (dominant versus subordinate). Hence, they would show more errors than controls for each type of word, and show either no differences in N400 from controls, or greater N400 to all sentence ending types regardless of whether it affirmed the dominant or subordinate homograph meaning.
2. Methods
2.1. Subjects
Procedures were approved by the local IRB, and all subjects gave informed consent. Eighteen right-handed male schizophrenic patients were recruited from the McLean inpatient units. All patients were screened for a negative history of electro-convulsive therapy, epilepsy or seizures, head trauma, hearing loss, alcohol dependence, alcohol abuse in the last 5 years, and any IV drug use. Clinical diagnoses were confirmed with chart review and SCID interview (Spitzer et al., 1990a).
Twenty-two healthy, right-handed men were recruited with newspaper advertisements from the local population. All control subjects were screened for a negative history of drug dependence, neurological disease or trauma, and psychopathology (SCID-NP; Spitzer et al., 1990b) as well as any immediate family history of psychopathology (by self-report).
All subjects were native English speakers. All subjects performed the mini-mental state examination (Folstein et al., 1975) to rule out any dementia or delirium and the information subscale of the WAIS-R (Wechsler, 1981) as an estimate of premorbid intelligence.
One schizophrenia patient was dropped from the study due to a corrupted EEG file. Three schizophrenia patients and two control subjects were dropped because they had too few trials to construct averaged ERPs after artifact rejection. Two schizophrenia patients and seven control subjects were dropped from further analyses to match the groups on scaled information scores, age, and parental-SES. Since information scores are thought to be relatively stable estimates of premorbid functioning, any group differences in experimental measures were likely not due to differences in intellectual or vocabulary skills. Subject characteristics and test scores are presented in Table 1. Schizophrenia subjects had generally lower social class ratings than controls, in accord with the debilitating effects of their illness. However, the social class ratings of the parents of each group did not differ, suggesting neither group was underprivileged and that rearing from different social strata was not a potential confound. The schizophrenia patients had less schooling than the controls, but all subjects were high school graduates or equivalent, and both groups, on average, had finished part college. Group performance on the mini-mental differed, but mean scores of the groups suggested that neither group was delirious nor demented at the time of testing.
Table 1.
Basic demographic, cognitive, and clinical measuresa
| Controls (n = 13) | Schizophrenics (n = 12) | P | |
|---|---|---|---|
| Age | 31.6 ± 9.8 | 35.3 ± 8.6 | >0.33 |
| Handednessb | 0.75 ± 0.24 | 0.66 ± 0.27 | >0.40 |
| SESc | 2.1 ± 0.8 | 3.4 ± 1.4 | =0.006 |
| Parental SES | 2.0 ± 1.2 | 2.8 ± 1.7 | >0.17 |
| Mini-mentald | 28.7 ± 1.1 | 27.1 ± 2.2 | =0.026 |
| WAIS-R informatione | 11.5 ± 1.9 | 10.4 ± 1.4 | >0.13 |
| Schooling (years) | 15.2 ± 1.2 | 13.2 ± 1.8 | =0.003 |
| Illness duration (years) | 9.1 ± 8.1 | ||
| GASf | 33.9 ± 10.0 | ||
| BPRSg | 44.3 ± 12.4 | ||
| Medicationh | 490.3 ± 763.8 |
Note: values are mean ± SD.
Oldfield (1971)−1 = left-handed; and 1 = right-handed.
Socio-economic status, Hollingshead (1965). 5 lowest; 1 highest.
Summed scores mini-mental state examination.Folstein et al. (1975). Range 0–30.
Summed scaled scores, Wechsler (1981).
Global assessment scale, Endicott et al. (1976).
Brief psychiatric rating scale, Overall and Gorman (1962).
Chlorpromazine equivalents.
2.2. Sentence paradigm
A total of 152 sentences were presented to the subjects, each four words long and reading ‘THE NOUN WAS ADJECTIVE/VERB’. One hundred and two sentences contained homographs, half of them affirming the dominant meaning, one-half the subordinate meaning. The remaining 50 sentences contained a noun with one meaning. The sentence-ending word (adjective/verb) was always congruent with the noun, and in the case of homographs constrained its meaning as either dominant or subordinate. Dominant meanings had probabilities of usage approximately three times greater than subordinate meanings (Chapman et al., 1964; Kausler and Kollasch, 1970; Nelson et al., 1980; Wollen et al., 1980; Onifer and Swinney, 1981; Gorfein et al., 1982).
Words were presented one at a time on a computer screen for 1 s with a stimulus onset asynchrony (SOA) of 1.25 s. The cue to respond (an imperative question ‘OK?’) appeared 250 ms after the offset of the last word and remained on screen for 2 s prior to the initiation of the next sentence. Subjects were required to indicate as quickly as possible during this interval whether or not the sentence made sense to them by performing a right thumb press if sensible or a left thumb response if nonsensical. Subjects sat 1 m from the computer screen.
2.3. Recording system
EEG activity was recorded from the scalp through 28 tin electrodes in pre-configured caps (ElectroCap International). Linked-earlobes were the reference, the forehead was the ground. Two electrodes located medially to the right eye, one above and one below, were used to monitor vertical eye movements and blinks. Electrodes placed at the outer canthi of the eyes were used to monitor horizontal eye movements. All electrode impedances were below 3 KOhms, and the ears were matched within 1 KOhm. The EEG amplifier bandpass was 0.15 (6 dB/octave rolloff) to 40 Hz (36 dB/octave rolloff). Single trial epochs were digitized at 3.9 ms/sample (256 Hz). Each epoch was of 1100 ms duration, including a 100 ms pre-stimulus baseline. Averaging and artifact rejection were done off-line. ERP responses were digitally low-pass filtered at 8.5 Hz with a 24 dB/octave rolloff to remove ambient electrical noise, muscle artifact, and alpha contamination. Epochs from each electrode site were baseline corrected by subtraction of the average pre-stimulus voltage, and corrected for eye movement artifact using regression-based weighting coefficients when the standard deviations of the corrections were <0.01 µV (Semlitsch et al., 1986). Trials were again base-line corrected after eye-correction. Subsequently, epochs which contained voltage exceeding ±75 µV at F7, F8, Fp1, or Fp2 were rejected. Averages were constructed for the last words of the sentences, which were disambiguating for homographs. Trials were separated for sentence endings that affirmed the dominant homograph meaning and those that affirmed the subordinate homograph meaning and whether the subject determined that the sentence was sensible or nonsensical. Because homographs were repeated, once with the dominant meaning and once with the subordinate meaning, ERP comparisons with one meaning nouns, which were not repeated, were not performed due to potential repetition priming effects on N400 and LPC.
2.4. Analyses
Behavioral responses were sorted by the sentence type (one meaning noun, dominant homograph, subordinate homograph). These three types were sorted as a function of subjective judgement. Further, responses that occurred less than 100 ms after the probe were rejected from analyses, and sentences to which no response was made were rejected from analyses. The total number for each word type/judgement was divided by the total number of valid sentences for that type, and multiplied by 100 to provide percent responses. Analysis of judgements was restricted to the percent of valid sentences judged nonsensical, as the measures were complementary (summing to 100%). Following analysis of all three word types, the base error rates of the patients and controls were corrected for by subtraction of the error rates to single meaning nouns from the error rates to homograph sentences. Reaction times were averaged separately for each of the six sentence/judgement combinations. (Note that a response rate of 0 is a valid measure, but a reaction time would be missing, as would ERP measures.)
Groups were compared on the amplitudes of N400 and LPC. Peak amplitudes were selected for the Cz electrode via automated detection. Based on the grand average Cz peak, the following latency ranges were selected for peak picking: controls: N400: 300–600 ms; LPC: 400–800; schizophrenia: N400: 400–700; LPC: 600–1000, with a rule that LPC follow N400. Each peak for each subject was verified by visual inspection and adjusted if necessary. N400 and LPC amplitude for each site were measured over a 50 ms bin centered about the peak latency, as in Salisbury et al. (2000). Averages could not be constructed for errors in comprehending the dominant homograph meanings in several subjects since no errors were committed. The mean number of trials in these averages for the remaining subjects was under three for the patients and under two for controls, questioning the validity of the ERP data for this condition. Hence, two separate repeated measures ANOVAs were conducted on the ERP data. First, ERP responses to only the subordinate meanings were analyzed with diagnostic group as the between-subjects factor (schizophrenia versus control) and judgement (sensible, nonsensical) as the within-subjects factor. Patients were predicted to show greater N400 to these sentences than controls if a bias was present, regardless of subjective comprehension. Second, the ERP responses to only sentences judged sensible were compared for dominant and subordinate homographs. If a bias was present, then the patients should show abnormal ERPs only to the subordinate sentences. Repeated-measures ANOVA with diagnostic group as the between-subjects factor (schizophrenia versus control) and word type (dominant homograph, subordinate homograph) as the within-subject factor were conducted. Effects were assessed separately along the traditional midline (Fz, Cz, Pz) and at temporal-parietal sites (TCP1 and TCP2, corresponding to Wernicke’s left and right). The Huynh-Feldt epsilon was used to correct d.f. for factors with more than two levels.
3. Results
3.1. Subjective judgements
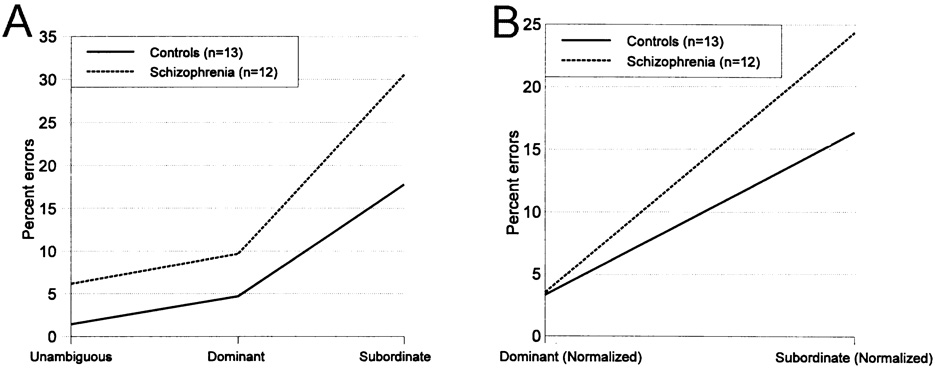
Comprehension error rates for the different sentences for the two groups are presented in Fig. 1. Schizophrenia patients made more errors to all sentences than did controls, F(1, 23) = 4.82, P < 0.04. All subjects made more errors to dominant homograph sentences than to one meaning sentences, and to subordinate sentences than to dominant homograph sentences, F(2, 46) = 87.17, P <0.001, ε = 0:77. Schizophrenia subjects, however, showed a predilection to misinterpret the subordinate homograph sentences, evinced by a group by word type interaction, F(2, 46) = 3.77, P < 0.05, ε =0.77. Schizophrenia subjects made approximately 5% more errors than controls to one meaning and dominant meaning sentences, but nearly 13% more errors to subordinate homograph meaning sentences. When error rates to the homographic sentences were corrected by subtracting the base error rate, estimated by errors to one meaning sentences, schizophrenia subjects no longer made more errors than the controls when collapsed over both types of homographic sentences, F(1,23) = 2.30, P > 0.14. All subjects made more errors to the subordinate homograph meaning sentences, F(1, 23) = 94.29, P < 0.001. Of primary importance, there was again a group by word type interaction, F(1, 23) = 4:927, P < 0.04. Schizophrenia subjects made essentially the same number of errors to dominant homo-graph sentences as controls when accounting for their base error rate (0.19% more), but disproportionately more errors to the subordinate sentences (7.96% more).
Fig. 1.
Percent of comprehension errors for the two groups for each type of sentence. Panel A shows that patients make more errors to all sentences, both groups make more errors in comprehending subordinate homograph meanings, and that patients make disproportionately more errors to subordinate sentences than controls. Panel B presents the error rates to homograph sentences controlling for the base error rate by subtraction of the error rate on one meaning noun sentences. The persistently increased error rate to subordinate sentences indicates a semantic bias.
3.2. Reaction times
Reaction times were averaged for each sentence type, separated by whether the subject interpreted the sentence as sensible or nonsensical, and are presented in Table 2. Because 11 (three patients, eight controls) of the 25 subjects made no errors to the one-meaning noun sentences, analyses of reaction times were performed in two ways. First, analyses of all three sentence types were restricted to just those judged sensible. The schizophrenia subjects took longer to judge a sentence as sensible than the controls, F(1, 23) = 8, 35, P < 0.01. All subjects took longer to assess the dominant homograph meaning sentences than the one meaning sentences, and longer to assess the subordinate homograph meaning sentences than the dominant homograph sentences, F(1, 23) = 37.31, P < 0.001, ε = 0.98. Thus, although slower, the pattern of reaction times for judging the different sentences as sensible was the same in the patients as in the controls. Second, analyses of reaction times to sentences judged sensible or nonsensical were restricted to the homograph sentences. One patient made no errors to the dominant homograph sentences. Schizophrenia subjects were marginally slower to respond than controls, F(1, 22) = 3.83, P = 0.063. All subjects took longer to decide that any sentence did not make sense to them, F(1, 23) = 65.04, P < 0.001, but patients showed less increase than controls at a trend-level, F(1,22) = 3.48, P = 0.076. Responses to the two different homograph sentences did not differ significantly, but word type did interact with subjective judgement, F(1, 23) = 10.39, P < 0.01. Both groups were quicker to judge a subordinate sentence as nonsensical relative to dominant sentences, and slower to judge a subordinate sentence as sensible relative to dominant sentences. Caution should be used in interpretation of this reaction time data: responses were made to a probe that followed the completion of the sentence some 1.25 s after presentation of the sentence ending. Thus, the degree to which the changes in RT reflect ongoing processes is unclear.
Table 2.
Reaction times for sentences and subjective judgementsa
| Controls (n = 13) | Schizophrenics (n = 12) | |
|---|---|---|
| Sensible | ||
| One meaning | 0.57 ± 0.1 | 0.76 ± 0.2 |
| Dominant homograph | 0.61 ± 0.1 | 0.81 ± 0.2 |
| Subordinate homograph | 0.74 ± 0.1 | 0.91 ± 0.2 |
| Nonsensical | ||
| One meaningb | 0.98 ± 0.4 | 1.16 ± 0.4 |
| Dominant homograph | 1.10 ± 0.2 | 1.16 ± 0.4 |
| Subordinate homograph | 0.98 ± 0.1 | 1.02 ± 0.3 |
Note: values are mean ± SD in s.
Means are based on nine schizophrenia patients, and five controls.
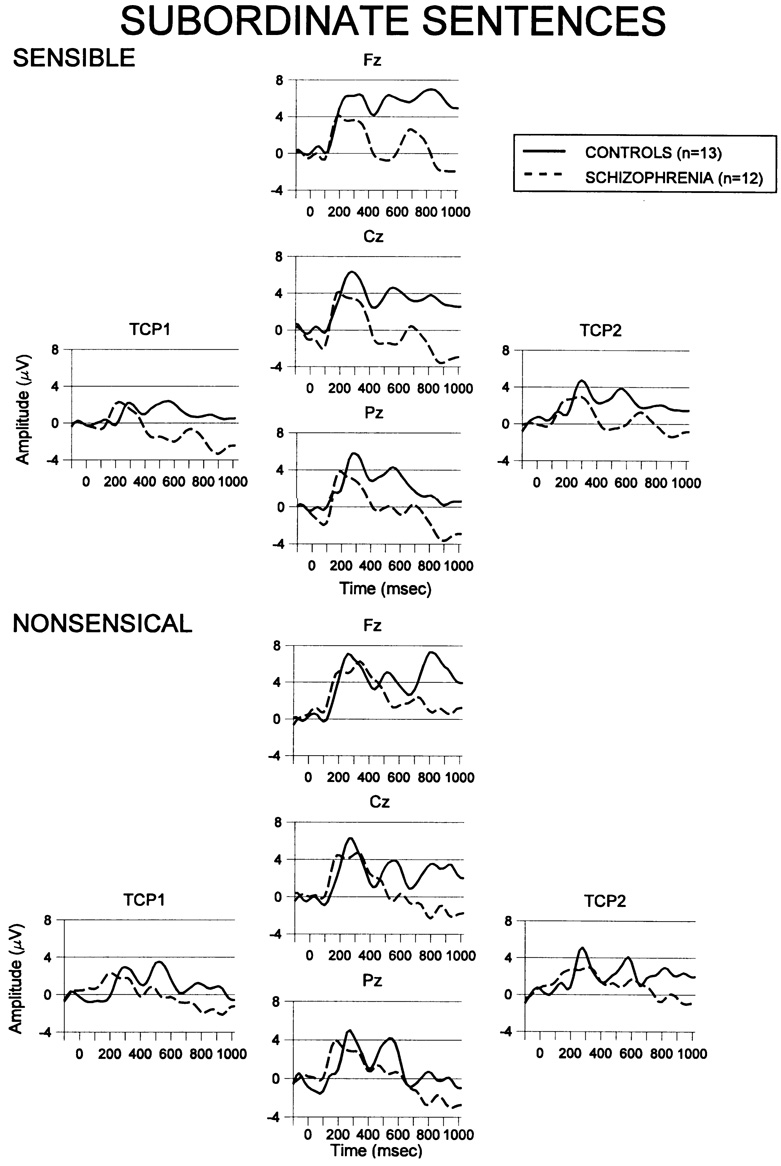
3.3. ERPs to subordinate homographs
Grand averaged ERP responses to the subordinate homograph sentence endings for each group are presented in Fig. 2, separated as a function of the subjective comprehension. Group mean interval amplitude and latency values for N400 and LPC to all sentences are presented in Table 3. Along the midline, N400 amplitudes did not differ between groups, P > 0.1. N400 amplitude displayed a centro-parietal gradient in both groups, F(2, 46) = 4.86, P < 0.03, ε = 0.67. Of primary importance was a group by sensibility interaction, F(1, 23) = 4.86, P < 0.04. Controls subjects showed an N400 effect, with greater N400 to sentences which they deemed nonsensical. In the patients, both sensible and nonsensical sentences evoked a large N400. Patients responded to the subordinate sentence endings with an N400 as large as that evoked in controls when the controls deemed the sentence nonsensical, regardless of whether they could correctly comprehend the sentence. For lateral sites, groups were not significantly different in overall N400 amplitude, P > 0.2, and the sensibility by group interaction reached only trend-level significance, F(1, 23) = 3.29, P = 0.083. In both groups, N400 was marginally more negative over the left hemisphere, F(1. 23) = 4.11, P =0.054. However, this lateral distribution was affected by the sensibility judgement, F(1, 23) = 4.95, P < 0.04. Sentences judged nonsensical were relatively more right-lateralized than sentences judged sensible, which were strongly left-lateralized.
Fig. 2.
Grand averaged waveforms to the last word of subordinate sentences from each group at sagittal midline electrode sites (Fz, Cz, Pz )and lateral sites (TCP1 and TCP2). Sensible: ERPs to subordinate homograph sentence endings judged sensible by the subjects. Nonsensical: ERPs to subordinate homograph sentence endings judged nonsensical by subjects.
Table 3.
ERP amplitudes and latenciesa
| Control (n =13) | Schizophrenia (n = 12) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| N400 | ||||||||||
| Amplitude | Fz | Cz | Pz | TCP1 | TCP2 | Fz | Cz | Pz | TCP1 | TCP2 |
| Dom, sense | 4.2 (5.9) | 1.6 (5.8) | 1.8 (5.4) | 0.5 (4.5) | 2.6 (3.7) | −2.2 (6.2) | −2.7 (5.1) | −1.9 (5.7) | −2.5 (5.3) | −1.1 (4.3) |
| Sub, sense | 3.8 (5.6) | 1.6 (4.9) | 1.9 (3.9) | 0.1 (3.8) | 1.7 (3.1) | −2.8 (8.7) | −3.8 (7.9) | −2.4 (7.4) | −3.3 (6.2) | −1.7 (5.9) |
| Sub, non | 1.9 (5.0) | −1.0 (4.3) | −0.7 (4.8) | −0.6 (4.2) | −0.6 (3.4) | −1.2 (8.8) | −2.8 (7.1) | −1.9 (7.7) | −2.2 (5.5) | −1.1 (5.9) |
| Latency | ||||||||||
| Dom, sense | 457.0 (104.3) | 503.7 (102.3) | ||||||||
| Sub, sense | 421.9 (78.5) | 544.4 (102.3) | ||||||||
| Sub, non | 421.9 (64.7) | 517.0 (83.4) | ||||||||
| LPC | ||||||||||
| Amplitude | Fz | Cz | Pz | TCP1 | TCP2 | Fz | Cz | Pz | TCP1 | TCP2 |
| Dom, sense | 8.3 (6.0) | 6.4 (6.1) | 4.4 (6.9) | 3.7 (5.3) | 5.2 (4.1) | 2.4 (6.8) | 1.7 (6.5) | 0.9 (6.5) | 0.2 (5.6) | 1.3 (4.8) |
| Sub, sense | 6.8 (5.3) | 5.3 (4.6) | 4.7 (4.5) | 2.7 (4.1) | 3.9 (3.2) | 3.5 (8.0) | 1.4 (7.7) | 0.9 (6.6) | −0.2 (5.8) | 2.1 (4.9) |
| Sub, non | 6.4 (4.3) | 5.9 (4.2) | 5.0 (3.4) | 3.9 (2.5) | 3.9 (3.2) | 5.3 (9.1) | 2.6 (7.4) | 1.7 (6.7) | 1.5 (6.0) | 2.3 (5.5) |
| Latency | ||||||||||
| Dom, sense | 630.3 (110.0) | 662.6 (107.5) | ||||||||
| Sub, sense | 561.8 (88.3) | 705.2 (118.0) | ||||||||
| Sub, non | 561.2 (52.0) | 668.2 (63.0) | ||||||||
Note: values are mean (SD). Amplitudes are µV, mean voltage over 50 ms interval centered about Cz peak latency. Latencies are ms. Dom, sense dominant homograph sentence judged sensible; Sub, sense subordinate homograph sentence judged sensible; and Sub, non subordinate homograph sentence judged nonsensical.
For responses to the subordinate endings, N400 peak latency at the vertex was prolonged in schizophrenia patients, F(1, 23) = 12.83, P < 0.01. N400 latencies were not significantly different for the different judgements.
For the subordinate sentences, midline LPC amplitude did not differ between groups, P < 0.1. LPC was larger frontally than posteriorly in both groups, F(2, 46) 7.32, P > 0.01, ε = 0.79. At lateral sites, groups did not differ in overall LPC amplitude, P > 0.1 Lateral LPC amplitudes were marginally more positive on the right in both groups, F(1, 23) = 4.18, P = 0.053. LPC lateral amplitudes were less lateralized when subjects judged the sentences as nonsensical, F(1, 23) = 7.95, P = 0.01.
For responses to the subordinate endings, LPC peak latency at the vertex was prolonged in schizophrenia patients, F(1, 23) = 20.50, P < 0.001. LPC latencies were not significantly different for the different judgements.
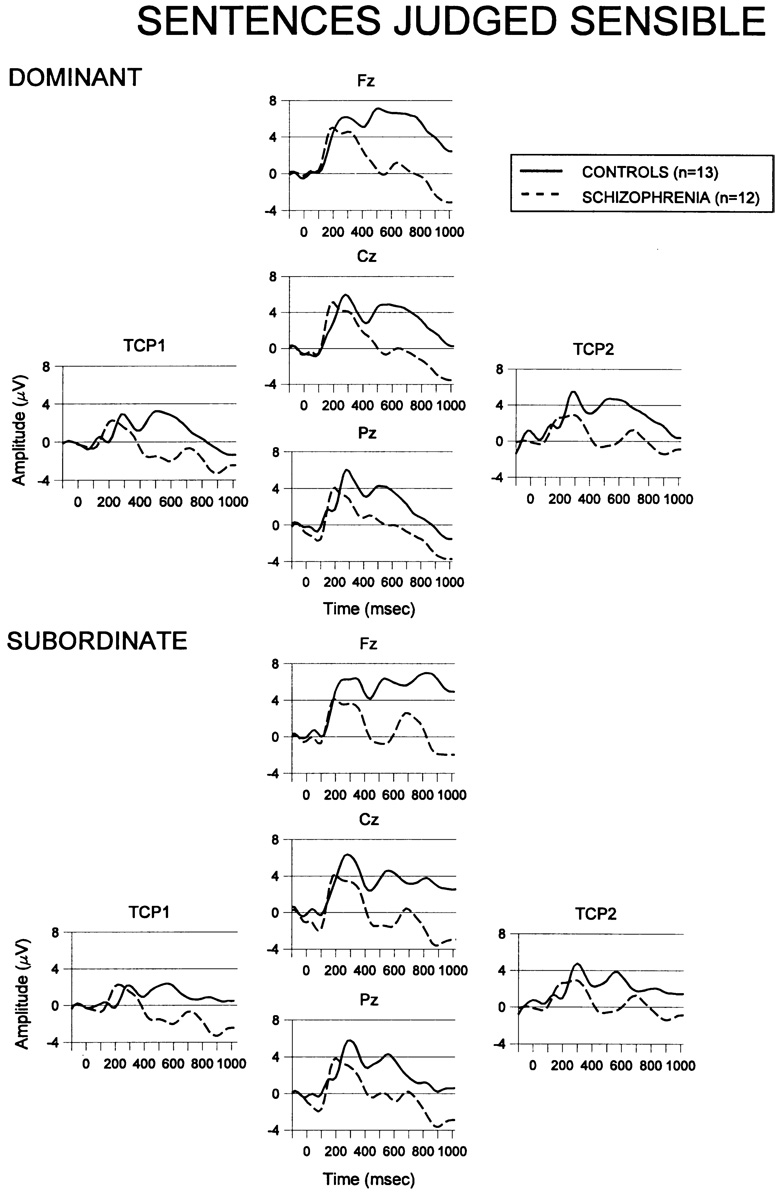
3.4. ERPs to homograph sentences judged sensible
To affirm that the greater N400 to subordinate homo-graphs judged sensible in the patients reflected an early stage semantic bias, it would be necessary to show a normal N400 to the dominant homographs in the patients, or at least a relative increase in N400 in the patients to the subordinate sentences. To assess this, ERPs were compared between the two homograph type sentences for correctly comprehended sentences. Grand averaged ERPs for these conditions are presented in Fig. 3. Mean interval amplitude and latency values are presented in Table 3. Schizophrenia patients showed greater N400 than controls regardless of the sentence type, F(1, 23) = 5.08, P < 0.04. N400 amplitude showed a marginal centro-posterior gradient in both groups, F(2, 46) = 3.68, P = 0.057, ε = 0.62. At lateral sites, schizophrenia patients showed marginally greater N400 negativity, F(1, 23) = 3.57, P = 0.071, and both groups showed greater negativity over the left hemisphere, F(1, 23) = 13.86, P = 0.001.
Fig. 3.
Grand averaged waveforms to the last word of sentences judged sensible from each group at sagittal midline electrode sites (Fz, Cz, Pz) and lateral sites (TCP1 and TCP2). Dominant: ERPs to dominant homograph sentence endings judged sensible by the subjects. Subordinate: ERPs to subordinate homograph sentence endings judged sensible by subjects. These subordinate ERPs are the same waveforms as in Fig. 2, presented for ease of comparison along this different dimension.
For responses to the correctly-judged homograph sentence endings, N400 latency at the vertex was prolonged in schizophrenia patients, F(1, 23) = 4.77, P < 0.04. N400 latencies were not significantly different for the different meanings.
LPC for the homograph sentences judged sensible was marginally smaller in the patients, F(1, 23) = 3.43, P = 0.077. LPC activity was unaffected by the different meanings. LPC was larger frontally than posteriorly in both groups, F(2, 46) = 7.07, P < 0.01, ε = 0.65. The same patterns were present at lateral sites: Patients were marginally smaller in LPC amplitude, F(1, 23) = 2.95, P = 0.099, and LPC was unaffected by the different meanings. Both groups showed larger LPC over the right hemisphere, F(1, 23) = 11.55, P < 0.01.
For responses to the correctly-judged homograph sentence endings, LPC latency at the vertex was prolonged in schizophrenia patients, F(1, 23) = 5.41, P < 0.03. LPC latencies were earlier in controls to the subordinate sentence than to the dominant sentences, but were unaffected in the patients, reflected in a group by word interaction on LPC latency, F(1, 23) 4.41, P < 0.05.
3.5. Correlations with cognitive and clinical measures
BPRS factors and items were correlated with ERP amplitudes and latencies to sentence endings in the schizophrenia group using Spearman’s rank-order correlations. The Hostility-suspiciousness factor correlated negatively with LPC latency to subordinate sentences judged nonsensical, r = −0.72, P < 0.01, driven mostly by the paranoia item, r = −0.60, P < 0.04. This factor tended to be positively correlated with N400 amplitude for all sentences: The higher the hostility-suspiciousness, the smaller the N400 negativity, again driven mostly by the paranoia item. The other factors (thinking disturbance, withdrawal-retardation, and anxious-depression) were uncorrelated with ERP measures, with the exception of the withdrawal-retardation factor, which was positively correlated with N400 latency to the dominant sentences judged sensible, r = 0.59, P < 0.05. The greater the negative symptoms, the longer the N400 latency to those sentences.
4. Discussion
This experiment was designed to contrast predictions from semantic bias theories in schizophrenia, wherein semantic processing is influenced by the strength of associates of the content of verbal memory (e.g. Chapman et al., 1964; Kwapil et al., 1990; Maher, 1983; Manschreck et al., 1988; Spitzer et al., 1993), from those executive dysfunction theories of schizophrenia where thought disorder arises independently from the content of verbal memory (e.g. Barch et al., 1996; Cohen et al., 1999; Schwartz, 1982). A semantic bias would lead to a selective comprehension bias of subordinate homograph sentences, and largest N400 to those sentences. By contrast, a pervasive late-stage cognitive abnormality would lead to increased errors to all sentences, and larger N400 to all sentences relative to controls. This study design was unique in that performance was not dependent on the use of context to order activations. The use of context-free sentences avoids the potential pitfall of biasing the performance of the subjects, since it is apparent that a verbal working memory problem exists in schizophrenia (e.g. Cohen et al., 1999; Nestor et al., 1998). When it is necessary to maintain context for a task to be completed, the effects related to a failure to maintain or utilize such context may overwhelm the effects related to a relatively short-lived semantic activation abnormality. Thus, this task, where the only context is the noun itself, may present a clearer picture of semantic activation processes themselves. On this task, schizophrenia subjects displayed an unequivocal predilection to misunderstand subordinate usages of homographs. The behavioral data quite clearly show a selective abnormality in addition to a general comprehension abnormality. Although the patients display a broad problem in interpreting any word, it is particularly marked for words related to subordinate homograph meanings. Thus, the pattern of the behavioral data suggests that the content of semantic memory influences comprehension errors in schizophrenia and is not random (e.g. Cohen et al., 1999). Further, theories of late executive attentional dysfunction as solely underlying schizophrenic thought disorder (e.g. Schwartz, 1982) can be dismissed.
Although the results of this experiment are unequivocal in showing that the pattern of comprehension abnormalities in schizophrenia is related to the content of verbal memory, it remains unclear whether these data suggest the presence of automatic semantic hyperpriming early in the processing stream or a later failure of controlled verbal memory utilization of context. To the degree that preselection of a strong meaning or associate (viz, a bias) would be reflected in N400 activity, then the ERP data from this experiment suggest that the selective comprehension errors cannot be accounted for by a faulty semantic priming mechanism. Because N400 activity is reduced by increased semantic priming, patients should have shown normal or at least less relative N400 activity to the dominant sentence endings, in addition to greater N400 to subordinate sentence endings. This was not the case. In fact, patients showed large N400 activity to all sentences, regardless of type. The N400 data do not support the hypothesis that schizophrenia subjects pre-select a dominant meaning and maintain this representation in verbal working memory. These results suggest a broader problem at the physiologic level that is independent of the content of verbal memory. Since the N400 to subordinate sentence judged sensible and to dominant sentences judged sensible was as large as the N400 to subordinate sentences judged nonsensical, yet the patients judged approximately 70 and 90% of the first sentence types correctly, one might speculate that the patients need to somehow compensate for their initial dysfunction reflected in the N400. A different mechanism must be proposed to explain the ERP and behavioral data of this experiment, one which does not rely on hyperpriming as an explanatory construct.
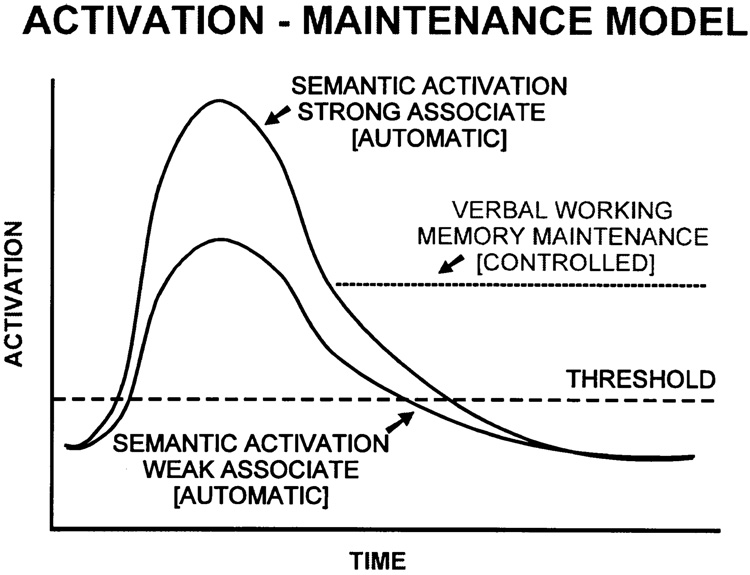
A potential confound of this design may provide a clue to a possible model. There are, in fact, two probabilities on these context-free homograph sentences. There is the over-all probability of the homograph meaning itself, which has been referred to as its dominant and subordinate meanings. For the sentences used herein, these are fairly well known, based on several published normative studies (Chapman et al., 1964; Kausler and Kollasch, 1970; Nelson et al., 1980; Wollen et al., 1980; Onifer and Swinney, 1981; Gorfein et al., 1982). Thus we can refer confidently to dominant versus subordinate sentences. However, the second probability is the cloze probability of a specific ending for any particular sentence. For the dominant sentences, endings will generally have a high cloze probability, and for subordinate sentences endings will generally have low cloze probabilities. That is, endings for dominant sentences will generally comprise strong associates and endings for subordinate meanings will comprise weak associates. Thus it is possible that the comprehension errors are due to some other factor related to the processing or maintenance in verbal memory of weak associates, rather than a bias to select strong associates actively. This ‘activation-maintenance’ model is illustrated in Fig. 4. If all associates of a given word are activated as a function of their associative strength, then it stands to reason, given a failure to maintain representations in verbal memory and a constant decay rate for different items in semantic memory, that strong associates will be activated for a longer period of time. Given the relatively long SOAs in these sentences between nouns and adjectives/ verb phrases, approximately 2.5 s, it is possible that the behavioral effect represents the longer duration of strong associate activation in semantic memory. The ERP effects, with N400 to all endings, may reflect a general verbal memory maintenance failure such that even the residual strong associate activation several seconds after initial activation is relatively weak. Our current studies aim to equate the cloze probability of dominant and subordinate associates to purely test network strengths to determine whether weak associates or subordinate networks are the primary failing point in schizophrenia.
Fig. 4.
Model of interaction between initial semantic activation and verbal memory maintenance of activations. Strong associates are initially activated to a greater degree than weak associates. A faulty maintenance function in schizophrenia would cause the content of verbal memory to be determined by the decay rate of the initial excitation. Given a constant decay rate behavior would be solely a function of the semantic relatedness of associates until the point where no initial activation remained, beyond which behavior would be random. A bias toward strong associates would be greatest in the interval where weak associate activation approaches zero but strong associate activation remains above threshold.
This maintenance failure model is reminiscent of a model of prefrontal cortex dysfunction and related memory maintenance failure in schizophrenia proposed by Cohen et al. (1996). In that model, degradation of context was exacerbated over time due to a decay process, with a reduction in the delay that can be tolerated as the disease progressed. Here we suggest that such a basic maintenance mechanism failure coupled with differential associate activation as a function of semantic relatedness (Collins and Loftus, 1975; Simpson, 1984; Swinney, 1979) can explain the apparent semantic bias in schizophrenia. It is important to note that the effects could operate purely as a function of an abnormality in the maintenance of context at an unconscious intermediate stage of information processing, with-out any necessity for an initial overactivation in semantic memory. However, given that the majority of studies show a hyperpriming effect in schizophrenia patients (e.g. Henik et al., 1995; Kwapil et al., 1990; Manschreck et al., 1988; Spitzer et al., 1993, 1994) one might assume that there is some amount of increased activation in the semantic network. The preliminary data of Mathalon et al. (2001) indicate that N400 is smaller in patients than controls at short presentation intervals, suggesting the presence of early hyperpriming in schizophrenia. Such over-activation would clearly exacerbate the bias towards strong associates in the maintenance decay model above.
Separation of the ERP responses as a function of comprehension revealed an interesting difference in ERP behavior in the patients and controls. When subordinate sentences were judged as nonsensical, controls responded with an increase in N400 activity. The responses of the patients, by contrast, showed large N400 activity regardless of whether they comprehended the sentence or not. The presence of N400 in the patients regardless of the subjective judgement of the sentence is tantalizing, because it is entirely consistent with the sentence-ending word being incongruent with a previously selected dominant meaning, regardless of any subsequent operations that make the sentence sensible. However, the failure to show less N400 to dominant usages of the homographs makes it unlikely that the dominant meaning was preselected by the patients. As outlined above, it remains possible that the effects of this study represent a failure to maintain sentential context over several seconds in the patients, where a steady rate of decay of semantic activation leads to greater yet still weak residual activation of strong associates over the time interval, reflected in both the behavioral increase in misunderstanding subordinate sentences, and N400 activity to all sentence types.
One caveat is that patients and comparison subjects may have inferred to different degrees from the presence of a response button indicating that a particular sentence was nonsensical that some subset of the sentences would be nonsensical, and hence the patients made more errors because they assumed a priori that some sentences would be invalid. One might suggest that if due to a lack of attention then such an increase in errors should be broad, rather than restricted to one type of sentence, yet the possibility cannot be ruled out that subordinate sentence were judged nonsensical because of an expectation that some sentences should be nonsense. Current investigations are examining this possibility by including incongruent sentence endings balanced with valid sentence endings.
The N400 elicited on this task is more negative over the left hemisphere, in contrast to the N400 generally reported to be more negative on the right (e.g. Kutas and Hillyard, 1982). This same pattern was present in controls in our previous report (Salisbury et al., 2000) and appears robust. It is possible that this effect may reflect the relatively little amount of context on these sentences, by contrast with the relatively strong context established in studies of semantic incongruity. One might speculate that strong sentential context might involve comparisons to and integrations with representations of the gist of sentences and passages, and may thus rely more heavily on right hemisphere functions, as originally proposed by Kutas and Hillyard (1982). The sentences here, since they involve no context except the noun itself, may in fact be more sensitive to left hemisphere semantic memory activations. The finding that controls tended to show relatively more N400 activity on the right when they failed to comprehend these sentences suggests this may be the case. Further research might help to clarify this topographical characteristic of N400 on this protocol.
The delay in N400 and LPC latency in patients confirms the most robust finding across studies of language-related ERPs in patients. The processes related to language processing and comprehension are clearly delayed in these patients.
In contrast to the results of Salisbury et al. (2000), there was no significant correlation between the degree of thinking disturbance as measured from the BPRS and N400 activity to the disambiguating sentence ending word of subordinate homograph sentences. It is unclear whether the effect is robust. In this sample, associations were present between the Hostility-suspiciousness factor and N400 for each of the sentences, driven mostly by the paranoia item. Essentially, the greater the hostility-suspiciousness, the more normal the N400 response. It remains unclear why this might be. Future research using longer sentences to dissociate disambiguating word-based ERP activity from sentence-ending ERP activity and word pairs at fast and slow presentation rates might help to clarify the associations between symptoms and ERPs.
The use of classical cognitive measures of RT and judgements concomitantly with electrophysiological measures of brain activity provide a powerful multifaceted investigation of the behavior of subjects from several different vantage points of complexity. The approaches are complementary, and provide data that mutually constrain explanatory models. The results here suggest that subordinate meanings of homographs are particularly misinterpreted by schizophrenic patients, disproportionately from their general comprehension abnormality, and thus provide evidence that the content of verbal memory exerts effects on the performance of schizophrenia patients. The ERP data, however, do not indicate any bias towards selection of strong associates at the relatively long 2.5 s SOA used. As suggested above, the combined behavioral and ERP results of this study can be modelled by a failure to maintain activation of associates in verbal memory, with performance in patients a function of the greater decay rate of material in verbal working memory. Further research using short SOAs should indicate whether any evidence of hyperpriming is observed in ERP activity, and whether the processing abnormality is related to associate strengths rather than homograph meaning strengths.
Acknowledgements
We are grateful to Andrea Sherwood, Iris Fischer, Paola Mazzoni, Deirdre Farrell, and Carlye Griggs for technical assistance. This work was supported by a grant from NARSAD (DFS); by NIMH 40799 and the Medical Research Service/Schizophrenia Research Center of the Department of Veterans Affairs (RWM).
References
- Barch D, Cohen JD, Servan-Schreiber D, Steingard S, Steinhauer SS, Van Kammen DP. Semantic priming in schizophrenia: An examination of spreading activation using word pronunciation and multiple SOAs. J Abnorm Psychol. 1996;105:592–601. doi: 10.1037//0021-843x.105.4.592. [DOI] [PubMed] [Google Scholar]
- Besson M, Macar F. An event-related potential analysis of incongruity in music and other non-linguistic contexts. Psychophysiology. 1987;24:14–25. doi: 10.1111/j.1469-8986.1987.tb01853.x. [DOI] [PubMed] [Google Scholar]
- Chapman L, Chapman J, Miller G. A theory of verbal behavior in schizophrenia. In: Maher B, editor. Progress in Experimental Personality Research. Vol. 1. New York: Academic Press; 1964. pp. 49–77. [PubMed] [Google Scholar]
- Cohen JD, Braver TS, O’Reilly RC. A computational approach to prefrontal cortex, cognitive control, and schizophrenia: recent developments and current challenges. Philos Trans R Soc Lond B. 1996;1996:1515–1527. doi: 10.1098/rstb.1996.0138. [DOI] [PubMed] [Google Scholar]
- Cohen JD, Barch DM, Carter C, Servan-Schreiber D. Context-processing deficits in schizophrenia: converging evidence from three theoretically motivated cognitive tasks. J Abnorm Psychol. 1999;108:120–133. doi: 10.1037//0021-843x.108.1.120. [DOI] [PubMed] [Google Scholar]
- Collins A, Loftus E. A spreading-activation theory of semantic processing. Psychological Review. 1975;82:407–428. [Google Scholar]
- Endicott J, Spitzer RL, Fleiss J, Cohen J. The global assessment scale (GAS): a procedure for measuring overall severity of psychiatric disturbance. Arch Gen Psychiatry. 1976;33:766–772. doi: 10.1001/archpsyc.1976.01770060086012. [DOI] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. Mini-mental state: a practical method for grading the state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Gorfein DS, Viviani JM, Leddo J. Norms as a tool for the study of homography. Mem Cognit. 1982;10:503–509. doi: 10.3758/bf03197654. [DOI] [PubMed] [Google Scholar]
- Grunze HCR, Rainnie DG, Hasselmo ME, Barkai E, Hearn EF, McCarley RW, Greene RW. NMDA-dependent modulation of CA1 local circuit inhibition. J Neurosci. 1996;16:2034–2043. doi: 10.1523/JNEUROSCI.16-06-02034.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halgren E. Insights from evoked potentials into the neuropsychological mechanisms of reading. In: Scheibel AB, Wechsler AF, editors. Neurobiology of Higher Function. New York: Guilford Press; 1990. pp. 103–150. [Google Scholar]
- Henik A, Nissimov E, Priel B, Umansky R. Effects of cognitive load on semantic priming in patients with schizophrenia. J Abnorm Psychol. 1995;104:576–584. doi: 10.1037//0021-843x.104.4.576. [DOI] [PubMed] [Google Scholar]
- Hollingshead AB. Two-factor Index of Social Position. New Haven, CT: Yale Station; 1965. [Google Scholar]
- Katayama J, Yagi A. Negative brain potentials elicited by an unexpected color patch or word. Electroenceph clin Neurophysiol. 1992;83:248–253. doi: 10.1016/0013-4694(92)90118-2. [DOI] [PubMed] [Google Scholar]
- Kausler DH, Kollasch SF. Word associations to homographs. J Verbal Learn Verbal Behav. 1970;9:444–449. [Google Scholar]
- Kutas M, Hillyard S. Reading senseless sentences: brain potentials reflect semantic incongruity. Science. 1980;207:203–205. doi: 10.1126/science.7350657. [DOI] [PubMed] [Google Scholar]
- Kutas M, Hillyard S. The lateral distribution of event-related potentials during sentence processing. Neuropsychologia. 1982;20:579–590. doi: 10.1016/0028-3932(82)90031-8. [DOI] [PubMed] [Google Scholar]
- Kutas M, Hillyard S. An electrophysiological probe of incidental semantic association. J Cogn Neurosci. 1989;1:38–49. doi: 10.1162/jocn.1989.1.1.38. [DOI] [PubMed] [Google Scholar]
- Kwapil T, Hegley D, Chapman L, Chapman J. Facilitation of word recognition by semantic priming in schizophrenia. J Abnorm Psychol. 1990;99:215–221. doi: 10.1037//0021-843x.99.3.215. [DOI] [PubMed] [Google Scholar]
- Maher BA. The language of schizophrenia: a review and interpretation. Br J Psychiatry. 1972;120:3–17. doi: 10.1192/bjp.120.554.3. [DOI] [PubMed] [Google Scholar]
- Maher BA. A tentative theory of schizophrenic utterance. In: Maher BA, Maher WB, editors. Progress in Experimental Personality Research: Psychopathology. Vol. 12. New York: Academic Press; 1983. pp. 1–52. [PubMed] [Google Scholar]
- Manschreck TC, Maher BA, Milavetz JJ, Ames D, Weisstein CC, Schneyer ML. Semantic priming in thought disordered schizophrenic patients. Schizophr Res. 1988;1:61–66. doi: 10.1016/0920-9964(88)90041-2. [DOI] [PubMed] [Google Scholar]
- Mathalon DH, Ford JM, Faustman WO. Delusions in schizophrenia: an event-related brain potential study. Schizophr Res. 2001;49:206. doi: 10.1016/s0006-3223(01)01166-0. [DOI] [PubMed] [Google Scholar]
- Nelson DL, McEvoy CL, Walling JR, Wheeler JW., Jr The University of South Florida homograph norms. Behav Res Methods Instrum. 1980;12:16–37. [Google Scholar]
- Nestor PG, Akdag SJ, O’Donnell BF, Niznikiewicz M, Law S, Shenton ME, McCarley RW. Word recall in schizophrenia: a connectionist model. Am J Psychiatry. 1998;155:1685–1690. doi: 10.1176/ajp.155.12.1685. [DOI] [PubMed] [Google Scholar]
- Nigam A, Hoffman JE, Simons RF. N400 to semantically anomalous pictures and words. J Cogn Neurosci. 1992;4:15–22. doi: 10.1162/jocn.1992.4.1.15. [DOI] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: the Edinburgh Inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Onifer W, Swinney D. Accessing lexical ambiguities during sentence comprehension: effects of frequency of meaning and contextual bias. Mem Cognit. 1981;9:225–236. [Google Scholar]
- Overall JE, Gorman DR. The brief psychiatric rating scale. Psychol Rep. 1962;10:799–812. [Google Scholar]
- Polich J. Semantic categorization and event-related potentials. Brain Language. 1985;26:304–321. doi: 10.1016/0093-934x(85)90045-8. [DOI] [PubMed] [Google Scholar]
- Pratarelli ME. Semantic processing of pictures and spoken words: evidence from event-related brain potentials. Brain Cognit. 1994;24:137–157. doi: 10.1006/brcg.1994.1008. [DOI] [PubMed] [Google Scholar]
- Salisbury DF, O’Donnell BF, McCarley RW, Nestor PG, Shenton ME. Event-related potentials elicited during a context-free homograph task in normal versus schizophrenic subjects. Psychophysiology. 2000;37:456–463. [PMC free article] [PubMed] [Google Scholar]
- Schwartz S. Is there a schizophrenic language? Behav Brain Sci. 1982;5:579–626. [Google Scholar]
- Semlitsch HV, Anderer P, Schuster P, Presslich O. A solution for reliable and valid reduction of ocular artifacts, applied to the P300 ERP. Psychophysiology. 1986;23:695–703. doi: 10.1111/j.1469-8986.1986.tb00696.x. [DOI] [PubMed] [Google Scholar]
- Simpson GB. Lexical ambiguity and its role in models of word recognition. Psychol Bull. 1984;96:316–340. [PubMed] [Google Scholar]
- Spitzer R, Williams J, Gibbon M, First M. The Structured Clinical Interview for DSM-IIIR (SCID) Washington, DC: American Psychiatric Association; 1990a. [Google Scholar]
- Spitzer R, Williams J, Gibbon M, First M. The structured clinical interview for DSM-IIIR (SCID-NP)-Non-Patient Edition. Washington, DC: American Psychiatric Association; 1990b. [Google Scholar]
- Spitzer M, Braun U, Hermle L, Maier S. Associative semantic network dysfunction in thought-disordered schizophrenic patients: direct evidence from indirect semantic priming. Biol Psychiatry. 1993;34:864–877. doi: 10.1016/0006-3223(93)90054-h. [DOI] [PubMed] [Google Scholar]
- Spitzer M, Weisker I, Winter M, Maier S, Hermle L, Maher BA. Semantic and phonological priming in schizophrenia. J Abnorm Psychol. 1994;103:864–877. doi: 10.1037//0021-843x.103.3.485. [DOI] [PubMed] [Google Scholar]
- Swinney DA. Lexical access during sentence comprehension: (re)consideration of context effects. J Verbal Learn Verbal Behav. 1979;18:645–659. [Google Scholar]
- Truscott I. Contextual constraint and schizophrenic language. J Consult Clin Psychol. 1970;35:189–194. doi: 10.1037/h0030122. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Manual for the Wechsler Adult Intelligence Scale –Revised. Cleveland, OH: The Psychological Corp; 1981. [Google Scholar]
- Wollen K, Cox S, Coahran M, Shea D, Kirby R. Frequency of occurrence and concreteness ratings of homograph meanings. Behav Res Methods Instrument. 1980;12:8–15. [Google Scholar]