Abstract
Aging results in a general decline in the response to external insults, including acute inflammatory challenges. In young animals, the inflammatory response requires activation of the sympathetic system, including neurotransmitters such as ATP, and catecholamines (epinephrine and norepinephrine). To test whether aging affects activation of this axis, and whether this in turn might affect cytokine release, we administered lipopolysaccharide (LPS) i.p. to adult, middle-aged and aged Fisher 344 rats (6, 15 and 23-month old, respectively) and evaluated the early (0–12 hours) serum levels of Neuropeptide-Y (NP-Y), ATP and vanillyl mandelic acid (VMA, as an indirect measurement of catecholamine levels). In addition, we evaluated the association between these factors and serum levels of the cytokines tumor necrosis factor-alpha (TNFα)3 and interleukin-10 (IL-10). Induction of both ATP and NP-Y was markedly reduced in the serum of aged animals, when compared to their younger counterparts, while induction of VMA was not affected by age. In spite of these changes, serum levels of TNFα and IL-10 were strongly hyper induced and delayed in aged rats. The results suggest that during aging there is a dysregulation in sympathetic neurotransmitter regulatory mechanisms, and this might play a role in the impairment of the inflammatory response.
1. Introduction
Aging is associated with a general decline in the ability of the organism to respond to external insults, including acute inflammatory challenges (Yoshikawa, 2000). It has been suggested that changes in sympathoadrenal function might provide a possible explanation (Seals and Esler, 2000). The sympathetic nervous system, whose major neurotransmitters are catecholamines [epinephrine (E) and norepinephrine (NE)], adenosine triphosphate (ATP), and neuropeptide Y (NPY) (Burnstock and Knight, 2004), is a key modulator of cardiovascular, metabolic and other physiological functions in humans, and it plays a critical role in the maintenance of vascular tone both under basal conditions and in response to acute stress (Burnstock and Knight, 2004; Seals and Esler, 2000). Thus, it has been suggested that the sympathoadrenal system may be involved in the increase in cardiovascular disease observed in elderly populations (Marin and Rodriguez-Martinez, 1999; Seals and Esler, 2000).
The sympathoadrenal system also plays an important role as an integrative interface between the nervous and immune systems (Black, 1994; Elenkov et al., 2000). Sympathetic nerves innervate secondary lymphoid organs, and are able to deliver sympathetic neurotransmitters (s-NT) in the close vicinity of immune cells where they are recognized by specific s-NT receptors present in those cells (Elenkov et al., 2000; Sanders and Straub, 2002; Webster et al., 2002). These s-NT regulate the immune response, acting both as inhibitors or activators, depending on cell type and available receptors (Bergmann and Sautner, 2002; Burnstock, 2006b; Burnstock, 2007; Prod’homme et al., 2006; Straub et al., 2001)
The interplay between aging and the sympathetic system has been understudied. In humans, healthy aged individuals show elevated basal levels of epinephrine under resting conditions. It has been shown that this effect is mediated by changes in clearance and spillover, rather than changes at the level of synthesis or secretion (Madden et al., 1998; Marin and Rodriguez-Martinez, 1999; Seals and Esler, 2000). In contrast, both increased and decreased serum levels of NE have been described in response to several stressors in the aged compared to younger counterparts (Seals and Esler, 2000). On the other hand, both NPY and adenosine levels have barely been studied in aging. A decrease in the number of NPY positive nerves has been described in several tissues from aging mice (Marin and Rodriguez-Martinez, 1999). A diminished functional response to adenosine nucleotides has also been reported. However, nucleotide levels have not been studied (Burnstock, 2007; Marin and Rodriguez-Martinez, 1999).
Changes in sympathetic activity and disruption of neuroimmune communication during aging could lead to functional alterations in the inflammatory response in the elderly, such as deregulation of cytokine production (Bruunsgaard et al., 2001; Effros et al., 1991; Gomez et al., 2005; Grimble, 2003; Krabbe et al., 2004). Supporting a potential role of neurotransmitters on the deregulated inflammatory response, immune cells from aged individuals show a differential response to exogenous administration of s-NTs. In adult mice, for example, NPY and NE separately exhibit an inhibitory effect on the Con A-induced proliferative response, either alone or jointly with LPS (Medina et al., 2000). Similarly, Puerto et al. (2005) described an intricate regulatory effect of neurotransmitters on TNFα production by macrophages in vitro: while in adults NPY has an inhibitory effect on TNFα production, this effect was not observed in macrophages derived from old mice. In contrast, NE decreased TNFα levels only in old animals (Puerto et al., 2005). Similar studies in vivo have not been reported.
Our aim was to analyze whether there is a relationship between s-NTs and cytokines in a model of systemic inflammation in vivo. Therefore we examined serum levels of several s-NTs [NPY, catecholamines {detected as vanillyl mandelic acid (VMA), ATP and its metabolites adenosine, ADP and AMP}] as well as cytokines (TNFα and IL-10), in rats of three different ages, given either phosphate buffer saline (PBS) or LPS. The results indicate that aged rats show a significantly impaired release of ATP and NPY, with no change in VMA or ATP metabolites relative to younger counterparts. In spite of this decrease in activating NTs, the response at the level of both cytokines, TNFα and IL-10 was found to be exacerbated in aged rats in response to LPS. Our work revealed an age-related loss in the complex relationship between neurotransmitters and cytokines, a dysfunction that should be a contributing factor to the altered inflammatory response during aging.
2. Materials and Methods
Animals and treatment
Male Fisher 344 rats of different ages were obtained from the National Institute of Aging colony at Harlan Laboratories (Indianapolis, IN). The animals were housed individually in a specific-pathogen free facility at the Lankenau Institute for Medical Research, and they were fed adlibitum with laboratory chow. Rats were sacrificed either at the age of 6 months (adult), 15 months (middle-aged) or 22–23 months (aged). All animal protocols were approved by the ethical committee of the Lankenau Institute for Medical Research. Five rats of each age were intraperitoneally (i.p.) injected with 2 mg/kg LPS from Pseudomonas aeruginosa serotype 10 (Sigma Chemical Co., St. Louis, MO, lot number 99H4059), dissolved in sterile PBS at 5 mg/mL as described previously (Acuna-Castillo et al., 2006). The LPS dose used in these experiments did not produced mortality in any of the age groups, at least within the 12 h of the experiments. Control rats received a similar volume of sterile PBS and were sacrificed immediately. LPS-injected animals were sacrificed 0.5, 1, 2, 4, 6, and 12 h after endotoxin administration, blood was collected and allowed to clot during 10 min. Serum was obtained after standard centrifugation, and serum aliquots of 105 μL were stored at −80 °C.
Determination of serum cytokines and neurotransmitters
TNFα and IL-10 were detected using enzyme-linked immunosorbent assay (ELISA) OptEIA kits (Cat. Nos.: 2697KI; 2611KI; respectively, BD Pharmingen, San Diego, CA, USA), according to the manufacturer’s instructions. The detection limits were 31.3 pg/mL for TNFα, and 15.6 pg/mL for IL-10. Microplates were read at 450 nm and 570 nm, the latter for optical imperfection correction.
Determination of serum sympathetic neurotransmitters
Thirty μL of serum were used to measure catecholamines, NPY, ATP, and their respective metabolites. Twenty pmol of 3,4-dihydroxybenzylamine (DHBA) as internal standard were added to 30 μl of serum, then the samples were concentrated by Sep-Pak cartridge (Waters); elution separated nucleotides, NE and its metabolites, and NPY. The samples were dried by speedvac, and stored at −20 °C.
Catecholamines detection
Samples were reconstituted in 200 μl of HPLC-grade water, and catecholamines were quantified by HPLC coupled to an electrochemical detector (Donoso et al., 1997). Calibration yielded linearity from 1 to 300 pmol/mL.
Detection of Purines
Total purines eluted from Sep-Pak and dried were reconstituted in 220 μL of HPLC water, and detected by fluorescence emission after chemical reaction with chloroacetaldehyde to form a fluorescent adduct. This was a modification of a method by Levitt et al., 1984, who originally described a procedure for the detection of 1,N6-etheno-derivatives of purines (Levitt et al., 1984). In brief, 100 μL of citrate phosphate buffer (pH 4) was added to 200 μL of reconstituted sample. In a fume hood 10 μL of 2-chloroacetaldehyde were added to the samples; and the samples were heated in a dry-bath for 40 min at 80 °C. The purines were separated by using an intelligent model L-6210 inert pump gradient system (Merck-Hitachi) using a LiChrospher 100 RP-18 (Merck) HPLC column in phosphate buffer (pH 3), and detected using a fluorescence spectrophotometer detector (260 nm excitation, and 410 nm emission).
NPY detection
The samples containing NPY were reconstituted in 370 μL of buffer RIA and quantified by RIA as described (Donoso et al., 1997). The linearity of the RIA ranged from 1 to 100 fmol/assay tube. The inflammatory status of the animals was determined by measuring serum levels of acute phase proteins (alpha-2 macroglobulin, alpha-1 acid glycoprotein, and haptoglobin), which were barely detectable. Overall, the absence of these mediators discarded an ongoing inflammatory process in the animals.
Data acquisition and Analysis
Point-to-point analysis was carried out by means of the Mann Whitney test. Curve analysis was made using the Friedman and Quad test, with Dunn’s post test. Correlation analysis was performed with a contingency table, through Fisher’s exact test. In order to confirm the existence of statistical analysis association, contingency tables were made only for factors that were induced in at least two age groups, and a Fisher exact test was then applied. Curve normalization was analyzed only where kinetic inductions were observed. Statistical analysis and correlation analysis were carried out considering the average value obtained for the maximal TNFα induction in young rats serum (<3000 ng/mL), lower IL-10 induced levels (<500 pg/mL), basal levels of VMA in all age groups (<12 pmol/mL), and ATP age average induced levels (<3 pmol/mL) in aged rats, ADO basal levels, ADP basal levels and NPY average basal levels in all age groups. All the data were analyzed using the GraphPad Prism Version 2.0 statistical package (San Diego, CA, USA). Data were expressed as mean ± SEM, obtained form at least 3–5 different animals. Statistical differences were considered with p < 0.05.
3. Results
Age-related changes in neurotransmitter induction in response to LPS
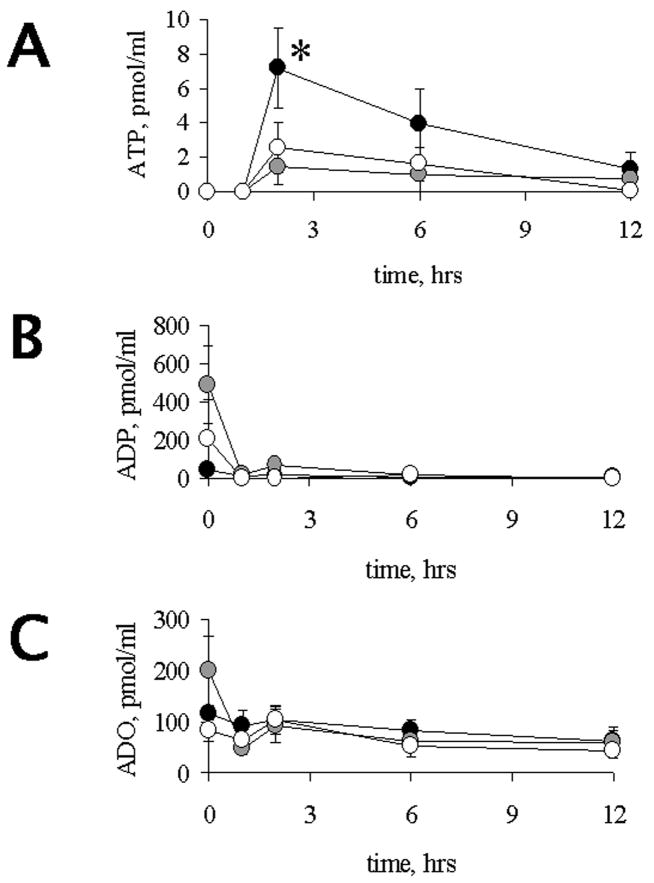
Our first aim was to find out whether aging affects the release of NTs in response to an i.p. challenge with LPS. Regardless of age, an increase in ATP levels was observed at 2 h after treatment (Figure 1A). This increase was more pronounced in adult rats than in either of the other age groups examined (p < 0.05). In all 3 groups, serum ATP levels returned to basal within 12 h post LPS. Regardless of age or treatment, serum levels of ADP and adenosine did not show significant changes (Figure 1B and 1C, respectively).
Figure 1.
Time-course of LPS-induced ATP (A), ADP (B) and ADO (C) levels in rats of different age groups. ADO, ADP and ATP were determined in serum of adult (black circles), 1middle-aged (grey circles) and 23 mo (open circles) old rats at various times after LPS challenge as shown, LPS was administered at 0 h time point. †,*, # indicate significant differences (p < 0.05) between adults (6 mo) and middle aged (15 mo), adults and aged (23 mo), or middle aged and aged, respectively. Each point was studied in 3–5 animals from the different age groups. Values, Means ± SEM.
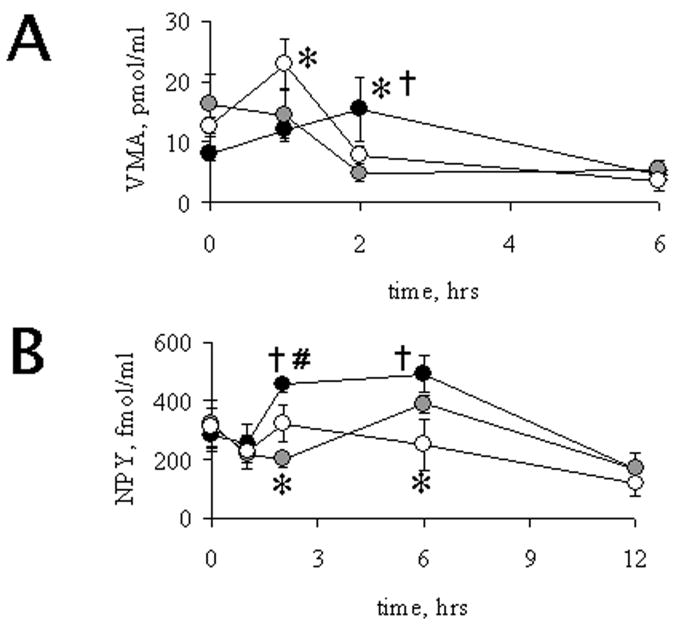
Catecholamines were detected by HPLC coupled to an electrochemical detector. The experimental retention time observed did not correspond to NE or E, and it was determined to correspond to VMA, a major metabolite of catecholamines (Eisenhofer et al., 1996). Basal levels of VMA were detectable in all age-groups and no age-related differences were found (p = 0.15). Following LPS administration, serum VMA levels were induced in adult and aged rats, and decreased after 6 h post LPS (Figure 2A). Interestingly, VMA was maximally induced at 2 h in adult rats, however, an earlier induction was observed in aged rats, with a peak at 1 h after LPS. Relative to the other age groups receiving LPS, middle-aged rats did not show changes in serum VMA when compared to their controls given PBS, and indeed a 60 % decrease was observed at 2 h after LPS. As shown in Figure 2B, adult animals had an increase in serum levels of NPY with a maximum at 2 h post LPS treatment. However, neither middle-aged nor aged animals showed significant changes in NPY levels. These results suggest that following LPS administration, advanced age inhibits the production of NPY, but has no effect on catecholamine production.
Figure 2.
Time-course of LPS-induced VMA (A) and NPY (B) levels in rats of different age groups. Rats aged 6 mo (black circles), 15 mo (grey circles) and 23 mo (open circles) were i.p. injected with LPS 2 m/kg and sacrificed after various times post challenge, LPS challenge was given at 0 h time point. Blood was collected and serum was obtained as described under methods. †,*, # indicate significant differences (p < 0.05) between adults (6 mo), middle-aged (15 mo) and aged (23 mo), or middle-aged and aged, respectively. Each point was studied in 3–5 animals from the different age groups. Values, Mean ± SEM.
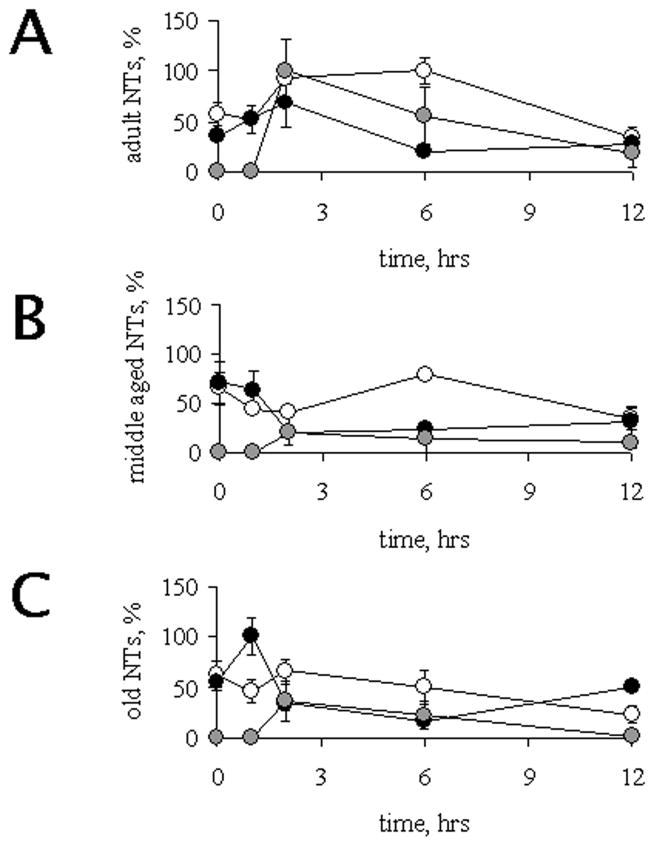
In summary, our results show an impairment of s-NT induction associated with advanced age. Figure 3 shows the same data, but graphed by age and normalized against the value observed in adult animals 2 h after LPS, which is the time of maximum induction of all neurotransmitters in this age group (Figure 3A). In middle-aged animals (Figure 3B), neither VMA nor NPY were induced in response to LPS, and ATP induction was less pronounced but faster (maximum at 1 h post LPS) than in younger rats. Finally, in (Figure 3C), NPY was also not induced, while catecholamine levels were transiently induced 1 h post-stimulus, and the maximum induction of ATP was found 2 h after LPS administration.
Figure 3.
Serum levels VMA, ATP and NPY. Data obtained from NPY (white circles), ATP (gray circles) and VMA (black circles) were normalized with respect to maximum values obtained among all age groups studied. Those correspond to values obtained from adult animals at 2h after LPS administration for NPY and ATP, and at 1h following LPS for aged rats for VMA. The normalized results were plotted in a time course according to age for adults (panel A), middle-aged (panel B) and aged (panel C) individuals. Values, Mean ± SEM.
Expression of TNFα and IL-10 in response to LPS is deregulated during aging
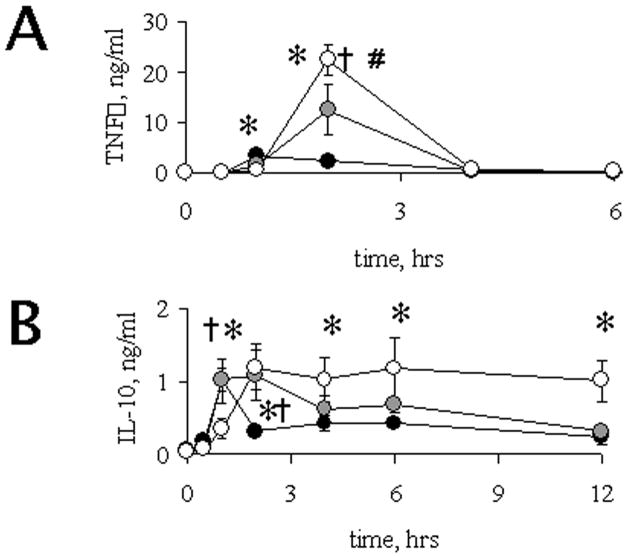
TNFα is a pro-inflammatory cytokine whose overproduction after inflammatory challenges has been described in advanced age in aged humans (Bruunsgaard et al., 2001) and mice (Tateda et al., 1996). However, the mechanism that explains this increase has not been clarified. LPS treatment led to an increase in serum levels of TNFα in rats of all ages (Figure 4A), and maximal induction was significantly higher in rats of advanced age (4-fold in middle-aged and 10-fold in aged rats), when compared to adult animals (p < 0.05). In addition, there were significant differences in the time course of induction. In adult rats, TNFα levels reached a maximum 1 h after LPS administration, while in middle-aged and aged rats the increase in TNFα was delayed, reaching its maximum 2 h after LPS treatment. In all cases, serum levels of TNFα returned to basal 4 h after endotoxin administration. These results show that, associated with aging, there is an elevation of maximum expression of TNFα, and a time delay in the induction of this cytokine in response to LPS administration.
Figure 4.
Time-course of LPS-induced TNFα (A) and IL-10 (B) levels in rats of different age groups. TNFα and IL-10 were determined in serum of 6 mo (black circles), 15 mo (grey circles) and 23 mo (open circles) old rats at various times after LPS challenge as shown, LPS challenge was given at 0 h in x axis. †,*, # indicate significant differences (p < 0.05) between adults (6 mo) and middle aged (15 mo), adults and aged (23 mo), or middle aged and aged, respectively. Each point was studied in 3–5 animals from the different age groups. Values, Means ± SEM.
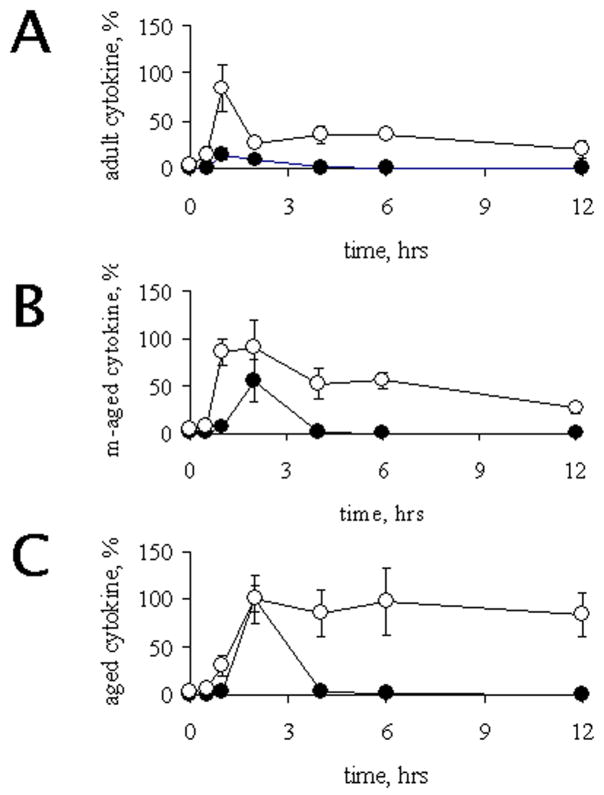
IL-10, a potent negative regulator of TNFα induction, has also been shown to be deregulated during aging in response to an inflammatory stimulus (Rajasingh et al., 2006; Tateda et al., 1996). To confirm this, we evaluated serum levels of IL-10 after LPS administration. Adult rats (Figure 4B) showed a transient induction of IL-10 at 1 h after LPS, returning to basal already by 2 h. In contrast, middle-aged and aged rats showed a delayed induction (by 2 h), but these levels remained high for longer, especially in the aged group. In summary, the induction of TNFα and IL-10 show similar kinetics, with maximum expression at either 1 h post LPS treatment (in young) or 2 h post LPS (in aged animals). Non-coordinated expression of the cytokines was observed in middle-aged rats. This is relevant since it has been proposed that IL-10 can lead to a lower production of TNFα (Rajasingh et al., 2006), and this regulatory circuit might be intact (though delayed) in aged animals (see Figure 5 compare A and C), as it has been proposed elsewhere (Tateda et al., 1996).
Figure 5.
Age related time-course of TNFα and IL-10. Data obtained from TNFα (black circles) and IL-10 (open circles) were normalized relative to maximum values obtained among all age groups studied (corresponding to old rats 2 hours in both cases). These results were plotted in a time course according to age. Panels show the summary of cytokines obtained for adults (panel A), middle-aged (panel B) and aged (panel C) individuals. Values, Mean ± SEM.
Catecholamines have been described as having a negative effect on the production of pro-inflammatory cytokines during inflammatory response, however the effects of aging on this association still are not evaluated. We first evaluated a possible correlation between TNFα and VMA. As shown in figures 3 and 5, a similar kinetics of induction was observed for VMA with TNFα. However, in both middle-aged and aged rats VMA was early induced, when compared to TNFα. In order to evaluate an association between the age-related defects in the serum levels of TNFa and VMA, their curves were superposed point-by-point, and a correlation was observed (R2= 0.425, p < 0.05). In order to analyze a possible relationship between the different effectors, we conducted Fisher exact tests on the various possible combinations. Analysis was developed using the maximum induction of TNFα and the lowest VMA levels as cut-off points, and the data was arranged in a contingence table, thereby the analysis includes animals showing serum levels of VMA y TNFα defined by the cut-off point described in Table 1. The data show a strong negative relationship between these two molecules (P<0.05). In contrast, no effect was observed between VMA and IL-10, or between any of the other NTs and either of the cytokines (data not shown). Finally, we examined the relationship between IL-10 and TNFα after LPS induction, and again, we found no correlation (Table 2). The complexity of the present results makes difficult to conclude age-related effects in the regulatory mechanisms of cytokine production. These changes could be related either with the levels of pro-inflammatory cytokines or with neurohumoral mechanisms.
Table 1.
Correlation between TNFα levels and VMA acid levels in serum. The thresholds were defined considering higher TNFα concentration from adult animals and VMA basal levels in adult animals
| VMA pmol/mL | |||
|---|---|---|---|
| <12 | ≥ 12 | ||
| TNFα ng/mL | < 3000 | 8 | 8 |
| > 3000 | 9 | 0 | |
|
|
|||
p< 0.05
Table 2.
Correlation between TNFα and IL-10 levels in serum. The thresholds were defined considering the highest TNFα concentrations and the half value of maximum IL-10 induction in adult animals
| IL-10 pg/mL | |||
|---|---|---|---|
| < 500 | ≥ 500 | ||
| TNFα ng/mL | < 3000 | 8 | 4 |
| > 3000 | 12 | 8 | |
|
|
|||
p > 0.05
4. Discussion
In this work we have established that the release of sympathetic NT induced by an acute inflammatory stress is deregulated in aged rats. The induction of ATP and NPY was hampered in old animals, while age had no effect on the kinetics of induction of serum catecholamines. In spite of this imbalance, we confirmed previous observations that indicate that aged rodents have a significantly exacerbated production of TNFα in response to LPS (Tateda et al., 1996). Furthermore, serum levels of TNFα did not appear to be negatively regulated by IL-10, since induction of IL-10 was sustained in time in old animals, compared to young ones, and yet TNFα levels were higher in aged rats. We conclude that the fine-tuning of cytokine production by the sympathetic nervous system is lost in advanced age. This is the first demonstration of an age-related impairment in the induction of catecholamines, ATP and its derivatives, and NPY, following an inflammatory challenge.
The neuroendocrine system plays a regulatory role in many physiological functions, and loss of this control could lead to imbalances in immune responses, vascular tone and in other systems and responses, which are negatively affected by advancing age. Indeed, several of the changes in immune response observed in the elderly resemble those seen following either chronic stress or glucocorticoid treatment, and the effect of both stress and aging upon the innate immune system shows a direct correlation with the functional integrity of the hypothalamus–pituitary–adrenal (HPA) axis [reviewed by (Bauer, 2005) (Elenkov et al., 2000)].
Dehydroepiandrosterone (DHEA) and catecholamines (Webster et al., 2002) have been described as candidates with a possible role during inflammation, however, the effect of aging on these inter relations is not an area of active research. It has been speculated that hypersecretion of glucocorticoids can impact the immune response by suppressing the release of pro-inflammatory cytokines such as IL-1β and TNF-α (Yeager et al., 2004). However, we and others have not observed changes in corticosterone levels in response to LPS as a function of age, thus hampering the possibility of its active role on the are-related defects of the inflammatory response (Tateda et al., 1996), (Gomez, C.R., Kovacs E.J., and Sierra, F., unpublished observations), however our results support the idea that catecholamines may be involved in the inflammatory dysregulation associated with aging. In contrast with other anti-inflammatory mediators associated with the HPA axis, we observed a negative correlation between TNFα and catecholamine levels, an observation that is in agreement with previous reports in which similar effects were described in the absence of the age variable (Bergmann and Sautner, 2002; Elenkov et al., 2000). Connor et al. have reported the existence of a regulatory mechanism by which catecholamines can negatively regulate TNFα production in an IL-10-independent pattern (Connor et al., 2005). Both NPY and adenosine nucleotide derivatives have also been shown to modulate the inflammatory response. For example, macrophages from NPY Y1 receptor deficient mice produce lowers levels of TNFα in response to LPS challenge relative to wild type mice given LPS (Wheway et al., 2007). In contrast, there does not appear to be a regulatory role of NPY over IL-10 production. In the case of adenosine nucleotide derivatives, it has been shown that ATP stimulates TNFα induction in macrophages throughout P2X7 receptors, while adenosine derivates induce more complex interactions with P2X receptors depending of the cell type and P2 receptor involved [Reviewed by (Burnstock, 2006a; Burnstock, 2006b; Di Virgilio et al., 2001)]. Finally, ATP and/or a metabolite released from LPS-activated microglia may induce IL-10 expression through P2Y purinergic receptors (Seo et al., 2008).
Catecholamines are known to exert inhibitory effects on the immune response and specifically on TNFα production (Beck et al., 2004). The fact that we measured a catecholamine metabolite (VMA) rather than intact catecholamines is a limitation of our studies. It is likely that our extraction method was responsible for our inability to observe intact catecholamines, because both adrenaline and noradrenalin levels are notoriously influenced by the sampling method (Grouzmann et al., 2003). Independent of the molecular source of VMA in our study (EPI, NE, or even dopamine), the fact that in adult animals we detected induction of VMA at the same time as NPY and ATP suggests a sympathetic origin, even though other sources (non- nervous) cannot be ruled out at the present time (Vizi and Elenkov, 2002).
In addition to the role of the sympathetic system described here, it has recently been established that the parasympathetic nervous system can also exert an anti-inflammatory effect. The discovery that cholinergic neurons can inhibit acute inflammation has qualitatively expanded our understanding of how the nervous system modulates immune responses. Stimulation of the vagus nerve significantly inhibits TNFα release in animals receiving lethal doses of endotoxin, and the authors established that the effect is attributable to acetylcholine (ACh), the main neurotransmitter of the vagus nerve [review by (Tracey, 2007)]. In addition, parasympathetic system has been described to be affected during aging (De Meersman and Stein, 2007), however possible changes on modulation of the immune response has not been studied. Our study supports previous findings showing age-related alterations in cytokine induction after an inflammatory challenge (Bruunsgaard et al., 2001; Tateda et al., 1996). This behavior is not exclusive for TNFα, and similar over-expression has been reported by us and others for other proinflammatory cytokines such as IL-6 (Gomez et al., 2006b; Tateda et al., 1996), and IL-1β (Tateda et al., 1996). While monocytes/macrophages are the main source of TNFα, many other cell types including adipocytes, microglia, Kupffer cells, keratinocytes, natural killer, and both B and T lymphocytes are able to produce this cytokine in response to several stimuli (Tracey and Cerami, 1993). Consequently, it is not clear which cells are responsible for the over-production of this cytokine in old animals.
The overexpression of these proinflammatory cytokines suggests a loss in upstream regulatory mechanisms during aging. While the major anti-inflammatory mechanism used to control the inflammatory status is IL-10 (Donnelly et al., 1999; Marchant et al., 1994; Rajasingh et al., 2006), the deregulation of TNFα seen in the elderly does not appear to be related to changes in the induction of IL-10 (Figure 4). Indeed, our data further suggests that aging is associated with a loosening of the ability of IL-10 to efficiently control TNFα induction. It has been proposed that the inhibitory effect of IL-10 over TNF-α occurs via inhibition of MAPK pathways, mainly p38 (Rajasingh et al., 2006). We find a correlation between p38 activity in whole spleen homogenates and splenic TNFα mRNA, but this correlation is lost as the animals get older (Acuña-Castillo C., Gomez CR., and Sierra F., unpublished observations). In summary, our results indicate that while adult animals appear to maintain IL-10-dependent anti-inflammatory control, the process of aging gradually leads to a loss of IL-10-mediated control of the expression of TNFα. A similar conclusion was reached by Tateda et al, though in their case no middle-aged animals were present (Tateda et al., 1996).
In a previous report we have shown that humoral factors that differ in the serum from young or old rats can modulate the ability of macrophages to produce TNFα in response to an LPS stimulus in vitro (Gomez et al., 2006a). Our current results are in agreement, and bring into possible play an additional set of humoral factors, namely, NTs released by the sympathetic nervous system. Our findings are only correlative and descriptive in nature, so no mechanistic conclusions can be drawn. However, these observations should pave the way for further experimentation, using pharmacological approaches and genetically modified rodents into the possible roles of NTs in the modulation of the immune response in the elderly. Considering the aging of current societies and the considerable loss in immune responsiveness observed in the elderly, this is a venue that will require further exploration in the near future.
Acknowledgments
We appreciate the assistance of Dr. Hugo Cárdenas with the statistical analysis and Dr. Ravi Shankar Ph.D. for critical reading of the manuscript. This work was supported by a Bicentenario para la Inserción de Jóvenes Investigadores a la Academia Grant; FONDECYT 11070177 (CAC); DICYT Grants (PN, KM, CAC, MI); FONDAP and MIFAB ( CC, DH, JO, JHT); and NIH/NIA AG13902 (VP, CT, CG, FS). This work was done in memory of Ricardo Burgos and Santiago Fernández.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Acuna-Castillo C, Leiva-Salcedo E, Gomez CR, Perez V, Li M, Torres C, Walter R, Murasko DM, Sierra F. T-kininogen: a biomarker of aging in Fisher 344 rats with possible implications for the immune response. J Gerontol A Biol Sci Med Sci. 2006;61:641–9. doi: 10.1093/gerona/61.7.641. [DOI] [PubMed] [Google Scholar]
- Bauer ME. Stress, glucocorticoids and ageing of the immune system. Stress. 2005;8:69–83. doi: 10.1080/10253890500100240. [DOI] [PubMed] [Google Scholar]
- Beck G, Brinkkoetter P, Hanusch C, Schulte J, van Ackern K, van der Woude FJ, Yard BA. Clinical review: immunomodulatory effects of dopamine in general inflammation. Crit Care. 2004;8:485–91. doi: 10.1186/cc2879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergmann M, Sautner T. Immunomodulatory effects of vasoactive catecholamines. Wien Klin Wochenschr. 2002;114:752–61. [PubMed] [Google Scholar]
- Black PH. Immune system-central nervous system interactions: effect and immunomodulatory consequences of immune system mediators on the brain. Antimicrob Agents Chemother. 1994;38:7–12. doi: 10.1128/aac.38.1.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruunsgaard H, Pedersen M, Pedersen BK. Aging and proinflammatory cytokines. Curr Opin Hematol. 2001;8:131–6. doi: 10.1097/00062752-200105000-00001. [DOI] [PubMed] [Google Scholar]
- Burnstock G. Historical review: ATP as a neurotransmitter. Trends Pharmacol Sci. 2006a;27:166–76. doi: 10.1016/j.tips.2006.01.005. [DOI] [PubMed] [Google Scholar]
- Burnstock G. Pathophysiology and therapeutic potential of purinergic signaling. Pharmacol Rev. 2006b;58:58–86. doi: 10.1124/pr.58.1.5. [DOI] [PubMed] [Google Scholar]
- Burnstock G. Physiology and pathophysiology of purinergic neurotransmission. Physiol Rev. 2007;87:659–797. doi: 10.1152/physrev.00043.2006. [DOI] [PubMed] [Google Scholar]
- Burnstock G, Knight GE. Cellular distribution and functions of P2 receptor subtypes in different systems. Int Rev Cytol. 2004;240:31–304. doi: 10.1016/S0074-7696(04)40002-3. [DOI] [PubMed] [Google Scholar]
- Connor TJ, Brewer C, Kelly JP, Harkin A. Acute stress suppresses pro-inflammatory cytokines TNF-alpha and IL-1 beta independent of a catecholamine-driven increase in IL-10 production. J Neuroimmunol. 2005;159:119–28. doi: 10.1016/j.jneuroim.2004.10.016. [DOI] [PubMed] [Google Scholar]
- De Meersman RE, Stein PK. Vagal modulation and aging. Biol Psychol. 2007;74:165–73. doi: 10.1016/j.biopsycho.2006.04.008. [DOI] [PubMed] [Google Scholar]
- Di Virgilio F, Chiozzi P, Ferrari D, Falzoni S, Sanz JM, Morelli A, Torboli M, Bolognesi G, Baricordi OR. Nucleotide receptors: an emerging family of regulatory molecules in blood cells. Blood. 2001;97:587–600. doi: 10.1182/blood.v97.3.587. [DOI] [PubMed] [Google Scholar]
- Donnelly RP, Dickensheets H, Finbloom DS. The interleukin-10 signal transduction pathway and regulation of gene expression in mononuclear phagocytes. J Interferon Cytokine Res. 1999;19:563–73. doi: 10.1089/107999099313695. [DOI] [PubMed] [Google Scholar]
- Donoso MV, Brown N, Carrasco C, Cortes V, Fournier A, Huidobro-Toro JP. Stimulation of the sympathetic perimesenteric arterial nerves releases neuropeptide Y potentiating the vasomotor activity of noradrenaline: involvement of neuropeptide Y-Y1 receptors. J Neurochem. 1997;69:1048–59. doi: 10.1046/j.1471-4159.1997.69031048.x. [DOI] [PubMed] [Google Scholar]
- Effros RB, Svoboda K, Walford RL. Influence of age and caloric restriction on macrophage IL-6 and TNF production. Lymphokine Cytokine Res. 1991;10:347–51. [PubMed] [Google Scholar]
- Eisenhofer G, Aneman A, Hooper D, Rundqvist B, Friberg P. Mesenteric organ production, hepatic metabolism, and renal elimination of norepinephrine and its metabolites in humans. J Neurochem. 1996;66:1565–73. doi: 10.1046/j.1471-4159.1996.66041565.x. [DOI] [PubMed] [Google Scholar]
- Elenkov IJ, Wilder RL, Chrousos GP, Vizi ES. The sympathetic nerve--an integrative interface between two supersystems: the brain and the immune system. Pharmacol Rev. 2000;52:595–638. [PubMed] [Google Scholar]
- Gomez CR, Acuna-Castillo C, Nishimura S, Perez V, Escobar A, Salazar-Onfray F, Sabaj V, Torres C, Walter R, Sierra F. Serum from aged F344 rats conditions the activation of young macrophages. Mech Ageing Dev. 2006a;127:257–63. doi: 10.1016/j.mad.2005.10.002. [DOI] [PubMed] [Google Scholar]
- Gomez CR, Boehmer ED, Kovacs EJ. The aging innate immune system. Curr Opin Immunol. 2005;17:457–62. doi: 10.1016/j.coi.2005.07.013. [DOI] [PubMed] [Google Scholar]
- Gomez CR, Goral J, Ramirez L, Kopf M, Kovacs EJ. Aberrant acute-phase response in aged interleukin-6 knockout mice. Shock. 2006b;25:581–5. doi: 10.1097/01.shk.000029553.39081.ec. [DOI] [PubMed] [Google Scholar]
- Gomez CR, Nomellini V, Faunce DE, Kovacs EJ. Innate immunity and aging. Exp Gerontol. 2008;43:718–728. doi: 10.1016/j.exger.2008.05.0168.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimble RF. Inflammatory response in the elderly. Curr Opin Clin Nutr Metab Care. 2003;6:21–9. doi: 10.1097/00075197-200301000-00005. [DOI] [PubMed] [Google Scholar]
- Krabbe KS, Pedersen M, Bruunsgaard H. Inflammatory mediators in the elderly. Exp Gerontol. 2004;39:687–99. doi: 10.1016/j.exger.2004.01.009. [DOI] [PubMed] [Google Scholar]
- Levitt B, Head RJ, Westfall DP. High-pressure liquid chromatographic-fluorometric detection of adenosine and adenine nucleotides: application to endogenous content and electrically induced release of adenyl purines in guinea pig vas deferens. Anal Biochem. 1984;137:93–100. doi: 10.1016/0003-2697(84)90352-x. [DOI] [PubMed] [Google Scholar]
- Madden KS, Thyagarajan S, Felten DL. Alterations in sympathetic noradrenergic innervation in lymphoid organs with age. Ann N Y Acad Sci. 1998;840:262–8. doi: 10.1111/j.1749-6632.1998.tb09566.x. [DOI] [PubMed] [Google Scholar]
- Marchant A, Bruyns C, Vandenabeele P, Ducarme M, Gerard C, Delvaux A, De Groote D, Abramowicz D, Velu T, Goldman M. Interleukin-10 controls interferon-gamma and tumor necrosis factor production during experimental endotoxemia. Eur J Immunol. 1994;24:1167–71. doi: 10.1002/eji.1830240524. [DOI] [PubMed] [Google Scholar]
- Marin J, Rodriguez-Martinez MA. Age-related changes in vascular responses. Exp Gerontol. 1999;34:503–12. doi: 10.1016/s0531-5565(99)00029-7. [DOI] [PubMed] [Google Scholar]
- Medina S, Del Rio M, Hernanz A, De la Fuente M. Age-related changes in the neuropeptide Y effects on murine lymphoproliferation and interleukin-2 production. Peptides. 2000;21:1403–9. doi: 10.1016/s0196-9781(00)00284-9. [DOI] [PubMed] [Google Scholar]
- Prod’homme T, Weber MS, Steinman L, Zamvil SS. A neuropeptide in immune-mediated inflammation, Y? Trends Immunol. 2006;27:164–7. doi: 10.1016/j.it.2006.02.003. [DOI] [PubMed] [Google Scholar]
- Puerto M, Guayerbas N, Alvarez P, De la Fuente M. Modulation of neuropeptide Y and norepinephrine on several leucocyte functions in adult, old and very old mice. J Neuroimmunol. 2005;165:33–40. doi: 10.1016/j.jneuroim.2005.03.021. [DOI] [PubMed] [Google Scholar]
- Rajasingh J, Bord E, Luedemann C, Asai J, Hamada H, Thorne T, Qin G, Goukassian D, Zhu Y, Losordo DW, Kishore R. IL-10-induced TNF-alpha mRNA destabilization is mediated via IL-10 suppression of p38 MAP kinase activation and inhibition of HuR expression. Faseb J. 2006;20:2112–4. doi: 10.1096/fj.06-6084fje. [DOI] [PubMed] [Google Scholar]
- Sanders VM, Straub RH. Norepinephrine, the beta-adrenergic receptor, and immunity. Brain Behav Immun. 2002;16:290–332. doi: 10.1006/brbi.2001.0639. [DOI] [PubMed] [Google Scholar]
- Seals DR, Esler MD. Human ageing and the sympathoadrenal system. J Physiol. 2000;528:407–17. doi: 10.1111/j.1469-7793.2000.00407.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sebastian C, Espia M, Serra M, Celada A, Lloberas J. MacrophAging: a cellular and molecular review. Immunobiology. 2005;210:121–6. doi: 10.1016/j.imbio.2005.05.006. [DOI] [PubMed] [Google Scholar]
- Seo DR, Kim SY, Kim KY, Lee HG, Moon JH, Lee JS, Lee SH, Kim SU, Lee YB. Cross talk between P2 purinergic receptors modulates extracellular ATP-mediated interleukin-10 production in rat microglial cells. Exp Mol Med. 2008;40:19–26. doi: 10.3858/emm.2008.40.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straub RH, Cutolo M, Zietz B, Scholmerich J. The process of aging changes the interplay of the immune, endocrine and nervous systems. Mech Ageing Dev. 2001;122:1591–611. doi: 10.1016/s0047-6374(01)00289-5. [DOI] [PubMed] [Google Scholar]
- Tateda K, Matsumoto T, Miyazaki S, Yamaguchi K. Lipopolysaccharide-induced lethality and cytokine production in aged mice. Infect Immun. 1996;64:769–74. doi: 10.1128/iai.64.3.769-774.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tracey KJ. Physiology and immunology of the cholinergic antiinflammatory pathway. J Clin Invest. 2007;117:289–96. doi: 10.1172/JCI30555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tracey KJ, Cerami A. Tumor necrosis factor: an updated review of its biology. Crit Care Med. 1993;21:S415–22. [PubMed] [Google Scholar]
- Vizi ES, Elenkov IJ. Nonsynaptic noradrenaline release in neuro-immune responses. Acta Biol Hung. 2002;53:229–44. doi: 10.1556/ABiol.53.2002.1-2.21. [DOI] [PubMed] [Google Scholar]
- Webster JI, Tonelli L, Sternberg EM. Neuroendocrine regulation of immunity. Annu Rev Immunol. 2002;20:125–63. doi: 10.1146/annurev.immunol.20.082401.104914. [DOI] [PubMed] [Google Scholar]
- Wheway J, Herzog H, Mackay F. The Y1 receptor for NPY: a key modulator of the adaptive immune system. Peptides. 2007;28:453–8. doi: 10.1016/j.peptides.2006.09.030. [DOI] [PubMed] [Google Scholar]
- Wu D, Meydani SN. Age-associated changes in immune and inflammatory responses: impact of vitamin E intervention. J Leukoc Biol. 2008 doi: 10.1189/jlb.0108023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeager MP, Guyre PM, Munck AU. Glucocorticoid regulation of the inflammatory response to injury. Acta Anaesthesiol Scand. 2004;48:799–813. doi: 10.1111/j.1399-6576.2004.00434.x. [DOI] [PubMed] [Google Scholar]
- Yoshikawa TT. Epidemiology and unique aspects of aging and infectious diseases. Clin Infect Dis. 2000;30:931–3. doi: 10.1086/313792. [DOI] [PubMed] [Google Scholar]