Abstract
Context
Recently asymptomatic middle-aged adult children of patients with Alzheimer Disease (AD) were found to exhibit fMRI deficits in the mesial temporal lobe during an encoding task. Whether this effect will be observed on other fMRI tasks is not yet known. This study examines the neural substrates of self-appraisal in people at risk for AD. Accurate appraisal of deficits is a problem for many AD patients, and prior fMRI studies of healthy young adults indicates that brain areas vulnerable to AD such as the anterior mesial temporal lobe and posterior cingulate are involved during self appraisal tasks.
Objective
To determine whether parental family history of AD (FH) or the ε4 allele of the Apolipoprotein E gene (APOE4) exert independent effects on brain function during self-appraisal.
Design
Cross-sectional factorial design in which APOE4 status (present/absent) was one factor, and FH status was the other. All participants received cognitive testing, genotyping and an fMRI task that required subjective self-appraisal (SA) decisions regarding trait adjective words in comparison to semantic decisions about the same words.
Setting
An academic medical center with a research-dedicated 3.0 Tesla MRI facility.
Participants
Cognitively normal middle-aged adults (N=110): 51 +FH; 59 −FH.
Outcome measure
Blood oxygen-dependent contrast measured with T2* weighted echo-planar imaging.
Results
FH and APOE4 status interacted in the posterior cingulate as well as left superior and medial frontal regions. There were main effects of FH (−FH > +FH) in left hippocampus, and ventral posterior cingulate. There were no main effects of APOE.
Conclusion
These results suggest that a parental history of AD may influence brain function during subjective self-appraisal in regions commonly affected by AD. Although these participants were asymptomatic and middle-aged, the findings suggest there may be subtle alterations in brain function attributable to AD risk factors.
Neuropathology studies of people at risk for Alzheimer disease (AD) suggest that AD may be preceded by a silent preclinical phase in which the brain incurs neuropathological change.1–4 Both the apolipoprotein E gene (APOE) ε4 allele (APOE4) and first-degree family history (FH)5 are risk factors for developing late onset AD.6 Identifying initial brain changes in at-risk individuals with non-invasive functional imaging may help elucidate pre-clinical presence and progression of early AD.
Most prior fMRI studies in populations at risk for AD have focused on the hippocampus with episodic memory tasks that draw on encoding or retrieval processes7–15 because memory symptoms are among the earliest to occur in AD. We have recently shown that asymptomatic middle-aged adults (mean age 55) who have a parent with AD exhibit reduced BOLD responses in the mesial and ventral temporal lobe during an encoding task12 and this effect was not explained by APOE. The findings suggested that memory-related brain changes attributable to FH may be occurring in regions vulnerable to AD approximately two decades prior to the typical age of onset in sporadic AD.
Other early symptoms of AD may involve high-level executive functions such as metacognitive appraisal. Studies on executive functions have often operationalized the construct to the cognitive control processes that subserve executive function, including imperviousness to distraction, inhibition of prepotent responses, working memory, and mental flexibility and speed. These well-studied functions have generally been attributed to dorsolateral prefrontal cortex.16 The metacognitive aspects of executive function are less well studied and include processes such as self-monitoring, self appraisal (the focus of the present report), planning prospective action, social tuning of one’s behavior for adaptive functioning in the world of people (judgment), and in making inferential or subjective decisions.
Prior studies have implicated cortical midline structures including the anterior medial prefrontal cortex (AMPFC) and posterior cingulate for metacognitive processes,17–19 particularly the processes of self-monitoring of actions and bodily states, self-regulation of affect, self-reflection on abilities and traits, and social cognition, such as making inferences about the mental states of others.20 The hippocampus has also been implicated in cognitive self-appraisal.21 Neuroimaging studies of the posterior cingulate have found decreased cerebral metabolic rate of glucose metabolism (rCMRglu),22–24 cerebral atrophy, 22, 25 and amyloid binding 22, 26 in people with AD. In two studies, people at risk for AD also exhibited reductions in CMRglu in the posterior cingulate.4, 24 Furthermore, in AD, “resting state” abnormalities have been found in the posterior cingulate and medial frontal lobe as well as hippocampus.22, 27, 28
No studies have yet examined metacognitive brain function in healthy people at risk for AD. This may be a useful avenue of study since AD patients often exhibit deficits in metacognitive abilities29 such as appreciating the extent or severity of their deficits. The purpose of the present study was to determine whether we could observe AD risk-associated differences in BOLD activity during a self-referential decision task that consistently evokes BOLD activity from the posterior cingulate, medial frontal lobe and mesial temporal lobes across prior studies of healthy adults.19, 30–32 We examined brain activation in 110 physically and cognitively asymptomatic middle-aged adults who differed on the presence of parental family history (FH) and APOE4 genotype. A 2 × 2 factorial design was used to examine the relative contribution of APOE and FH on the cerebral response. Based on our prior findings, we expected that parental family history of AD would have an effect on cerebral activation that was separable from APOE in cortical midline brain regions and hippocampus.
Methods
Participants
One hundred ten subjects underwent fMRI scanning and cognitive testing (Table 1). Fifty-one (mean age 53.6; SD 6.4) had at least one parent with AD (+FH) and were recruited from the Wisconsin Registry for Alzheimer’s Prevention,33 a longitudinal registry of cognitively normal adults between the ages of 40–65 (at entry) who have at least one parent with sporadic AD. To verify the diagnosis of AD in the parent, parental medical records were obtained and reviewed by a multidisciplinary diagnostic consensus panel. Typically, the clinical work-up and diagnosis in the parent were conducted at the University of Wisconsin Memory Clinics and the adult children were then approached for participation. The average age of symptom onset in the affected parent was 73. All subjects in the +FH group underwent baseline neuropsychological evaluations and laboratory tests that included APOE genotyping using PCR and sequencing. Fifty-three percent were ε4 positive (ε3/ε3=24 ; ε3/ε4=20; ε4/ε4=7).
Table 1.
Demographic Neuropsychological and Performance Data for Each Group
| Negative Family history | Positive Family history | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| ε4 neg | ε4 pos | ε4 neg | ε4 pos | ||||||
| mean | SD | mean | SD | mean | SD | mean | SD | ||
| N | 47 | -- | 12 | -- | 24 | -- | 27 | -- | -- |
| Age | 55.2 | 6.3 | 55.7 | 5.8 | 52.2 | 6.6 | 54.9 | 6.0 | NS |
| Education | 16.2 | 2.6 | 17.1 | 2.2 | 16.3 | 2.5 | 16.8 | 2.3 | NS |
| Gender M / F | 9 / 38 | -- | 7 / 5 | -- | 11 / 13 | -- | 13 / 14 | -- | a |
| Neuropsychological Function | |||||||||
| WRAT-Reading | 109.0 | 8.3 | 108.5 | 7.6 | 108.3 | 8.9 | 108.15 | 7.6 | NS |
| RAVL1-5 | 50.2 | 7.3 | 49.1 | 7.3 | 51.7 | 7.2 | 47.3 | 8.7 | NS |
| RAVL7 | 10.0 | 2.6 | 9.8 | 2.3 | 10.7 | 3.0 | 8.9 | 2.9 | NS |
| Trail Making A | 27.0 | 8.2 | 31.5 | 8.6 | 24.9 | 6.5 | 29.5 | 7.5 | b |
| Trail Making B | 63.3 | 21.4 | 60.1 | 21.5 | 50.5 | 13.3 | 64.3 | 20.8 | NS |
| Controlled Oral word | 43.6 | 10.0 | 48.2 | 11.6 | 43.0 | 10.8 | 44.6 | 10.0 | NS |
| Boston Naming Test | 57.3 | 3.1 | 57.7 | 3.6 | 57.5 | 2.5 | 57.5 | 1.9 | NS |
| CES-Depression Scale | 5.0 | 5.8 | 6.3 | 5.1 | 3.9 | 4.0 | 5.5 | 5.6 | NS |
| Hemoglobin | 13.87 | .81 | 14.00 | 1.34 | 14.00 | 1.08 | 14.12 | .87 | NS |
| Systolic blood pressure | 130.4 | 16.3 | 127.6 | 17.0 | 129.7 | 14.9 | 133.5 | 15.9 | NS |
| Performance in the Scanner | |||||||||
| Reaction time Self Appraisal | 1.58 | .21 | 1.61 | .19 | 1.60 | .29 | 1.62 | .22 | NS |
| Reaction time Semantic | 1.69 | .26 | 1.77 | .21 | 1.70 | .31 | 1.74 | .27 | NS |
* Chi Square statistic p<.05.
significant main effect of APOE p<.05.
WRAT=Wide Range Achievement Test-III; RAVL=Rey Auditory Verbal Learning Test; CES=Center for Epidemiologic Studies
A group of 59 participants (mean age 55.3; SD 6.2) with no family history of AD (−FH) were recruited from the community and matched to the demographic characteristics of the +FH sample. Absence of first-degree family history of AD was determined through self-report of the participant through phone interview as well as on a detailed medical history questionnaire. To be included in the −FH group, both parents had to survive to at least age 70 (most were well beyond this) and not carry a diagnosis of dementia or exhibit frank symptoms of dementia of any kind. Twelve (20%) of the controls were ε4 positive (ε3/ε4 = 11; ε4/ε4 = 1); 47 were ε4 negative (all ε3/ε3).
The demographics of the ε4 positive and negative subgroups are shown in Table 1 along with neuropsychological and fMRI task performance. We only included participants who had the ε4 or ε3 allele of APOE (21 participants with ε2 alleles were excluded). This was done in order to control for potential heterogeneity among genotypes. The proportion of women in the cells differed; therefore gender was used as a covariate in the fMRI data analysis. Exclusions for this imaging study included MRI scanner incompatibility, history of major psychiatric disease (e.g., schizophrenia; substance dependence; current or recent major depression) or major medical conditions (e.g., history of neurological disorders including prior head trauma with loss of consciousness, cancer requiring chemotherapy or radiation, insulin dependent diabetes) or abnormal structural MRI or neuropsychological testing as part of study participation. Most psychoactive drugs were excluded, though we did allow low dose selective serotonin reuptake inhibitors if stable for more than three months.
All subjects in this study received another fMRI task of episodic encoding that has been reported on previously.12 Participation in this study was contingent on signed informed consent, and the study was conducted in accordance with the Declaration of Helsinki.
fMRI task
The fMRI paradigm has been described in detail in prior studies with healthy young adults30 and subjects with MCI.34 Briefly, the task requires participants to make yes/no decisions based on individually presented trait adjectives across two conditions: referential self-appraisal (SA) and non-referential affective/semantic decision (SEM). The same set of 30 adjectives was presented in the SA and SEM conditions in counterbalanced order. In the SA condition participants decided whether or not adjectives described their own personal traits and abilities, whereas in the non-referential SEM condition, they decided whether or not adjectives in the set were of positive valence. First presentation of each adjective was counterbalanced across conditions such that novelty was not confounded with condition order. Two equivalent forms of the task were presented sequentially (counterbalanced), each using separate adjective sets. Within each task run, each of the two conditions was presented in five pseudo-randomized cycles. Words were presented every 4 s (3 s on screen and 1 s interstimulus interval) in blocks of 6 per condition.
Imaging procedures
After higher order shimming, T2* weighted gradient-echo echoplanar images (EPI) were obtained: echo time 30 ms; repetition time (TR) = 2000 ms; flip angle = 90°; acquisition matrix = 64 × 64 voxels; field of view (FOV) = 240 mm. Thirty sagittal slices of the brain were acquired within the TR at each time point, with voxel resolution of 3.75 × 3.75 × 4 mm, 1 mm skip between slices. In both 4 min 8 s scanning trials, 124 time points were collected, of which 3 images acquired during the first 6 s were discarded (for a total of 242 reconstructed time points).
Residual magnetic field (Bo) inhomogeneity resulting in regional distortions are common with EPI. We corrected these by measuring 3D field maps across the brain (co-planar with the fMRI slices). This was accomplished by measuring the phase of non-EPI gradient echo images at 2 echo times (7 and 10 ms). The phase difference between the two echo images is proportional to the static field inhomogeneity.35 The warp calculation and correction36 was performed using the FMRIB Software Library (FSL3.2). Anatomic T1-weighted volumes and T2 weighted axial slices were also acquired using parameters previously described.12
Anatomic Imaging and VBM analysis
Axial T1 and T2 weighted images were acquired after the functional runs. A 3D IR-prepped fast gradient echo pulse sequence provided high-resolution T1-weighted structural images with the following parameters: inversion time = 600 ms, fast gradient echo read-out with TR/TE/flip = 9 ms/1.8 ms/20°; acquisition matrix = 256 × 192 × 124 (interpolated to 256 × 256 × 124); field of view = 240 mm; slice thickness = 1.2 mm (124 slices); ± 16 kHz receiver bandwidth.
A Fast Recovery Fast Spin Echo 2D T2-weighted axial sequence was also acquired with the same start and stop locations as the T1 weighted images. The parameters were: field of view = 240 mm, matrix 256 × 256, TR = 9000 ms, TE = 93 ms, flip angle = 90. Seventy slices were acquired; slice thickness = 1.7 mm with 0.3 mm skip. An experienced neuroradiologist examined all images prior to the analysis for clinical evidence of any neurovascular disease or structural abnormality that would exclude the subjects from the analysis.
Data Analysis
Other preprocessing steps and statistical analysis were accomplished with Statistical Parametric Mapping SPM2 software (www.fil.ion.ucl.ac.uk/spm). The image time-series was motion-corrected, field map corrected as described above, normalized into the Montreal Neurological Institute (MNI) standard space using the T2* weighted template provided through SPM2 (written out with 2×2×2 mm voxel resolution), and then smoothed with an 8 mm full-width at half-maximum Gaussian kernel.
The time-series data for individual participants were analyzed using a boxcar model convolved with the canonical hemodynamic response function (HRF) as implemented in SPM2.37 The statistical model included high frequency signal filtering (high pass filter = 128 seconds) and the AR1 method of estimating temporal autocorrelation. The SA versus SEM contrast was computed for each participant and entered into second level analyses.
The average effect of task (SA vs SEM), collapsed across groups, was first computed and thresholded at punc <.005 which corresponded to pFDR-corrected< .04. This map was written out (see Fig 1) and used to constrain the subsequent analyses of group differences to only those brain voxels that were relevant to the task. With ANCOVA, a 2×2 factorial analysis was conducted that examined between group effects of FH, APOE, and FH*APOE interactions. Gender was used as a covariate. This same design was also applied to the demographic and neuropsychological data. For the fMRI factorial analyses, statistical significance was also assessed at a voxel-level threshold of p < .005 uncorrected.
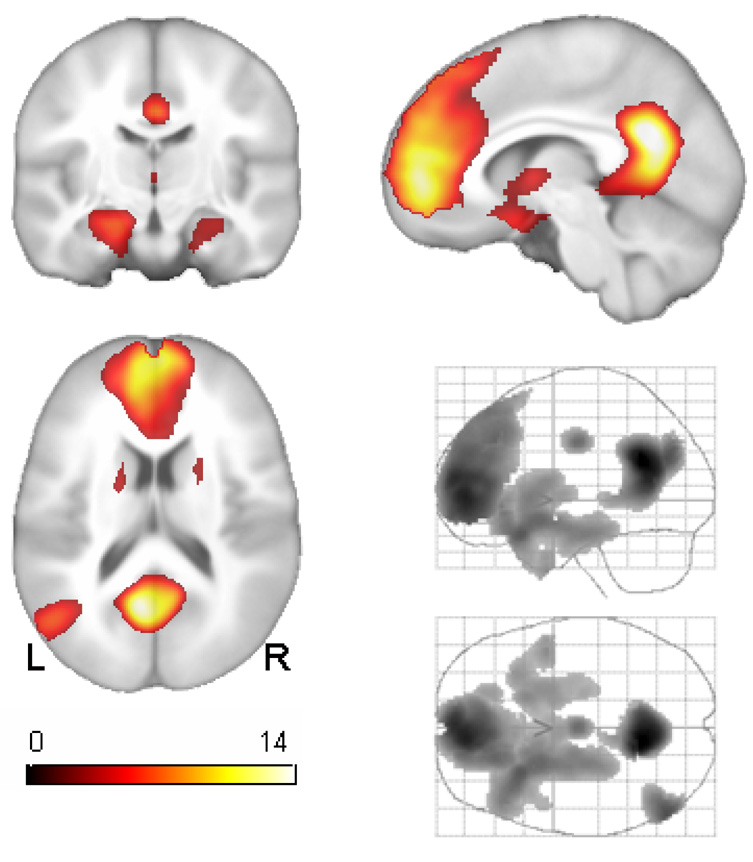
Figure 1.
Main effect of task across all 110 cognitively normal participants. Orthographic coronal, sagittal and axial views are shown as well as lateral and transverse maximum intensity projections of the result. Left is on left. The map is thresholded at pFDR <.05 corresponding to t>2.57. The main effect of task in this comparison was used to restrict inference on subsequent comparisons of risk status. See Table 2 for cluster locations and statistical details.
Anatomic analyses
In order to determine whether group activation differences were due to anatomical differences in gray matter volume, we conducted voxel-based morphometry (VBM) in the same search space and using the same model as the fMRI ANCOVA, but with the additional covariate of total gray matter volume. The procedures we used have been described in detail elsewhere.12, 38, 39
Results
Demographic characteristics and task performance are shown in Table 1. The Chi-Square statistic indicated the proportion of men and women differed in one of the groups and therefore gender was used as a covariate in subsequent behavioral and fMRI ANCOVAs. Factorial ANCOVA of demographic and neuropsychological indicated no APOE*FH interactions. Tests for main effects of APOE and FH in these healthy asymptomatic subjects were also non-significant with the exception of Trail Making Test part A on which the −ε4 subjects performed 3.7 s faster than the +ε4 subjects. There were no differences in the fMRI behavioral data with regard to reaction time and response bias.
Functional imaging findings
The Effect of Task
The average response to the task (SA > SEM) is shown in Figure 1 with statistics and MNI locations reported in Table 2. Active regions included two prominent midline clusters; the posterior cluster spans the ventral PCC and retrosplenial cortex (RSC).40 The large AMPFC cluster spans the medial surface of the superior frontal gyrus and rostral anterior cingulate. Also two large bilateral clusters were observed in the anterior mesial temporal lobe spanning the hippocampus and amygdala, and extending contiguously to the ventral forebrain and basal ganglia and thalamus. All comparisons in the subsequent factorial ANCOVA analysis were constrained to only those regions that were active in Figure 1. This procedure reduced the search region to 9.3% of the original number of voxels in the common brain mask. This was implemented to reduce the potential vulnerability to false positive errors and to ensure that subsequent results from group comparisons were interpretable with regard to the cognitive task.
Table 2.
Average Effect of the Appraisal Task
| Number of 2×2×2mm voxels | Voxel -T | x,y,z (MNI) | Location |
|---|---|---|---|
| 3649 | 15.92 | −10 −54 26 | posterior cingulate |
| 10149 | 13.17 | −6 54 2 | AMPFC |
| 742 | 9.86 | −52 −70 26 | left lateral parietal |
| 8943 | 9.52 | −28 20 −22 | left posterior orbital |
| 8.40 | −22 10 −14 | left nucleus acumbens | |
| 6.80 | −22 −18 −22 | left hippocampus | |
| 6.82 | −16 14 2 | left caudate | |
| 5.94 | 18 16 4 | right caudate | |
| 5.61 | 24 6 −12 | right nucleus acumbens | |
| 5.25 | 32 22 −24 | right lateral orbital | |
| 3.83 | 24 −10 −20 | right hippocampus | |
| 304 | 8.12 | 0 −10 36 | mid-cingulate |
| 87 | 6.49 | 28 −80 −40 | right cerebellum |
Note: All voxel-level t values are significant at punc <.005 (critical t 2.62) corresponding to pFDR<.04.
AMPFC—anterior medial prefrontal cortex
MNI—Montreal Neurological Institute standard space
The effect of risk factors
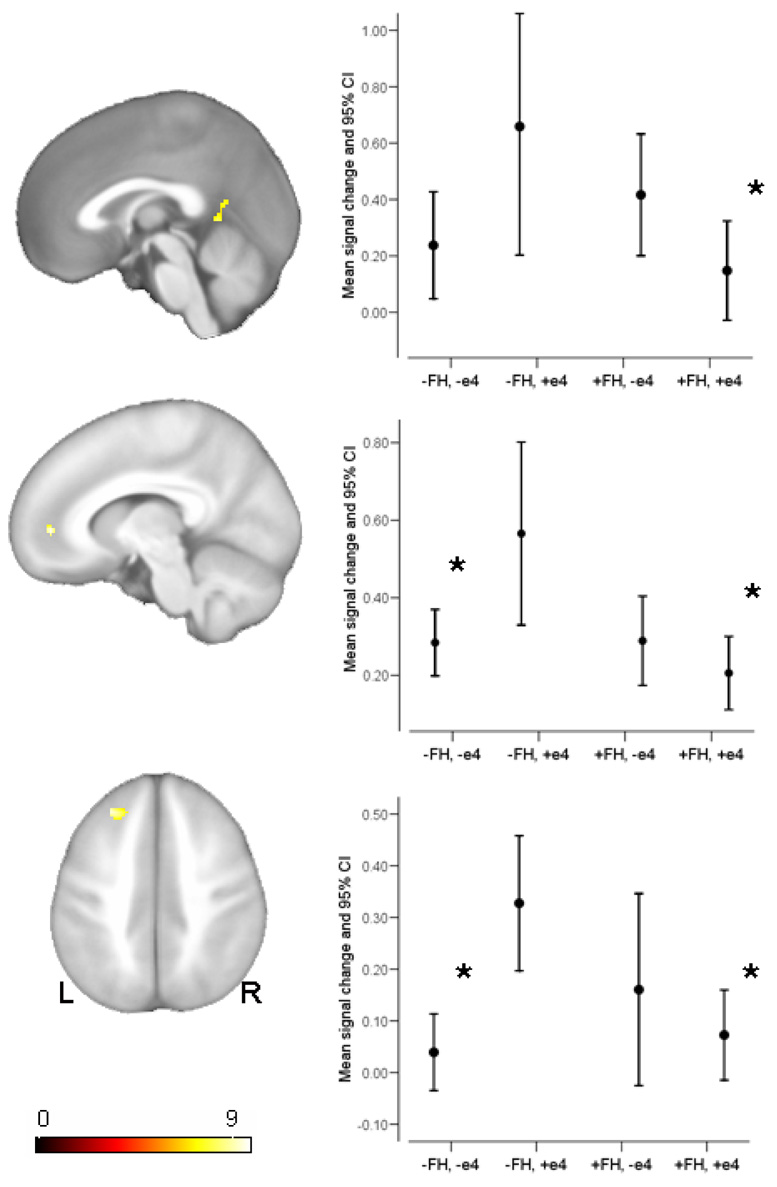
Using factorial ANCOVA, an F-test for the interaction between FH and APOE yielded prefrontal clusters at voxel location −26, 36, 36 (F=9.73, punc=.002; 102 voxels) in the left superior frontal gyrus, and at voxel location −10, 48, 2 (F=9.71, punc =.002; 26 voxels) in the left anterior cingulate. A third small cluster was found in the retrosplenial area of the posterior cingulate at voxel location 0, −50, 4 (F=8.26, punc =.005, 33 voxels). These clusters and associated plots of signal change are shown in Figure 2. Post-hoc analyses were conducted and significant mean differences are indicated on the plots. Group differences were only significant relative to the −FH,+e4 group. The other three groups did not differ from each other.
Figure 2.
The interaction between FH and APOE4 status. The three clusters that reached statistical significance are shown in sagittal and axial views. The corresponding plots depict the mean for the four groups (error bars 95% confidence interval), derived from the first eigenvariate of each subject across the entire cluster. A * indicates the mean differed significantly from the −FH,+e4 group (p<.005).
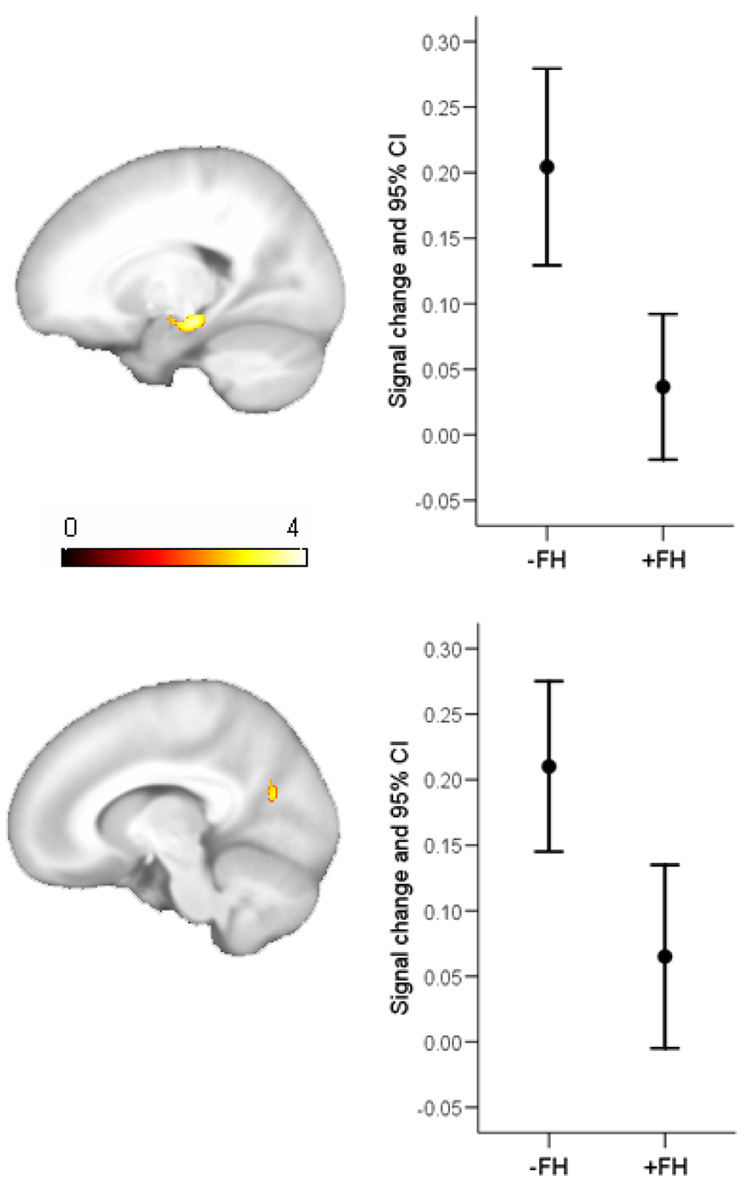
The main effects of FH and APOE were tested next. Because main effects are not readily interpretable in the presence of interactions, voxels that were identified as significant in the interaction map of Figure 2 were not considered in the analysis of main effects (this was achieved using the “mask with other contrasts” option in SPM2). The main effect of −FH > +FH was significant in the left hippocampus (x,y,z; −16 −22 −14; T=4.05; punc<.00001; 233 voxels) and the left ventral posterior cingulate (−14 −66 20; T=3.33; punc=.001; 50 voxels). These results are shown in Figure 3. No significant results were found in the reverse comparison (+FH > −FH). The effect of APOE was tested with the contrast +ε4 > −ε4 (and its reverse). Significant voxels in the interaction were again excluded. The results revealed no significant voxels in either contrast.
Figure 3.
Statistical parametric map of the main effect of first-degree family history. The figure shows regions where the −FH groups activate more on average than the +FH groups. Select brain slices are shown (left is on left) depicting the result in the posterior cingulate and subiculum with corresponding plots of the mean signal change, derived from the first eigenvariate across the cluster (error bars 95% confidence interval).
Anatomical Analysis
Using VBM no group differences in gray matter volume were found, suggesting that the fMRI findings above were not attributable to atrophy.
Discussion
This study examined the cerebral response during a metacognitive task, appraisal of the self on trait adjectives. We used this task in people at risk for AD because converging research has indicated that the regions normally responsive on this task 30–32 appear to overlap with brain regions affected by AD. Our analyses indicated differences in task-related activation associated with FH as well as regions where APOE and FH risk factors interacted. Parental FH of AD had the effect of diminishing the cerebral response in the ventral posterior cingulate and the left mesial temporal lobe. Although there were no main effects of APOE4, this risk factor interacted with FH in left dorsolateral prefrontal cortex, AMPFC, and retrosplenial posterior cingulate; plots indicated that e4 carriers who were −FH exhibited greater signal change to the task. The observed effects were not due to gray matter atrophy, nor global cognitive function.
The medial parietal cortex has been implicated in memory retrieval and recognition22, 41, 42 as well as metacognitive appraisals.19, 31, 32, 43–46 Several recent studies report medial parietal hypometabolism47 or hypoperfusion48 in individuals with Mild Cognitive Impairment (MCI), a diagnosis that confers considerable risk for developing AD. Longitudinal studies also indicate that posterior cingulate metabolism and regional blood flow discriminate between individuals with MCI who soon develop AD and those MCI patients who remain stable.49–51 Reiman and colleagues 4, 24 found that the medial parietal lobe including the posterior cingulate (PCC) and precuneus were hypometabolic for glucose in cognitively normal APOE4 carriers relative to noncarriers (the effect of FH was not tested in these earlier studies). The medial parietal findings observed in the present study during a cognitive challenge appear to be generally consistent with these prior results, and suggest that this region may be beginning to exhibit dysfunction in these asymptomatic middle-aged adults at risk for AD. As further evidence of this possibility, Ries et al studied amnestic MCI patients with the same paradigm reported here and with ratings of anosognosia. A significant positive correlation was found between insight and activation; MCI subjects who exhibit diminished insight for their cognitive impairment also exhibit diminished responses in the posterior cingulate and mesial frontal lobe.34 The data from Ries et al. and the present study suggest that risk factors for AD are influencing systems supporting metacognition, which may eventually become part of the symptom picture of AD.
Areas of the left MTL were also differentially active in −FH subjects on this task. In a young adult sample we have recently shown that this region of the hippocampus exhibits task-dependent functional connectivity with the anterior medial prefrontal cortex on this same task.30 The hippocampus and subiculum are densely connected to the medial frontal lobe 52, 53 in rhesus monkeys. Phillips et al21 include the hippocampus in the dorsal axis of an emotion appraisal model (also involving the dorsomedial and dorsolateral frontal lobe) that receives biasing self-relevant input from ventral structures including the amygdala, nucleus accumbens and ventral medial frontal lobe.17 Interestingly, amyloid burden in the mesial temporal lobe has been found to be related to the degree of anosognosia in patients with AD.54 The role of the hippocampus in affective and cognitive appraisal and how this might relate to the symptom picture of AD is not completely understood and deserving of much more study.
In this sample, APOE4 and FH interacted (Figure 2), but there were no APOE4 main effects. The interaction was largely due to the finding that e4 positive, but FH negative subjects exhibited the greatest activation. An intriguing study by Mondadori et al55 points out several salutary affects of APOE4 on the brain in early life, and they present fMRI results with a memory encoding task indicating that young adult ε3/ε4 carriers exhibit hippocampal learning-related signal adaptation but young non-carriers do not. There is still much to learn about the effect of APOE4 across the lifespan, but in the context of the recent literature and the findings in the present report, it is likely that interactions are occurring between APOE4 and age and/or other AD risk factors which manifests as a putative salutary effect early in life, but a deleterious effect later in life.
The results in this report are consistent with a prior report of an episodic encoding task with most of these same subjects12 in which we found a robust effect of FH in the hippocampus and ventral temporal lobes during object encoding. In that prior report we also found that the −FH,+ ε4 group again exhibited the greatest cerebral response in the hippocampus, while +FH, +ε4 subjects had the least. A similar finding was observed when subjects possessing the ε2 allele were removed.15
It is noteworthy that at least two other recent studies have reported first-degree family history effects. In a behavioral experiment of odor identification it was found that siblings of AD patients exhibited reduced accuracy relative to controls. This effect was more pronounced in siblings who were APOE4 positive.56 With fMRI, Bassett et al7 examined first-degree family history and APOE in a large sample (n=195) and found FH affected brain activation during a paired-associate encoding task, whereas APOE did not.
There remains a fundamental issue regarding fMRI group differences in cognitively normal versus at-risk or cognitively impaired populations. Some studies have reported risk-associated 8, 57–59 and disease-associated 10, 60 increases in cerebral activity, while other studies report decreases in cerebral response with increased risk 12, 14, 55 or cognitive impairment.13, 61–63 Although there are many sources of noise and variability with fMRI, some possible reasons for these study differences may be: A) task difficulty. Increased difficulty has the effect of increasing fMRI activation;64, 65 B) choice of comparison condition from which BOLD signal change is measured. It has been shown that a resting low level baseline such as rest or cross hair fixation versus an active cognitively challenging baseline produce very different results;66, 67; and/or C) choice of analysis methods and statistical model—for example spatially normalizing to a standard space versus native space,68 or counting of suprathreshold voxels within a region versus statistical parametric mapping.10 Given the variability across studies, researchers that develop fMRI tasks for use in clinical and at-risk populations should adopt a task-specific psychometric approach to measuring brain activation. Such an approach might include parametrically varying difficulty, comparison to normative data,55 and characterizing tasks across larger samples and across a range of demographic (e.g. age) and clinical parameters (e.g. genes, cognitive status61).
In conclusion, these data suggest that first-degree family history of AD may influence brain function many years prior to typical disease onset. The genetic and environmental factors that embody family history are still largely unknown and further study is required. The results highlight the idea that factors beyond APOE contribute to AD and should be included when possible in studies of AD risk. Although memory dysfunction is a core feature of AD and is typically one of the first noticeable symptoms, these findings with a self-referential decision task suggest that brain areas underlying metacognitive functions may also show compromise in people at risk, and may correspond, in part, to the metacognitive deficits that are observed in symptomatic AD.
Acknowledgement
This study was supported by R01 AG21155 (SCJ) and a Merit Review grant from the Department of Veterans Affairs. The first author had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. The assistance of Britta Jabbar, Shelly Fitzgerald, Gemma Gliori, Lisa Newman, Allison Wichmann, Taylor Schmitz, Mehul Trivedi, PhD, Michael Anderle, and Ron Fisher is greatly appreciated.
References
- 1.Corder EH, Ghebremedhin E, Taylor MG, Thal DR, Ohm TG, Braak H. The biphasic relationship between regional brain senile plaque and neurofibrillary tangle distributions: modification by age, sex, and APOE polymorphism. Ann N Y Acad Sci. 2004 Jun;1019:24–28. doi: 10.1196/annals.1297.005. [DOI] [PubMed] [Google Scholar]
- 2.Braak E, Griffing K, Arai K, Bohl J, Bratzke H, Braak H. Neuropathology of Alzheimer's disease: what is new since A. Alzheimer? Eur Arch Psychiatry Clin Neurosci. 1999;249 Suppl 3:14–22. doi: 10.1007/pl00014168. [DOI] [PubMed] [Google Scholar]
- 3.Ohm TG, Muller H, Braak H, Bohl J. Close-meshed prevalence rates of different stages as a tool to uncover the rate of Alzheimer's disease-related neurofibrillary changes. Neuroscience. 1995 Jan;64(1):209–217. doi: 10.1016/0306-4522(95)90397-p. [DOI] [PubMed] [Google Scholar]
- 4.Reiman EM, Chen K, Alexander GE, et al. Functional brain abnormalities in young adults at genetic risk for late-onset Alzheimer's dementia. Proc Natl Acad Sci U S A. 2004 Jan 6;101(1):284–289. doi: 10.1073/pnas.2635903100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fratiglioni L, Ahlbom A, Viitanen M, Winblad B. Risk factors for late-onset Alzheimer's disease: a population-based, case-control study. Ann Neurol. 1993 Mar;33(3):258–266. doi: 10.1002/ana.410330306. [DOI] [PubMed] [Google Scholar]
- 6.Green RC, Cupples LA, Go R, et al. Risk of dementia among white and African American relatives of patients with Alzheimer disease. Jama. 2002 Jan 16;287(3):329–336. doi: 10.1001/jama.287.3.329. [DOI] [PubMed] [Google Scholar]
- 7.Bassett SS, Yousem DM, Cristinzio C, et al. Familial risk for Alzheimer's disease alters fMRI activation patterns. Brain. 2006 May;129(Pt 5):1229–1239. doi: 10.1093/brain/awl089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bondi MW, Houston WS, Eyler LT, Brown GG. fMRI evidence of compensatory mechanisms in older adults at genetic risk for Alzheimer disease. Neurology. 2005 Feb 8;64(3):501–508. doi: 10.1212/01.WNL.0000150885.00929.7E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fleisher AS, Houston WS, Eyler LT, et al. Identification of Alzheimer disease risk by functional magnetic resonance imaging. Arch Neurol. 2005 Dec;62(12):1881–1888. doi: 10.1001/archneur.62.12.1881. [DOI] [PubMed] [Google Scholar]
- 10.Dickerson BC, Salat DH, Greve DN, et al. Increased hippocampal activation in mild cognitive impairment compared to normal aging and AD. Neurology. 2005 Aug 9;65(3):404–411. doi: 10.1212/01.wnl.0000171450.97464.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Johnson SC, Baxter L, Susskind-Wilder L, Connor DJ, Sabbagh MN, Caselli RJ. Hippocampal adaptation to face repetition in healthy elderly and mild cognitive impairment. Neuropsychologia. 2004;42(7):980–989. doi: 10.1016/j.neuropsychologia.2003.11.015. [DOI] [PubMed] [Google Scholar]
- 12.Johnson SC, Schmitz TW, Trivedi MA, et al. The influence of Alzheimer disease family history and apolipoprotein E epsilon4 on mesial temporal lobe activation. J Neurosci. 2006 May 31;26(22):6069–6076. doi: 10.1523/JNEUROSCI.0959-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Johnson SC, Schmitz TW, Moritz CH, et al. Activation of brain regions vulnerable to Alzheimer's disease: The effect of mild cognitive impairment. Neurobiol Aging. 2006;27(11):1604–1612. doi: 10.1016/j.neurobiolaging.2005.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lind J, Persson J, Ingvar M, et al. Reduced functional brain activity response in cognitively intact apolipoprotein E {varepsilon}4 carriers. Brain. 2006 Mar 14; doi: 10.1093/brain/awl054. [DOI] [PubMed] [Google Scholar]
- 15.Trivedi MA, Schmitz TW, Ries ML, et al. Reduced hippocampal activation during episodic encoding in middle-aged individuals at genetic risk of Alzheimer's disease: a cross-sectional study. BMC Med. 2006;4:1. doi: 10.1186/1741-7015-4-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Royall DR, Lauterbach EC, Cummings JL, et al. Executive control function: a review of its promise and challenges for clinical research. A report from the Committee on Research of the American Neuropsychiatric Association. J Neuropsychiatry Clin Neurosci. 2002 Fall;14(4):377–405. doi: 10.1176/jnp.14.4.377. [DOI] [PubMed] [Google Scholar]
- 17.Schmitz TW, Johnson SC. Relevance to self: a brief review and framework of neural systems underlying appraisal. Neuroscience and Biobehavioral Reviews. doi: 10.1016/j.neubiorev.2006.12.003. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Northoff G, Bermpohl F. Cortical midline structures and the self. Trends Cogn Sci. 2004 Mar;8(3):102–107. doi: 10.1016/j.tics.2004.01.004. [DOI] [PubMed] [Google Scholar]
- 19.Northoff G, Heinzel A, de Greck M, Bermpohl F, Dobrowolny H, Panksepp J. Self-referential processing in our brain-A meta-analysis of imaging studies on the self. Neuroimage. 2006 Feb 5; doi: 10.1016/j.neuroimage.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 20.Amodio DM, Frith CD. Meeting of minds: the medial frontal cortex and social cognition. Nat Rev Neurosci. 2006 Apr;7(4):268–277. doi: 10.1038/nrn1884. [DOI] [PubMed] [Google Scholar]
- 21.Phillips ML, Drevets WC, Rauch SL, Lane R. Neurobiology of emotion perception I: The neural basis of normal emotion perception. Biol Psychiatry. 2003 Sep 1;54(5):504–514. doi: 10.1016/s0006-3223(03)00168-9. [DOI] [PubMed] [Google Scholar]
- 22.Buckner RL, Snyder AZ, Shannon BJ, et al. Molecular, structural, and functional characterization of Alzheimer's disease: evidence for a relationship between default activity, amyloid, and memory. J Neurosci. 2005 Aug 24;25(34):7709–7717. doi: 10.1523/JNEUROSCI.2177-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Alexander GE, Chen K, Pietrini P, Rapoport SI, Reiman EM. Longitudinal PET Evaluation of Cerebral Metabolic Decline in Dementia: A Potential Outcome Measure in Alzheimer's Disease Treatment Studies. Am J Psychiatry. 2002 Jun;159(5):738–745. doi: 10.1176/appi.ajp.159.5.738. [DOI] [PubMed] [Google Scholar]
- 24.Reiman EM, Caselli RJ, Yun LS, et al. Preclinical evidence of Alzheimer's disease in persons homozygous for the epsilon 4 allele for apolipoprotein E. N Engl J Med. 1996;334(12):752–758. doi: 10.1056/NEJM199603213341202. Eng. [DOI] [PubMed] [Google Scholar]
- 25.Scahill RI, Schott JM, Stevens JM, Rossor MN, Fox NC. Mapping the evolution of regional atrophy in Alzheimer's disease: unbiased analysis of fluid-registered serial MRI. Proc Natl Acad Sci U S A. 2002 Apr 2;99(7):4703–4707. doi: 10.1073/pnas.052587399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Price JC, Klunk WE, Lopresti BJ, et al. Kinetic modeling of amyloid binding in humans using PET imaging and Pittsburgh Compound-B. J Cereb Blood Flow Metab. 2005 Jun 8;25(11):1528–1547. doi: 10.1038/sj.jcbfm.9600146. [DOI] [PubMed] [Google Scholar]
- 27.Lustig C, Snyder AZ, Bhakta M, et al. Functional deactivations: change with age and dementia of the Alzheimer type. Proc Natl Acad Sci U S A. 2003 Nov 25;100(24):14504–14509. doi: 10.1073/pnas.2235925100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Greicius MD, Srivastava G, Reiss AL, Menon V. Default-mode network activity distinguishes Alzheimer's disease from healthy aging: evidence from functional MRI. Proc Natl Acad Sci U S A. 2004 Mar 30;101(13):4637–4642. doi: 10.1073/pnas.0308627101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Barrett AM, Eslinger PJ, Ballentine NH, Heilman KM. Unawareness of cognitive deficit (cognitive anosognosia) in probable AD and control subjects. Neurology. 2005 Feb 22;64(4):693–699. doi: 10.1212/01.WNL.0000151959.64379.1B. [DOI] [PubMed] [Google Scholar]
- 30.Schmitz TW, Johnson SC. Self-appraisal decisions evoke dissociated dorsal-ventral aMPFC networks. NeuroImage. 2006 Dec 1;30:1050–1058. doi: 10.1016/j.neuroimage.2005.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Johnson SC, Baxter LC, Wilder LS, Pipe JG, Heiserman JE, Prigatano GP. Neural correlates of self-reflection. Brain. 2002 Aug;125(Pt 8):1808–1814. doi: 10.1093/brain/awf181. [DOI] [PubMed] [Google Scholar]
- 32.Schmitz TW, Kawahara-Baccus TN, Johnson SC. Metacognitive evaluation, self-relevance, and the right prefrontal cortex. Neuroimage. 2004 Jun;22(2):941–947. doi: 10.1016/j.neuroimage.2004.02.018. [DOI] [PubMed] [Google Scholar]
- 33.Sager MA, Hermann B, La Rue A. Middle-Aged Children of Persons With Alzheimer's Disease: APOE Genotypes and Cognitive Function in the Wisconsin Registry for Alzheimer's Prevention. J Geriatr Psychiatry Neurol. 2005 Dec;18(4):245–249. doi: 10.1177/0891988705281882. [DOI] [PubMed] [Google Scholar]
- 34.Ries ML, Jabbar B, Schmitz TW, et al. Anosognosia in mild cognitive impairment: relationship to activation of cortical midline structures involved in self-appraisal. Journal of the International Neuropsychological Society. doi: 10.1017/S1355617707070488. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jezzard P, Balaban RS. Correction for geometric distortion in echo planar images from B0 field variations. Magn Reson Med. 1995 Jul;34(1):65–73. doi: 10.1002/mrm.1910340111. [DOI] [PubMed] [Google Scholar]
- 36.Jenkinson M. Fast, automated, N-dimensional phase-unwrapping algorithm. Magn Reson Med. 2003 Jan;49(1):193–197. doi: 10.1002/mrm.10354. [DOI] [PubMed] [Google Scholar]
- 37.Friston KJ. Commentary and opinion: II. Statistical parametric mapping: ontology and current issues. Journal of Cerebral Blood Flow & Metabolism. 1995;15(3):361–370. doi: 10.1038/jcbfm.1995.45. [DOI] [PubMed] [Google Scholar]
- 38.Trivedi M, Wichmann A, Jabbar B, et al. Structural MRI discriminates individuals with mild cognitive impairment from age-matched controls: a combined neuropsychological and voxel based morphometry study. Alzheimer’s and Dementia: The Journal of the Alzheimer’s Association. 2007;2:296–302. doi: 10.1016/j.jalz.2006.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Good CD, Johnsrude IS, Ashburner J, Henson RN, Friston KJ, Frackowiak RS. A voxel-based morphometric study of ageing in 465 normal adult human brains. Neuroimage. 2001;14(1 Pt 1):21–36. doi: 10.1006/nimg.2001.0786. [DOI] [PubMed] [Google Scholar]
- 40.Vogt BA, Vogt L, Laureys S. Cytology and functionally correlated circuits of human posterior cingulate areas. Neuroimage. 2006 Jan 15;29(2):452–466. doi: 10.1016/j.neuroimage.2005.07.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Valenstein E, Bowers D, Verfaellie M, Heilman KM, Day A, Watson RT. Retrosplenial amnesia. Brain. 1987 Dec;110(Pt 6):1631–1646. doi: 10.1093/brain/110.6.1631. [DOI] [PubMed] [Google Scholar]
- 42.Wagner AD, Shannon BJ, Kahn I, Buckner RL. Parietal lobe contributions to episodic memory retrieval. Trends Cogn Sci. 2005 Sep;9(9):445–453. doi: 10.1016/j.tics.2005.07.001. [DOI] [PubMed] [Google Scholar]
- 43.Johnson SC, Schmitz TW, Kawahara-Baccus TN, et al. The Cerebral Response during Subjective Choice with and without Self-reference. J Cogn Neurosci. 2005 Dec;17(12):1897–1906. doi: 10.1162/089892905775008607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kelley WM, Macrae CN, Wyland CL, Caglar S, Inati S, Heatherton TF. Finding the self? An event-related fMRI study. J Cogn Neurosci. 2002 Jul 1;14(5):785–794. doi: 10.1162/08989290260138672. [DOI] [PubMed] [Google Scholar]
- 45.Lane RD, Fink GR, Chau PM, Dolan RJ. Neural activation during selective attention to subjective emotional responses. Neuroreport. 1997;8(18):3969–3972. doi: 10.1097/00001756-199712220-00024. [DOI] [PubMed] [Google Scholar]
- 46.Gusnard DA, Akbudak E, Shulman GL, Raichle ME. Medial prefrontal cortex and self-referential mental activity: Relation to a default mode of brain function. Proc Natl Acad Sci U S A. 2001;20:20. doi: 10.1073/pnas.071043098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nestor PJ, Fryer TD, Ikeda M, Hodges JR. Retrosplenial cortex (BA 29/30) hypometabolism in mild cognitive impairment (prodromal Alzheimer's disease) Eur J Neurosci. 2003 Nov;18(9):2663–2667. doi: 10.1046/j.1460-9568.2003.02999.x. [DOI] [PubMed] [Google Scholar]
- 48.Johnson NA, Jahng GH, Weiner MW, et al. Pattern of cerebral hypoperfusion in Alzheimer disease and mild cognitive impairment measured with arterial spin-labeling MR imaging: initial experience. Radiology. 2005 Mar;234(3):851–859. doi: 10.1148/radiol.2343040197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chetelat G, Desgranges B, De La Sayette V, Viader F, Eustache F, Baron JC. Mild cognitive impairment: Can FDG-PET predict who is to rapidly convert to Alzheimer's disease? Neurology. 2003 Apr 22;60(8):1374–1377. doi: 10.1212/01.wnl.0000055847.17752.e6. [DOI] [PubMed] [Google Scholar]
- 50.Huang C, Wahlund LO, Svensson L, Winblad B, Julin P. Cingulate cortex hypoperfusion predicts Alzheimer's disease in mild cognitive impairment. BMC Neurol. 2002 Sep 12;2(1):9. doi: 10.1186/1471-2377-2-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kogure D, Matsuda H, Ohnishi T, et al. Longitudinal evaluation of early Alzheimer's disease using brain perfusion SPECT. J Nucl Med. 2000 Jul;41(7):1155–1162. [PubMed] [Google Scholar]
- 52.Arikuni T, Sako H, Murata A. Ipsilateral connections of the anterior cingulate cortex with the frontal and medial temporal cortices in the macaque monkey. Neurosci Res. 1994 Nov;21(1):19–39. doi: 10.1016/0168-0102(94)90065-5. [DOI] [PubMed] [Google Scholar]
- 53.Cavada C, Company T, Tejedor J, Cruz-Rizzolo RJ, Reinoso-Suarez F. The anatomical connections of the macaque monkey orbitofrontal cortex. A review. Cereb Cortex. 2000 Mar;10(3):220–242. doi: 10.1093/cercor/10.3.220. [DOI] [PubMed] [Google Scholar]
- 54.Marshall GA, Kaufer DI, Lopez OL, Rao GR, Hamilton RL, DeKosky ST. Right prosubiculum amyloid plaque density correlates with anosognosia in Alzheimer's disease. J Neurol Neurosurg Psychiatry. 2004 Oct;75(10):1396–1400. doi: 10.1136/jnnp.2003.030007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mondadori CR, de Quervain DJ, Buchmann A, et al. Better Memory and Neural Efficiency in Young Apolipoprotein E {varepsilon}4 Carriers. Cereb Cortex. 2006 Oct 31; doi: 10.1093/cercor/bhl103. [DOI] [PubMed] [Google Scholar]
- 56.Handley OJ, Morrison CM, Miles C, Bayer AJ. ApoE gene and familial risk of Alzheimer's disease as predictors of odour identification in older adults. Neurobiol Aging. 2006 Oct;27(10):1425–1430. doi: 10.1016/j.neurobiolaging.2005.08.001. [DOI] [PubMed] [Google Scholar]
- 57.Mondadori CR, Buchmann A, Mustovic H, et al. Enhanced brain activity may precede the diagnosis of Alzheimer's disease by 30 years. Brain. 2006 Nov;129(Pt 11):2908–2922. doi: 10.1093/brain/awl266. [DOI] [PubMed] [Google Scholar]
- 58.Han SD, Houston WS, Jak AJ, et al. Verbal paired-associate learning by APOE genotype in non-demented older adults: fMRI evidence of a right hemispheric compensatory response. Neurobiol Aging. 2007 Feb;28(2):238–247. doi: 10.1016/j.neurobiolaging.2005.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bookheimer SY, Strojwas MH, Cohen MS, et al. Patterns of brain activation in people at risk for Alzheimer's disease. N Engl J Med. 2000;343(7):450–456. doi: 10.1056/NEJM200008173430701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Dickerson BC, Salat DH, Bates JF, et al. Medial temporal lobe function and structure in mild cognitive impairment. Ann Neurol. 2004 Jul;56(1):27–35. doi: 10.1002/ana.20163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Celone KA, Calhoun VD, Dickerson BC, et al. Alterations in memory networks in mild cognitive impairment and Alzheimer's disease: an independent component analysis. J Neurosci. 2006 Oct 4;26(40):10222–10231. doi: 10.1523/JNEUROSCI.2250-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Small S, Perera GM, DeLaPaz R, Mayeux R, Stern Y. Differential regional dysfunction of the hippocampal formation among elderly with memory decline and Alzheimer's disease. Ann Neurol. 1999;45(4):466–472. doi: 10.1002/1531-8249(199904)45:4<466::aid-ana8>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 63.Machulda MM, Ward HA, Borowski B, et al. Comparison of memory fMRI response among normal, MCI, and Alzheimer's patients. Neurology. 2003 Aug 26;61(4):500–506. doi: 10.1212/01.wnl.0000079052.01016.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Demb JB, Desmond JE, Wagner AD, Vaidya CJ, Glover GH, Gabrieli JD. Semantic encoding and retrieval in the left inferior prefrontal cortex: A functional MRI study of task difficulty and process specificity. Journal of Neuroscience. 1995;15(9):5870–5878. doi: 10.1523/JNEUROSCI.15-09-05870.1995. Eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Rao SM, Bandettini PA, Binder JR, et al. Relationship between finger movement rate and functional magnetic resonance signal change in human primary motor cortex. J Cereb Blood Flow Metab. 1996;16(6):1250–1254. doi: 10.1097/00004647-199611000-00020. [DOI] [PubMed] [Google Scholar]
- 66.Stark CE, Squire LR. When zero is not zero: the problem of ambiguous baseline conditions in fMRI. Proc Natl Acad Sci U S A. 2001 Oct 23;98(22):12760–12766. doi: 10.1073/pnas.221462998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Morcom AM, Fletcher PC. Does the brain have a baseline? Why we should be resisting a rest. Neuroimage. 2006 Oct 16; [Google Scholar]
- 68.Sandstrom CK, Krishnan S, Slavin MJ, Tran TT, Doraiswamy PM, Petrella JR. Hippocampal atrophy confounds template-based functional MR imaging measures of hippocampal activation in patients with mild cognitive impairment. AJNR Am J Neuroradiol. 2006 Sep;27(8):1622–1627. [PMC free article] [PubMed] [Google Scholar]