Abstract
PURPOSE
To determine the roles of glial fibrillary acidic protein (GFAP) and vimentin in Müller cell reactivity.
METHODS
Retinal detachments were created in mice deficient for GFAP and vimentin (GFAP-/-vim-/-) and age-matched wild-type (wt) mice. The reactivity of the retina was studied by immunofluorescence and electron microscopy.
RESULTS
Müller cell morphology was different and glutamine synthetase immunoreactivity was reduced in the undisturbed GFAP-/-vim-/- retinas. After retinal detachment, Müller cells formed subretinal glial scars in the wt mice. In contrast, such scars were not observed in GFAP-/-vim-/- mice. Müller cells, which normally elongate and thicken in response to detachment, appeared compressed, thin, and “spikey” in the GFAP-/-vim-/- mice. The end foot region of Müller cells in the GFAP-/-vim-/- mice often sheared away from the rest of the retina during detachment, corroborating earlier results showing decreased resistance of this region in GFAP-/-vim-/- retinas to mechanical stress. In regions with end foot shearing, ganglion cells showed intense neurite sprouting, as revealed by anti-neurofilament labeling, a response rarely observed in wt mice.
CONCLUSIONS
Müller cells are subtly different in the GFAP-/-vim-/- mouse retina before detachment. The end foot region of these cells may be structurally reinforced by the presence of the intermediate filament cytoskeleton, and our data suggest a critical role for these proteins in Müller cell reaction to retinal detachment and participation in subretinal gliosis.
Astrocyte reactivity, including increased thickness of cellular processes and increased intermediate filament protein expression, is a hallmark of the central nervous system (CNS) response to injury.1-4 Müller cells, the radial glial cells of vertebrate retina, react similarly to a variety of ocular injuries, including retinal detachment, which is separation of the neural retina from the adjacent pigmented epithelium.5 Retinal detachment is a serious retinal injury that can result in significant loss of vision even after successful surgical repair.6-9 Although the retina is a part of the central nervous system, it differs from the brain and spinal cord in several important ways in its reactions to and its ability to recover from injury. Retinal reattachment surgery has been used for decades and demonstrates the capacity of the retina for functional recovery. In the brain and spinal cord, two types of glial cells, astrocytes and oligodendrocytes, are present. In the retina, Müller cells form regular radial columns through the retinal tissue and have some of the functional attributes of astrocytes. Astrocytes exist only in the nerve fiber layer, and no oligodendrocytes are present. The retina is also clearly organized into alternating layers of neurons and synapses; therefore, changes in overall organization or loss of cells are relatively easy to recognize. The retina is vulnerable to injury because of its location and the fragile nature of the very thin layer of retinal tissue. A blow to the eye or head, penetrating ocular injury, high myopia, and diseases such as diabetic retinopathy and age-related macular degeneration can all lead to retinal detachment and loss of vision.
In recent years, the Müller cells have begun to receive attention, comparable to that given astrocytes in the brain and spinal cord, as major contributors to the injury response of the retina. Similarly, neuronal remodeling or plasticity in response to injury and its potential role in functional recovery in the retina received almost no attention until recently, when the remodeling of retinal neurons in response to detachment was first reported.10 Observations of tissue samples suggest that gliosis and neuronal remodeling are significant components of the retina's response to injury in humans.11-15 Müller cell reactivity plays a major role in one of the most devastating pathophysiological consequences of detachment, the formation of abnormal “cellular membranes” (scars) on the retinal surface.5 Thus, understanding Müller cell reactivity is important for understanding the injury response of the retina and, by extension, may contribute to insight into gliosis elsewhere in the CNS.
Experimental data suggest that the reactions of Müller cells and neurons are similar in cats, rabbits, mice, and humans (for reviews, see Fisher et al.16 and Jones and Marc17). Müller cell reactivity is associated with an increased expression of vimentin or GFAP (or both), cell proliferation, and growth. The expansion of Müller processes into the subretinal space forms fibrotic tissue that blocks the regeneration of outer segments but not anatomic reattachment.18 Müller cell growth into the vitreous plays an important role in proliferative vitreoretinopathy that can result in re-detachment of the retina and in blindness.19 Moreover, neurites that grow from retinal neurons after detachment seem to have a particular affinity for glial scars formed by reactive Müller cells.20 Although there is a temporal correlation between the hypertrophy of Müller cells and their increased expression of intermediate filament proteins,21 an actual functional link between the two has not been clearly defined.
It has been shown that injury to the brain or spinal cord in GFAP-/-vim-/- mice results in altered astrocyte scar formation and changes in the wound-healing response.22,23 Retinas of GFAP-/-vim-/- mice were shown to be less resistant to severe mechanical injury, and hypoxia-triggered pathologic vascularization into the vitreous was reduced in these mice.24 In this study, we compared retinas in wt and GFAP-/-vim-/-mice to determine the roles of these two molecules in the response of Müller cells and associated neurons to retinal detachment.
MATERIALS AND METHODS
Retinal Detachment
Adult (12-16 weeks of age) male GFAP-/-vim-/- (n = 12) and age-matched wt (n - 12) mice were used in this study. Both wt and GFAP-/-vim-/- mice were on a C57BL6 to 129SV-129Ola-SF2J mixed genetic background. Retinal detachments were induced in the right eyes, as previously reported, with minor modification.25 Briefly, mice were anesthetized with a mixture of 12.5 mg/kg xylazine and 62.5 mg/kg ketamine (both from Phoenix Pharmaceutical, St. Joseph, MO) and were placed on a warm pad on the operating table. The pupil was then dilated with a drop of 1% cyclopentolate and 2.5% phenylephrine hydrochloride (Akorn, Buffalo Grove, IL). With the use of an operating microscope to visualize the retina, a scleral puncture was made at the supranasal equator with a 30-gauge needle. One drop of hydroxypropyl methylcellulose (Goniosoft; OcuSoft Inc., Richmond, TX) was placed on the cornea to assist in the adherence of a glass coverslip, allowing for visualization of the retina. The tip of a small-bore (100-μm), custom-pulled glass pipette held by a micromanipulator was inserted through the pilot hole and moved through the retina, at which time a solution of 0.25% sodium hyaluronate (Healon; Pharmacia & Upjohn, Uppsala, Sweden) was infused between the photoreceptor cells and the RPE (subretinal space), creating detachment. Mice that received scleral punctures without subretinal injections served as controls. All experimental procedures and use of animals followed the protocol approved by the Animal Care and Use Committee of University of California at Santa Barbara and the Schepens Eye Research Institute and conformed to the standards in the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research.
Tissue Preparation
At 7 and 28 days after detachment surgery, the animals were killed with CO2, and the eyes were gently enucleated and placed in 4% paraformaldehyde in sodium phosphate buffer (0.1 M; pH 7.4) for immunohistochemistry (IHC) or in buffered 1% glutaraldehyde/1% paraformaldehyde for electron microscopy. After overnight fixation, the cornea and lens were removed from the eye, and the tissue was processed for IHC or electron microscopy.
Immunohistochemistry
For IHC, the eyes were rinsed in phosphate-buffered saline (PBS) for a minimum of 1 hour, embedded in low-melt agarose (5%; Sigma, St. Louis, MO) at 41°C, and sectioned at 100 μm with tissue-sectioning equipment (Vibratome; Leica, Lumberton, NJ). Sections were incubated in blocking serum (normal donkey serum 1:20 in PBS, 0.5% bovine serum albumin [BSA], 0.1% Triton X-100, and 0.1% azide [PBTA]) overnight on a rotator at 4°C. Primary antibodies in PBTA were added the following day and placed on a rotator overnight at 4°C. Antibodies to the following proteins were used: GFAP (1:400, rabbit polyclonal; Dako, Carpinteria, CA), vimentin (1:500, goat polyclonal; Santa Cruz Biotechnology, Santa Cruz, CA), S100 (1:1000, rabbit polyclonal; Dako), glutamine synthetase (GS; 1:500, mouse monoclonal; BD Biosciences, San Jose, CA), rod opsin (1:500, mouse monoclonal; gift from Robert Molday, University British Columbia, Vancouver, BC, Canada), heavy and light subunits of neurofilament protein (1:500, mouse monoclonal; Biomeda, Hayward, CA), protein kinase C alpha (PKC, rabbit polyclonal, 1:100; Biomol Research Laboratories, Plymouth Meeting, PA), and laminin (1:25, rabbit polyclonal; Sigma, St. Louis, MO). The following day, primary antibodies were rinsed in PBTA and corresponding secondary antibodies, diluted 1:200, were added and incubated overnight on a rotator at 4°C. Secondary antibodies included donkey-anti-mouse or anti-rabbit conjugated to Cy2 or Cy3 (Jackson ImmunoResearch Laboratories, West Grove, PA). On the last day, sections were rinsed in PBTA and mounted in 5% n-propyl gallate in glycerol on glass slides and sealed with a coverslip. The slides were viewed with a laser scanning confocal microscope (FluoView 500; Olympus, Center Valley, PA). During any session of observation and image collection for a given antibody, black and gain levels were kept constant to allow comparisons of labeling intensity.
Electron Microscopy
After the initial fixation, the tissue was postfixed in osmium tetroxide (2% in 0.068 M phosphate buffer) for 1 hour, dehydrated in a graded ethanol series, and embedded in Spurrs resin (EMS, Hatfield, PA). Tissue blocks were sectioned on an ultramicrotome at 90-nm thickness. Sections were stained with 1% aqueous uranyl acetate and Reynolds lead citrate and were viewed with a transmission electron microscope (JEM 1230; JEOL, Tokyo, Japan).
Quantitation
To compare variations in the overall organization of the outer nuclear layer (ONL) after detachment in the wt and GFAP-/-vim-/- animals, we calculated a “distortion index” (DI) from data derived from the confocal images.26 The DI measures the covariation in cell density and the thickness of the ONL along a length of tissue within a vertical section through the retina. The ONL of a retinal section from a healthy animal cut at right angles to the plane of the retinal layers is uniform over any given length; thus, the distortion index is low (<0.1). Twisting or contortion of the retina, migration of cells, or abnormal expansion of some cells produced variability in the width of the ONL and the apparent number of nuclei, resulting in an increase in DI. A simple change in cutting angle that was uniform along the length of a tissue section would not produce an increase in this index.
RESULTS
Intermediate Filament Proteins
Vimentin and GFAP are upregulated in Müller cells after detachment in wt mice (Figs. 1A, normal; 1B, 7-day detached). In the attached wt mouse retina, anti-vimentin labeling appears as thin wisps or streaks running across the retina from the vitreal border (inner limiting membrane [ILM]; Fig. 1A) to the outer limiting membrane (OLM). As reported by others (for a review, see Sarthy and Ripps27), detectable labeling with anti-GFAP in the normal mouse retina occurs only in the astrocytes of the nerve fiber layer (NFL, Fig. 1A, green) on the border between the retina and the vitreous. Astrocytes, however, are not labeled with anti-vimentin. Both antibodies also lightly labeled horizontal cells in the outer plexiform layer (OPL, Figs. 1A, 1B). After 7 days of detachment, Müller cells labeled across their entire lengths with both antibodies (Fig. 1B). After 28 days, the labeling intensity for both was increased, and the labeled processes within the retina appeared slightly thickened (Fig. 1C). Anti-vimentin- and anti-GFAP-labeled Müller cell processes were occasionally observed extending beyond the OLM and into the subretinal space by this time (Fig. 1C, arrow).
FIGURE 1.
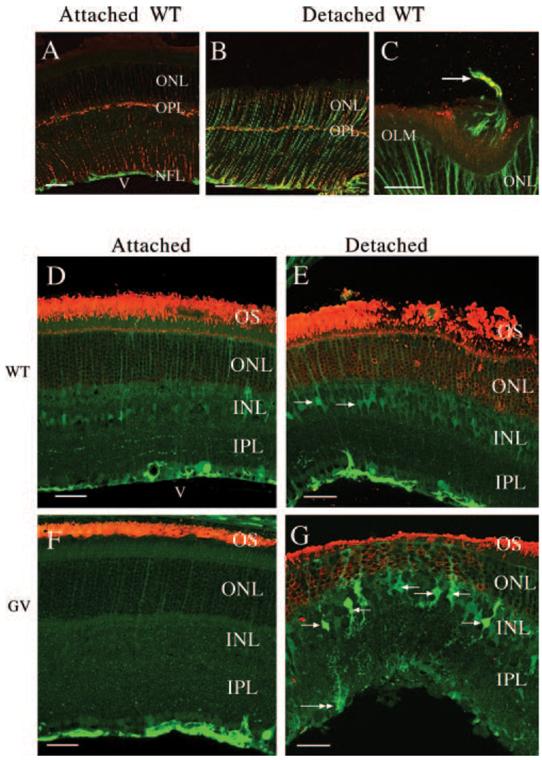
Laser scanning confocal images of immunolabeled retinal sections showing Müller cell reactivity and rod opsin distribution. (A-C) Wild-type attached (A) and detached (B, C) retinas labeled with anti-vimentin (red) and anti-GFAP (green). A GFAP/vimentin-positive Müller cell process (arrow) extends into the subretinal space in (C). (D, F) Attached; (E, G) detached wt and GFAP-/-vim-/- (GV) retinas labeled with anti-rod opsin (red) and anti-S-100 (green). (E) Transition zone between attached (left) and detached (right) retinas. Anti-S-100 lightly labels Müller cells in the attached retinas (D, F) and increases slightly with detachment (E, G). The morphology of the Müller cells in the detached wt and GFAP-/-vim-/- animals is distinctively different. White arrows: Müller cell bodies. White double arrow: Müller cell end foot. INL, inner nuclear layer; IPL, inner plexiform layer; NFL, nerve fiber layer; OLM, outer limiting membrane; ONL, outer nuclear layer; OPL, outer plexiform layer; v, vitreous. Scale bars: (A, B, D-G) 50 μm; (C) 20 μm.
Müller Cells
Anti-S100 Labeling
Müller cells in the GFAP-/-vim-/-mice were visualized by labeling with anti-S100.24 In wt animals, anti-S100 labeling occurred in parallel thin streaks, similar to the images obtained with anti-vimentin (Fig. 1D). There was no clearly defined change in this pattern after detachment (Fig. 1E). The pattern of labeling in the attached GFAP-/-vim-/-mouse retina (Fig. 1F) appeared similar to that in wt animals (Fig. 1D), though the overall labeling intensity was less. After detachment, the morphology of these Müller cells was dramatically changed (Fig. 1G). Strikingly, the cell bodies and major processes that emerged from them appeared more irregular (compare Figs. 1E, 1G). The main trunk of the Müller cells appeared slightly thickened, and within the inner plexiform layer (IPL) they had many small “spikey” lateral protrusions. Müller cells have small lateral processes in all species,28 but these do not generally label with the intermediate filament or S100 antibodies. The end foot also appeared more rounded or clublike after detachment in the mutant mice. Although anti-S100 labeled outer Müller cell processes to the OLM in the GFAP-/-vim-/- animals (Fig. 1G), labeled processes were never observed to extend into the subretinal space after detachment as they did in wt retinas.
Anti-Glutamine Synthetase Labeling
To more readily examine the end foot region of the Müller cells, we used an antibody to glutamine synthetase (GS; Fig. 2A). This antibody does not stain the astrocytes in the nerve fiber layer (as does anti-S100), allowing for better visualization of end foot morphology. In the wt retinas, the end feet form a continuous layer along the vitreal interface (Fig. 2A). As shown previously in other species,29,30 there is a decreased expression of GS after detachment, but the end feet always appear as a continuous layer (Fig. 2C, day 7). In the attached regions of the GFAP-/-vim-/- retinas (Fig. 2B), the level of anti-GS labeling was reduced, appearing comparable to wt retinas after 7 days of detachment (Fig. 2C). Moreover, the end foot labeling often appeared discontinuous. In some regions, faint staining of fine Müller cell end feet extensions created the appearance of a “gap” between the adjacent end feet (Fig. 2B, arrow), whereas in other regions, the end feet truly appeared to be discontinuous, a fact confirmed by electron microscopy. When the GFAP-/-vim-/- retinas were detached, the difference from wt retinas became even more exaggerated (Fig. 2D, day 7). Here the irregular appearance of the vitreal border is probably a result of both reduced labeling (as in Fig. 2B) and some shearing away of portions of the Müller cell cytoplasm.
FIGURE 2.
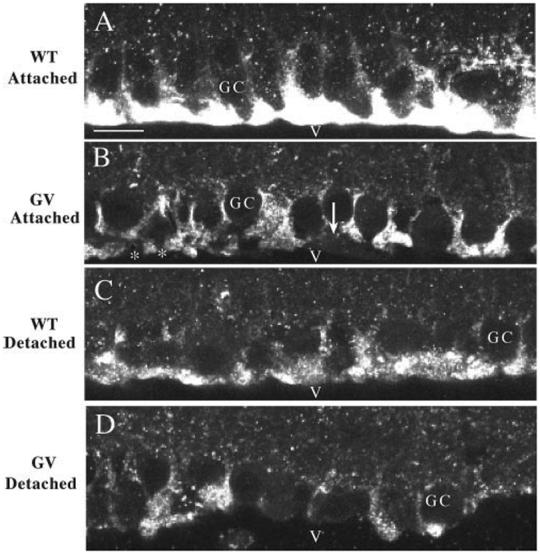
Laser scanning confocal images of the vitreal border of retinal sections labeled with anti- glutamine synthetase. In all cases, the heaviest labeling with this antibody is observed in the layer of Müller cell end feet. Detaching the wt retina results in reduced expression in the end feet (A, C); however, the end feet still form a continuous border along the retina. Antibody labeling is decreased in the attached GFAP-/-vim-/- (GV) retinas (compare A and B), and the layer of end feet appears to be discontinuous. In some areas (*) there appear to be spaces between the end feet, whereas in others the end foot cytoplasm is still visible but unlabeled (B, arrow). Labeling is reduced further in the end feet after retinal detachment (D). GC, ganglion cell bodies; v, vitreous. Scale bars, (A-D)20 μm.
Anti-Laminin
Because of the reported fragility of the end foot region of Müller cells in the GFAP-/-vim-/- mice,24 an antibody to laminin (a major component of the basal lamina) was used to visualize the basement membrane at the vitreo-retinal interface. In wt attached and detached retinas and in attached regions in GFAP-/-vim-/- retinas, there was a continuous border of labeling between the neural retina and the vitreous (data not shown). After detachment in the GFAP-/-vim-/- retinas, many areas contained labeled basal lamina (Fig. 3A, BM), but frequently areas in which the basal lamina could be observed was separated from the neural retina (Fig. 3A, double arrowhead). The basal lamina in Figure 3A appeared to vary in thickness, but this was because portions of it were torn away from the retina and had become slightly twisted and distorted in the thick vibratome section. Splitting across the end foot was reported to occur during dissection of the eyes in earlier experiments24; therefore, we took special care during the dissection and fixation processes to leave the globe as undisturbed as possible.
FIGURE 3.
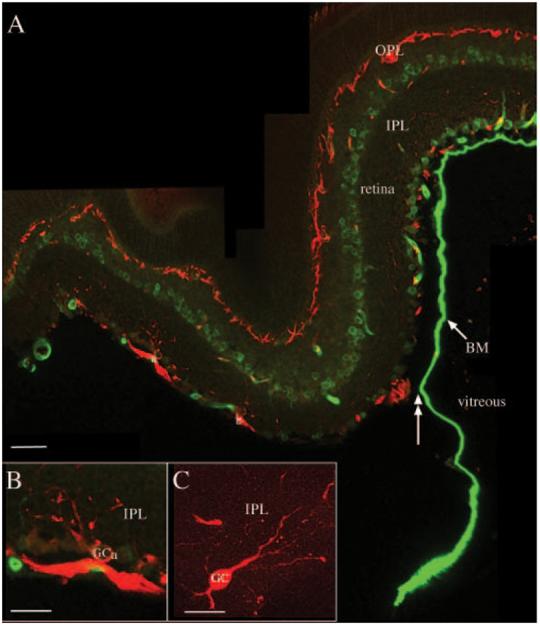
Shearing of Müller cell end feet and ganglion cell reactivity. Laser scanning confocal images from sections of GFAP-/-vim-/-retinas detached for 7 days and labeled with antibodies to neurofilament protein (red) and laminin (green). (A) Anti-laminin most prominently labels the basement membrane (BM) of the inner limiting membrane, between the retina and the vitreous. Shearing of the end feet and BM is shown (double-headed arrow). Anti-neurofilament labeling in the intact retina occurs in bundles of optic axons and horizontal cells in the outer plexiform layer (OPL) and rarely in dendrites of ganglion cells in the inner plexiform layer (IPL). In the regions of shearing, some ganglion cell bodies and dendrites label intensely with the anti-neurofilament antibody (A, a, b). In regions in which the Müller cell end feet are sheared away from the retina and the basement membrane floats freely in the vitreous, it appears to vary in thickness as the basement membrane is twisted and distorted in the thick vibratome section. (B, C) Higher magnification images of anti-neurofilament-labeled ganglion cells in regions of end foot/ILM shearing. GCa, ganglion cell “a” in (A). Scale bars: (A)50 μm; (B, C)20 μm.
Electron Microscopy
Müller cell end feet were distinctly triangular in cross-section in wt attached (not shown) and detached (Fig. 4A) retinas. Together with the basal lamina, they formed a continuous border between the neural retina and the vitreous. In attached regions of the GFAP-/-vim-/- animals, the Müller cell end feet appeared more amorphous and as thin threads of cytoplasm along the vitreoretinal border (Fig. 4B). Müller cell end feet from detached regions of GFAP-/-vim-/- eyes appeared as clublike or as blunt, rounded, vacuolated structures lying between the ganglion cells instead of spreading along the vitreoretinal border (Fig. 4C). In other regions of the detachments, the end feet were completely unidentifiable, giving a “ragged” appearance to the vitreoretinal border and exposing ganglion cell bodies directly to the vitreous (Fig. 4D).
FIGURE 4.
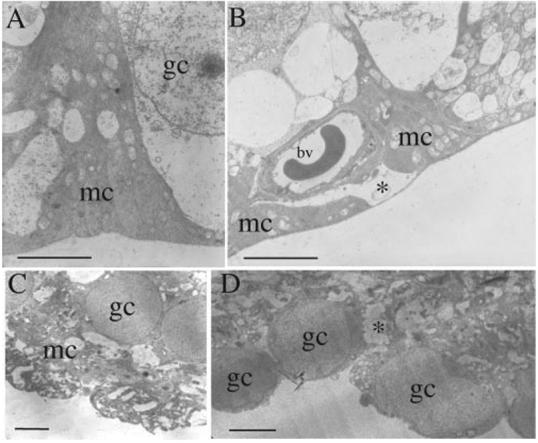
Electron micrographs of Müller cell end feet (mc) from wt (A) and GFAP-/-vim-/- (B-D) retinas. In wt retinas (whether attached or detached), the end feet assume a smooth pyramidal shape along the vitreal surface of the retina. Their cytoplasm is always recognizable as more electron dense than that of adjacent cells. In the attached GFAP-/-vim-/- retinas, the end feet have a “flatter” shape and stretch thinly across the retinal surface (B), and there are distinctive gaps where the end feet are missing (*). (C) Detached GFAP-/-vim-/- retina, where shearing of the end feet did not occur. (D) Detached GFAP-/-vim-/- retina with end foot shearing. In both cases, the Müller cell cytoplasm between ganglion cells appeared vacuolated with areas that appeared stretched or torn (*). In the region of shearing (D), the actual end foot is missing, and ganglion cells are directly exposed to vitreous. bv, blood vessel; gc, ganglion cell nuclei; mc, Müller cell end foot cytoplasm. Scale bars, (A-D)5 μm.
Retinal Neurons
Photoreceptors
After detachment in both the wt and the mutant retinas, the outer segments degenerated to varying degrees, and there was a concomitant redistribution of rod opsin to the plasma membrane of cell bodies in the ONL, as has been shown previously31-33 (Figs. 1E, 1G, red), with no apparent differences between the two groups.
Nakazawa et al.34 have recently reported that attenuation of Müller cell reactivity may decrease apoptotic cell death in GFAP-/-vim-/- animals. In our experiments, the decline in photoreceptor cell number was variable from animal to animal. However, after 7 days of detachment, the ONL always changed its structural organization. The change could have resulted from a localized loss of cells (see Fig. 1G for an example), withdrawal of synaptic terminals, hypertrophy of Müller cell processes in the ONL, folding of the retina, and movement of photoreceptor cells into the subretinal space.10,18,35 These structural changes are easily recognized but not so easily described. Therefore, the DI was used as a way to quantitatively describe the overall morphologic change. The DI was calculated from a total of 7.52, 5.35, 8.74, and 8.56 mm of retinal length of wt, wt 7-day detached, GFAP-/-vim-/-, and GFAP-/-vim-/- 7-day detached retinas, respectively (Fig. 5). The wt and GFAP-/-vim-/- attached retinas have essentially identical, low DI. In the GFAP-/-vim-/- retinas, the index increases by approximately 3.5-fold at 7 days of detachment. In the wt retinas, the change was slightly less than twofold. Overall, the DI confirmed our impressions from light and electron microscopic observations that the ONL of the GFAP-/-vim-/- retinas were structurally more irregular after detachment than were those of the wt animals. These results might occur if the intermediate filament cytoskeleton in the reactive Müller cells helps to stabilize the outer retina as it undergoes changes associated with photoreceptor degeneration.
FIGURE 5.
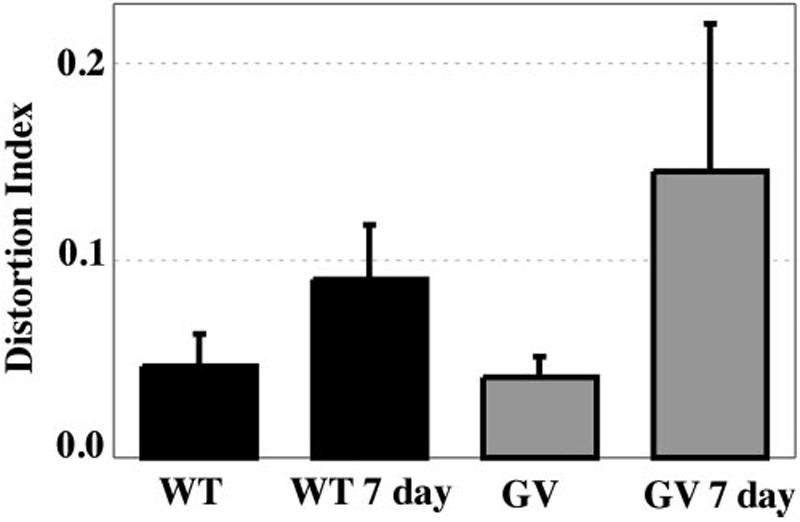
Distortion index (DI). The wt and GFAP-/-vim-/- (GV) retinas have equivalent DIs when attached to the RPE. Detachment in both results in significant distortion of the ONL (P < 0.001) by comparison with the attached retina, but the distortion that occurs in the detached GFAP-/-vim-/- retinas is significantly greater (P < 0.001) than that in the detached wt retinas. Error bars = SD.
Horizontal and Rod Bipolar Cells
These two classes of retinal interneuron respond to detachment by sprouting neurites,10,36 sometimes growing across the ONL and into glial scars in the subretinal space.10,20 Seven days after detachment, neurites labeled with antibodies to both PKCα (rod bipolar cells) and neurofilament protein (horizontal cells) were observed growing into the ONL of both the wt and the GFAP-/-vim-/- retinas (Fig. 6). There were no obvious differences between the two strains.
FIGURE 6.
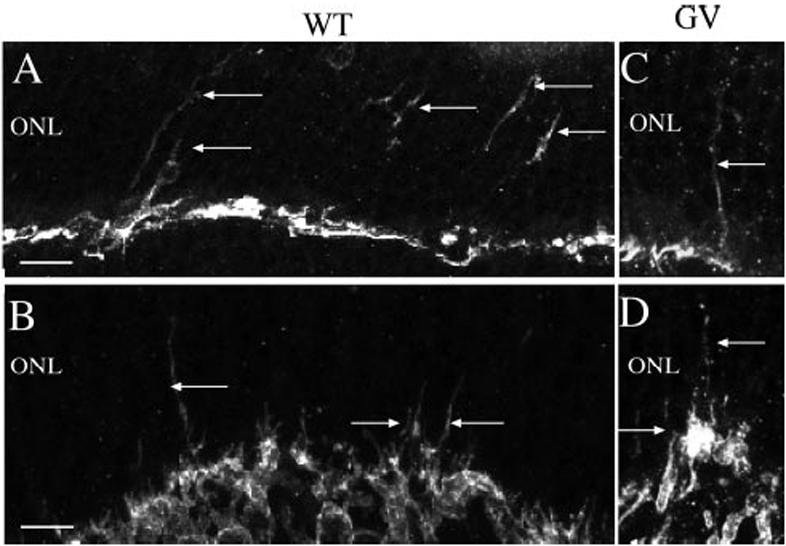
Neuronal remodeling. Laser scanning confocal images from sections of detached wild-type (A, B) and GFAP-/-vim-/- (GV) retinas (C, D) labeled with antibodies to neurofilament protein (A, C) and the α subunit of PKC (B, D). In wt and GFAP-/-vim-/- retinas, neurite outgrowths (arrows) occurred from horizontal (anti-NF; A, C) and rod bipolar (anti-PKC; B, D) cells in response to retinal detachment. Scale bars: (A, C)20 μm; (B, D)10 μm.
Ganglion Cells
The neurofilament antibody used in these studies consistently labels axons, rarely dendrites, and never the somata of ganglion cells in the wt retinas. In feline retina, a subpopulation of large ganglion cells begins to heavily label with the antibody 3 to 7 days after detachment.37 After detachment in wt animals and in the GFAP-/-vim-/- animals, in which the Müller cell end feet and basement membrane remained intact, there were more labeled ganglion cell dendrites in the inner plexiform layer (Figs. 3A-C). Where the ILM and Müller cell end feet stripped away from the retina, there were frequent, intensely labeled ganglion cell dendrites and cell bodies (Figs. 3A-C). Fine processes, presumably neurites that had sprouted in response to detachment, were observed on the basal cell body or along the axons of these cells (Fig. 3C). By examining large expanses of the nondetached GFAP-/-vim-/- retinas, we occasionally found areas of basement membrane and end feet shearing, but these areas did not have neurofilament-positive ganglion cell somata.
DISCUSSION
The absence of GFAP and vimentin appears to have little effect on the overall morphology of Müller cells or the organization of the retina under normal conditions. This suggests that intermediate filaments formed by them are not essential for the development or the structural maintenance of these complex cells,24 though, interestingly, the expression of two cytosolic proteins was consistently reduced in the GFAP-/-vim-/- mice. Data on the distribution of GFAP and vimentin in different species suggest similar conclusions. Müller cells in adult mouse, ground squirrel, and rabbit express high levels of vimentin across nearly the entire width of the retina, whereas in nonreactive feline and human Müller cells vimentin and GFAP expression are limited almost exclusively to the end foot (for a review, see Ref. 21). Electron microscopy shows that intermediate filaments are rare outside the end foot region of the normal feline retina.38 As Müller cells react to injury, there is an increase in intermediate filament protein expression concurrent with their hypertrophy (marked thickening and expansion into the subretinal space; for a review, see Lewis and Fisher21). In the GFAP-/-vim-/- mice, anti-GS labeling and electron microscopy show subtle but consistent differences in the shape of the end feet, with frequent gaps between adjacent end feet suggesting that they may not effectively cover the retinal surface.
Although increased expression of vimentin and GFAP (and a concurrent increase in the intermediate filaments) is part of the overall reactive response of Müller cells to injury, in the feline retina a subpopulation of Müller cells with more vimentin than GFAP in the apical cytoplasm grows into the subretinal space20,21 and Müller cells with more GFAP than vimentin in the end foot grow into the vitreous, where they can form fibrotic scar tissue characteristic of proliferative vitreoretinopathy.20 Thus, there appear to be functional links between the behavior of Müller cells and the differential and compartmentalized expression of these two proteins. Astrocytes in the brain and spinal cord of the GFAP-/-vim-/- mice produce less compact glial scars,22 but in our experiments the retinal Müller cells did not form subretinal scars after detachment. These data all suggest that intermediate filaments are mechanistically involved in glial scar formation. The greater distortion index in the GFAP-/-vim-/- mice (Fig. 5) may also reflect the inability of Müller cells to provide a stable structural framework for the outer retina in the face of morphologic changes occurring during the degeneration and loss of photoreceptors. Although the intermediate filament cytoskeleton may not be an essential element in the development or maintenance of Müller cell morphology, it does seem to be a critical component of the cells' ability to respond to injury.
The Müller cell end foot represents a structurally and functionally distinct compartment within the Müller cell.38-40 The presence of a robust intermediate filament cytoskeleton may protect this region from mechanical damage, perhaps by forming a tethering complex that links various structural proteins in the cell. Lundkvist et al.24,41 showed that the lack of intermediate filament proteins made the end foot fragile and susceptible to damage when the eye was removed and fixed. In our experiments, end foot shearing frequently occurred in the GFAP-/-vim-/- retinas with detachments. We believe our data indicate that the fracturing occurred at the time of detachment or during the period of detachment as the elevated and folded retina flexed because of its lack of anchoring to the RPE. The presence of reactive, neurofilament-labeled ganglion cells only in the regions of shearing in the detached retinas suggests that the damage did not occur during dissection because the increased expression of protein molecules could not occur in the few minutes between enucleation and fixation.
Structural reinforcement of the end foot region with intermediate filaments may also be essential for protection from mechanical damage given that the vitreous gel is firmly attached to the laminin-rich basement membrane of the Müller cells (the ILM) and fibers from the vitreous attach directly to the Müller cell end feet. Green and Sebag42 suggest that this adherence is mechanically strong and likely to result in physical damage to the end feet when the ILM is surgically removed. Such strong mechanical linkage would explain why we find immunolabeling characteristic of basement membrane, Müller cell cytoplasm, and ganglion cell neurites in specimens of cellular “membranes” surgically removed from the vitreous of human patients.19,43 This interface is subjected to constant changes in mechanical force by the ever-changing movement of the vitreous gel relative to the retina during normal eye movement,42 and it is undoubtedly subjected to strong mechanical shock during the lifetime of an animal. The presence of an intermediate filament cytoskeleton in the end feet of all species may provide protection against damage from such forces.
An interesting byproduct of these data is the suggestion that Müller cells have the capacity to survive after extensive plasma membrane damage. We did not observe any degenerating or dying Müller cells where end foot shearing was obvious, even though the shearing most likely occurred days before the tissue was harvested.
The fact that ganglion cells show an increased expression of neurofilament protein and neurite sprouting in the areas of end foot shearing is probably a good indication that they are damaged by this process and is consistent with reports that the retinal and CNS environment of the mutant mice is permissive for neurite extension and regeneration.3,44-46
Although there are no gross phenotypic abnormalities in Müller cells in the GFAP-/-vim-/- retinas, the cells appear altered in subtle ways. We have previously suggested that the absence of GFAP and vimentin could affect retinal detachment-induced phosphorylation of Erk and c-fos in Müller cells, perhaps because of disabled subcellular translocation of these signaling proteins.34 Studies using the GFAP-/-vim-/- mice seem to point clearly to a role for GFAP and vimentin in stabilizing and strengthening the specialized end foot compartment of the cell, which may help protect the vitreoretinal interface from mechanical damage. Additionally, intermediate filaments appear to be essential for the wound-healing response of Müller cells, specifically subretinal glial scar formation. By extrapolation, this presumably extends to vitreal scar formation by Müller cells. Thus, the short-term inhibition of intermediate filament reorganization and synthesis during an episode of detachment or other retinal injury may help to reduce the incidence or severity of subretinal fibrosis or proliferative vitreoretinopathy.
Acknowledgments
Supported in part by National Institutes of Health/National Eye Institute Grant EY00888 (SKF), National Science Foundation Grant ITR-0331697 (SKF), the Department of Defense (D-FC), the Massachusetts Lions Eye Research Fund (D-FC), Research to Prevent Blindness (D-FC), the American Health Foundation (D-FC), the Swedish Medical Research Council (11548), the Swedish Stroke Foundation, the Torsten and Ragnar Söderberg Foundations, the region of Västra Götaland (RUN), the Frimurare Foundation, Hjärnfonden, ALF Göteborg (MP), and NCRR Shared Instrumentation Grant 1S10RR017753-01 (SKF).
Footnotes
Disclosure: M.R. Verardo, None; G.P. Lewis, None; M. Takeda, None; K.A. Linberg, None; J. Byun, None; G. Luna, None; U. Wilhelmsson, None; M. Pekny, None; D.-F. Chen, None; S.K. Fisher, None
References
- 1.Profyris C, Cheema SS, Zang D, Azari MF, Boyle K, Petratos S. Degenerative and regenerative mechanisms governing spinal cord injury. Neurobiol Dis. 2004;15:415–436. doi: 10.1016/j.nbd.2003.11.015. [DOI] [PubMed] [Google Scholar]
- 2.Eng LF, Ghirnikar RS. GFAP and astrogliosis. Brain Pathol. 1994;4:229–237. doi: 10.1111/j.1750-3639.1994.tb00838.x. [DOI] [PubMed] [Google Scholar]
- 3.Wilhelmsson U, Bushlong EA, Price DL, et al. Redefining the concept of reactive astrocytes as cells that remain within their unique domains upon reaction to injury. Proc Natl Acad Sci U S A. 2006;103:17513–17518. doi: 10.1073/pnas.0602841103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pekny M, Nilsson M. Astrocyte activation and reactive gliosis. Glia. 2005;50:427–434. doi: 10.1002/glia.20207. [DOI] [PubMed] [Google Scholar]
- 5.Fisher SK, Lewis GP. Cellular effects of detachment on the neural retina and the retinal pigment epithelium. In: Ryan SJ, Wilkinson CP, editors. Retina. 4th ed Vol. 3. Mosby; St. Louis, MO: 2006. pp. 1991–2012. [Google Scholar]
- 6.Burton TC. Recovery of visual acuity after retinal detachment involving the macula. Trans Am Ophthalmol Soc. 1982;80:475–497. [PMC free article] [PubMed] [Google Scholar]
- 7.Tani P, Robertson DM, Langworthy A. Rhegmatogenous retinal detachment without macular involvement treated with scleral buckling. Am J Ophthalmol. 1980;90:503–508. doi: 10.1016/s0002-9394(14)75019-6. [DOI] [PubMed] [Google Scholar]
- 8.Tani PD, Robertson DM, Langworthy A. Prognosis for central vision and anatomic reattachment in rhegmatogenous retinal detachment with macula detached. Am J Ophthalmol. 1981;92:611–620. doi: 10.1016/s0002-9394(14)74651-3. [DOI] [PubMed] [Google Scholar]
- 9.Chang SD, Kim IT. Long-term visual recovery after scleral buckling procedure of rhegmatogenous retinal detachment involving the macula. Korean J Ophthalmol. 2000;14:20–26. doi: 10.3341/kjo.2000.14.1.20. [DOI] [PubMed] [Google Scholar]
- 10.Lewis GP, Linberg KA, Fisher SK. Neurite outgrowth from bipolar and horizontal cells after experimental retinal detachment. Invest Ophthalmol Vis Sci. 1998;39:424–434. [PubMed] [Google Scholar]
- 11.Kroll AJ. Secondary retinal detachment: electron microscopy of retina and pigment epithelium. Am J Ophthalmol. 1969;68:223–237. [PubMed] [Google Scholar]
- 12.Okada M, Matsumura M, Ogino N, Honda Y. Müller cells in detached human retina express glial fibrillary acidic protein and vimentin. Graefes Arch Clin Exp Ophthalmol. 1990;228:467–474. doi: 10.1007/BF00927264. [DOI] [PubMed] [Google Scholar]
- 13.Chang CH, Lai WW, Edward DP, Tso MO. Apoptotic photoreceptor cell death after traumatic retinal detachment in humans. Arch Ophthalmol. 1995;113:880–886. doi: 10.1001/archopht.1995.01100070054025. [DOI] [PubMed] [Google Scholar]
- 14.Li ZY, Klajavin IJ, Milam AH. Rod photoreceptor neurite sprouting in retinitis pigmentosa. J Neurosci. 1995;15:5429–5438. doi: 10.1523/JNEUROSCI.15-08-05429.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sethi CS, Lewis GP, Fisher SK, et al. Glial remodeling and neural plasticity in human retinal detachment with proliferative vitreoretinopathy. Invest Ophthalmol Vis Sci. 2005;46:329–342. doi: 10.1167/iovs.03-0518. [DOI] [PubMed] [Google Scholar]
- 16.Fisher SK, Lewis GP, Linberg KA, Verardo MR. Cellular remodeling in mammalian retina: results from studies of experimental retinal detachment. Prog Retin Eye Res. 2005;24:395–431. doi: 10.1016/j.preteyeres.2004.10.004. [DOI] [PubMed] [Google Scholar]
- 17.Jones BW, Marc RE. Retinal remodeling during retinal degeneration. Exp Eye Res. 2005;81:123–137. doi: 10.1016/j.exer.2005.03.006. [DOI] [PubMed] [Google Scholar]
- 18.Anderson DH, Guerin CJ, Erickson PA, Stern WH, Fisher SK. Morphological recovery in the reattached retina. Invest Ophthalmol Vis Sci. 1986;27:168–183. [PubMed] [Google Scholar]
- 19.Charteris DG, Downie J, Aylward GW, Sethi C, Luthert P. Intraretinal and periretinal pathology in anterior proliferative vitreoretinopathy. Graefe Arch Clin Exp Ophthalmol. 2007;245:93–100. doi: 10.1007/s00417-006-0323-5. [DOI] [PubMed] [Google Scholar]
- 20.Lewis GP, Fisher SK. Retinal plasticity and interactive cellular remodeling in retinal detachment and reattachment. In: Pinaud R, Tremere L, Weerd PD, editors. Plasticity in the Visual System: From Genes to Circuits. Springer; New York: 2005. pp. 55–78. [Google Scholar]
- 21.Lewis GP, Fisher SK. Upregulation of GFAP in response to retinal injury: its potential role in glial remodeling and a comparison to vimentin expression. In: Jeon KW, editor. Int Rev Cytol: A Survey of Cell Biology. Vol. 230. Elsevier Academic Press; San Diego, CA: 2003. pp. 263–290. [DOI] [PubMed] [Google Scholar]
- 22.Pekny M, Johansson CB, Eliasson C, et al. Abnormal reaction to central nervous system injury in mice lacking glial fibrillary acidic protein and vimentin. J Cell Biol. 1999;145:503–514. doi: 10.1083/jcb.145.3.503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wilhelmsson U, Li L, Pekney M, et al. Absence of glial fibrillary acidic protein and vimentin prevents hypertrophy of astrocytic processes and improves posttraumatic regeneration. J Neurosci. 2004;24:5016–5021. doi: 10.1523/JNEUROSCI.0820-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lundkvist A, Reichenbach A, Betsholtz C, Carmeliet P, Wolburg H, Pekny M. Under stress, the absence of intermediate filament proteins from Müller cells in the retina has structural and functional consequences. J Cell Sci. 2004;117:3481–3488. doi: 10.1242/jcs.01221. [DOI] [PubMed] [Google Scholar]
- 25.Yang L, Bula D, Arroyo JG, Chen DF. Preventing retinal detachment-associated photoreceptor loss in Bax-deficient mice. Invest Ophthalmol Vis Sci. 2004;45:648–654. doi: 10.1167/iovs.03-0827. [DOI] [PubMed] [Google Scholar]
- 26.Byun J, Verardo MR, Vu N, et al. Quantifying structural distortions in retinal tissue before and after injury. Workshop on Multiscale Biological Imaging, Data Mining and Informatics; September 7-8, 2006; Santa Barbara, CA: Presented at. [Google Scholar]
- 27.Sarthy V, Ripps H. Reactive gliosis. In: Blakemore C, editor. Perspectives in Vision Research. Kluwer Academic/Plenum Publishers; New York: 2001. pp. 198–215. [Google Scholar]
- 28.Dreher Z, Robinson SR, Distler C. Müller cells in vascular and avascular retinae: a survey of seven mammals. J Comp Neurol. 1992;323:59–80. doi: 10.1002/cne.903230106. [DOI] [PubMed] [Google Scholar]
- 29.Lewis GP, Erickson PA, Guerin CJ, Anderson DH, Fisher SK. Changes in the expression of specific Müller cell proteins during long term retinal detachment. Exp Eye Res. 1989;49:93–111. doi: 10.1016/0014-4835(89)90079-1. [DOI] [PubMed] [Google Scholar]
- 30.Lewis GP, Guerin CJ, Anderson DH, Matsumoto B, Fisher SK. Experimental retinal detachment causes rapid changes in the expression of glial cell proteins. Am J Ophthalmol. 1994;118:368–376. doi: 10.1016/s0002-9394(14)72962-9. [DOI] [PubMed] [Google Scholar]
- 31.Jansen H, Sanyal S, De Grip WJ, Schalken JJ. Development and degeneration of retina in rds mutant mice: ultra-immunohisto-chemical localization of opsin. Exp Eye Res. 1987;44:347–361. doi: 10.1016/s0014-4835(87)80170-7. [DOI] [PubMed] [Google Scholar]
- 32.Nir I, Papermaster DS. Immunocytochemical localization of opsin in degenerating photoreceptors of RCS rats, and rd and rds mice. Prog Clin Biol Res. 1989;314:251–264. [PubMed] [Google Scholar]
- 33.Lewis GP, Erickson PA, Anderson DH, Fisher SK. Opsin distribution and protein incorporation in photoreceptors after experimental retinal detachment. Exp Eye Res. 1991;53:629–640. doi: 10.1016/0014-4835(91)90223-2. [DOI] [PubMed] [Google Scholar]
- 34.Nakazawa T, Takeda M, Lewis GP, et al. Attenuated glial cell reactions and photoreceptor degeneration after retinal detachment in mice deficient in glial fibrillary acidic protein and vimentin. Invest Ophthalmol Vis Sci. 2007;48:2760–2768. doi: 10.1167/iovs.06-1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Erickson PA, Fisher SK, Anderson DH, Stern WH, Borgula GA. Retinal detachment in the cat: the outer nuclear and outer plexi-form layers. Invest Ophthalmol Vis Sci. 1983;24:927–942. [PubMed] [Google Scholar]
- 36.Linberg KA, Lewis GP, Matsumoto B, Fisher SK. Immunocytochemical evidence that rod-connected horizontal cell axon terminals remodel in response to experimental retinal detachment in the cat. Mol Vis. 2206;12:1674–1686. [PubMed] [Google Scholar]
- 37.Coblentz FE, Radeke MJ, Lewis GP, Fisher SK. Evidence that ganglion cells react to retinal detachment. Exp Eye Res. 2003;76:333–342. doi: 10.1016/s0014-4835(02)00305-6. [DOI] [PubMed] [Google Scholar]
- 38.Erickson PA, Fisher SK, Guerin CJ, Anderson DH, Kaska DD. Glial fibrillary acidic protein increases in Müller cells after retinal detachment. Exp Eye Res. 1987;44:37–48. doi: 10.1016/s0014-4835(87)80023-4. [DOI] [PubMed] [Google Scholar]
- 39.Erickson PA, Feinstein SC, Lewis GP, Fisher SK. Glial fibrillary acidic protein and its mRNA: ultrastructural detection and determination of changes after CNS injury. J Structural Biol. 1992;108:148–161. doi: 10.1016/1047-8477(92)90014-2. [DOI] [PubMed] [Google Scholar]
- 40.Newman E, Reichenbach A. The Müller cell: a functional element of the retina. Trends Neurosci. 1996;19:307–312. doi: 10.1016/0166-2236(96)10040-0. [DOI] [PubMed] [Google Scholar]
- 41.Li L, Lundkvist A, Andersson D, et al. Protective role of reactive astrocytes in brain ischemia. J Cereb Blood Flow Metab. 2008;28:468–481. doi: 10.1038/sj.jcbfm.9600546. [DOI] [PubMed] [Google Scholar]
- 42.Green WR, Sebag J. Vitreoretinal interface. In: Ryan SJ, Wilkinson CP, editors. Retina. 4th ed Vol. 3. Mosby; St. Louis, MO: 2006. pp. 1921–1989. [Google Scholar]
- 43.Lewis GP, Betts KE, Sethi CS, et al. Identification of ganglion cell neurites in human sub- and epiretinal membranes. Br J Ophthalmol. 2007;91:1234–1238. doi: 10.1136/bjo.2006.104612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kinouchi R, Takeda M, Yang L, et al. Robust neural integration from retinal transplants in mice deficient in GFAP and vimentin. Nat Neurosci. 2003;6:863–868. doi: 10.1038/nn1088. [DOI] [PubMed] [Google Scholar]
- 45.Menet V, Prieto M, Privat A, Giménez, Ribotta M. Axonal plasticity and functional recovery after spinal cord injury in mice deficient in both glial fibrillary acidic protein and vimentin genes. Proc Natl Acad Sci USA. 2003;100:8999–9004. doi: 10.1073/pnas.1533187100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Larsson A, Faijerson J, Wilhelmsson U, et al. Increased neurogenesis and astrogenesis from neural progenitor cells grafted in the hippocampus of GFAP-/-vim-/- mice. Stem Cells. 2007;25:2619–2627. doi: 10.1634/stemcells.2007-0122. [DOI] [PubMed] [Google Scholar]


