Abstract
Emerging evidence suggests that current fluoroquinolone dosing strategies may be inadequate to treat bloodstream infections caused by organisms classified as sensitive. This study sought to determine if differences in MICs for levofloxacin-susceptible gram-negative organisms correlate with differences in patient outcomes. A retrospective cohort study evaluated patients treated with levofloxacin for bloodstream infections caused by susceptible gram-negative organisms. Patients infected with gram-negative organisms for which MICs indicated susceptibility were categorized into three groups: those with organisms for which MICs were low (≤0.25 mg/liter), intermediate (0.5 mg/liter), and high (1 or 2 mg/liter). Patients were evaluated for baseline similarity, all-cause mortality, and measurements of morbidity. A total of 404 patients with bloodstream infections caused by gram-negative organisms were identified. Of these patients, 312 were treated with levofloxacin and included in the analysis. No significant difference in all-cause mortality among the three groups was observed. The high-MIC group had a significantly longer average hospital stay postculture than the low- and intermediate-MIC groups (16.4 days versus 7.3 and 7.9 days; P < 0.01) and a significantly longer duration of infection (2.1 days versus 1.0 and 1.2 days; P < 0.001). Multivariate analysis adjusting for covariates revealed that a high MIC was associated with an increase of 5.67 days (95% confidence interval, 0.77 to 10.62 days; P = 0.02) in the mean length of stay postculture compared to the mean length of stay for the low-MIC group. Patients treated with levofloxacin for bloodstream infections caused by gram-negative organisms for which MICs were elevated, yet still in the susceptible category, had worse outcomes than similar patients infected with organisms for which MICs were lower. In vitro susceptibility classifications of fluoroquinolones for the treatment of bloodstream infections caused by gram-negative organisms require further study.
The pharmacokinetic (PK) and pharmacodynamic (PD) profiles of fluoroquinolones have been well-described, and susceptibility classifications are the main determinant of their use in clinical practice. Expert guidelines contain susceptibility breakpoints to aid clinicians with the interpretation of in vitro data. These breakpoints are based on the MIC distribution for a large number of microorganisms, the observed clinical response in patients treated with usual doses, and the PK-PD properties of the drug (8, 16, 16a, 22, 24, 25). The breakpoints are often used, in conjunction with susceptibility results, to predict clinical outcome. However, emerging data highlight the lack of association between in vitro susceptibility breakpoints and outcomes (3, 30).
Fluoroquinolones are considered to be concentration dependent, with both the area under the concentration-time curve from 0 to 24 h (AUC0-24) divided by the MIC (the AUC0-24/MIC ratio) and the maximum serum drug concentration (Cmax) divided by the MIC (the Cmax/MIC ratio) as predictors of clinical outcome (13, 24). Numerous studies have attempted to discern the parameter best associated with clinical outcome. Studies favoring the Cmax/MIC ratio have shown that maximum serum fluoroquinolone concentrations reaching 8 to 12 times the MIC are associated with favorable outcomes (4, 13, 26). However, evidence for the AUC0-24/MIC ratio is convincing if the colinearity of the Cmax/MIC and AUC0-24/MIC ratios is removed (27). Evaluations have shown previously that AUC0-24/MIC ratios greater than 125 are associated with optimal outcomes for infections with gram-negative organisms (13, 17, 23).
The aforementioned studies were completed largely in vivo and did not account for protein binding (with the exception of one trial [4]). One can anticipate that slightly lower concentrations are needed when free drug is considered. As predicted, lower AUC0-24/MIC ratios are required when only free-drug values are used in calculations, and extrapolating free-drug concentrations from the data in the aforementioned clinical studies results in an optimal free-drug AUC0-24/MIC ratio between 61 and 105 (2, 5, 14).
As currently defined by the Clinical and Laboratory Standards Institute (CLSI) and the European Committee on Antimicrobial Susceptibility Testing, the MIC breakpoint of levofloxacin for aerobic and facultative gram-negative organisms is 2 mg/liter (8, 16a; Levaquin package insert [http://www.levaquin.com]). The achievable AUC0-24 for levofloxacin is 54.6 mg·h/liter for multiple 500-mg doses taken every 24 h (Levaquin package insert [http://levaquin.com/levaquin/shared/pi/levaquin.pdf]). After accounting for protein binding of 30%, one can assume a mean AUC0-24 of 38.2 mg·h/liter. Simple division shows that a MIC of less than 0.5 mg/liter is necessary to achieve a free-drug AUC0-24/MIC ratio of at least 75 mg·hr/liter, which is a conservative target supported by efficacy data. Hence, it has been suggested previously that the PD breakpoint for levofloxacin should be 0.25 mg/liter (24). Because achievable AUC0-24 values for levofloxacin are predicted to be suboptimal for MICs of ≥0.5 mg/liter, we reviewed MICs for our gram-negative pathogens in infections treated with levofloxacin. A pilot study at Northwestern Memorial Hospital, Chicago, IL, identified elevated fluoroquinolone MICs for Escherichia coli and Klebsiella pneumoniae (29). We hypothesized that among patients treated with a fluoroquinolone, those with bloodstream infections caused by gram-negative organisms for which levofloxacin MICs were elevated, yet still in the susceptible category, would fare worse than those infected with organisms for which levofloxacin MICs were lower.
(This work was presented at the 46th Interscience Conference on Antimicrobial Agents and Chemotherapy, San Francisco, CA, 27 to 30 September 2006 [10].)
MATERIALS AND METHODS
A retrospective cohort analysis was carried out using an existing case mix database (28) from a previous study completed at Northwestern Memorial Hospital, an 897-bed academic medical center. All blood cultures isolating gram-negative organisms (Enterobacteriaceae and non-Enterobacteriaceae, including Pseudomonas spp.) between 1 January 2001 and 30 November 2003 were considered for inclusion. The study was reviewed and approved by the Northwestern University institutional review board.
Microbiology and antibiotic therapy.
All clinical isolates were identified to the species level using the Vitek 2 system (bioMerieux, St. Louis, MO). When Vitek 2 was unable to identify the species, identification was performed based on manual biochemical reactions. In vitro susceptibilities for levofloxacin were required to be quantified as MICs (≤0.25, 0.5, 1, or ≥2 mg/liter). MICs of all antibiotics were obtained by the Vitek 2 or Etest (AB Biodisk, Solna, Sweden) methodology and were interpreted according to the 2007 CLSI antimicrobial susceptibility testing requirements (8). Treatment was defined as described previously (28). Briefly, antibiotics were considered active for patient courses if they were received on the day of the culture or within 24 h of the initial culture, and the infecting organism was considered susceptible by CLSI criteria as described above. Pharmacy dispensing records for all antibiotics active against gram-negative bacilli were matched to the in vitro susceptibility results for all study patients. Patients who received any antimicrobial active against the organism, in combination with levofloxacin, were documented as having other active antimicrobials.
All cultures were considered clinically significant, as the isolation of a gram-negative organism from the blood is rarely consistent with contamination. Patients with blood cultures positive for two or more different organisms recovered during the same hospitalization were excluded from the analysis. In addition, patients were excluded if susceptibility results were not obtained.
Study group assignment based on PK-PD parameters.
Patients were categorized into three groups: the low-MIC group, the intermediate-MIC group, and the high-MIC group. The low-MIC group consisted of patients treated with levofloxacin with an MIC less than or equal to 0.25 mg/liter. The intermediate-MIC group consisted of patients treated with levofloxacin with an MIC equal to 0.5 mg/liter. The high-MIC group consisted of patients treated with levofloxacin with an MIC equal to 1 or 2 mg/liter. Therefore, all patients included in this evaluation had organisms for which the MIC indicated susceptibility by current CLSI guidelines (8).
Outcome measures.
The three groups were assessed for baseline demographic characteristics, including age, sex, and the infecting organism (E. coli, K. pneumoniae, Pseudomonas aeruginosa, or other). The Deyo modification of the Charlson comorbidity score was used as a marker for the severity of illness (6, 11). Patients were stratified based on three categories of Charlson scores: 0, 1 to 2, and ≥3. Admission to an intensive care unit (ICU) within 24 h of the blood culture showing gram-negative organisms, admission to a surgical service with sepsis as a diagnosis code, and nosocomial infection, defined by a positive-culture date more than 2 days after the hospital admission date, were noted as additional measures of infection severity (28). Data on the preculture length of stay, the preculture length of fluoroquinolone treatment, and the prescription of active antimicrobials in addition to levofloxacin were also collected to further describe the groups.
Patient outcomes were evaluated in comparisons of the three groups. The primary outcome in the study was all-cause inpatient mortality. Markers of morbidity were assessed secondarily and included the duration of infection (time to resolution as demonstrated by blood culture), the postculture length of stay, and the length of fluoroquinolone treatment postculture. The duration of infection was measured by using available blood cultures. Serial sampling of blood cultures in cases of bloodstream infection caused by gram-negative organisms is generally the standard of care at our hospital. Patients who died in the hospital were excluded from the analyses of the length of stay postculture, the length of levofloxacin therapy postculture, and the duration of infection.
Statistical analysis.
Statistical analysis was performed with SPSS version 16.0 (SPSS, Inc., Chicago, IL). The association of inpatient mortality with the MIC classification (high, intermediate, or low) was tested using the χ2 test. χ2 tests were also used to test the association of categorical independent variables (sex, organism type, nosocomial versus community-acquired infection, Charlson score level, ICU admission, surgical admission, the presence of sepsis, and treatment with another active microbial) and the MIC classification. The association between patient age and the MIC category was tested by analysis of variance, and the associations of the MIC category and other nonnormally distributed continuous independent variables (the preculture length of stay, preculture length of levofloxacin treatment, and duration of infection) were analyzed using the nonparametric Kruskal-Wallis test. When three-group comparisons revealed statistical differences, between-group comparisons of the high-MIC group and all others were performed by the Mann-Whitney U test for the nonparametric comparison of two groups. Noncontinuous measures were evaluated with χ2 tests dichotomously separating the high-MIC group from the combined intermediate- and low-MIC group.
In addition to univariate analyses, multiple linear regression (analysis of covariance) was used to test the simultaneous significance of associations between MIC groups and the postculture length of stay, controlling for all the covariates listed above with the exception of the preculture length of levofloxacin treatment, which was very highly correlated with the preculture length of stay. The duration of infection was also evaluated as a dependent variable; however, the results are not detailed, as this analysis did not produce an improved regression model as evaluated by using R2.
RESULTS
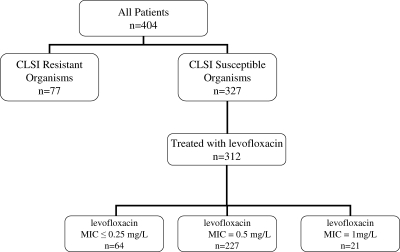
A total of 404 patients treated with fluoroquinolones for bloodstream infections caused by gram-negative organisms were identified (Fig. 1). Of these patients, 312 were treated with levofloxacin and included in the analysis. Patients excluded from the analysis were 77 patients who had a bloodstream infection that was resistant to fluoroquinolone antibiotics and 15 patients who were treated with a fluoroquinolone other than levofloxacin. Of the 312 patients treated with levofloxacin, 64 were in the low-MIC group, 227 were in the intermediate-MIC group, and 21 were in the high-MIC group. Demographic data for all patients are presented in Table 1. There were no significant differences in age, sex, or Charlson score among the three groups. The organisms identified as causative pathogens included E. coli (53%), Klebsiella spp. (28%), and Pseudomonas spp. (5%). Other organisms accounted for the remaining 14% and included most commonly Enterobacter cloacae, Proteus mirabilis, Serratia marcescens, and Acinetobacter baumannii. The three groups differed in the percentage of infections due to E. coli (50 versus 57.3 versus 9.5% for the low-, intermediate-, and high-MIC groups; P < 0.001) and Pseudomonas spp. (0% versus 3.5 and 38.1%; P < 0.001). The groups also differed in the preculture in length of stay (2.7 versus 2.0 versus 11.7 days; P < 0.001) and the length of levofloxacin treatment preculture (0.2 versus 0.5 versus 8.8 days; P < 0.001). There were a total of 55 patients with a nosocomial bloodstream infection, and the percentages of these patients among the three groups were significantly different (38.1 versus 15.6 and 17%; P = 0.04). These covariates were all assessed as potential confounders. There was no difference among the three groups in ICU or surgical admissions at the time of initial therapy, in the number of patients receiving active antimicrobial agents in addition to levofloxacin, or in the number of patients with a diagnosis code of sepsis.
FIG. 1.
Patient groups.
TABLE 1.
Characteristics of patients with bloodstream infections caused by gram-negative organisms in low-, intermediate-, and high-MIC groups
| Characteristica | Value for patients (n = 312) grouped according to:
|
P valuee | ||
|---|---|---|---|---|
| Low MICb (n = 64) | Intermediate MICc (n = 227) | High MICd (n = 21) | ||
| Male sex | 29 (45.3) | 105 (46.3) | 13 (61.9) | 0.37 |
| Age (yr)f | 58.6 (19.2) | 61.1 (18.0) | 60.2 (16.7) | 0.62g |
| Infecting organism | ||||
| P. aeruginosa | 0 (0) | 8 (3.5) | 8 (38.1)h | <0.001 |
| E. coli | 32 (50) | 130 (57.3) | 2 (9.5)h | <0.001 |
| K. pneumoniae | 19 (29.7) | 61 (26.9) | 8 (38.1) | 0.53 |
| Other | 13 (20.3) | 28 (12.3) | 3 (14.3) | 0.27 |
| Nosocomial infection | 10 (15.6) | 37 (16.3) | 8 (38.1)h | 0.04 |
| Charlson score | 0.96 | |||
| 0 | 29 (45.8) | 104 (45.8) | 9 (42.9) | |
| 1-2 | 28 (43.8) | 91 (40.1) | 9 (42.9) | |
| ≥3 | 7 (10.9) | 32 (14.1) | 3 (14.3) | |
| ICU admission | 16 (25) | 58 (25.6) | 8 (38.1) | 0.44 |
| Surgical admission | 42 (65.6) | 136 (59.9) | 13 (61.9) | 0.71 |
| Sepsis diagnosis code | 14 (21.9) | 67 (29.5) | 6 (27.9) | 0.48 |
| Treatment with another active antimicrobial | 26 (40.6) | 98 (43.2) | 11 (52) | 0.64 |
| Preculture length of stay (days)f | 2.7 (6.9) | 2.0 (6.0) | 11.7 (25.1)h | 0.01i |
| Preculture length of levofloxacin treatment (days)f | 0.2 (0.9) | 0.5 (4.7) | 8.8 (23.8)j | 0.08i |
All data are presented as the number (percentage) of patients unless otherwise indicated.
A low MIC was defined as a levofloxacin MIC of ≤0.25 mg/liter.
An intermediate MIC was defined as a levofloxacin MIC of 0.5 mg/liter.
A high MIC was defined as a levofloxacin MIC of 1 or 2 mg/liter.
Determined by a χ2 test unless otherwise indicated.
Data are presented as the mean (standard deviation).
Determined by analysis of variance.
Results of Mann-Whitney U nonparametric test for two groups, the high-MIC group versus all others: group with infections caused by P. aeruginosa, P < 0.001; group with infections caused by E. coli, P < 0.001; and group with nosocomial infections, P < 0.011.
Determined by a Kruskal-Wallis nonparametric test for three groups.
Results of Mann-Whitney U nonparametric test for two groups, the high-MIC group versus all others: P = 0.007.
Mortality and morbidity data.
Bivariate mortality and morbidity data are presented in Table 2. While there was no significant difference in mortality among the three groups, other outcome variables showed differences among the MIC categories. Specifically, the postculture lengths of stay (7.3 versus 7.9 and 16.4 days; P = 0.001), the lengths of levofloxacin treatment postculture (4.8 versus 6.2 and 13.3 days; P < 0.001), and the durations of infection (1.0 versus 1.2 and 2.1 days; P < 0.001) differed among the groups. When the high-MIC category was compared to the low and intermediate categories combined, the average length of stay postculture (P < 0.01), the length of levofloxacin treatment postculture (P = 0.02), and the duration of infection (P < 0.001) were significantly longer for the high-MIC group.
TABLE 2.
Comparisons of lengths of stay and treatment outcomes for surviving patients with bloodstream infections caused by gram-negative organisms in the low-, intermediate-, and high-MIC groups
| Outcomea | Value for patients (n = 275)f grouped according to:
|
P valuee | ||
|---|---|---|---|---|
| Low MICb (n = 56) | Intermediate MICc (n = 201) | High MICd (n = 18) | ||
| Mortalityf | 8 (12.5%) | 26 (11.5%) | 3 (14.3%) | 0.91g |
| Length of stay postculture (days) | 7.3 (6.8) | 7.9 (8.8) | 16.4 (20.6)h | 0.02 |
| Length of levofloxacin treatment postculture (days) | 4.8 (4.7) | 6.2 (6.2) | 13.3 (17.4)i | 0.01 |
| Duration of infection (days) | 1.0 (0.1) | 1.2 (0.8) | 2.1 (1.9)j | <0.001 |
All data are presented as the mean (standard deviation) unless otherwise indicated.
A low MIC was defined as a levofloxacin MIC of ≤0.25 mg/liter.
An intermediate MIC was defined as a levofloxacin MIC of 0.5 mg/liter.
A high MIC was defined as a levofloxacin MIC of 1 or 2 mg/liter.
Determined by the Kruskal-Wallis test unless otherwise indicated.
The total number of patients including those who died was 312; patients who died were not considered in analyses of outcomes other than mortality.
Determined by a χ2 test.
Results of Mann-Whitney U nonparametric test for two groups, the high-MIC group versus all others: P = 0.006.
Results of Mann-Whitney U nonparametric test for two groups, the high-MIC group versus all others: P = 0.02.
Results of Mann-Whitney U nonparametric test for two groups, the high-MIC group versus all others: P < 0.001.
Multivariate analysis.
Logistic regression indicated no significant difference among groups for the likelihood of inpatient death (results not shown). Multiple linear regression analysis controlling for the infecting organism and the severity of illness (Table 3) revealed that a high MIC was associated with an increase of 5.67 days (P = 0.02) in the postculture length of stay compared to the mean length of stay for the low-MIC group. In addition, ICU care at the time of initial treatment (associated with a 6.39-day increase; P < 0.001), the preculture length of stay (associated with 0.27-day increase for every additional day of preculture stay; P < 0.001), and the use of another active antimicrobial (associated with a 2.97-day increase; P = 0.007) were all independently associated with the length of stay among survivors. Pseudomonas infection was associated with a decrease (by 4.69 days; P = 0.004) in the postculture length of stay among survivors.
TABLE 3.
Characteristics associated with increasing length of stay postculture for surviving patientsa
| Characteristic | β value (days) | 95% Confidence interval | P value |
|---|---|---|---|
| High MICb,c | 5.67 | 0.77 to 10.62 | 0.02 |
| Intermediate MICc,d | 0.73 | −1.69 to 3.17 | 0.55 |
| Age in years | 0.02 | −0.04 to 0.07 | 0.53 |
| Male sex | 1.04 | −0.99 to 3.07 | 0.31 |
| Charlson scoree | |||
| 1-2 | 1.06 | −1.02 to 3.14 | 0.32 |
| ≥3 | 2.85 | −0.54 to 6.24 | 0.10 |
| Sepsis diagnosis code | −1.67 | −3.94 to 0.60 | 0.15 |
| ICU admission | 6.39 | 3.73 to 9.05 | <0.001 |
| Nosocomial infection | 2.04 | −1.43 to 5.52 | 0.25 |
| Days of preculture stay | 0.27 | 0.13 to 0.41 | <0.001 |
| Infecting organismf | |||
| P. aeruginosa | −6.67 | −12.72 to −0.63 | 0.03 |
| E. coli | −1.48 | −4.62 to 1.66 | 0.35 |
| K. pneumoniae | 0.09 | −3.20 to 3.39 | 0.96 |
| Treatment with another active antimicrobial | 2.97 | 0.82 to 5.13 | 0.007 |
Data for 275 patients were analyzed (r2 = 0.36). P values were determined by multiple linear regression; statistically significant values are in bold.
A high MIC was defined as a levofloxacin MIC of 1 or 2 mg/liter.
Patients in the high- and intermediate-MIC groups were compared to those in the low-MIC group (a low MIC was defined as a levofloxacin MIC of ≤0.25 mg/liter).
An intermediate MIC was defined as a levofloxacin MIC of 0.5 mg/liter.
Patients were compared to those with a Charlson score of 0.
Patients were compared to those infected with other organisms.
DISCUSSION
These findings indicate that although there was no difference found in inpatient mortality among patients infected with organisms for which fluoroquinolone MICs varied, there was a significantly longer duration of hospitalization (by approximately 6 days) for surviving patients infected with organisms for which MICs were high but in the sensitive range, even after the analysis controlled for the severity of illness and the infecting organism. This significant difference in morbidity supports the belief that current MIC breakpoint values for fluoroquinolones used to treat bloodstream infections caused by gram-negative organisms may be too high.
Bloodstream infections are associated with significant mortality and morbidity and high health care costs (31). The emergence of antimicrobial-resistant pathogens may be one of the most common causes of inactive therapy and the driver of more stringent MIC breakpoint determinations. A previous publication highlighted the history of MIC breakpoint selection methods and emphasized the use of PK-PD theory in the classification of susceptible and resistant organisms (24). Other recent studies have challenged current susceptibility breakpoints for gram-negative pathogens causing bloodstream infections. For patients with such infections treated with cefepime, MICs of ≥8 μg/ml proved to be an independent predictor of mortality compared to lower MICs within the susceptible category (3). Likewise, Tam et al. found that reduced susceptibility to empirical piperacillin-tazobactam therapy for pseudomonal bacteremias was associated with increased mortality (30).
The failure of current MIC breakpoints to predict clinical outcomes has led to the suggestion that breakpoints should account for interindividual PK variation. To this end, Drusano et al. have proposed Monte Carlo simulation as a means of determining breakpoints based on population PKs and microbiological susceptibilities (12, 15). Such methods for setting breakpoints have been adopted by both the European Committee on Antimicrobial Susceptibility Testing and the CLSI (1, 20). One study using these techniques compared the CLSI MIC breakpoints to achievable PK profiles and showed that fluoroquinolone breakpoints derived from PK-PD theory for aerobic gram-negative bacteria were lower than current CLSI susceptibility breakpoints (18). Another recent study by Kiser and colleagues evaluated levofloxacin PKs-PDs in severe burn injury (21). Using a Monte Carlo simulation, they showed that for patients infected with gram-negative organisms for which MICs were ≤0.5 μg/ml, the probability of achieving an AUC0-24/MIC ratio of ≥87 was 100%, but for patients infected with organisms for which MICs were 1 or 2 μg/ml, which are classified as susceptible by CLSI standards, the probability was 55 or 0%, respectively. Results from our evaluation provide empirical support for these theoretical studies and evidence that the CLSI breakpoints for fluoroquinolones may need to be reevaluated. While this is a retrospective review, it is the first clinical study to our knowledge that provides evidence of suboptimal outcomes in patients who are treated with fluoroquinolones for bloodstream infections caused by organisms for which MICs are high yet within the susceptible category.
Possible explanations for treatment failure include the existence of organisms with a genetic point mutation increasing proclivity to the ultimate result of full phenotypic fluoroquinolone resistance. Such scenarios would be consistent with the treatment failures associated with first-step mutations of parC or gyrA in Streptococcus pneumoniae and Salmonella enterica (9, 19). Unfortunately, we were not able to evaluate the hypothesis of resistance progression, as our study was retrospective and, generally, susceptibility profiles are generated only for the first isolate from a patient from whom a series of organisms is obtained.
The primary end point of this study was all-cause mortality, the rates of which among the three study groups were not significantly different. This finding is not surprising, given the insensitivity of the end point and the large sample size needed to detect significant factors that affect mortality. However, if one considers only the 177 patients (57%) who received levofloxacin monotherapy (no other active treatment), an association may emerge. Essentially, 38 patients remained in the low-MIC category, 129 patients remained in the intermediate-MIC category, and 10 patients remained in the high-MIC category. Mortality rates were 3, 4, and 20%, respectively. The high-MIC group had significantly more frequent death than the other two groups (P = 0.05) (results not shown). However, with the small numbers of patients who died, it is difficult to determine if this finding represents a true effect.
Perhaps strengthening the argument, the postculture length of stay was determined to be increased by 5.7 days for surviving patients in the high-MIC group after the analysis controlled for multiple covariates known to affect mortality and baseline differences among the groups, including concomitant therapy (Table 3). This increased length of stay for the high-MIC group may indicate greater morbidity in our population due to inadequate antimicrobial dosing or improper agent selection. We believe that the length of stay is a fair marker for morbidity and probably detects subtle changes in patient outcomes that are otherwise not readily apparent in looking solely for differences in mortality. However, it should be noted that any classification of the length of stay in retrospective analyses is subject to bias. We chose to remove data for patients that ultimately died to prevent bias in the data toward shorter lengths of stay for patients with the direst outcome.
Because multivariate results did not differ for predictors of mortality, only univariate results are reported for mortality across stratifications of MICs. After our initial analysis described in Materials and Methods, we attempted to define an MIC (appropriately adjusted for confounders) that was associated with increased mortality by using a binary recursive partitioning analysis. The results of this method (not shown) were inconclusive, as our data did not lend itself well to this type of analysis (such as that performed by Bhat et al. [3]). Our data set did not have as favorable a distribution of MICs as the data in the previous work. Specifically, the MICs in our study were limited to a range of ≤0.25 to 2 mg/liter whereas previous data represented a greater numerical spread. This factor may pose problems for future studies attempting to discern breakpoints by using this methodology.
This study examined all patients hospitalized at Northwestern Memorial Hospital with a bloodstream infection caused by gram-negative organisms between 1 January 2001 and 30 November 2003. This study population included patients who may have been less critically ill than others at the time of infection. Differences in the pathogenicity of the infecting organisms also exist. Compared to other bloodstream infections caused by gram-negative pathogens, P. aeruginosa bacteremias have been shown to be associated with increased mortality, while E. coli bacteremias have been shown to have decreased likelihood of fatality (28). In the present study, the high-MIC group had a higher proportion of infections due to Pseudomonas spp. (38.1% versus 0 and 3.5%) and a lower proportion of infections due to E. coli (9.5% versus 50 and 57.3%) than the intermediate- and low-MIC groups. This factor may have had an effect on the length of stay and the mortality in the study according to univariate analysis; however, these differences were equilibrated in the multivariate analysis, and the high MIC remained a significant predictor of the length of stay postculture. We are unable to explain why patients in our data set infected with Pseudomonas spp. had a decreased length of stay. This finding may be an artifact of the small number of evaluable patients (n = 11) infected with Pseudomonas spp. in the length-of-stay model. Additionally, we speculate that infections with Pseudomonas spp. may have been disproportionately confined to a urinary nidus. Bloodstream infections secondary to primary infections in the lung and peritoneum and unidentified sources of infection are also associated with increased morbidity and mortality (28). Since it was not possible to determine the sources of the bloodstream infections from the microbiological database used, it is unclear whether the sources of infection differed between MIC stratifications.
At the time of data collection for this study, levofloxacin was the primary fluoroquinolone used at Northwestern Memorial Hospital. We excluded ciprofloxacin-treated patients since they accounted for a mere 4.6% (15 of 327) of the total number of patients receiving fluoroquinolones. Including these patients would likely introduce excess variability with little improved study power. The data were also collected prior to the FDA approval of levofloxacin doses of 750 mg daily (Levaquin package insert [http://www.levaquin.com]). However, even these doses may not be adequate for bloodstream infections caused by gram-negative organisms for which MICs are high, given the PK-PD relationships at high MICs. Such results can be predicted based on the subtherapeutic serum drug concentrations obtained (7), but future clinical work will need to decipher if the predictions are in fact true. Also, because the antibiotic data in this study came from pharmacy dispensing records, it was assumed that all doses dispensed were administered to the patient and that all doses were given in a timely fashion. Patients at Northwestern Memorial Hospital receive FDA-approved doses of antibiotics with appropriate renal function adjustments. This is ensured by prospective clinical pharmacist participation in the care of each patient. Any deviations from this standard procedure are likely random.
In conclusion, patients treated with a fluoroquinolone for bloodstream infections caused by gram-negative organisms for which MICs were elevated, yet within the susceptible category, had a longer stay postculture than similar patients infected with organisms for which MICs were lower. This study provides the first clinical evidence to suggest that lower MIC breakpoints may be necessary for patients with bloodstream infections treated with fluoroquinolones.
Acknowledgments
We declare no financial interests relevant to this subject or this paper.
Footnotes
Published ahead of print on 15 December 2008.
REFERENCES
- 1.Ambrose, P. G. 2006. Monte Carlo simulation in the evaluation of susceptibility breakpoints: predicting the future: insights from the society of infectious diseases pharmacists. Pharmacotherapy 26:129-134. [DOI] [PubMed] [Google Scholar]
- 2.Ambrose, P. G., S. M. Bhavnani, C. M. Rubino, A. Louie, T. Gumbo, A. Forrest, and G. L. Drusano. 2007. Pharmacokinetics-pharmacodynamics of antimicrobial therapy: it's not just for mice anymore. Clin. Infect. Dis. 44:79-86. [DOI] [PubMed] [Google Scholar]
- 3.Bhat, S. V., A. Y. Peleg, T. P. Lodise, Jr., K. A. Shutt, B. Capitano, B. A. Potoski, and D. L. Paterson. 2007. Failure of current cefepime breakpoints to predict clinical outcomes of bacteremia caused by gram-negative organisms. Antimicrob. Agents Chemother. 51:4390-4395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blaser, J., B. B. Stone, M. C. Groner, and S. H. Zinner. 1987. Comparative study with enoxacin and netilmicin in a pharmacodynamic model to determine importance of ratio of antibiotic peak concentration to MIC for bactericidal activity and emergence of resistance. Antimicrob. Agents Chemother. 31:1054-1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Booker, B. M., P. F. Smith, A. Forrest, J. Bullock, P. Kelchlin, S. M. Bhavnani, R. N. Jones, and P. G. Ambrose. 2005. Application of an in vitro infection model and simulation for reevaluation of fluoroquinolone breakpoints for Salmonella enterica serotype Typhi. Antimicrob. Agents Chemother. 49:1775-1781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Charlson, M. E., P. Pompei, K. L. Ales, and C. R. MacKenzie. 1987. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J. Chronic Dis. 40:373-383. [DOI] [PubMed] [Google Scholar]
- 7.Chow, A. T., C. Fowler, R. R. Williams, N. Morgan, S. Kaminski, and J. Natarajan. 2001. Safety and pharmacokinetics of multiple 750-milligram doses of intravenous levofloxacin in healthy volunteers. Antimicrob. Agents Chemother. 45:2122-2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Clinical and Laboratory Standards Institute. 2007. Performance standards for antimicrobial susceptibility testing; 17th informational supplement. CLSI document M100-S17. Clinical and Laboratory Standards Institute, Wayne, PA.
- 9.Crump, J. A., K. Kretsinger, K. Gay, R. M. Hoekstra, D. J. Vugia, S. Hurd, S. D. Segler, M. Megginson, L. J. Luedeman, B. Shiferaw, S. S. Hanna, K. W. Joyce, E. D. Mintz, and F. J. Angulo. 2008. Clinical response and outcome of infection with Salmonella enterica serotype Typhi with decreased susceptibility to fluoroquinolones: a United States FoodNet multicenter retrospective cohort study. Antimicrob. Agents Chemother. 52:1278-1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.DeFife, R., M. H. Scheetz, J. M. Feinglass, M. J. Postelnick, and K. K. Scarsi. 2006. Abstr. 46th Intersci. Conf. Antimicrob. Agents Chemother., abstr. 2488.
- 11.Deyo, R. A., D. C. Cherkin, and M. A. Ciol. 1992. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J. Clin. Epidemiol. 45:613-619. [DOI] [PubMed] [Google Scholar]
- 12.Drusano, G. L., D. Z. D'Argenio, S. L. Preston, C. Barone, W. Symonds, S. LaFon, M. Rogers, W. Prince, A. Bye, and J. A. Bilello. 2000. Use of drug effect interaction modeling with Monte Carlo simulation to examine the impact of dosing interval on the projected antiviral activity of the combination of abacavir and amprenavir. Antimicrob. Agents Chemother. 44:1655-1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Drusano, G. L., D. E. Johnson, M. Rosen, and H. C. Standiford. 1993. Pharmacodynamics of a fluoroquinolone antimicrobial agent in a neutropenic rat model of Pseudomonas sepsis. Antimicrob. Agents Chemother. 37:483-490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Drusano, G. L., S. L. Preston, C. Fowler, M. Corrado, B. Weisinger, and J. Kahn. 2004. Relationship between fluoroquinolone area under the curve: minimum inhibitory concentration ratio and the probability of eradication of the infecting pathogen, in patients with nosocomial pneumonia. J. Infect. Dis. 189:1590-1597. [DOI] [PubMed] [Google Scholar]
- 15.Drusano, G. L., S. L. Preston, C. Hardalo, R. Hare, C. Banfield, D. Andes, O. Vesga, and W. A. Craig. 2001. Use of preclinical data for selection of a phase II/III dose for evernimicin and identification of a preclinical MIC breakpoint. Antimicrob. Agents Chemother. 45:13-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dudley, M. N., and P. G. Ambrose. 2000. Pharmacodynamics in the study of drug resistance and establishing in vitro susceptibility breakpoints: ready for prime time. Curr. Opin. Microbiol. 3:515-521. [DOI] [PubMed] [Google Scholar]
- 16a.European Committee on Antimicrobial susceptibility Testing. 19 June 2008, posting date. Fluoroquinolones—EUCAST clinical MIC breakpoints (version 2.5). ESCMID, Basel, Switzerland. http://www.srga.org/eucastwt/MICTAB/MICquinolones.htm.
- 17.Forrest, A., D. E. Nix, C. H. Ballow, T. F. Goss, M. C. Birmingham, and J. J. Schentag. 1993. Pharmacodynamics of intravenous ciprofloxacin in seriously ill patients. Antimicrob. Agents Chemother. 37:1073-1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Frei, C. R., N. P. Wiederhold, and D. S. Burgess. 2008. Antimicrobial breakpoints for gram-negative aerobic bacteria based on pharmacokinetic-pharmacodynamic models with Monte Carlo simulation. J. Antimicrob. Chemother. 61:621-628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fuller, J. D., and D. E. Low. 2005. A review of Streptococcus pneumoniae infection treatment failures associated with fluoroquinolone resistance. Clin. Infect. Dis. 41:118-121. [DOI] [PubMed] [Google Scholar]
- 20.Kahlmeter, G., D. F. Brown, F. W. Goldstein, A. P. MacGowan, J. W. Mouton, A. Osterlund, A. Rodloff, M. Steinbakk, P. Urbaskova, and A. Vatopoulos. 2003. European harmonization of MIC breakpoints for antimicrobial susceptibility testing of bacteria. J. Antimicrob. Chemother. 52:145-148. [DOI] [PubMed] [Google Scholar]
- 21.Kiser, T. H., D. W. Hoody, M. D. Obritsch, C. O. Wegzyn, P. C. Bauling, and D. N. Fish. 2006. Levofloxacin pharmacokinetics and pharmacodynamics in patients with severe burn injury. Antimicrob. Agents Chemother. 50:1937-1945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.MacGowan, A. P., and R. Wise. 2001. Establishing MIC breakpoints and the interpretation of in vitro susceptibility tests. J. Antimicrob. Chemother. 48(Suppl. 1):17-28. [DOI] [PubMed] [Google Scholar]
- 23.Madaras-Kelly, K. J., B. E. Ostergaard, L. B. Hovde, and J. C. Rotschafer. 1996. Twenty-four-hour area under the concentration-time curve/MIC ratio as a generic predictor of fluoroquinolone antimicrobial effect by using three strains of Pseudomonas aeruginosa and an in vitro pharmacodynamic model. Antimicrob. Agents Chemother. 40:627-632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mouton, J. W. 2003. Impact of pharmacodynamics on breakpoint selection for susceptibility testing. Infect. Dis. Clin. N. Am. 17:579-598. [DOI] [PubMed] [Google Scholar]
- 25.NCCLS. 2001. Development of in vitro susceptibility testing criteria and quality control parameters; approved guideline, 2nd ed. NCCLS document M23-A2. NCCLS, Wayne, PA.
- 26.Preston, S. L., G. L. Drusano, A. L. Berman, C. L. Fowler, A. T. Chow, B. Dornseif, V. Reichl, J. Natarajan, and M. Corrado. 1998. Pharmacodynamics of levofloxacin: a new paradigm for early clinical trials. JAMA 279:125-129. [DOI] [PubMed] [Google Scholar]
- 27.Scaglione, F., J. W. Mouton, R. Mattina, and F. Fraschini. 2003. Pharmacodynamics of levofloxacin and ciprofloxacin in a murine pneumonia model: peak concentration/MIC versus area under the curve/MIC ratios. Antimicrob. Agents Chemother. 47:2749-2755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Scarsi, K. K., J. M. Feinglass, M. H. Scheetz, M. J. Postelnick, M. K. Bolon, and G. A. Noskin. 2006. Impact of inactive empiric antimicrobial therapy on inpatient mortality and length of stay. Antimicrob. Agents Chemother. 50:3355-3360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Scheetz, M. H., M. K. Bolon, T. R. Zembower, J. Warren, and G. A. Noskin. 2005. Real-time, unit-specific antimicrobial susceptibility identifies significant differences among gram-negative bacteria, abstr. 472. 43rd Ann. Meet. Infect. Dis. Soc. Am., San Francisco, CA, 6 to 9 October 2005.
- 30.Tam, V. H., E. A. Gamez, J. S. Weston, L. N. Gerard, M. T. Larocco, J. P. Caeiro, L. O. Gentry, and K. W. Garey. 2008. Outcomes of bacteremia due to Pseudomonas aeruginosa with reduced susceptibility to piperacillin-tazobactam: implications on the appropriateness of the resistance breakpoint. Clin. Infect. Dis. 46:862-867. [DOI] [PubMed] [Google Scholar]
- 31.Weinstein, M. P., M. L. Towns, S. M. Quartey, S. Mirrett, L. G. Reimer, G. Parmigiani, and L. B. Reller. 1997. The clinical significance of positive blood cultures in the 1990s: a prospective comprehensive evaluation of the microbiology, epidemiology, and outcome of bacteremia and fungemia in adults. Clin. Infect. Dis. 24:584-602. [DOI] [PubMed] [Google Scholar]