Abstract
Population pharmacokinetic analysis demonstrated that renal function, as assessed by creatinine clearance (CLCR), was the patient characteristic that had a clinically relevant impact on ceftobiprole pharmacodynamics. Dosing adjustments based on CLCR for subjects with renal impairment should provide ceftobiprole exposure similar to that in patients with normal renal function.
Ceftobiprole is a broad-spectrum cephalosporin with activity against methicillin-resistant Staphylococcus aureus and other clinically relevant gram-positive and gram-negative pathogens (1). In phase III trials among patients with complicated skin and skin structure infections (cSSSI) due to gram-positive bacteria, ceftobiprole (500 mg every 12 h; 1-h infusion) was noninferior to vancomycin (5) while early treatment or empirical therapy with ceftobiprole (500 mg every 8 h; 2-h infusion) was as effective as vancomycin plus ceftazidime in patients with cSSSI due to gram-positive and/or gram-negative pathogens (4). Interindividual variability in pharmacokinetics may potentially be sufficient to influence pharmacodynamics and therefore require adjustment to dosing regimens. The present study used a population analysis approach to identify patient characteristics that result in pharmacokinetic variability of ceftobiprole and evaluated the likely clinical significance of these sources of variability among patients with cSSSI.
The study population analyzed comprised 595 subjects (162 healthy volunteers and 433 patients, from whom 5,185 plasma ceftobiprole concentration measurements were obtained) participating in eight phase I (n = 162), one phase II (n = 27), and two phase III (n = 406) clinical trials. Ceftobiprole was administered intravenously at various doses (125 to 1,000 mg) infused over a period of 0.5 to 2 h as a single dose, twice or three times daily for up to 12 days in the phase I and II studies. Blood samples were taken according to a rich-blood-sampling scheme in the phase I and II studies, while they were taken according to both rich- and sparse-blood-sampling schemes (samples taken on day four of treatment at 2 and 6 h postinfusion) in the phase III studies.
A sequence of compartmental models for characterization of the pharmacokinetic profiles was tested using the first-order conditional estimation method in the NONMEM software program (version V; Icon, Ellicott City, MD). A model consisting of a central compartment plus deep and shallow peripheral compartments with first-order elimination provided the best fit for the ceftobiprole plasma concentration-versus-time data. The parameters used in the model were volumes of distribution (V, defined as V1, V2, and V3, respectively, for the central, shallow peripheral, and deep peripheral compartments); intercompartmental flow between the central and shallow peripheral compartments (Q2); and intercompartmental flow between the central and deep peripheral compartments (Q3). The interindividual variability of Q2 and Q3 was negligible and hence was set to zero. A ceftobiprole medocaril-to-ceftobiprole conversion model was not necessary because the conversion rate is rapid, which was supported by the fact that only negligible levels of ceftobiprole medocaril were detectable in plasma and urine after ceftobiprole medocaril administration. A stepwise forward addition, followed by a backward elimination covariate search, was performed.
The model had good predictability for the observed concentrations during an internal evaluation step (prediction error = 5.54%; absolute prediction error = 13.2%). Visual predictive checks of the pharmacokinetic model confirmed its suitability. Thus, all the pharmacokinetic parameters were reestimated after the model building and validation data sets were combined (Table 1).
TABLE 1.
Parameter estimates and standard errors for the final population pharmacokinetic model
| Parameter | Value for population
|
Magnitude of between-subject variability (% CV)
|
||
|---|---|---|---|---|
| Mean, final estimate | % SEM | Final estimate | % SEM | |
| CL (liters/h)a | 5.36 | 1.06 | 20.45 | 19.93 |
| V in central compartment (V1, liters)b | 7.40 | 2.96 | 46.48 | 26.06 |
| Distribution clearance between shallow and central compartments (Q2, liters/h) | 11.20 | 6.52 | NAf | NA |
| V in shallow peripheral compartment (V2, liters)c | 6.10 | 3.43 | 5.99 | 28.13 |
| Distribution clearance between deep and central compartments (Q3, liters/h) | 0.76 | 6.96 | NA | NA |
| V in deep peripheral compartment (V3, liters)d | 3.27 | 4.10 | 12.17 | 47.91 |
| Exponent for CL (normalized by 120 ml/min) | 0.82 | 4.07 | NA | NA |
| Coefficient for V2 due to health status | 0.57 | 15.90 | NA | NA |
| Coefficient for V3 due to sex | −0.18 | 9.46 | NA | NA |
| Coefficient for clearance due to health status | 0.20 | 12.16 | NA | NA |
| Exponent for V1 (normalized by 75 kg) | 1.05 | 12.95 | NA | NA |
| Coefficient for V1 due to health status | 0.45 | 18.00 | NA | NA |
| Coefficient for V2 due to sex | −0.13 | 35.89 | NA | NA |
| Residual variability: sparsely sampled | 36.06% CVe | 14.08 | ||
| Residual variability: intensively sampled | 13.42% CV | 7.28 | ||
| Between-observation variability (CL) | 28.88 | 20.86 | ||
| Between-observation variability (V1) | 8.73 | 23.75 | ||
| Correlation coefficient (CL vs V1) | 0.68 | |||
For a healthy volunteer with CLCR of 120 ml/min.
For a healthy volunteer with 75 kg body weight.
For a male healthy volunteer.
For a male subject.
CV, coefficient of variance.
NA, not applicable.
V1 was affected by weight, which may reflect the increased extravascular volume associated with increased body weight. Body weight and creatinine clearance (CLCR) are confounded, as in the Cockcroft-Gault equation (2), and body weight itself did not exert a sufficient effect to necessitate dose adjustment. The effect of sex on V2 is consistent with the lower V in women, which is attributable to their lower body weight; when corrected for body weight, there was no significant sex effect.
A dosing regimen of ceftobiprole (500 mg, given as a 2-h intravenous infusion administered every 8 h) was used in simulations to assess the clinical implications of the patient characteristics identified as influencing the pharmacokinetics of ceftobiprole (i.e., sex, weight, CLCR, and health status). Using the simulated time courses of free ceftobiprole concentrations, the pharmacodynamic index of the percentage of time the drug concentration was greater than the MIC during a dosing interval (%T>MIC) was calculated to evaluate the clinical effect of the covariate. In the simulations, the MIC was set at 4 μg/ml since ceftobiprole has an MIC at which 90% of organisms are inhibited (MIC90) of 0.5 μg/ml against methicillin-susceptible S. aureus, an MIC90 of 2 μg/ml against methicillin-resistant S. aureus, and an MIC90 of 4 μg/ml against the majority of the Enterobacteriaceae (1). Of the covariates that explained the interindividual variability, CLCR was the only factor that had a clinically relevant effect.
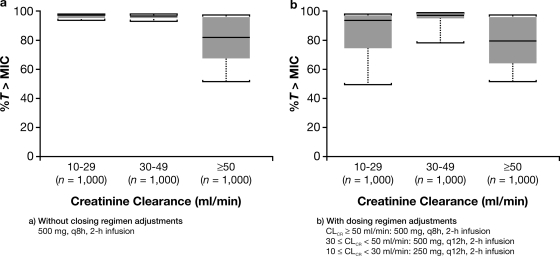
To assess the impact of dosing regimens adjusted according to renal status (for normal function or mild impairment, ceftobiprole 500 mg, 2-h infusion every 8 h; for moderate renal impairment, ceftobiprole 500 mg, 2-h infusion every 12 h; and for severe renal impairment, ceftobiprole 250 mg, 2-h infusion every 12 h), a simulation of %T>MIC was performed using 1,000 male patients with body weights of 75 kg and assuming the MIC target of 4 μg/ml. The simulations showed that without dosage adjustment, the %T>MICs were higher for patients with moderate and severe renal impairment than for patients with normal function or mild renal impairment (Fig. 1a). However, dosage adjustment, according to the degree of renal impairment, resulted in %T>MICs closer to those for patients with normal renal function or mild renal impairment (Fig. 1b). The significance of renal function is reflective of glomerular filtration being predominantly responsible for the removal of free ceftobiprole from the systemic circulation (7). Other elimination processes, such as metabolic clearance, are limited; 80 to 90% of the dose is recovered as unchanged ceftobiprole from urine following single or multiple doses (3, 6).
FIG. 1.
Simulation of the %T>MIC for ceftobiprole at 500 mg infused over 2 h once every 8 h (q8h), according to the degree of renal function, defined by CLCR, in 1,000 male virtual patients without (a) or with (b) dosing regimen adjustment.
In summary, CLCR was the factor that had a clinically relevant impact on ceftobiprole pharmacodynamics, as evaluated by %T>MICs. Dosing adjustments based on CLCR for subjects with renal impairment should provide ceftobiprole exposure similar to that in patients with normal renal function.
Acknowledgments
All authors are employees of Johnson & Johnson Pharmaceutical Research & Development, LLC, Raritan, NJ.
The analyses and studies described in this report were funded by Johnson & Johnson Pharmaceutical Research & Development.
Footnotes
Published ahead of print on 15 December 2008.
REFERENCES
- 1.Amsler, K. M., T. A. Davies, W. Shang, M. R. Jacobs, and K. Bush. 2008. In vitro activity of ceftobiprole against pathogens from two phase 3 clinical trials of complicated skin and skin structure infections. Antimicrob. Agents Chemother. 52:3418-3423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cockcroft, D. W., and M. H. Gault. 1976. Prediction of creatinine clearance from serum creatinine. Nephron 16:31-41. [DOI] [PubMed] [Google Scholar]
- 3.Murthy, B., and A. Schmitt-Hoffmann. 2008. Pharmacokinetics and pharmacodynamics of ceftobiprole, an anti-MRSA cephalosporin with broad-spectrum activity. Clin. Pharmacokinet. 47:21-33. [DOI] [PubMed] [Google Scholar]
- 4.Noel, G. J., K. Bush, P. Bagchi, J. Ianus, and R. S. Strauss. 2008. A randomized, double-blind trial comparing ceftobiprole medocaril with vancomycin plus ceftazidime for the treatment of patients with complicated skin and skin-structure infections. Clin. Infect. Dis. 46:647-655. [DOI] [PubMed] [Google Scholar]
- 5.Noel, G. J., R. S. Strauss, K. Amsler, M. Heep, R. Pypstra, and J. S. Solomkin. 2008. Results of a double-blind, randomized trial of ceftobiprole treatment of complicated skin and skin structure infections caused by gram-positive bacteria. Antimicrob. Agents Chemother. 52:37-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schmitt-Hoffmann, A., L. Nyman, B. Roos, M. Schleimer, J. Sauer, N. Nashed, T. Brown, A. Man, and E. Weidekamm. 2004. Multiple-dose pharmacokinetics and safety of a novel broad-spectrum cephalosporin (BAL5788) in healthy volunteers. Antimicrob. Agents Chemother. 48:2576-2580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schmitt-Hoffmann, A., B. Roos, M. Schleimer, J. Sauer, A. Man, N. Nashed, T. Brown, A. Perez, E. Weidekamm, and P. Kovacs. 2004. Single-dose pharmacokinetics and safety of a novel broad-spectrum cephalosporin (BAL5788) in healthy volunteers. Antimicrob. Agents Chemother. 48:2570-2575. [DOI] [PMC free article] [PubMed] [Google Scholar]