Abstract
Efflux proteins have been shown to greatly affect the uptake of antiretroviral drugs by cells and to hamper their access to the human immunodeficiency virus type 1 replication site. This study evaluated the factors that may lead to drug-drug interactions between emtricitabine (FTC), tenofovir (TFV), and efavirenz (EFV), including the modulation of efflux transporter expression and function. Peripheral blood mononuclear cells from healthy volunteers were used to determine whether or not an interaction between antiretroviral drugs and target cells occurred in any combination of FTC, TFV, EFV, FTC-TFV, TFV-EFV, or FTC-TFV-EFV. Following 20 h of treatment, intracellular drug concentrations were measured by liquid chromatography-tandem mass spectrometry. Efflux transporter functionality and inhibitor drug properties were assessed by measuring fluorescent dye efflux. ABCB1 (P-glycoprotein), ABCC 1 to 6 (multidrug resistance-associated protein), and OAT (organic anion transporter) expression in response to the treatments was quantified by semiquantitative real-time PCR. Cells treated with a double combination (FTC-TFV or TFV-EFV) or the triple combination (FTC-TFV-EFV) produced higher FTC and TFV intracellular concentrations than cells treated with FTC or TFV alone. However, no change in the EFV intracellular concentration was observed. FTC tended to induce abcc5 mRNA expression and EFV tended to induce abcc1 and abcc6 mRNA expression, whereas TFV tended to reduce mdr1, abcc1, abcc5, and abcc6 mRNA expression. Under these conditions, a decrease in the functionality of ABCC was observed, and this decrease was associated with the direct inhibitory actions of these drugs. This in vitro study reveals a benefit of the combination FTC-TFV-EFV in terms of the intracellular FTC and TFV concentrations and highlights the pharmacological mechanisms that lead to this effect.
The combined administration of at least three anti-human immunodeficiency virus (anti-HIV) drugs from different drug classes as highly active antiretroviral therapy has been shown to slow the progression of disease, improve survival, and result in better virologic and immunologic responses (10). The USA Panel of the International AIDS Society recommends combination therapies that comprise a nonnucleoside reverse transcriptase inhibitor (NNRTI) or a protease inhibitor boosted with low-dose ritonavir, each combined with two nucleoside reverse transcriptase inhibitors (NRTIs) or nucleotide reverse transcriptase inhibitors (NtRTIs), for the treatment of HIV infection in adults (18). Atripla (Bristol-Myers Squibb and Gilead Sciences), which contains the NNRTI efavirenz (EFV), the NRTI emtricitabine (FTC), and the NtRTI tenofovir disoproxil fumarate (an oral prodrug of tenofovir [TFV]), is the first once-daily single-tablet regimen (29).
Viral resistance, a lack of adherence to the treatment regimen, and pharmacological factors may contribute to anti-HIV treatment failure. Efforts to predict treatment failure currently focus on the measurement of plasma antiretroviral drug concentrations (38). However, this is not the only relevant biological variable. Drug efflux transport systems, such as P-glycoprotein (Pgp) and multidrug resistance-associated proteins (MRPs; or ABCCs), that result in low intracellular levels of parental antiretroviral drugs or their active derivatives, are of particular importance (26). The efficacy of a combination therapy depends on the level of activation of the targeted cells and on drug interactions which may limit drug access to the target sites of HIV replication.
ABCCs and Pgp belong to the ATP-binding cassette (ABC) superfamily of membrane transporter proteins (20, 30). They are present in lymphocytes and monocytes (1, 32) and on physiological barriers (e.g., the blood-brain barrier [36]), where they are involved in the active efflux of a wide variety of drugs (17). Transport substrates of Pgp are mostly hydrophobic. ABCC substrates are represented by amphiphilic anions, like conjugates of lipophilic compounds with glutathione, glucuronate, or sulfate (31).
The interactions of protease inhibitors with Pgp and ABCCs have been extensively studied, and it has been shown that they are either substrates or modulators of efflux transporters (9, 15, 23, 33, 42, 48, 54). However, data concerning the interaction of NNRTIs or NRTIs with efflux transporters are sparse and conflicting (7, 9, 13, 46, 49, 50, 57).
Some studies report that NNRTIs are neither Pgp substrates (13, 50) nor Pgp modulators (7). Others indicate the potential of long-term treatment with EFV to induce Pgp expression in peripheral blood mononuclear cells (PBMCs) (9) or in the LS180 cell line (57). EFV, but not TFV and FTC, seems to inhibit Pgp in MDCKII mdr1 cells (46, 49). A recent publication describing a study performed with the LS180 cell line showed that long-term incubation with high concentrations of FTC, but not TFV, can increase mdr1 mRNA expression and Pgp function (57).
The interaction of these drugs with ABCCs has been reported. The role of ABCC4 in the renal elimination of TFV has been demonstrated in ABCC4-knockout mice (24). In ABCC2- and ABCC4-overexpressing MDCKII cells, Ray et al. (46) showed that ABCC4, but not ABCC2, transports TFV. They also reported that TFV does not interact with ABCC2-mediated calcein accumulation. However, in a study with the same cell line, Weiss et al. (56) demonstrated that TFV, EFV, and FTC interact with ABCC1, ABCC2, and ABCC3. The interaction of TFV with the human organic anion transporters (OATs) hOAT1 and hOAT3, two members of the solute carrier (SLC) family, has also been reported in a study with the transfected HEK293 cell line (52).
In vivo, combinations of TFV and lamivudine or FTC appear to provide improved virologic responses (3). A regimen of tenofovir disoproxil fumarate-FTC and EFV demonstrates a superior durability of viral load suppression and an improved safety and morphological profile over those achieved with zidovudine-lamivudine and EFV (2, 43). Drug combinations are aimed at achieving synergism between the compounds and reducing the likelihood of the development of drug resistance.
The present study assessed whether and how dual or triple combinations of FTC, TFV, and EFV may lead to higher intracellular drug concentrations. Particular emphasis was given to the modulation of transporter expression and function or direct transporter inhibition by antiretroviral agents.
MATERIALS AND METHODS
Subjects and cell treatment.
PBMCs from healthy donors (Etablissement Français du Sang, Rungis, France) were isolated and treated with vehicle control (0.2% dimethyl sulfoxide [DMSO]; Sigma Aldrich, St. Quentin-Fallavier, France) or with FTC (a generous gift from Gilead Sciences), TFV (a generous gift from Gilead Sciences), EFV (a generous gift from Bristol-Myers Squibb), alone or in combination (5 μM each), in RPMI 1640 medium (Gibco, Cergy Pontoise, France) supplemented with 10% fetal bovine serum, 2 mmol/liter glutamine, 50 μg/ml penicillin, 50 μg/ml streptomycin, and 100 μg/ml neomycin (Gibco) for 20 h at 37°C in a humid atmosphere with 5% CO2.
Quantitation of FTC, TFV, and EFV in PBMCs by liquid chromatography-tandem mass spectrometry.
Following a 20-h incubation with the drugs, the analytes were extracted from the PBMCs, samples, quality controls, and standards, as reported previously (44). A 40-μl fraction of the remaining solution was injected into a high-pressure liquid chromatograph-tandem mass spectrometer, as described previously (35), that was adapted for FTC and EFV detection. Briefly, the chromatographic separation was achieved on a Synergi Polar-RP column (particle size, 4 μm; 50 by 2 mm; Phenomenex, Le Pecq, France) thermostated at 40°C with a mobile phase consisting of 0.5% formic acid and a methanol gradient delivered at a flow rate of 0.3 ml/min from 2 to 80%. Detection by mass spectrometry was performed with a triple-quadrupole tandem mass spectrometer (Quantum Discovery) with an electrospray ionization source (Thermo-Fisher Scientific) in the positive ionization mode for TFV and FTC and in the negative ionization mode for EFV. The PBMCs in each sample (n = 12) were counted by using a validated biochemical test, as described previously (6).
Pgp or ABCC function.
In order to explain the differences in drug quantitation, the functionalities of Pgp and ABCC were assessed by measuring fluorescent dye efflux (0.1 μmol/liter calcein-acetoxymethylester) in the presence or the absence of specific inhibitors (2 μM cyclosporine [Sigma Aldrich] and 30 μM MK571 [Calbiochem, VWR, Fontenay-sous-Bois, France]) for 30 min at 37°C. Cyclosporine at a concentration of 2 μM is a specific Pgp inhibitor (34), and MK571 is a specific inhibitor of ABCC-associated drug resistance (i.e., ABCCs 1, 2, and 3) (16). The cells were then washed twice in cold phosphate-buffered saline, fixed with CellFix fixative (1:10, 400 μl, 4°C), and analyzed by flow cytometry.
The fluorescence due to calcein was plotted as a histogram of fluorescence staining in channel 1 (FL1). Transporter function was quantified as described previously (45) by using the following equation: activity (percent) = [100 − (Gmeancalcein/Gmeaninhibitor)] × 100, where Gmeancalcein is the geometric mean fluorescence of calcein in the samples tested, and Gmeaninhibitor is the geometric median fluorescence of calcein in the presence of inhibitor.
Each experiment was performed with PBMCs from seven different donors.
Transporter mRNA expression.
In order to explain the effect of antiretroviral therapy on the functionality of ABCC, we carried out further experiments on mRNA transporter expression. Following the 20-h antiretroviral treatment, the efflux and the influx of transporter expression were determined by real-time PCR (n = 3).
RNA was isolated with a GenElute mammalian total RNA kit (Sigma Aldrich). The total RNA concentration and the purity of the RNA were then determined by measuring the absorbance at 260 nm and 280 nm. The A260/A280 ratio ranged from 1.8 to 2. A sample of 0.5 μg of total RNA was converted to cDNA with random primers in a total volume of 10 μl by using an RT2 first-strand kit (Superarray Bioscience Corporation, Frederick, MD). The cDNA was diluted with distilled water to a volume of 100 μl. Each primer in the primer set was used at a concentration of 0.4 μM for the specific RT2 Profiler PCR array, according to the manufacturer's protocol. The plates used for the analysis were ABC (abcb1, abcc1, abcc2, abcc4, abcc5, abcc6, abcg2) and SLC (slc22A6, slc22A8) transporter PCR arrays (catalogue number CAPH-0468). Relative expression values were calculated as 2−ΔCT, where ΔCT is the difference in the threshold cycle (CT) values for the genes of interest and the housekeeping gene (hypoxanthine-guanine phosphoribosyltransferase). If the CT was greater than 35, we considered the expression level too low to be applicable.
Direct inhibitory effects of drugs on calcein accumulation.
Experiments to determine the direct inhibitory effects of the drugs were carried out in order to highlight the pharmacological mechanisms that lead to intracellular drug concentrations. PBMCs (5 × 105 cells/ml) from healthy donors (n = 6) were incubated with the ABCC inhibitor MK571 (30 μM) as a positive control or with different concentrations of FTC, TFV, or EFV (0.5 to 10 μM, which is the range of soluble and noncytotoxic concentrations, as determined previously [49]) for 30 min at 37°C in 1 ml complete RPMI 1640 medium. Drug accumulation was initiated by the addition of calcein-AM (0.1 μM per 5-ml tube). Following 1 h of incubation, the cells were centrifuged (500 × g, 5 min, 4°C), washed twice in cold phosphate-buffered saline, and then fixed with CellFix fixative (1:10, 400 μl, 4°C) and analyzed by flow cytometry. The fluorescence due to calcein was monitored in channel 1 (FL1) and was plotted as a histogram of FL1 staining. The level of calcein accumulation after incubation with calcein-AM should be increased by the presence of the ABCC substrate or inhibitor because of competition or inhibition of calcein-AM efflux.
Statistics.
The data are expressed as the means ± standard deviations (SDs). GraphPad Prism (version 3.0) software (GraphPad Software, San Diego, CA) was used to perform statistical analysis to highlight any statistically significant differences between the data groups. The significance of differences between groups and controls was evaluated by using a one-way analysis of variance (ANOVA) with Dunnett's posttest or a two-tailed Student's t test. Differences between means were considered significant when the P value was less than 0.05.
RESULTS
Cytotoxicity assay.
None of the compounds tested (FTC, TFV, and EFV) exerted cytotoxic effects at the concentrations used (0.5, 5, and 10 μM), as evaluated with a 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide-based in vitro toxicology assay kit (Sigma Aldrich).
In vitro intracellular accumulation of antiretroviral drugs in PBMCs treated for 20 h in vitro.
Drug concentrations were measured following the 20-h incubation with 5 μM of each drug. Compared with the concentration achieved with FTC alone, the FTC concentration increased by 42.4% ± 6.3% when it was combined with TFV (P = 0.0002) and 61.1% ± 13.7% when it was combined with TFV-EFV (P = 0.0007) (Fig. 1A). Compared with the concentration achieved with TFV alone, the TFV concentration increased by 30.0% ± 8.4% when it was combined with FTC (P = 0.004), 38.8% ± 9.4% when it was combined with EFV (P = 0.003), and 56.1 ± 19.1% when it was combined with FTC-EFV (P = 0.014) (Fig. 1B). Compared with the concentration achieved with EFV alone, the EFV concentration did not change statistically significantly when it was combined with TFV or FTC-TFV (Fig. 1C).
FIG. 1.
Intracellular concentrations (pmol/106 cells) of FTC (A), TFV (B), or EFV (C), used alone or in combination. Data are expressed as means ± SDs (n = 12). The P values (*, P < 0.05; **, P < 0.01; ***, P < 0.001) were determined by ANOVA with Dunnett's multiple-comparison test for post hoc comparison of the results with those for the vehicle control.
Effect of 20 h of treatment on lymphocyte multidrug resistance transporter function.
In order to explain the differences seen by quantitation of the drugs used alone or in combination, we investigated efflux transporter function following the 20-h cell treatments. The percentage of ABCC and Pgp activity was evaluated by measuring the effects of selective inhibitors of Pgp and ABCCs (MK571 and cyclosporine, respectively) on the level of calcein-AM accumulation. The percent inhibition caused by 30 μM MK571 or 2 μM cyclosporine is summarized in Table 1.
TABLE 1.
Relative activity of ABC transporter measured by the effect of a selective inhibitor (cyclosporine or MK571) on calcein-AM accumulationa
| Treatmentb | % Inhibition of:
|
|
|---|---|---|
| Pgp activity with 2 μM cyclosporine inhibition | ABCC activity with 30 μM MK571 inhibitionb | |
| Vehicle (0.2% DMSO) | 31.8 ± 9.0 | 32.2 ± 9.6 |
| FTC | 32.3 ± 10.5 | 21.0 ± 8.5** |
| TFV | 32.0 ± 11.8 | 17.4 ± 9.2** |
| EFV | 29.8 ± 11.0 | 16.6 ± 4.9** |
| FTC-TFV | 29.9 ± 14.1 | 19.6 ± 7.6* |
| TFV-EFV | 30.0 ± 10.7 | 9.7 ± 4.5** |
| FTC-TFV-EFV | 27.5 ± 13.5 | 10.9 ± 9.6*** |
PBMCs were treated with 5 μM FTC, 5 μM TFV, and 5 μM EFV, alone or in combination, for 20 h. Data are represented as means ± SDs (n = 7).
The significance of differences between the treated groups and the vehicle control group was evaluated by Student's t test. A P value of <0.05 was considered significant. *, P < 0.05; **, P < 0.01; ***, P < 0.001.
Cyclosporine at a concentration of 2 μM caused no statistically significant change in the level of calcein-AM accumulation in any of the treated cells.
For all drugs used in combination or alone, the decreases in ABCC function were statistically significant: the P values were 0.004 for FTC treatment, 0.001 for TFV treatment, 0.0048 for EFV treatment, 0.014 for FTC-TFV treatment, 0.002 for TFV-EFV treatment, and 0.0001 for FTC-TFV-EFV treatment.
Expression of ABC and OAT transporter mRNA in response to cell treatment with antiretroviral drugs.
Following the 20-h incubation with an antiretroviral regimen, the level of transporter mRNA expression was investigated by semiquantitative real-time PCR (Fig. 2). We evaluated the expression of mdr1, abcc1, abcc2, abcc4, abcc5, abcc6, slc22A6, and slc22A8, which encode Pgp, ABCC1, ABCC2, ABCC4, ABCC5, ABCC6, OAT1, and OAT3, respectively. All these transporters are thought to be involved in antiretroviral efflux. The CT values for the housekeeping gene (hypoxanthine-guanine phosphoribosyltransferase) were not significantly modified by the treatments.
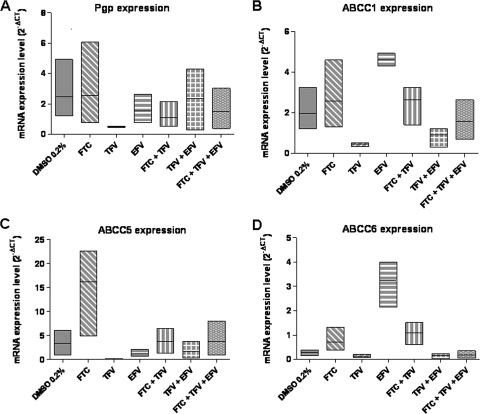
FIG. 2.
Levels of expression of Pgp (A), ABCC1 (B), ABCC5 (C), and ABCC6 (D) mRNA in PBMCs treated with FTC, TFV, or EFV, alone or in combination. Data are expressed as a low-high bar graph; the horizontal line represents the mean (n = 3).
Treated or untreated cells had very low levels of abcc2, abcc4, slc22A6, and slc22A8 mRNA. For these genes, the CT values were greater than 35.
EFV alone at a concentration of 5 μM tended to induce abcc1 and abcc6, which encode xenobiotic efflux transporters. For abcc1, the mean mRNA level increased from 2.0 ± 0.6 (control cells) to 4.6 ± 0.2 (cells treated with EFV). The geometric mean ratio was 2.6 (Fig. 2B). For abcc6, the mean mRNA level increased from 0.3 ± 0.1 (control) to 3.2 ± 0.6 (cells treated with EFV). The geometric mean ratio was 1.7 (Fig. 2D).
FTC alone at a concentration of 5 μM tended to induce abcc5, which encodes the nucleoside/nucleotide efflux transporter ABCC5. The mean mRNA level increased from 3.3 ± 1.4 (control cells) to 16.2 ± 5.6 (cells treated with FTC) (P = 0.002). The geometric mean ratio was 5 (Fig. 2C).
For all the transporters studied (Pgp, ABCC1, ABCC5, and ABCC6), TFV tended to reduce the level of mRNA expression. The mean Pgp mRNA level decreased from 2.5 ± 1.2 (control cells) to 0.5 ± 0.02 (cells treated with TFV). The mean ABCC1 mRNA level decreased from 2.0 ± 0.6 (control cells) to 0.45 ± 0.05 (cells treated with TFV). The mean ABCC5 mRNA level decreased from 3.3 ± 1.5 (control cells) to 0.1 ± 0.05 (cells treated with TFV). The mean ABCC6 mRNA level decreased from 0.3 ± 0.1 (control cells) to 0.1 ± 0.03 (cells treated with TFV). The geometric mean ratios were 0.27, 0.25, 0.05, and 0.07 for Pgp, ABCC1, ABCC5, and ABCC6 mRNA levels, respectively.
Direct inhibitory effect of drug on calcein accumulation.
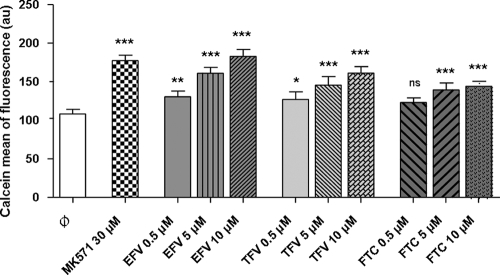
The direct inhibitory effects of the drugs on calcein-AM extrusion were determined by measuring the calcein fluorescence with or without MK571, as a positive control, or different doses of FTC, TFV, and EFV (Fig. 3).
FIG. 3.
Calcein assay assessing the concentration-dependent increase in intracellular fluorescence (mean of calcein fluorescence in arbitrary units [au]) in lymphocytes treated with EFV, TFV, and FTC. The negative control ( ) was 0.2% DMSO, and the positive control was 30 μM MK571. Data are expressed as mean ± SDs (n = 6). The P values (*, P < 0.05; **, P < 0.01; ***, P < 0.001; ns, nonsignificant) were determined by ANOVA with Dunnett's multiple-comparison test for post hoc comparison of the results with those for the vehicle control.
) was 0.2% DMSO, and the positive control was 30 μM MK571. Data are expressed as mean ± SDs (n = 6). The P values (*, P < 0.05; **, P < 0.01; ***, P < 0.001; ns, nonsignificant) were determined by ANOVA with Dunnett's multiple-comparison test for post hoc comparison of the results with those for the vehicle control.
With 30 μM MK571, the level of calcein accumulation was 63.86% higher than that for the control.
EFV increased the intracellular level of calcein accumulation in a concentration-dependent manner. EFV seemed to be the better inhibitor and had an effect at 10 μM similar to that of 30 μM MK571 (68.95% higher than that for the control). TFV increased the intracellular level of calcein accumulation to 48.89% with 10 μM TFV (n = 6) in a concentration-dependent manner. FTC increased the intracellular level of calcein accumulation to 33.18% with 10 μM FTC (n = 6) in a concentration-dependent manner.
DISCUSSION
Drug interactions in patients receiving highly active antiretroviral therapy are often caused by more than one mechanism, such as a mutation in the HIV reverse transcriptase or protease; drug metabolism, such as by phosphorylation; or efflux transporter modulation. This shows the importance of knowing all the potential targets involved and considering their complex interplay for dose individualization (56). Very few studies have considered the effect of the combination of FTC, TFV, and EFV on their intracellular concentrations and ABC multidrug transporter modulation.
Cell treatment for 20 h produced significant increases in FTC and TFV levels when the combinations FTC-TFV, TFV-EFV, and FTC-TFV-EFV were used compared with the levels seen with each individual drug. Furthermore, dose-response curves were generated for each drug in the presence of rising concentrations of the other drugs. We showed increasing intracellular concentrations of TFV and FTC with rising doses of EFV. The FTC concentration increased with a rise in the concentration of TFV and vice versa. In all cases, the intracellular nucleoside concentration reached a plateau beginning at a concentration of 1 μM EFV or 5 μM the other nucleosides (data not shown).
These results differ from those of Borroto-Esoda et al. (8) regarding the TFV concentration in PBMCs when TFV was combined with FTC. A potential explanation for this inconsistency could be the differences in transporter expression (5, 53) and phosphorylated metabolite levels between PBMCs stimulated with phytohemagglutinin and interleukin-2 (8) and inactivated PBMCs. Probably due to the very slow phosphorylation in our experimental system with inactivated PBMCs, the intracellular FTC and TFV triphosphorylated metabolite concentrations were at about the lower limit of quantitation of our assay method used for NRTI triphosphates (44).
Regarding EFV, there was no change in the level of EFV when it was alone used or in combination with the other drugs tested. This might be due to the intracellular concentration/extracellular concentration ratio, which was very high compared with those for the other drugs tested (about 44-fold higher), possibly saturating efflux transporters. Alternatively, EFV is possibly not a Pgp or ABCC substrate (13, 50).
In order to explain the increasing FTC and TFV concentrations in combination therapies, we suggest an ABC transporter interaction. We investigated the Pgp and ABCC functionalities following the 20-h treatment with drug combinations. The ABCC and Pgp activities were measured (in percent) by determination of the effects of selective inhibitors of Pgp and ABCCs (MK571 and cyclosporine, respectively) on calcein-AM accumulation. Calcein-AM is a well-established Pgp and ABCC index substrate (41, 55). It is a fluorogenic, highly lipid-soluble dye that rapidly penetrates the plasma membrane. Inside the cell, endogenous esterases produce the hydrophilic and fluorescent dye calcein, which cannot leave the cell via the plasma membrane (21). Whereas calcein-AM is a substrate of Pgp and ABCC, calcein is not. Cyclosporine at a concentration of 2 μM is a specific Pgp inhibitor (34), and 30 μM MK571 is a specific inhibitor of ABCC-associated drug resistance (16). Using a functionality test with one substrate and two specific inhibitors, the present study provides evidence for a significant decrease in the ABCC functionality but not the Pgp functionality in lymphocytes treated with FTC, TFV, and EFV, alone or in combination, for 20 h (Table 1).
ABCC transporters could play a role in intracellular drug accumulation. However, they are not the only cause. For example, treatments with FTC, TFV, and FTC-TFV had the same effect on the ABCC functionality, but the FTC and TFV concentrations were higher when the combination therapy was used. Since nucleoside/nucleotide analogues are hydrophilic, a hypothesis could be that they enter cells by influx transporters instead of by passive diffusion. These influx transporters, like members of the SLC family, could be modulated by drug combination therapies.
In order to investigate the effect of antiretroviral therapy on the ABCC functionality, we carried out further experiments on mRNA transporter expression and on direct transporter efflux inhibition by antiretroviral drugs.
In our experimental system, mdr1, abcc1, abcc5, and abcc6 mRNAs were quantifiable, whereas abcc2, abcc4, bcrp, oat1, and oat3 mRNAs were not detectable. The mRNAs of mdr1, abcc1, and abcc5 have been demonstrated in PBMCs (monocytes and lymphocytes) (1, 26, 32). The levels of expression of the other genes were very low under our experimental conditions. However, our findings cannot exclude the possibility of a rapid increase in the level of mRNA or the degradation of mRNA after cell treatment.
The reduced functionality of ABCC was not related to the decreased expression of abcc1, abcc5, or abcc6 mRNAs, except for TFV. A significant dissociation between expression and activity has been reported (1, 12, 39, 57).
Given that only three repetitions of the experiments were performed, no statistical test would be relevant. Even if we have to remain cautious owing to the small amount of data obtained for the different treatment groups, we could observe some trends for increases or decreases in the levels of mRNA expression.
The nucleoside analogue FTC tended to induce the expression of abcc5 mRNA, which encodes the nucleoside and nucleotide transporter ABCC5 (22, 47, 58). EFV tended to induce abcc1 and abcc6 mRNA expression; both of these genes encode xenobiotic transporters (4, 11). TFV tended to alter the levels of mdr1, abcc1, abcc5, and abcc6 mRNA expression.
These results suggest different mechanisms of regulation between transporters. Nuclear receptors, such as the constitutive androstane receptor (CAR) and the pregnane X receptor (PXR), play important roles in the transcriptional regulation and induction of several genes like mdr1 and abcc2 (27, 51). In contrast, only a few studies have evaluated abcc1; furthermore, the results have been controversial (28, 37). The abcc1 and abcc6 gene promoters contain an Sp1-binding site, which is involved in the regulation of their transcription (25, 40). The nuclear factor E2 p45-related factor (Nrf-2), a transcription factor for the antioxidant-responsive element, can be required for both the constitutive and the inducible expression of ABCC1 mRNA (19). PXR is expressed in PBMCs (1); the transcription factors Sp1 and Nrf-2 are ubiquitously expressed in mammalian cells. EFV has recently been demonstrated to act as a strong ligand of CAR and PXR (14). No data are available for the other drugs.
Combination therapies did not affect transporter mRNA expression, probably due to regulation compensations between the different drugs. The use of EFV alone increased the level of abcc1 or abcc6 mRNA expression, but the use of TFV alone decreased it; and use of the combinations (TFV-EFV or FTC-TFV-EFV) resulted in a compensation for the effect seen with EFV or TFV alone. The same effect was observed with FTC. The use of FTC alone increased the level of abcc5 mRNA expression, but the use of TFV alone decreased it; and use of the combinations (FTC-TFV or FTC-TFV-EFV) resulted in a compensation for the effect seen with FTC or TFV alone. Of course, all these observations deserve confirmation.
The effects of the drugs seen on ABCC function were not caused by ABCC mRNA expression modulation. Therefore, we propose that the drugs could have a direct effect on ABCC transport. FTC, TFV, and EFV at concentrations ranging from 0.5 μM to 10 μM increased the levels of calcein-AM accumulation in a concentration-dependent manner (Fig. 3), suggesting the interaction of these compounds with ABCC transporters, as described previously (46, 56).
In conclusion, this in vitro study reveals a benefit from the use of combination therapy, like that achieved with Atripla, in terms of the FTC and TFV intracellular drug concentrations. However, there is always a balance between efficacy and toxicity/adverse effects, and a good combination therapy may require patient therapeutic drug monitoring. This effect on drug concentration seems to be partly due to a decrease in ABCC functionality and particularly to the direct interaction of the drugs with members of the ABCC family. We also provide evidence for different pathways of transcriptional expression modulation between efflux transporters.
Acknowledgments
We thank the Agence Nationale de Recherche sur le SIDA for financial support.
Footnotes
Published ahead of print on 15 December 2008.
REFERENCES
- 1.Albermann, N., F. H. Schmitz-Winnenthal, K. Z'graggen, C. Volk, M. M. Hoffmann, W. E. Haefeli, and J. Weiss. 2005. Expression of the drug transporters MDR1/ABCB1, MRP1/ABCC1, MRP2/ABCC2, BCRP/ABCG2, and PXR in peripheral blood mononuclear cells and their relationship with the expression in intestine and liver. Biochem. Pharmacol. 70:949-958. [DOI] [PubMed] [Google Scholar]
- 2.Arribas, J. R., A. L. Pozniak, J. E. Gallant, E. DeJesus, B. Gazzard, R. E. Campo, S. S. Chen, D. McColl, C. B. Holmes, J. Enejosa, J. J. Toole, and A. K. Cheng. 2007. Tenofovir disoproxil fumarate, emtricitabine, and efavirenz compared with zidovudine/lamivudine and efavirenz in treatment-naive patients: 144-week analysis. J. Acquir. Immune. Defic. Syndr. 47:74-78. [DOI] [PubMed] [Google Scholar]
- 3.Bartlett, J. A., S. S. Chen, and J. B. Quinn. 2007. Comparative efficacy of nucleoside/nucleotide reverse transcriptase inhibitors in combination with efavirenz: results of a systematic overview. HIV Clin. Trials 8:221-226. [DOI] [PubMed] [Google Scholar]
- 4.Belinsky, M. G., Z. S. Chen, I. Shchaveleva, H. Zeng, and G. D. Kruh. 2002. Characterization of the drug resistance and transport properties of multidrug resistance protein 6 (MRP6, ABCC6). Cancer Res. 62:6172-6177. [PubMed] [Google Scholar]
- 5.Belliard, A. M., S. Tardivel, R. Farinotti, B. Lacour, and C. Leroy. 2002. Effect of hr-IL2 treatment on intestinal P-glycoprotein expression and activity in Caco-2 cells. J. Pharm. Pharmacol. 54:1103-1109. [DOI] [PubMed] [Google Scholar]
- 6.Benech, H., F. Theodoro, A. Herbet, N. Page, D. Schlemmer, A. Pruvost, J. Grassi, and J. R. Deverre. 2004. Peripheral blood mononuclear cell counting using a DNA-detection-based method. Anal. Biochem. 330:172-174. [DOI] [PubMed] [Google Scholar]
- 7.Berruet, N., S. Sentenac, D. Auchere, F. Gimenez, R. Farinotti, and C. Fernandez. 2005. Effect of efavirenz on intestinal P-glycoprotein and hepatic p450 function in rats. J. Pharm. Pharm. Sci. 8:226-234. [PubMed] [Google Scholar]
- 8.Borroto-Esoda, K., J. E. Vela, F. Myrick, A. S. Ray, and M. D. Miller. 2006. In vitro evaluation of the anti-HIV activity and metabolic interactions of tenofovir and emtricitabine. Antivir. Ther. 11:377-384. [PubMed] [Google Scholar]
- 9.Chandler, B., L. Almond, J. Ford, A. Owen, P. Hoggard, S. Khoo, and D. Back. 2003. The effects of protease inhibitors and nonnucleoside reverse transcriptase inhibitors on P-glycoprotein expression in peripheral blood mononuclear cells in vitro. J. Acquir. Immune. Defic. Syndr. 33:551-556. [DOI] [PubMed] [Google Scholar]
- 10.Chearskul, P., C. Rongkavilit, H. Al-Tatari, and B. Asmar. 2006. New antiretroviral drugs in clinical use. Indian J. Pediatr. 73:335-341. [DOI] [PubMed] [Google Scholar]
- 11.Choudhuri, S., and C. D. Klaassen. 2006. Structure, function, expression, genomic organization, and single nucleotide polymorphisms of human ABCB1 (MDR1), ABCC (MRP), and ABCG2 (BCRP) efflux transporters. Int. J. Toxicol. 25:231-259. [DOI] [PubMed] [Google Scholar]
- 12.De Moerloose, B., C. Dhooge, and J. Philippe. 1999. Discordance of P-glycoprotein expression and function in acute leukemia. Adv. Exp. Med. Biol. 457:107-118. [DOI] [PubMed] [Google Scholar]
- 13.Dirson, G., C. Fernandez, P. Hindlet, F. Roux, M. German-Fattal, F. Gimenez, and R. Farinotti. 2006. Efavirenz does not interact with the ABCB1 transporter at the blood-brain barrier. Pharm. Res. 23:1525-1532. [DOI] [PubMed] [Google Scholar]
- 14.Faucette, S. R., T. C. Zhang, R. Moore, T. Sueyoshi, C. J. Omiecinski, E. L. LeCluyse, M. Negishi, and H. Wang. 2007. Relative activation of human pregnane X receptor versus constitutive androstane receptor defines distinct classes of CYP2B6 and CYP3A4 inducers. J. Pharmacol. Exp. Ther. 320:72-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ford, J., E. R. Meaden, P. G. Hoggard, M. Dalton, P. Newton, I. Williams, S. H. Khoo, and D. J. Back. 2003. Effect of protease inhibitor-containing regimens on lymphocyte multidrug resistance transporter expression. J. Antimicrob. Chemother. 52:354-358. [DOI] [PubMed] [Google Scholar]
- 16.Gekeler, V., W. Ise, K. H. Sanders, W. R. Ulrich, and J. Beck. 1995. The leukotriene LTD4 receptor antagonist MK571 specifically modulates MRP associated multidrug resistance. Biochem. Biophys. Res. Commun. 208:345-352. [DOI] [PubMed] [Google Scholar]
- 17.Gottesman, M. M., C. Cardarelli, S. Goldenberg, T. Licht, and I. Pastan. 1998. Selection and maintenance of multidrug-resistant cells. Methods Enzymol. 292:248-258. [DOI] [PubMed] [Google Scholar]
- 18.Hammer, S. M., M. S. Saag, M. Schechter, J. S. Montaner, R. T. Schooley, D. M. Jacobsen, M. A. Thompson, C. C. Carpenter, M. A. Fischl, B. G. Gazzard, J. M. Gatell, M. S. Hirsch, D. A. Katzenstein, D. D. Richman, S. Vella, P. G. Yeni, and P. A. Volberding. 2006. Treatment for adult HIV infection: 2006 recommendations of the International AIDS Society—USA Panel. Top. HIV Med. 14:827-843. [PubMed] [Google Scholar]
- 19.Hayashi, A., H. Suzuki, K. Itoh, M. Yamamoto, and Y. Sugiyama. 2003. Transcription factor Nrf2 is required for the constitutive and inducible expression of multidrug resistance-associated protein 1 in mouse embryo fibroblasts. Biochem. Biophys. Res. Commun. 310:824-829. [DOI] [PubMed] [Google Scholar]
- 20.Higgins, C. F. 1992. ABC transporters: from microorganisms to man. Annu. Rev. Cell Biol. 8:67-113. [DOI] [PubMed] [Google Scholar]
- 21.Hollo, Z., L. Homolya, T. Hegedus, and B. Sarkadi. 1996. Transport properties of the multidrug resistance-associated protein (MRP) in human tumour cells. FEBS Lett. 383:99-104. [DOI] [PubMed] [Google Scholar]
- 22.Homolya, L., A. Varadi, and B. Sarkadi. 2003. Multidrug resistance-associated proteins: export pumps for conjugates with glutathione, glucuronate or sulfate. Biofactors 17:103-114. [DOI] [PubMed] [Google Scholar]
- 23.Huisman, M. T., J. W. Smit, H. R. Wiltshire, R. M. Hoetelmans, J. H. Beijnen, and A. H. Schinkel. 2001. P-glycoprotein limits oral availability, brain, and fetal penetration of saquinavir even with high doses of ritonavir. Mol. Pharmacol. 59:806-813. [DOI] [PubMed] [Google Scholar]
- 24.Imaoka, T., H. Kusuhara, M. Adachi, J. D. Schuetz, K. Takeuchi, and Y. Sugiyama. 2007. Functional involvement of multidrug resistance-associated protein 4 (MRP4/ABCC4) in the renal elimination of the antiviral drugs adefovir and tenofovir. Mol. Pharmacol. 71:619-627. [DOI] [PubMed] [Google Scholar]
- 25.Jiang, Q., Y. Matsuzaki, K. Li, and J. Uitto. 2006. Transcriptional regulation and characterization of the promoter region of the human ABCC6 gene. J. Investig. Dermatol. 126:325-335. [DOI] [PubMed] [Google Scholar]
- 26.Jorajuria, S., N. Reuddre-Bosquet, F. Becher, S. Martin, F. Porcheray, A. Garrigues, A. Mabondzo, H. Benech, J. Grassi, S. Orlowski, D. Dormont, and P. Clayette. 2004. ATP binding cassette multidrug transporters limit the anti-HIV activity of zidovudine and indinavir in infected human macrophages. Antivir. Ther. 9:519-528. [PubMed] [Google Scholar]
- 27.Kast, H. R., B. Goodwin, P. T. Tarr, S. A. Jones, A. M. Anisfeld, C. M. Stoltz, P. Tontonoz, S. Kliewer, T. M. Willson, and P. A. Edwards. 2002. Regulation of multidrug resistance-associated protein 2 (ABCC2) by the nuclear receptors pregnane X receptor, farnesoid X-activated receptor, and constitutive androstane receptor. J. Biol. Chem. 277:2908-2915. [DOI] [PubMed] [Google Scholar]
- 28.Kauffmann, H. M., S. Pfannschmidt, H. Zoller, A. Benz, B. Vorderstemann, J. I. Webster, and D. Schrenk. 2002. Influence of redox-active compounds and PXR-activators on human MRP1 and MRP2 gene expression. Toxicology 171:137-146. [DOI] [PubMed] [Google Scholar]
- 29.Killingley, B., and A. Pozniak. 2007. The first once-daily single-tablet regimen for the treatment of HIV-infected patients. Drugs Today (Barcelona) 43:427-442. [DOI] [PubMed] [Google Scholar]
- 30.Klein, I., B. Sarkadi, and A. Varadi. 1999. An inventory of the human ABC proteins. Biochim. Biophys. Acta 1461:237-262. [DOI] [PubMed] [Google Scholar]
- 31.Krishna, R., and L. D. Mayer. 2000. Multidrug resistance (MDR) in cancer. Mechanisms, reversal using modulators of MDR and the role of MDR modulators in influencing the pharmacokinetics of anticancer drugs. Eur. J. Pharm. Sci. 11:265-283. [DOI] [PubMed] [Google Scholar]
- 32.Laupeze, B., L. Amiot, L. Payen, B. Drenou, J. M. Grosset, G. Lehne, R. Fauchet, and O. Fardel. 2001. Multidrug resistance protein (MRP) activity in normal mature leukocytes and CD34-positive hematopoietic cells from peripheral blood. Life Sci. 68:1323-1331. [DOI] [PubMed] [Google Scholar]
- 33.Lee, C. G., M. M. Gottesman, C. O. Cardarelli, M. Ramachandra, K. T. Jeang, S. V. Ambudkar, I. Pastan, and S. Dey. 1998. HIV-1 protease inhibitors are substrates for the MDR1 multidrug transporter. Biochemistry 37:3594-3601. [DOI] [PubMed] [Google Scholar]
- 34.Legrand, O., G. Simonin, J. Y. Perrot, R. Zittoun, and J. P. Marie. 1998. Pgp and MRP activities using calcein-AM are prognostic factors in adult acute myeloid leukemia patients. Blood 91:4480-4488. [PubMed] [Google Scholar]
- 35.Levi, M., and A. Pruvost. 2006. Sensitive HPLC-ESI-MS/MS method for the simultaneous quantitative determination of abacavir, lamivudine and tenofovir in human plasma, abstr. 69. Abstr. 7th Int. Workshop Clin. Pharmacol. HIV Ther.
- 36.Loscher, W., and H. Potschka. 2005. Blood-brain barrier active efflux transporters: ATP-binding cassette gene family. NeuroRx 2:86-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Magnarin, M., M. Morelli, A. Rosati, F. Bartoli, L. Candussio, T. Giraldi, and G. Decorti. 2004. Induction of proteins involved in multidrug resistance (P-glycoprotein, MRP1, MRP2, LRP) and of CYP 3A4 by rifampicin in LLC-PK1 cells. Eur. J. Pharmacol. 483:19-28. [DOI] [PubMed] [Google Scholar]
- 38.Masuhr, A., M. Mueller, V. Simon, T. Zwingers, M. Kurowski, H. Jessen, E. Lauenroth-Mai, A. Moll, D. Schranz, C. Moecklinghoff, and K. Arasteh. 2002. Predictors of treatment failure during highly active antiretroviral therapy (racing trial). Eur. J. Med. Res. 7:341-346. [PubMed] [Google Scholar]
- 39.Meaden, E. R., P. G. Hoggard, S. H. Khoo, and D. J. Back. 2002. Determination of P-gp and MRP1 expression and function in peripheral blood mononuclear cells in vivo. J. Immunol. Methods 262:159-165. [DOI] [PubMed] [Google Scholar]
- 40.Muredda, M., K. Nunoya, R. A. Burtch-Wright, E. U. Kurz, S. P. Cole, and R. G. Deeley. 2003. Cloning and characterization of the murine and rat mrp1 promoter regions. Mol. Pharmacol. 64:1259-1269. [DOI] [PubMed] [Google Scholar]
- 41.Ozben, T. 2006. Mechanisms and strategies to overcome multiple drug resistance in cancer. FEBS Lett. 580:2903-2909. [DOI] [PubMed] [Google Scholar]
- 42.Perloff, M. D., L. L. Von Moltke, J. E. Marchand, and D. J. Greenblatt. 2001. Ritonavir induces P-glycoprotein expression, multidrug resistance-associated protein (MRP1) expression, and drug transporter-mediated activity in a human intestinal cell line. J. Pharm. Sci. 90:1829-1837. [DOI] [PubMed] [Google Scholar]
- 43.Pozniak, A. L., J. E. Gallant, E. DeJesus, J. R. Arribas, B. Gazzard, R. E. Campo, S. S. Chen, D. McColl, J. Enejosa, J. J. Toole, and A. K. Cheng. 2006. Tenofovir disoproxil fumarate, emtricitabine, and efavirenz versus fixed-dose zidovudine/lamivudine and efavirenz in antiretroviral-naive patients: virologic, immunologic, and morphologic changes—a 96-week analysis. J. Acquir. Immune. Defic. Syndr. 43:535-540. [DOI] [PubMed] [Google Scholar]
- 44.Pruvost, A., F. Theodoro, L. Agrofoglio, E. Negredo, and H. Benech. 2008. Specificity enhancement with LC-positive ESI-MS/MS for the measurement of nucleotides: application to the quantitative determination of carbovir triphosphate, lamivudine triphosphate and tenofovir diphosphate in human peripheral blood mononuclear cells. J. Mass Spectrom. 43:224-233. [DOI] [PubMed] [Google Scholar]
- 45.Rautio, J., J. E. Humphreys, L. O. Webster, A. Balakrishnan, J. P. Keogh, J. R. Kunta, C. J. Serabjit-Singh, and J. W. Polli. 2006. In vitro P-glycoprotein inhibition assays for assessment of clinical drug interaction potential of new drug candidates: a recommendation for probe substrates. Drug Metab. Dispos. 34:786-792. [DOI] [PubMed] [Google Scholar]
- 46.Ray, A. S., T. Cihlar, K. L. Robinson, L. Tong, J. E. Vela, M. D. Fuller, L. M. Wieman, E. J. Eisenberg, and G. R. Rhodes. 2006. Mechanism of active renal tubular efflux of tenofovir. Antimicrob. Agents Chemother. 50:3297-3304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ritter, C. A., G. Jedlitschky, S. H. Meyer Zu, M. Grube, K. Kock, and H. K. Kroemer. 2005. Cellular export of drugs and signaling molecules by the ATP-binding cassette transporters MRP4 (ABCC4) and MRP5 (ABCC5). Drug Metab. Rev. 37:253-278. [DOI] [PubMed] [Google Scholar]
- 48.Seelig, A., X. L. Blatter, and F. Wohnsland. 2000. Substrate recognition by P-glycoprotein and the multidrug resistance-associated protein MRP1: a comparison. Int. J. Clin. Pharmacol. Ther. 38:111-121. [DOI] [PubMed] [Google Scholar]
- 49.Storch, C. H., D. Theile, H. Lindenmaier, W. E. Haefeli, and J. Weiss. 2007. Comparison of the inhibitory activity of anti-HIV drugs on P-glycoprotein. Biochem. Pharmacol. 73:1573-1581. [DOI] [PubMed] [Google Scholar]
- 50.Stormer, E., L. L. Von Moltke, M. D. Perloff, and D. J. Greenblatt. 2002. Differential modulation of P-glycoprotein expression and activity by non-nucleoside HIV-1 reverse transcriptase inhibitors in cell culture. Pharm. Res. 19:1038-1045. [DOI] [PubMed] [Google Scholar]
- 51.Synold, T. W., I. Dussault, and B. M. Forman. 2001. The orphan nuclear receptor SXR coordinately regulates drug metabolism and efflux. Nat. Med. 7:584-590. [DOI] [PubMed] [Google Scholar]
- 52.Uwai, Y., H. Ida, Y. Tsuji, T. Katsura, and K. Inui. 2007. Renal transport of adefovir, cidofovir, and tenofovir by SLC22A family members (hOAT1, hOAT3, and hOCT2). Pharm. Res. 24:811-815. [DOI] [PubMed] [Google Scholar]
- 53.Veau, C., L. Faivre, S. Tardivel, M. Soursac, H. Banide, B. Lacour, and R. Farinotti. 2002. Effect of interleukin-2 on intestinal P-glycoprotein expression and functionality in mice. J. Pharmacol. Exp. Ther. 302:742-750. [DOI] [PubMed] [Google Scholar]
- 54.Vishnuvardhan, D., L. L. Moltke, C. Richert, and D. J. Greenblatt. 2003. Lopinavir: acute exposure inhibits P-glycoprotein; extended exposure induces P-glycoprotein. AIDS 17:1092-1094. [DOI] [PubMed] [Google Scholar]
- 55.Weiss, J., S. M. Dormann, M. Martin-Facklam, C. J. Kerpen, N. Ketabi-Kiyanvash, and W. E. Haefeli. 2003. Inhibition of P-glycoprotein by newer antidepressants. J. Pharmacol. Exp. Ther. 305:197-204. [DOI] [PubMed] [Google Scholar]
- 56.Weiss, J., D. Theile, N. Ketabi-Kiyanvash, H. Lindenmaier, and W. E. Haefeli. 2007. Inhibition of MRP1/ABCC1, MRP2/ABCC2, and MRP3/ABCC3 by nucleoside, nucleotide, and non-nucleoside reverse transcriptase inhibitors. Drug Metab. Dispos. 35:340-344. [DOI] [PubMed] [Google Scholar]
- 57.Weiss, J., N. Weis, N. Ketabi-Kiyanvash, C. H. Storch, and W. E. Haefeli. 2008. Comparison of the induction of P-glycoprotein activity by nucleotide, nucleoside, and non-nucleoside reverse transcriptase inhibitors. Eur. J. Pharmacol. 579:104-109. [DOI] [PubMed] [Google Scholar]
- 58.Wijnholds, J., C. A. Mol, L. van Deemter, M. de Haas, G. L. Scheffer, F. Baas, J. H. Beijnen, R. J. Scheper, S. Hatse, E. De Clercq, J. Balzarini, and P. Borst. 2000. Multidrug-resistance protein 5 is a multispecific organic anion transporter able to transport nucleotide analogs. Proc. Natl. Acad. Sci. USA 97:7476-7481. [DOI] [PMC free article] [PubMed] [Google Scholar]