Abstract
Voriconazole is a potent triazole with broad-spectrum antifungal activity against clinically significant and emerging pathogens. The present population pharmacokinetic analysis evaluated voriconazole plasma concentration-time data from three studies of pediatric patients of 2 to <12 years of age, incorporating a range of single or multiple intravenous (i.v.) and/or oral (p.o.) doses. An appropriate pharmacokinetic model for this patient population was created using the nonlinear mixed-effect modeling approach. The final model described voriconazole elimination by a Michaelis-Menten process and distribution by a two-compartment model. It also incorporated a statistically significant (P < 0.001) influence of the CYP2C19 genotype and of the alanine aminotransferase level on clearance. The model was used in a number of deterministic simulations (based on various fixed, mg/kg of body weight, and individually adjusted doses) aimed at finding suitable i.v. and p.o. voriconazole dosing regimens for pediatric patients. As a result, 7 mg/kg twice a day (BID) i.v. or 200 mg BID p.o., irrespective of body weight, was recommended for this patient population. At these doses, the pediatric area-under-the-curve (AUC) distribution exhibited the least overall difference from the adult AUC distribution (at dose levels used in clinical practice). Loading doses or individual dosage adjustments according to baseline covariates are not considered necessary in administering voriconazole to children.
Voriconazole is a broad-spectrum triazole antifungal used for the treatment of invasive fungal infections. By selectively inhibiting the fungal enzyme 14-α-sterol demethylase, triazoles significantly disrupt fungal plasma membrane synthesis and sustained fungal growth (4). In vitro studies show that at clinically achievable concentrations, voriconazole exhibits potent activity against Aspergillus spp., Candida spp. (including Candida krusei and Candida glabrata, which are less susceptible to other azoles), and a broad range of rare and emerging fungal pathogens (with the notable exception of zygomycetes) (3, 12, 14, 24). It has been approved in both the United States and Europe for treatment of invasive aspergillosis, candidemia (in nonneutropenic patients), esophageal candidiasis (United States only), invasive candidiasis, and serious fungal infections caused by Scedosporium spp. and Fusarium spp.
Voriconazole is available for adult use, in both oral (p.o.) (tablets and suspension) and intravenous (i.v.) formulations. Given i.v. to adults, the recommended dosing regimen for voriconazole in the United States and Europe consists of two loading doses of 6 mg/kg of body weight 12 h apart, followed by 3 to 4 mg/kg twice a day (BID). Given p.o. to adults, two doses of 400 mg 12 h apart are followed by 200 mg BID, which can be increased to 300 mg in case of an inadequate patient response. Oral doses should be halved for adult patients with <40 kg of body weight. In the European Union, voriconazole is also licensed for pediatric use in patients of 2 to <12 years of age, with recommended dosages of 7 mg/kg BID (for the i.v. formulation) and 200 mg BID (for the oral suspension), without loading doses.
Following p.o. administration to adults, voriconazole is absorbed rapidly, with maximum plasma concentrations (Cmax) achieved 1 to 2 h after dosing in the fasted state. Absolute bioavailability is estimated to be 96%, which allows switching from i.v. to p.o. use when it is clinically indicated (16). Voriconazole is also distributed extensively into tissues, as evident from its volume of distribution at steady state (Vss) of approximately 4.6 liters/kg (15). In vitro data demonstrate that voriconazole is primarily metabolized by hepatic CYP2C19, CYP2C9, and CYP3A4 isoenzymes and that the drug is transformed into more than eight different metabolites, with the N-oxide accounting for 72% of metabolic products in plasma (7, 15). As with several other azoles that are hepatically metabolized by CYP450 isoenzymes, the pharmacokinetics of voriconazole are nonlinear (6, 18). Factors influencing voriconazole exposure have been studied extensively, and while they have demonstrated relatively low intrasubject variability, intersubject variability has been found to be high (15, 18).
The factors affecting intersubject variability in voriconazole exposure between adults include genetic differences, gender, and age, although none of these exerts a clinically relevant effect. For example, there are three main genotypes for the CYP2C19 enzyme, namely, homozygous poor metabolizers (PMs), heterozygous extensive metabolizers (HEMs), and homozygous extensive metabolizers (EMs). However, the CYP2C19 genotype by itself does not accurately predict voriconazole plasma concentrations in patients, and therefore dose adjustment based solely on this factor is not recommended (7). Similarly, data from therapeutic studies indicate that no dose adjustments are required on the basis of age or gender (15).
Candidates for systemic antifungal therapy with voriconazole also include pediatric patients (aged 2 to <12 years), often with underlying conditions such as hematological malignancies and/or transplant procedures (23). For example, U.S. data indicate a 0.4% annual incidence of invasive aspergillosis in hospitalized immunodeficient children, but incidences as high as 19% have been reported for certain pediatric subpopulations (13, 25). However, the optimum dosing regimen for children is under discussion. This report describes the results of a population pharmacokinetic analysis characterizing the interrelationships between voriconazole plasma concentrations and potential covariates in children, which further builds on a previously published report (23). The primary aim of our analysis was to construct a refined pharmacokinetic model for pediatric patients in order to derive suitable dosage recommendations for voriconazole in individuals of 2 to <12 years of age. Based on the assumption of similar exposure-response relationships in the different populations, the resultant dosing recommendations are intended to achieve voriconazole exposures in pediatric patients comparable to those in adults following the approved i.v. and p.o. dosing regimens.
MATERIALS AND METHODS
Study data.
Data from three open-label pediatric studies, with a total of 82 patients of 2 to <12 years of age, were incorporated into the analysis. These studies investigated the pharmacokinetics, safety, and tolerability of p.o. and/or i.v. voriconazole in children, examining a range of dosage regimens, including single doses of 3 and 4 mg/kg i.v. (study A; n = 11) as well as multiple doses of 3, 4, 6, and 8 mg/kg BID i.v. (study B and study C) followed by multiple oral suspension doses of 4 and 6 mg/kg BID (study C). Both multiple-dose studies followed a within-subject dose escalation methodology. In study B (n = 28), patients received loading doses of 6 mg/kg BID on day 1, followed by 3 mg/kg BID on days 2 to 4 and 4 mg/kg BID on days 4 to 8. In study C, one cohort received loading doses of 6 mg/kg BID i.v. on day 1, 4 mg/kg BID i.v. on days 2 to 4, 6 mg/kg BID i.v. on days 5 to 8, and then 4 mg/kg BID p.o. on days 9 to 12. A second cohort received 6 mg/kg BID i.v. on days 1 to 4, 8 mg/kg BID i.v. on days 5 to 8, and then 6 mg/kg BID p.o. on days 9 to 12. i.v. infusions were administered over 0.5 and up to and including 1.5 h (30% of all infusions), over 1.5 and up to and including 2.5 h (47%), or over 2.5 and up to and including 3.5 h (22%). The remaining 1% of infusions were outside these times.
Plasma voriconazole concentrations were determined using a previously validated high-performance liquid chromatography method (22). A total of 1,274 plasma samples—an average of 15.5 samples per individual—were analyzed for voriconazole concentration. Four plasma samples per patient, drawn at interval windows of 2 to 4 h, 4 to 8 h, 8 to 12 h, and 12 to 24 h postdose, were obtained in study A. For patients enrolled in study B, samples were drawn on days 1, 2, 4, and 8 at interval windows of 2 to 4 h, 4 to 8 h, 8 to 12 h, and 12 to 24 h postdose on each day designated for sample collection. In study C, six to eight samples per patient and per day were collected on days 4, 8, and 12 at prespecified time points, depending on whether patients received i.v. doses of 4 or 6 mg/kg (prior to dose, 2 min before the end of infusion, and 2, 4, 6, 8, and 12 h after the start of infusion) or 8 mg/kg (prior to dose, 2 min before the end of infusion, and 4, 6, 8, and 12 h after the start of infusion) or p.o. doses (prior to dose and 0.5, 1, 2, 4, 6, 8, and 12 h after dose).
The subsequent analysis considered a number of factors with a possible influence on voriconazole pharmacokinetics, including demographic factors (age, gender, weight, height, and ethnic origin), biochemistry (serum creatinine, aspartate aminotransferase, alanine aminotransferase [ALT], alkaline phosphatase [ALP], γ-glutamyltransferase, albumin, total bilirubin, and total protein levels), concomitant medications (CYP2C19 inhibitors, CYP2C9 inhibitors, CYP3A4 inhibitors, and CYP450 inducers), underlying disease (leukemia, bone marrow transplant, aplastic anemia, lymphoma, or other), and other factors (CYP2C19 genotype status and presence of mucositis). Data on these potential covariates were collected from each patient. In case of any missing covariate values for an individual, these values were replaced by the respective median value for the population. However, patients without CYP2C19 information were excluded from the analysis (four patients from study B and one patient from study C).
The patients' average body weight was 22.8 kg (range, 10.8 to 54.9 kg), with an average baseline ALT level of 40.7 IU/liter (range, 7 to 242 IU/liter) and an average ALP level of 135 IU/liter (range, 46 to 309 IU/liter). Some other characteristics of the study population are as follows: there were 47 males and 35 females; for CYP2C19 genotype, there were 58 EMs, 21 HEMs, and 3 PMs; there were 57 Caucasian patients, 6 black patients, 4 Asian patients, and 15 patients of other ethnic groups; there were 31 patients with leukemia, 39 with bone marrow transplantation, 2 with lymphoma, 1 with aplastic anemia, and 9 with other diseases; and mucositis was present in 57 patients and absent in 25 patients. Four, 23, and 18 patients received concomitant CYP2C19, CYP2C9, and CYP3A4 inhibitors, respectively, while 34 patients received concomitant CYP450 inducers.
Pharmacokinetic analysis.
Formal population pharmacokinetic analysis of the combined plasma concentration data was carried out by applying the nonlinear mixed-effect modeling approach. The software package NONMEM (version V, level 1.1) was used to derive the population mean and variance for all pharmacokinetic parameters (described below). Pharmacokinetic models were fitted to concentration-versus-time data through standard population pharmacokinetic methodology (2). All NONMEM analyses were carried out using the first-order conditional estimation method with the interaction option. The appropriateness of particular models was assessed by graphical diagnostics, using Xpose (version 3.1) software (8).
The model-building strategy was based on a previously published initiating model that was developed from a smaller data set than the one described here (i.e., results from study A and study B) (23). The initiating model for the present analysis used two-compartment disposition with Michaelis-Menten elimination parameterized in terms of clearance in the linear concentration range (CL), Michaelis-Menten constant (Km), volume of distribution of the central compartment (Vc), volume of distribution of the peripheral compartment (Vp), intercompartmental clearance rate (Q), and maximal rate of metabolism (Vmax). Vmax was obtained as CL multiplied by Km and expressed—along with the disposition parameters—as per kg of body weight. This model also used first-order absorption with estimated bioavailability, parameterized as the rate constant of absorption (Ka), and extent of bioavailability (F) but assumed no lag time for p.o. administration.
Interindividual variability terms, without covariances, were included on CL, Km, and F in the initial model and were described using exponential models. The latter model-building process also tested interindividual variability in other parameters, as well as covariance between parameters. The initiating model for residual error (RE) was an additive model for log-transformed concentration data, corresponding approximately to a proportional error on untransformed data. The RE magnitude was allowed to vary between subjects through incorporation of an interindividual variability term (10). Although it was not part of the initiating model, interoccasion variability was evaluated at later stages by a previously described method (11). The initiating model contained the following covariate relations for CL: CYP2C19 genotype (either EM or HEM/PM [as there were only three PMs in the data sets, they were merged with HEMs into one group]), log(ALT), and log(ALP).
The parameters CL, Vc, Q, and Vp were modeled as directly proportional to weight. The influence of further potential covariates, including age, on CL, Km, and F was investigated during the modeling process; candidate covariates were first identified through graphical analyses of covariates versus the empirical Bayes post hoc estimates for the relevant individual deviations of the model. In addition, some further covariates were tested for direct relationships in the model. For time-varying covariates, it was determined whether the relationship was adequately characterized by either the baseline covariate value or the change in covariate value from baseline. The statistical significance of including any such covariate relationship or an additional interindividual variability term into the NONMEM model was assessed by comparing the objective function values (OFVs) before and after inclusion. Additions to the model were retained in the model if their removal significantly (P < 0.001) increased OFV, i.e., the change in OFV was >10.8.
During model building, the internal validity of the population model was assessed by goodness-of-fit plots. Once a final model was identified, an internal validation was carried out. This included 10 case deletion diagnostics in order to demonstrate that the final model parameters were not influenced by data from any randomly selected group of patients. Each case deletion excluded the data set for a different, equally sized patient group (individuals were randomly assigned to one group per individual) and then reestimated the voriconazole pharmacokinetic parameters by using the final model.
Simulations.
Individual parameter estimates for subjects from study C were obtained from the final model and used in a number of deterministic simulations where area-under-the-curve (AUC) values were integrated from the individual concentration-time profile by use of the differential equation solver implemented in NONMEM V (ADVAN 6). The AUC distributions of voriconazole were obtained as percentile values following (i) 6, 7, 8, and 9 mg/kg BID i.v. for 14 days; (ii) 6, 7, 8, 9, 10, 11, and 12 mg/kg BID p.o. for 14 days; and (iii) 100, 150, 200, and 300 mg BID p.o. for 14 days. The resultant distributions based on individual parameters for the 48 subjects in study C were subsequently compared with the day 14 AUCs associated with the recommended adult dosing regimens (the “adult reference population”), i.e., either (i) 4 mg/kg BID i.v. or (ii and iii) 200 mg BID p.o. The average age of the adult reference population (15) was 35 years (range, 19 to 84 years), and the average weight was 70 kg (range, 51 to 97 kg), with 203 males and 33 females. Each simulation contrasted the 5, 10, 15, 20, 25, 50, 75, 80, 85, 90, and 95 percentile values between each pediatric simulation and its respective adult reference population. The same type of comparison was made for a range of individually adjusted (for relevant covariates) pediatric i.v. and p.o. regimens. Potential dosage regimens for which each resultant pediatric percentile value deviated no more than 50% from its respective adult percentile value were then identified. Once appropriate i.v. and p.o. dosage maintenance regimens for pediatrics had been established, the influence of a range of loading doses on the time course of attaining “stable” voriconazole exposures relative to that in adults was explored in further simulations.
RESULTS
Model building.
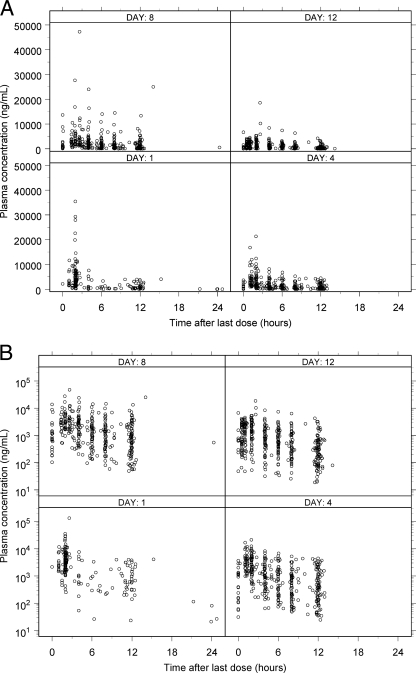
Figure 1 shows the observed voriconazole plasma concentrations following i.v. and p.o. administration versus time after last dose conditioned on the nominal study day.
FIG. 1.
Observed voriconazole plasma concentrations following i.v. (A) and p.o. (B) administration versus time after last dose, conditioned on the nominal study day. Day 1, all data for 0 to 48 h, corresponding to days 1 and 2, for all studies; day 4, all data for >48 to 96 h, corresponding to days 3, 4, and 5, for studies B and C only; day 8, all data for >96 to 192 h, corresponding to days 6, 7, 8, and 9, for studies B and C only; day 12, all data for >196 h, corresponding to >9 days, for study C only.
Table 1 details the parameter estimates from a number of models used in this analysis. For the final model, the absorption rate constant was estimated to be 0.849 h−1, and the extent of p.o. bioavailability was 44.6%. Replacing Michaelis-Menten elimination in the model with linear elimination resulted in a statistically significant (P < 0.001) OFV increase. A combined Michaelis-Menten and linear elimination did not improve the description of the data compared with a single Michaelis-Menten elimination. Distribution characteristics obtained from the final model for a typical pediatric individual weighing 22.8 kg were an 18.4-liter Vc, a 49.5-liter Vp, and a Q value of 13.9 liters/h.
TABLE 1.
Population pharmacokinetic parameter estimates for voriconazole for the initiating model, the final model, and the alternative final model, including RSE and deletion diagnostics coefficient of variation (CV)
| Parameter | Value
|
Final model RSE (%) | Final model deletion diagnostics CV (%) | ||
|---|---|---|---|---|---|
| Initiating model | Alternative final model | Final model | |||
| Km (ng/ml) | 5,400 | 2,970 | 3,030 | 45 | 38 |
| Vc (liters/kg) | 0.858 | 0.821 | 0.807 | 14 | 3 |
| Q (liters/h/kg) | 0.673 | 0.6 | 0.609 | 13 | 4 |
| Vp (liters/kg) | 1.8 | 2.13 | 2.17 | 11 | 4 |
| Ka (/h) | 2.5 | 0.853 | 0.849 | 40 | 14 |
| F | 42.8 | 43.2 | 44.6 | 14 | 6 |
| CL in EMs (liters/h/kg) | 0.482 | 0.631 | 0.582 | 19 | 9 |
| Decrease in CL for HEMs/PMs (%) | 31.8 | 36.6 | 35.5 | 29 | 10 |
| Decrease in CL with CYP2C9 inhibitors (%) | 26.8 | ||||
| Log(ALT) on CL (expressed as fractional change per unit change) | −0.153 | −0.108 | −0.0931 | 63 | 28 |
| Log(ALP) on CL (expressed as fractional change per unit change) | −0.292 | ||||
| CV(CL) (intersubject) | 61.6 | 57.9 | 52.8 | 48 | 12 |
| CV(CL) (interoccasion) | 41.7 | 43 | 21 | 6 | |
| CV(Km) | 113 | 127 | 131 | 31 | 9 |
| CV(F) | 40.7 | 72.5 | 69.7 | 41 | 7 |
| cor(CL,Km) | −0.721 | −0.685 | 51 | 11 | |
| cor(CL,F) | 0.61 | 0.66 | 35 | 7 | |
| cor(Km,F) | −0.645 | −0.646 | 37 | 13 | |
| Residual error (EMs) | 48.4 | 56.5 | 57.3 | 6.5 | 1 |
| Residual error (HEMs/PMs) | 29.7 | 29.9 | 14 | 4 | |
| CV(residual error) | 67.9 | 44.2 | 43 | 21 | 7 |
| OFV | 593.1 | 293.3 | 311.4 | ||
Neither Km, F, nor interindividual variability was significantly different between CYP2C19 genotypes. However, the RE magnitude was significantly lower for the PM/HEM group than for the EM group, with values of 29.9% and 57.3%, respectively (P < 0.001). While the ALP relationship with CL present in the initiating model was estimated to be close to zero and therefore omitted from the final model, the ALT relationship with CL remained significant and was retained. A new statistically significant covariate relationship (P < 0.001; ΔOFV = 18.4) was also detected: CYP2C9 inhibitors decreased CL 26.8% (90% confidence interval [CI], 13.6% to 40.0%) when such inhibitors were given concomitantly with voriconazole. In contrast, CL changes in response to cytochrome P450 inducers, as well as CYP3A4 and CYP2C19 inhibitors, were nonsignificant, being 13.5% (90% CI, −11.6% to 38.6%), −1.7% (90% CI, −23.8% to 27.2%), and 9.6% (90% CI, −24.5% to 43.7%), respectively. Allowing weight to influence F resulted in a decrease in OFV of 6.5 (P < 0.025), predicting lower F values for lighter patients. However, as this relationship did not reach the prespecified significance level (P < 0.001), it was not included in the final model. All other covariate relationships tested did not reach statistical significance.
Incorporating correlations between CL, Km, and F resulted in a 46-unit (P < 0.001) decrease in OFV compared with the initiating model, and introducing interoccasion variability in CL resulted in a significant (P < 0.001) decrease compared with a model without this variability. Since both changes were statistically significant, they were retained. Additional introductions of interindividual or interoccasion variability did not yield significant changes and/or resulted in unstable models.
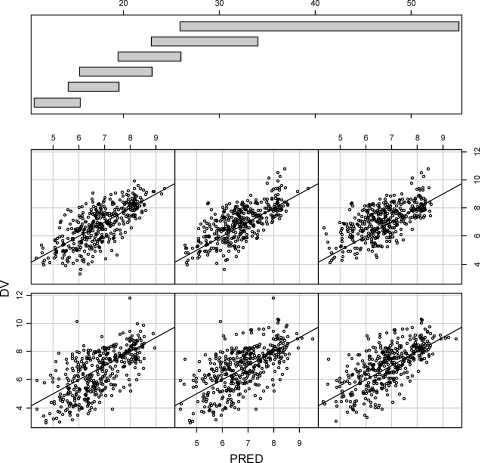
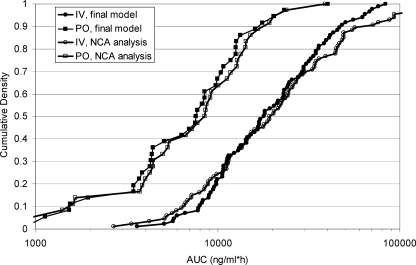
Goodness-of-fit plots for voriconazole plasma concentrations predicted from the final pharmacokinetic model are shown in Fig. 2. Figure 3 shows comparisons between the AUCs predicted from the final (compartmental) pharmacokinetic model and derived from the originating study noncompartmental analysis (trapezoidal rule). Table 2 compares the predicted CL, the covariate distribution, and the relative change in CL across different ranges of covariate (CYP2C19 status, ALT, and CYP2C9 inhibitor status) values for the alternative final model.
FIG. 2.
Logarithms of observed voriconazole concentrations versus population predictions split by patient weights (kg). Data for the lightest patients are shown to the lower left, and those for the heaviest patients are shown to the upper right. The weight ranges corresponding to each individual graph are indicated by the distribution bars at the top.
FIG. 3.
Cumulative distributions for voriconazole AUCs from study C following multiple dosing. AUCs were calculated for one dosing interval, using the linear trapezoidal rule (NCA analysis), or from the mixed-effect analysis (final model).
TABLE 2.
Predicted CL expressed as both a numerical value and a fraction of the CL in the typical individual, based on the alternative final model
| ALT level (IU/liter) | CYP2C19 genotype | CL (liters/h/kg)
|
No. of observations
|
CL (fraction of typical subject CLa)
|
|||
|---|---|---|---|---|---|---|---|
| Not taking CYP2C9 inhibitors | Taking CYP2C9 inhibitors | Not taking CYP2C9 inhibitors | Taking CYP2C9 inhibitors | Not taking CYP2C9 inhibitors | Taking CYP2C9 inhibitors | ||
| 10 | EM | 0.693 | 0.508 | 161 | 33 | 1.106 | 0.81 |
| HEM/PM | 0.44 | 0.322 | 47 | 27 | 0.701 | 0.513 | |
| 20 | EM | 0.646 | 0.473 | 218 | 76 | 1.031 | 0.754 |
| HEM/PM | 0.41 | 0.3 | 97 | 27 | 0.653 | 0.478 | |
| 40 | EM | 0.599 | 0.438 | 201 | 78 | 0.955 | 0.699 |
| HEM/PM | 0.38 | 0.278 | 50 | 25 | 0.606 | 0.443 | |
| 80 | EM | 0.552 | 0.404 | 113 | 24 | 0.88 | 0.644 |
| HEM/PM | 0.35 | 0.256 | 37 | 7 | 0.558 | 0.408 | |
| 160 | EM | 0.504 | 0.369 | 23 | 8 | 0.805 | 0.589 |
| HEM/PM | 0.32 | 0.234 | 22 | 0 | 0.51 | 0.373 | |
Typical subject characteristics were as follows: EM, 26.5 IU/liter ALT, not taking CYP2C9 inhibitors.
Deletion diagnostics.
Two of the parameters—Km and log(ALT) on CL—displayed larger variability than others across the 10 data sets during the deletion diagnostic tests. The change in Km upon omission of one of the groups was marked. However, exclusion of this group simultaneously resulted in a lower estimate of CL: the difference in the estimated elimination rate from the final model at 500, 1,000, 2,000, 4,000, and 8,000 ng/ml was −11%, −4%, 6%, 22%, and 41%, respectively. Omission of another data set rendered the ALT relationship smaller in magnitude, with a more uncertain estimate.
Simulations.
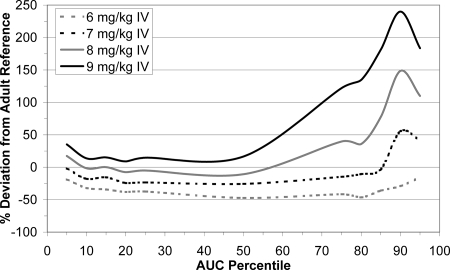
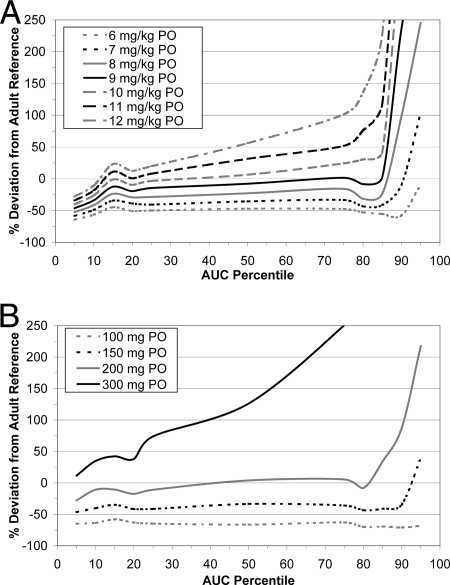
Figure 4 shows the percent deviations in the voriconazole AUC distribution within the pediatric population from the values for the reference adult population (following 4 mg/kg BID i.v.) at day 14 for the range of mg/kg i.v. dosages tested in the deterministic simulations. A dose of 8 mg/kg BID i.v. demonstrated the smallest deviation at the median exposure but higher relative exposure deviations of up to 150%. Exposure deviances also resulted following 7 mg/kg BID i.v., but they were of a much smaller magnitude. At this dosage, the relative deviations across all percentiles were almost entirely within a ±50% range of adult exposures. A 6-mg/kg BID i.v. dosage was associated with consistently lower exposures throughout the entire pediatric distribution than those for the adult reference population. Table 3 contrasts the simulated exposure deviations from the reference population between pediatric subjects receiving a dosage of 7 mg/kg BID i.v. and those receiving individually adjusted i.v. dosages. In comparing a dose of 7 mg/kg BID i.v. to doses individually adjusted according to CYP2C19 genotype or age, it appears that such adjustments offer no improvement over the unadjusted dose and can therefore not be justified on the basis of exposure distribution. Furthermore, the influence of a range of i.v. loading doses (6, 7, 8, 9, or 10 mg/kg BID on day 1) on pediatric voriconazole exposures preceding a maintenance regimen of 7 mg/kg BID i.v. starting on day 2 was considered. These loading-dose regimens appeared to have no important influence on the trajectory and timing by which “stable” voriconazole exposure was attained from day 2 onwards.
FIG. 4.
Percent deviations from the reference adult population AUC distribution (following 4 mg/kg BID i.v.) for a range of mg/kg i.v. pediatric doses.
TABLE 3.
Percent deviations from the reference adult population AUC distribution (following 4 mg/kg BID i.v.) for a range of individually adjusted i.v. pediatric doses according to CYP2C19 genotype or age compared with an unadjusted dose of 7 mg/kg
| Percentile | % Deviation from reference adult population AUC
|
|||||||
|---|---|---|---|---|---|---|---|---|
| Dose of 6 mg/kg for patients of ≤6 yr, else 7 mg/kg | Dose of 6 mg/kg for patients of ≤6 yr, else 8 mg/kg | Dose of 7 mg/kg for patients of ≤6 yr, else 6 mg/kg | Dose of 8 mg/kg for patients of ≤6 yr, else 6 mg/kg | Dose of 7 mg/kg for genotype EM, else 6 mg/kg | Dose of 8 mg/kg for genotype EM, else 6 mg/kg | Dose of 8 mg/kg for genotype EM, else 7 mg/kg | Dose of 7 mg/kg | |
| 5 | −18 | −18 | −14 | −5 | −2 | 17 | 17 | −2 |
| 10 | −26 | −26 | −21 | −18 | −18 | −2 | −2 | −18 |
| 15 | −29 | −28 | −30 | −17 | −16 | 0 | 0 | −16 |
| 20 | −31 | −22 | −26 | −19 | −24 | −9 | −8 | −24 |
| 25 | −31 | −23 | −31 | −22 | −26 | −12 | −6 | −24 |
| 50 | −39 | −38 | −40 | −26 | −34 | −25 | −11 | −26 |
| 75 | −26 | −20 | −28 | 9 | −26 | −3 | 20 | −15 |
| 80 | −31 | −20 | −24 | 10 | −30 | 11 | 17 | −10 |
| 85 | −26 | 16 | −15 | 7 | −7 | 17 | 35 | −3 |
| 90 | −9 | 23 | 2 | 36 | 25 | 82 | 104 | 55 |
| 95 | 39 | 110 | 13 | 78 | 39 | 110 | 110 | 42 |
Figure 5A and B illustrate the simulated deviations in voriconazole AUC distribution for a range of mg/kg and fixed p.o. dosages between the pediatric population and the reference adult population (200 mg BID p.o.) on day 14. These figures demonstrate that fixed-dose AUCs generally deviated much less (both positively and negatively) from the reference adult AUCs than did AUCs following p.o. mg/kg doses, especially above the 80th percentile. Figure 5B shows that for a 300-mg BID p.o. dose, high relative exposures (>50% deviation from the reference distribution) were frequent. Doses below 200 mg BID p.o. were associated with almost consistently lower exposures than those of the adult reference population. At the 200-mg BID p.o. dose, the relative deviation across all percentiles was almost entirely within a ±50% range of adult exposures. Table 4 contrasts the simulated exposure deviations and those of the reference population between pediatric subjects receiving a fixed dosage of 200 mg BID p.o. and those receiving individually adjusted p.o. dosages. In comparing a fixed dose of 200 mg BID p.o. to doses individually adjusted according to CYP2C19 genotype or age, it appears that such adjustments offer no improvement over the fixed dose and can therefore not be justified on the basis of exposure distribution. Furthermore, the influence of a range of p.o. loading doses (200, 300, 400, or 500 mg BID on day 1) preceding maintenance doses of 200 mg BID from day 2 onwards was considered. These loading-dose regimens appeared to have no important influence on the trajectory and timing by which “stable” voriconazole exposure was attained from day 2 onwards.
FIG. 5.
Percent deviations from the reference adult population AUC distribution (following 200 mg BID p.o.) for a range of mg/kg (A) and fixed (B) p.o. pediatric doses.
TABLE 4.
Percent deviations from the reference adult population AUC distribution (following 200 mg BID p.o.) for a range of individually adjusted p.o. pediatric doses according to CYP2C19 genotype or age compared with a fixed dose of 200 mg
| Percentile | % Deviation from reference adult population AUC
|
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Dose of 100 mg for patients of ≤6 yr, else 200 mg | Dose of 200 mg for patients of ≤6 yr, else 300 mg | Dose of 100 mg for patients with weight of ≤20 kg, else 200 mg | Dose of 150 mg for patients with weight of ≤20 kg, else 200 mg | Dose of 100 mg for genotype EM, else 100 mg | Dose of 200 mg for patients with weight of >20 kg or genotype EM, else 100 mg | Dose of 300 mg for patients with weight of >20 kg or genotype EM, else 200 mg | Dose of 100 mg for patients with weight of ≤20 kg and genotype PM/HEM, dose of 200 mg for patients with weight of ≤20 kg or genotype PM/HEM, else 300 mg | Dose of 200 mg | |
| 5 | −51 | −51 | −47 | −32 | −31 | −51 | −5 | −24 | −28 |
| 10 | −49 | −49 | −49 | −31 | −28 | −49 | −2 | −7 | −11 |
| 15 | −41 | −38 | −41 | −27 | −12 | −41 | 1 | −2 | −11 |
| 20 | −46 | −40 | −46 | −24 | −21 | −46 | 19 | −4 | −17 |
| 25 | −45 | −34 | −45 | −27 | −24 | −45 | 19 | 4 | −11 |
| 50 | −46 | −38 | −39 | −19 | −20 | −39 | 37 | 36 | 4 |
| 75 | −48 | −2 | −39 | −28 | −16 | −47 | 69 | 39 | 6 |
| 80 | −52 | −18 | −46 | −31 | −31 | −52 | 89 | 44 | −8 |
| 85 | −53 | −7 | −47 | −25 | −31 | −53 | 111 | 66 | 34 |
| 90 | −57 | 11 | −50 | 15 | −39 | −57 | 126 | 70 | 87 |
| 95 | 2 | 252 | 16 | 160 | −35 | −63 | 218 | 75 | 218 |
DISCUSSION
The present study used a population pharmacokinetic approach to identify potential sources of pharmacokinetic variability in children (2 to <12 years of age) receiving i.v. or p.o. voriconazole. The extent of variability attributable to these potential sources was also examined, and a compartmental model for pediatric voriconazole pharmacokinetics was developed. This model was then used in deterministic simulations aimed at establishing the most suitable i.v. and p.o. dosing regimens for voriconazole in young patients. Simulation outcomes suggest that exposures achieved with voriconazole dosages of 7 mg/kg BID i.v. and 200 mg BID p.o. in pediatric patients of 2 to <12 years of age are comparable to exposure levels observed in adult patients receiving approved dosing regimens. Based on the standard assumption that in the absence of any additional information a new subpopulation will derive consistent efficacy responses from drug levels similar to those in a patient population for which a dosage regimen has already been established, these voriconazole dosages can thus be recommended for pediatric patients.
The most recent pediatric studies used higher voriconazole doses than the earlier studies did, allowing a fuller characterization of its pharmacokinetics. Previous studies have demonstrated that the nonlinear, mixed-effect modeling approach used in this analysis is useful in analyzing both sparse pharmacokinetic data from patient studies (allowing pharmacokinetics to be assessed in the population in which a drug is to be used) (20) and rich data collected in experimental settings (1, 5, 21). Those studies also provided evidence that nonlinear, mixed-effect modeling using the first-order conditional estimation algorithm for population analyses offers superior detection and characterization of nonlinear pharmacokinetic processes in rich data obtained from few subjects, as was the case with most of the data included in the present analysis (9). Furthermore, this approach has been shown to allow better definition of how to use a drug in the clinical setting.
Important pharmacokinetic differences exist between adults and children receiving voriconazole. Although absorption rate constants were similar between children and adults (0.849 h−1 and 0.654 h−1, respectively), p.o. bioavailability of voriconazole in pediatric patients (44.6% bioavailability) was reduced 51.4% compared with that in adults (96% bioavailability, as determined by a NONMEM population pharmacokinetic analysis similar to the one described herein) (15). A potential mechanistic explanation could be that pediatric patients exhibit not only greater systemic metabolism but also greater first-pass metabolism than that of adults. This assumption is supported by several observations, as follows: (i) adults appear to reach saturation (nonlinearity) in pharmacokinetics at a lower voriconazole concentration; (ii) there is a strong negative correlation between Km and F, as well as between Vmax and F, indicating higher F values where saturation occurs at low concentrations; (iii) children on average have higher hepatic blood flow than that of adults per kilogram of body weight, leading to a higher dilution of voriconazole during absorption (for a 20-kg pediatric patient, hepatic extraction is approximately 55%, based on a hepatic plasma flow of 21.1 liters/h and a hepatic CL of 11.6 liters/h); and (iv) the elimination rate, even if not saturated, is sufficiently large that it can account for a hepatic first-pass effect of about 50%.
Logistical factors may also have contributed to this difference in p.o. bioavailability. Control over voriconazole administration relative to eating times during the pediatric studies might have been limited, while adult data were obtained under strict fasted conditions within a controlled phase I environment. Since voriconazole has been shown to display less bioavailability following fed conditions than after fasting (17), albeit with a lower magnitude of reduction than that documented here, study protocols for both adult and pediatric studies specified that p.o. dosing should not occur within 1 h of food consumption. However, given the nature of the patients enrolled in the pediatric studies, it is unlikely that fasting conditions identical to those of a controlled phase I environment were achieved. While mucositis was a relatively frequently observed adverse event in study C (it was not assessed in the other studies), the presence of this condition did not appear to influence voriconazole absorption and p.o. bioavailability.
Parameterization with CL and Km rather than Vmax and Km was chosen for a number of reasons, mainly due to offering more numerical stability when concentrations do not cover values much higher than the Km, similar to what has been published previously for the Emax model (19). In addition, when nonlinearity is not pronounced—such as in this analysis, where average voriconazole concentrations were typically below the Km—it is likely more clinically relevant to obtain parameter values for CL in the linear concentration range rather than those for Vmax. This choice of parameterization also allows retention of an exponential distribution model for CL in the linear range, which is more appropriate for mixed-effect models. Furthermore, when adding a nonlinear element to a linear model, retaining CL as a parameter allows the two models (linear and nonlinear) to be compared as nested models. Finally, with the majority of data below the Km, individual (post hoc) estimates of both Km and Vmax may be less informative than CL values, which can hamper model building and model evaluation efforts.
As in adult patients, voriconazole distribution in the present study was best described by a two-compartment model. The need to use a structural model describing elimination by a nonlinear Michaelis-Menten process was confirmed by the fact that a linear model resulted in a statistically significant OFV increase (P < 0.001). Due to a higher Km in children (3,030 ng/ml for EMs) than in adults (404 ng/ml for EMs), nonlinear elimination became apparent at higher doses for pediatric patients than for adults.
As in adults, elimination capacity in patients of 2 to <12 years of age was correlated with CYP2C19 genotype. However, while in children a difference in CL rates was seen only between the HEM/PM group and the EM group (35.5% reduction), in the adult population different estimates were obtained for PMs, HEMs, and EMs (15). This discrepancy between the adult and pediatric models may be due to the more limited voriconazole plasma concentration data available for pediatric PMs and HEMs. In children, the PM/HEM genotype exhibited a lower RE magnitude than the EM genotype did. A potential explanation for this could lie in the slower voriconazole elimination in the former group, which therefore had flatter concentration-time profiles that were less influenced by any potential inaccuracies in dosing or sampling times.
In addition to CYP2C19 genotype, the other significant pediatric covariates on CL were ALT level and coadministration of CYP2C9 inhibitors. While increased levels of ALT after initiation of voriconazole dosing were associated with lower CL values, the baseline level (i.e., prior to initiation of voriconazole dosing) of this covariate was not predictive of CL.
According to standard model-building criteria, the model that incorporated an influence of CYP2C9 inhibitors but was otherwise identical to the final model would ultimately have been chosen; this model is termed the “alternative final model” in Table 1. However, the main intended use of the final model was pediatric dose selection by simulation of different dosing schedules. Since the CYP2C9 inhibitor status changed for as many as 18 of the 82 patients during the course of therapy and since the difference in individual predictions between the two models was low (i.e., <1%, on average), it was deemed more appropriate to use the model not including a CYP2C9 inhibitor effect in the subsequent deterministic simulations.
These simulations were based on the data from study C (multiple-dose, i.v.-to-p.o.-switch dosing regimen) because this study provided the highest and longest duration of voriconazole exposure of the three studies and because the resultant simulations were interpolations within the observed concentration data. Furthermore, only study C obtained information following p.o. administration. Previous pharmacokinetic analyses of voriconazole pediatric data suggested that doses of 4 mg/kg BID i.v. would achieve median AUC values comparable to those for a reference adult population receiving 3 mg/kg BID i.v. (23). This observation likely reflects the higher elimination capacity in pediatric patients, due to a greater liver-mass-to-body-mass ratio than that of adults.
The present analysis shows that for pediatric patients, i.v. doses of 7 mg/kg BID are required to achieve median AUC values comparable to those of a reference adult population receiving 4 mg/kg BID i.v. (the maintenance dose most commonly used in clinical practice). This conclusion is supported by the simulation outcomes, despite the fact that this particular dose was not actually tested in the three pharmacokinetic studies that formed the basis of the final model. This dose differential between adult and pediatric populations can be explained by the different degrees of nonlinearity in voriconazole pharmacokinetics exhibited by each group. As shown in Fig. 4, 7 mg/kg BID i.v. appears to have the best “compromise” performance not only for the mid-range exposures but also across the entire distribution of exposures. In comparing a dose of 7 mg/kg BID i.v. to doses adjusted individually according to CYP2C19 genotype or age, it appears that such adjustments offer no improvement over the unadjusted dose and can therefore not be justified on the basis of exposure distribution. In addition, initiation of i.v. voriconazole therapy with doses other than 7 mg/kg had negligible outcomes in terms of the time course to attain stable exposure values.
Similarly, p.o. administration on a fixed rather than on a mg/kg of body weight basis minimizes deviations between pediatric and adult AUCs across the entire distribution of exposures. In this case, 200 mg BID p.o. deviated the least from the adult reference population at the median exposure, with relatively high deviation only at the 90th and 95th percentiles. Individual dose adjustments based on CYP2C19 genotype, age, or weight offered no improvement in terms of exposure distribution for a fixed dose of 200 mg BID p.o. Furthermore, initiating voriconazole therapy with p.o. doses other than 200 mg had only negligible effects on the time course required to attain stable exposure values. For p.o. voriconazole administration, the additional consideration of lower bioavailability in children is a further factor in explaining why higher dosages (on a mg/kg basis) are required for pediatric patients to achieve exposures consistent with those in adults following 200 mg voriconazole BID p.o.
The results of this population pharmacokinetic analysis and the subsequent deterministic simulations suggest that voriconazole doses of 7 mg/kg BID i.v. or 200 mg BID p.o. (irrespective of body weight) can be recommended for patients of 2 to <12 years of age for treatment of serious fungal infections. Covariate-based dose adjustments do not appear to offer substantial improvements over unadjusted dosing regimens in achieving exposures similar to those in adults. Similarly, loading doses offer little benefit, as they do not seem to reduce the length of time in which stable exposure values are attained. Voriconazole was in fact recently licensed in the European Union for use in this pediatric population in accordance with the above dosing recommendations. This pharmacokinetic study represents a substantial step forward in determining the most appropriate dosing regimen for voriconazole in children and could be complemented by future clinical studies.
Acknowledgments
This analysis and the underlying pharmacokinetic studies were sponsored by Pfizer Inc. M. Karlsson was a paid consultant to Pfizer Inc. in connection with these analyses and the preparation of the manuscript. Editorial support was provided by D. Wolf at Parexel and was funded by Pfizer Inc.
We thank Thomas J. Walsh (National Cancer Institute, Bethesda, MD) for his contribution to design and interpretation of the three pharmacokinetic studies.
Footnotes
Published ahead of print on 15 December 2008.
REFERENCES
- 1.Aarons, L., J. W. Mandema, and M. Danhof. 1991. A population analysis of the pharmacokinetics and pharmacodynamics of midazolam in the rat. J. Pharmacokinet. Biopharm. 19:485-496. [DOI] [PubMed] [Google Scholar]
- 2.Beal, S. L., and L. B. Sheiner (ed.). 1992. NONMEM users guides I-VIII. NONMEM Project Group, University of California San Francisco (UCSF), San Francisco, CA.
- 3.Carillo, A. J., and J. Guarro. 2001. In vitro activities of four novel triazoles against Scedosporium spp. Antimicrob. Agents Chemother. 45:2151-2153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chandrasekar, P. H., and E. Manavathu. 2001. Voriconazole: a second-generation triazole. Drugs Today 37:135-148. [DOI] [PubMed] [Google Scholar]
- 5.Graves, D. A., and I. Chang. 1990. Application of NONMEM to routine bioavailability data. J. Pharmacokinet. Biopharm. 18:145-160. [DOI] [PubMed] [Google Scholar]
- 6.Groll, A. H., S. C. Piscitelli, and T. J. Walsh. 1998. Clinical pharmacology of systemic antifungal agents: a comprehensive review of agents in clinical use, current investigational compounds, and putative targets for antifungal drug development. Adv. Pharmacol. 44:343-500. [DOI] [PubMed] [Google Scholar]
- 7.Hyland, R., B. C. Jones, and D. A. Smith. 2003. Identification of the cytochrome P450 enzymes involved in the N-oxidation of voriconazole. Drug Metab. Dispos. 31:540-547. [DOI] [PubMed] [Google Scholar]
- 8.Jonsson, E. N., and M. O. Karlsson. 1999. Xpose—an SPLUS based population pharmacokinetic-pharmacodynamic model building aid for NONMEM. Comput. Methods Programs Biomed. 58:51-64. [DOI] [PubMed] [Google Scholar]
- 9.Jonsson, E. N., J. R. Wade, and M. O. Karlsson. 2000. Nonlinearity detection: advantages of nonlinear mixed-effects modeling. AAPS PharmSci 2:E32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Karlsson, M. O., E. N. Jonsson, C. G. Wiltse, and J. R. Wade. 1998. Assumption testing in population pharmacokinetic models: illustrated with an analysis of moxonidine data from congestive heart failure patients. J. Pharmacokinet. Biopharm. 26:207-246. [DOI] [PubMed] [Google Scholar]
- 11.Karlsson, M. O., and L. B. Sheiner. 1993. The importance of modeling interoccasion variability in population pharmacokinetic analyses. J. Pharmacokinet. Biopharm. 21:735-750. [DOI] [PubMed] [Google Scholar]
- 12.Li, R. K., M. A. Ciblak, N. Nordoff, L. Pasarell, and D. W. Warnock. 2000. In vitro activities of voriconazole, itraconazole, and amphotericin B against Blastomyces dermatitidis, Coccidioides immitis, and Histoplasma capsulatum. Antimicrob. Agents Chemother. 44:1734-1736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Marr, K. A., R. A. Carter, F. Crippa, A. Wald, and L. Corey. 2002. Epidemiology and outcome of mould infections in hematopoietic stem cell transplant recipients. Clin. Infect. Dis. 34:909-917. [DOI] [PubMed] [Google Scholar]
- 14.Pfaller, M. A., S. A. Messer, R. J. Hollis, R. N. Jones, G. V. Doern, M. E. Brandt, and R. A. Hajjeh. 1998. In vitro susceptibilities of Candida bloodstream isolates to the new triazole antifungal agents BMS-207147, Sch 56592, and voriconazole. Antimicrob. Agents Chemother. 42:3242-3244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pfizer. 2001. FDA Antiviral Drugs Advisory Committee briefing document for voriconazole (oral and intravenous formulations). FDA, Rockville, MD. http://www.fda.gov/ohrms/dockets/AC/01/briefing/3792b2_01_Pfizer.pdf. Accessed 15 January 2008.
- 16.Purkins, L., N. Wood, P. Ghahramani, K. Greenhalgh, M. J. Allen, and D. Kleinermans. 2002. Pharmacokinetics and safety of voriconazole following intravenous- to oral-dose escalation regimens. Antimicrob. Agents Chemother. 46:2546-2553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Purkins, L., N. Wood, D. Kleinermans, K. Greenhalgh, and D. Nichols. 2003. Effect of food on the pharmacokinetics of multiple-dose oral voriconazole. Br. J. Clin. Pharmacol. 56(Suppl. 1):17-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Purkins, L., N. Wood, K. Greenhalgh, M. J. Allen, and S. D. Oliver. 2003. Voriconazole, a novel wide-spectrum triazole: oral pharmacokinetics and safety. Br. J. Clin. Pharmacol. 56(Suppl. 1):10-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schoemaker, R. C., J. M. van Gerven, and A. F. Cohen. 1998. Estimating potency for the Emax-model without attaining maximal effects. J. Pharmacokinet. Biopharm. 26:581-593. [DOI] [PubMed] [Google Scholar]
- 20.Sheiner, L. B., B. Rosenberg, and V. V. Marathe. 1977. Estimation of population characteristics of pharmacokinetic parameters from routine clinical data. J. Pharmacokinet. Biopharm. 5:445-479. [DOI] [PubMed] [Google Scholar]
- 21.Sheiner, L. B., and S. L. Beal. 1981. Evaluation of methods for estimating population pharmacokinetic parameters. ii. Biexponential model and experimental pharmacokinetic data. J. Pharmacokinet. Biopharm. 9:635-651. [DOI] [PubMed] [Google Scholar]
- 22.Stopher, D. A., and R. Gage. 1997. Determination of a new antifungal agent, voriconazole, by multidimensional high-performance liquid chromatography with direct plasma injection onto a size-exclusion column. J. Chromatogr. B 691:441-448. [DOI] [PubMed] [Google Scholar]
- 23.Walsh, T. J., M. O. Karlsson, T. Driscoll, A. G. Arguedas, P. Adamson, X. Saez-Llorens, A. J. Vora, A. C. Arrieta, J. Blumer, I. Lutsar, P. Milligan, and N. Wood. 2004. Pharmacokinetics and safety of intravenous voriconazole in children after single- or multiple-dose administration. Antimicrob. Agents Chemother. 48:2166-2172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wildfeuer, A., H. P. Seidl, I. Paule, and A. Haberreiter. 1998. In vitro evaluation of voriconazole against clinical isolates of yeasts, moulds and dermatophytes in comparison with itraconazole, ketoconazole, amphotericin B and griseofulvin. Mycoses 41:309-319. [DOI] [PubMed] [Google Scholar]
- 25.Zaoutis, T. E., K. Heydon, J. H. Chu, T. J. Walsh, and W. J. Steinbach. 2006. Epidemiology, outcomes, and costs of invasive aspergillosis in immunocompromised children in the United States, 2000. Pediatrics 117:711-716. [DOI] [PubMed] [Google Scholar]