Abstract
Candida infections represent a major threat in neonatal intensive care units. This is the first prospective study to obtain caspofungin plasma levels and safety data for neonates and very young infants. Patients of <3 months of age receiving intravenous amphotericin B for documented or highly suspected candidiasis were enrolled in a single-dose (n = 6) or subsequent multiple-dose (n = 12) panel; all received caspofungin at 25 mg/m2 once daily as a 1-hour infusion. Caspofungin plasma levels were measured by high-performance liquid chromatography and compared to historical data from adults. Patient chronological ages ranged from 1 to 11 weeks, and weights ranged from 0.68 to 3.8 kg. Gestational ages ranged from 24 to 41 weeks. Geometric mean (GM) peak (C1 h) and trough (C24 h) caspofungin levels were 8.2 and 1.8 μg/ml, respectively, on day 1, and 11.1 and 2.4 μg/ml, respectively, on day 4. GM ratios for C1 h and C24 h for neonates/infants relative to adults receiving caspofungin at 50 mg/day were 1.07 and 1.36, respectively, on day 1, and 1.18 and 1.21, respectively, on day 4. Clinical and laboratory adverse events occurred in 17 (94%) and 8 (44%) patients, respectively. Five patients (28%) had serious adverse events, none of which were considered drug related. Caspofungin at 25 mg/m2 once daily was well tolerated in this group of neonates/infants of <3 months of age and appears to provide relatively similar plasma exposure to that obtained in adults receiving 50 mg/day. However, the small number of patients studied precludes any definitive recommendations about caspofungin dosing for this group comprising a broad range of ages and weights.
Candida infections are a major concern in neonatal intensive care units, especially for infants with very low birth weights (<1,500 g). Late-onset sepsis develops in approximately 20% of critically ill neonates and low-birth-weight infants, and Candida species account for at least 10% of these infections (16). Antifungal regimens currently used in neonates are associated with increasing fungal resistance, notable toxicity, drug-drug interactions, or a limited spectrum of activity (4). Hence, there is a medical need to pursue the use of newer antifungal agents for the neonatal population.
Caspofungin is an echinocandin antifungal that is effective as primary therapy for esophageal candidiasis (1, 6, 19), candidemia (8), and other invasive Candida infections (5) in adults. In a pharmacokinetic study of 39 children and adolescents (2 to 17 years of age), a caspofungin dose of 50 mg/m2 daily (based on body surface area [BSA] dosing) resulted in plasma concentrations commensurate with the 50-mg daily dose used in adults (20). Data for younger children (3 to 24 months of age) who received caspofungin at 50 mg/m2 daily (11) show pharmacokinetic results similar to those seen for the 2- to 11-year-old cohort (20). Several reports suggest that caspofungin may be useful for the treatment of candidemia in neonates (10, 12, 15, 21); however, the appropriate dosage for this population has not been clearly defined. We conducted a prospective study to evaluate the safety, tolerability, and plasma levels of caspofungin in neonates/infants of <3 months of age given 25 mg/m2 daily.
MATERIALS AND METHODS
This was a multicenter, sequential-panel, open-label, noncomparative study conducted from July 2006 through October 2006 at eight investigator sites in the United States, South America, and India. The protocol was approved by the ethical review committee or institutional review board at each study site, and written informed consent was obtained from the legal guardian of each patient before any study procedures were performed. The protocol is registered at www.Clinicaltrials.gov (NCT00330395).
Study population.
Eligible patients were less than 3 months of age, weighed at least 500 g, and had a documented (culture confirmed) or highly suspected invasive Candida infection. Documented Candida infection was defined as the presence of at least one positive culture of Candida species from blood or another normally sterile, invasive body site which had been obtained within 4 days of study entry, either with or without the presence of clinical symptoms. Candida infection was highly suspected if (i) a patient had one or more Candida risk factors, (ii) a patient manifested one or more clinical symptoms at the time of study entry, and (iii) a bacterial etiology had been ruled out and antifungal therapy was being considered. Patients were excluded if they were hemodynamically unstable or if they were receiving rifampin, cyclosporine, phenytoin, carbamazepine, phenobarbital, an azole, or an echinocandin other than caspofungin. Patients were also ineligible if they had acute hepatitis or cirrhosis, documented human immunodeficiency virus infection, a hematocrit of <35% at screening (for patients with a body weight of ≤1,500 g), or elevated transaminases at study entry (aspartate transaminase [AST] or alanine aminotransferase [ALT] level of >2 times the upper limit of normal for that age).
Study design.
Patients were enrolled in one of two sequential panels, either panel A (single dose) or panel B (multiple dose). In panel A, patients received a single dose of caspofungin at 25 mg/m2 on day 1. In panel B, patients received caspofungin at 25 mg/m2 daily for at least 4 days and up to 28 days. Caspofungin was given as a single daily dose infused over a 1-hour period. All patients were also required to receive an intravenous amphotericin B formulation (amphotericin B deoxycholate or a lipid preparation of amphotericin B) at the time of study entry and to remain on the amphotericin B formulation for the duration of caspofungin therapy. Potential lipid preparations of amphotericin B included amphotericin B lipid complex (Abelcet; Enzon Pharmaceuticals), liposomal amphotericin B (Ambisome; Astellas Pharmaceuticals), and amphotericin B colloidal dispersion (Amphotec; Three Rivers Pharmaceuticals). Caspofungin monotherapy was not permitted. The dose chosen for this study was based on clinical experience with caspofungin as a salvage treatment for neonates and premature infants with invasive candidiasis, which suggested that caspofungin at 25 mg/m2 once daily would result in caspofungin exposures commensurate with those seen in adults receiving caspofungin at 50 mg/day (12).
Pharmacokinetic and safety measurements.
For the single-dose panel (panel A), blood for plasma sampling was collected predose (at screening), at 1 h post-caspofungin infusion (C1 h), and at 24 h post-caspofungin infusion (C24 h). For the multiple-dose panel (panel B), blood for plasma sampling was collected predose (at screening), at 1 h and 24 h post-caspofungin infusion on day 1, and at 1 h and 24 h post-caspofungin infusion on day 4. Assessment of the area under the curve (AUC) for plasma concentration versus time was not possible due to the small volume of blood available for collection in neonates/infants of <3 months of age. If a patient was to undergo any procedure that involved collecting cerebrospinal fluid (CSF) while the patient was on study therapy, an approximately 350-μl specimen of CSF was retained for measurement of caspofungin concentration.
Safety was evaluated through physical examination, vital signs, weight, serum chemistry, complete blood count, and urinalysis. Investigators identified the seriousness, causality, and action taken on caspofungin study therapy for all clinical and laboratory adverse events during the treatment period and for 14 days following treatment. Adverse events considered by the site investigator to be definitely, probably, or possibly related to study therapy were classified as drug related. All patients who received at least one dose of caspofungin were included in the safety analyses.
Bioanalytical and pharmacokinetic methods.
BSA regimens were calculated based on patients' heights and weights at the time of enrollment, using the Mosteller formula (9), as follows: BSA (m2) = {(height [cm] × weight [kg])/3,600}1/2. Plasma concentrations of caspofungin were determined at Merck Research Laboratories by high-pressure liquid chromatography with fluorescence detection as previously described (3, 14). The limit of quantitation was 125 ng/ml.
All available plasma levels collected on days 1 and 4 were included in the analyses. One subject was excluded from the day 4 trough concentration analysis because a day 4 trough sample was not collected. For the same subject, the day 4 peak sample was collected 4 min after the start of the infusion; this sample was used in the day 4 peak concentration analysis. Day 5 peak and trough samples were collected for this patient but were not included in the pharmacokinetic analysis. Given that the concentration values for both the day 4 4-min sample and the day 5 peak sample for this subject were within the range of day 4 peak concentration values observed for other subjects, it is expected that exclusion of the day 4 4-min concentration or replacement of the day 4 4-min concentration with the day 5 peak concentration in the pharmacokinetic analysis would not meaningfully change the results of this study.
Data from panel A and panel B were pooled for the statistical analysis and were compared with historical data from adults who received multiple doses of caspofungin at 50 mg daily for the treatment of esophageal/oropharyngeal candidiasis (1, 6, 19). In order to reduce the available multiple-dose pharmacokinetic data to a single-parameter set and to allow comparisons between pediatric patients and adults, for whom sampling on identical study days was not available, day 3 to 14 time-averaged values for C1 h and C24 h (in adults) were determined as the geometric means of all values for each individual parameter obtained during this time interval. If only one value was available during this interval, that value was used as the mean. Although true steady-state pharmacokinetics is not achieved until 2 to 3 weeks of dosing, much caspofungin accumulation occurs in the first few days of dosing (17). Thus, these day 3 to 14 time-averaged parameters are expected to be reasonably representative of steady state.
Statistical analyses.
Plasma caspofungin C24 h and C1 h were natural log transformed and analyzed using a mixed-effects model with fixed terms for age group, day, and age-by-day interaction and with subject as a random effect. Two-sided 90% confidence intervals (CIs) for the true arithmetic mean differences on day 1 and day 4 (values for neonates/infants receiving 25 mg/m2 minus values for adults receiving 50 mg) were calculated using the appropriate components of variance from the aforementioned model and referencing a t distribution. These limits were then subjected to exponentiation to obtain CIs for the true geometric mean ratios (GMRs) on day 1 and day 4. Assuming a true between-subject variance of 0.1591 for ln C24 h on day 1 and day 4 and a true between-subject variance of 0.0597 for ln C1 h on day 1 and day 4, the probabilities that the 90% CIs for the true GMRs (those for neonates/infants receiving 25 mg/m2 to those for adults receiving 50 mg) would lie within the bounds of 0.70 to 1.50 were 0.84, 0.81, 0.99, and 0.99 for day 1 C24 h, day 4 C24 h, day 1 C1 h, and day 4 C1 h, respectively, given that the true GMR was 1.0. The interval 0.7 to 1.5 was used previously in the caspofungin development program to define a clinically significant alteration in caspofungin pharmacokinetic parameters. The lower bound of 0.7 is based on data from a population pharmacokinetic analysis involving patients with esophageal or oropharyngeal candidiasis. In this analysis, there was a suggested association of reduced efficacy against Candida infections with reductions in caspofungin AUC or trough (C24 h) concentrations of >30%. No association between an increased risk of a dose-limiting adverse event or laboratory abnormality and concentration increases of >50% has been established for this program. Rather, the 1.5-fold limit for the confidence interval was obtained from the ratio of the mean plasma concentrations at a higher adult dose, studied with multiple dosing, than the recommended adult dose (i.e., 70 mg daily relative to 50 mg daily), which extensive clinical experience in adults suggests is well tolerated.
The caspofungin plasma levels (C24 h and C1 h) obtained for neonates/infants in this study were also compared with plasma levels in adults receiving caspofungin at 50 mg/day (after a 70-mg loading dose on day 1) for the treatment of invasive candidiasis (8), in 2- to 11-year-old children given caspofungin at 50 mg/m2/day (20), in 12- to 17-year-old adolescents given caspofungin at 50 mg/m2/day (20), and in 3- to 24-month-old children given caspofungin at 50 mg/m2/day (11). For each analysis, C24 h and C1 h were analyzed (separately) in a similar fashion and the 90% CIs for the true GMRs were calculated as described above.
RESULTS
Demographics and baseline characteristics.
Eighteen patients were enrolled in the study and received caspofungin therapy: 6 were enrolled in the single-dose panel (panel A) and 12 were enrolled in the multiple-dose panel (panel B). The majority (72%) of patients had a gestational age of ≤36 weeks at birth (Table 1). BSA ranged from 0.0758 to 0.2365 m2 (mean, 0.1529 m2). The mean duration of caspofungin therapy for patients enrolled in panel B was 8.7 days (range, 4 to 36 days; median, 5 days). The majority of patients (9/12 patients) in panel B received between 4 and 7 days of treatment; only 2 patients received caspofungin for more than 14 days.
TABLE 1.
Baseline patient characteristics
| Characteristic | No. (%) of patients with characteristic
|
||
|---|---|---|---|
| Single dose of caspofungin (25 mg/m2) (n = 6) | Multiple doses of caspofungin (25 mg/m2) (n = 12) | Total (n = 18) | |
| Gender | |||
| Male | 3 (50.0) | 9 (75.0) | 12 (66.7) |
| Female | 3 (50.0) | 3 (25.0) | 6 (33.3) |
| Race | |||
| American Indian or Alaska Native | 0 (0.0) | 1 (8.3) | 1 (5.6) |
| Asian | 2 (33.3) | 2 (16.7) | 4 (22.2) |
| Black, of African heritage | 0 (0.0) | 1 (8.3) | 1 (5.6) |
| Multiracial | 4 (66.7) | 8 (66.7) | 12 (66.7) |
| Gestational age at birth (wk) | |||
| <28 | 1 (16.7) | 0 (0.0) | 1 (5.6) |
| 28 to 31 | 4 (66.7) | 4 (33.3) | 8 (44.4) |
| 32 to 36 | 1 (16.7) | 3 (25.0) | 4 (22.2) |
| >36 | 0 (0.0) | 5 (41.7) | 5 (27.8) |
| Chronological age (wk) | |||
| ≤2 | 3 (50.0) | 2 (16.7) | 5 (27.8) |
| 3 to 4 | 0 (0.0) | 7 (58.3) | 7 (38.9) |
| 5 to 8 | 3 (50.0) | 1 (8.3) | 4 (22.2) |
| 9 to 12 | 0 (0.0) | 2 (16.7) | 2 (11.1) |
| Mean | 3.8 | 4.9 | 4.6 |
| Median | 3.5 | 4.0 | 4.0 |
| Range | 1-7 | 2-11 | 1-11 |
| Weight at enrollment (kg) | |||
| ≤1 | 2 (33.3) | 2 (16.7) | 4 (22.2) |
| 1.01 to 1.50 | 1 (16.7) | 2 (16.7) | 3 (16.7) |
| 1.51 to 2.00 | 1 (16.7) | 1 (8.3) | 2 (11.1) |
| 2.01 to 2.50 | 1 (16.7) | 2 (16.7) | 3 (16.7) |
| >2.50 | 1 (16.7) | 5 (41.7) | 6 (33.3) |
| Invasive Candida infection | |||
| Documented | 2 (33.3) | 6 (50.0) | 8 (44.4) |
| Highly suspected | 4 (66.7) | 6 (50.0) | 10 (55.6) |
Caspofungin pharmacokinetics.
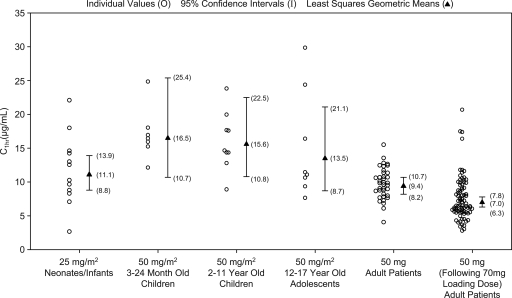
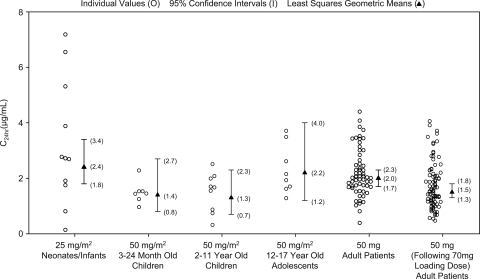
On day 1 and day 4, caspofungin peak concentrations (C1 h) for the neonates/infants were similar to those for adults with esophageal or oropharyngeal candidiasis, while caspofungin trough concentrations (C24 h) were slightly elevated in neonates/infants relative to those in adults (Table 2). Since the neonates/infants in this study had documented or highly suspected invasive candidiasis, a comparison to adults with invasive candidiasis was also conducted. On day 4, the peak and trough caspofungin levels were moderately elevated in neonates/infants relative to those in adults with invasive candidiasis (Table 2).
TABLE 2.
Caspofungin plasma concentrations in neonates/infants of <3 months of age and adults with esophageal/oropharyngeal candidiasis or invasive candidiasis
| Disease, day, and parameter | Neonates and infants
|
Historical adult patients
|
Ratio (neonates and infants/adults)
|
|||||
|---|---|---|---|---|---|---|---|---|
| n | GMa | 95% CI | n | GMa | 95% CI | GMR | 90% CI | |
| Esophageal/oropharyngeal candidiasis | ||||||||
| Day 1 | ||||||||
| C1 h | 18 | 8.2 | 6.8, 10.0 | 38 | 7.7 | 6.7, 8.7 | 1.07 | 0.89, 1.30 |
| C24 h | 18 | 1.8 | 1.4, 2.4 | 33 | 1.3 | 1.1, 1.6 | 1.36 | 1.04, 1.78 |
| Day 4b | ||||||||
| C1 h | 12 | 11.1 | 8.8, 13.9 | 38 | 9.4 | 8.2, 10.7 | 1.18 | 0.95, 1.46 |
| C24 h | 11 | 2.4 | 1.8, 3.4 | 60 | 2.0 | 1.7, 2.3 | 1.21 | 0.90, 1.63 |
| Invasive candidiasis | ||||||||
| Day 4b | ||||||||
| C1 h | 12 | 10.9 | 8.2, 14.6 | 86 | 7.0 | 6.3, 7.8 | 1.56 | 1.21, 2.01 |
| C24 h | 11 | 2.3 | 1.6, 3.5 | 90 | 1.5 | 1.3, 1.8 | 1.53 | 1.09, 2.17 |
Geometric mean, based on least-squares mean from a mixed-effect model performed on natural-log-transformed values.
For the adult patients, day 4 values were calculated by time averaging data for days 3 to 14.
Compared to children of 2 to 11 years of age, adolescents of 12 to 17 years of age, and young children of 3 to 24 months of age who received caspofungin at 50 mg/m2/day, the caspofungin peak concentrations in neonates/infants were somewhat reduced on days 1 and 4 (Table 3). Conversely, trough concentrations in the neonates/infants at doses of 25 mg/m2/day were somewhat increased on both day 1 and day 4 relative to the trough concentrations achieved in older pediatric populations at doses of 50 mg/m2/day.
TABLE 3.
Caspofungin plasma concentrations in neonates/infants of <3 months of age compared with those in children of 2 to 11 years of age, adolescents of 12 to 17 years of age, and children of 3 to 24 months of age
| Day and parameter | Ratio (neonates and infants/2- to 11-year-old children)
|
Ratio (neonates and infants/12- to 17-year-old adolescents)
|
Ratio (neonates and infants/3- to 24-month-old children)
|
|||
|---|---|---|---|---|---|---|
| GMRa | 90% CI | GMRa | 90% CI | GMRa | 90% CI | |
| Day 1 | ||||||
| C1 h | 0.59 | 0.41, 0.85 | 0.92 | 0.59, 1.43 | 0.48 | 0.32, 0.72 |
| C24 h | 1.77 | 1.00, 3.13 | 1.49 | 0.79, 2.81 | 1.37 | 0.72, 2.62 |
| Day 4 | ||||||
| C1 h | 0.71 | 0.48, 1.06 | 0.83 | 0.52, 1.32 | 0.67 | 0.43, 1.04 |
| C24 h | 1.85 | 1.00, 3.43 | 1.11 | 0.58, 2.14 | 1.67 | 0.86, 3.24 |
Based on least-squares means from ANOVA performed on natural-log-transformed values.
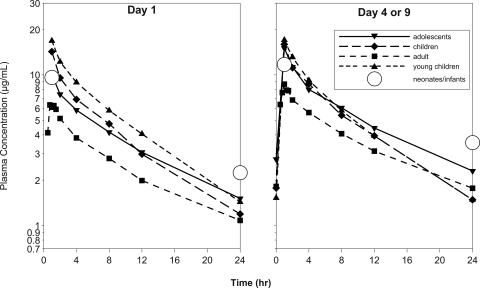
Peak and trough caspofungin levels on day 4 for the various patient age groups studied to date, including data from the prior pediatric pharmacokinetic studies (11, 20), are shown in Fig. 1 and 2. The plasma exposure, clearance, and terminal half-life for neonates/infants could not be calculated in the current study due to the limited sampling available from each patient. In the absence of these data, comparison of the peak and trough concentrations for neonates/infants to historical mean caspofungin concentration-time profiles for older pediatric patients and adults can provide some insight into how caspofungin pharmacokinetics in neonates/infants may compare to other historical patient populations (Fig. 3).
FIG. 1.
Day 4 C1 h (peak) caspofungin levels in neonates and infants (< 3 months of age), young children (3 to 24 months), older children (2 to 11 years), adolescents (12 to 17 years), and adults. Geometric means and corresponding 95% CI for all groups were generated from an analysis of variance (ANOVA) model with the neonates/infants and each particular group. The geometric mean and corresponding 95% CI for the neonate/infant group were calculated from the ANOVA model addressing the primary comparison with adults. The first column of adult data (50 mg daily) is for patients with esophageal and/or oropharyngeal candidiasis, and the second column of adult data (50 mg daily following 70 mg on day 1) is for patients with invasive candidiasis.
FIG. 2.
Day 4 C24 h (trough) caspofungin levels in neonates and infants (<3 months of age), young children (3 to 24 months), older children (2 to 11 years), adolescents (12 to 17 years), and adults. Geometric means and corresponding 95% CI for all groups were generated from an ANOVA model with the neonates/infants and each particular group. The geometric mean and corresponding 95% CI for the neonate/infant group were calculated from the ANOVA model addressing the primary comparison with adults. The first column of adult data (50 mg daily) is for patients with esophageal and/or oropharyngeal candidiasis, and the second column of adult data (50 mg daily following 70 mg on day 1) is for patients with invasive candidiasis.
FIG. 3.
Comparison of mean caspofungin plasma concentrations in neonates and infants (25 mg/m2; n = 18 on day 1 and n = 11 or 12 on day 4) to mean caspofungin plasma concentrations in adults (50 mg/day; n = 6), young children (3 to 24 months) (50 mg/m2; n = 6 on day 1 and n = 7 on day 4), older children (2 to 11 years) (50 mg/m2; n = 8), and adolescents (12 to 17 years) (50 mg/m2; n = 10).
A CSF sample was available for one patient in this study. The caspofungin concentration in this sample, collected on day 1, was below the quantifiable range (limit of quantification = 10 ng/ml). Caspofungin plasma concentrations in this patient were 10.3 and 1.8 μg/ml at peak and trough, respectively.
Safety.
One or more clinical adverse events were reported for 17 patients (94.4%) during caspofungin therapy or in the 14-day follow-up period (Table 4). The most common adverse events, noted in three patients (16.7%) each, were pyrexia, hyperventilation, and hypertension. Serious clinical adverse events were reported for five patients (27.8%), and three of these patients died. Caspofungin study therapy was discontinued in one patient (in the multiple-dose panel), due to worsening intestinal perforation, necrotizing enterocolitis, and sepsis. None of the clinical adverse events were considered by the investigator to be related to caspofungin. Eight patients (44.4%) had one or more laboratory adverse events (Table 4). The most common laboratory adverse event was decreased hemoglobin (16.7%). Three patients had additional laboratory tests not required by the protocol; increased alkaline phosphatase and lactate dehydrogenase were noted in all three patients, along with increased gamma glutamyltransferase in two and increased ALT and AST levels in two. None of the laboratory adverse events were serious or drug related or caused the patient to discontinue caspofungin therapy.
TABLE 4.
Clinical and laboratory adverse eventsa
| Event | No. (%) of patients with event
|
||
|---|---|---|---|
| Single-dose group (n = 6) | Multiple-dose group (n = 12) | Total (n = 18) | |
| Clinical adverse events | 6 (100) | 11 (91.7) | 17 (94.4) |
| Sinus bradycardia | 0 (0.0) | 2 (16.7) | 2 (11.1) |
| Sinus tachycardia | 0 (0.0) | 2 (16.7) | 2 (11.1) |
| Pyrexia | 0 (0.0) | 3 (25.0) | 3 (16.7) |
| Bacterial conjunctivitis | 1 (16.7) | 1 (8.3) | 2 (11.1) |
| Bronchopulmonary dysplasia | 1 (16.7) | 1 (8.3) | 2 (11.1) |
| Hyperventilation | 0 (0.0) | 3 (25.0) | 3 (16.7) |
| Pulmonary congestion | 1 (16.7) | 1 (8.3) | 2 (11.1) |
| Hypertension | 1 (16.7) | 2 (16.7) | 3 (16.7) |
| Laboratory adverse events | 0 (0.0) | 8 (66.7) | 8 (44.4) |
| Increased ALT level | 0 (0.0) | 2 (16.7) | 2 (11.1) |
| Increased aspartate aminotransferase level | 0 (0.0) | 2 (16.7) | 2 (11.1) |
| Increased absolute neutrophil count | 0 (0.0) | 2 (16.7) | 2 (11.1) |
| Decreased hematocrit | 0 (0.0) | 2 (16.7) | 2 (11.1) |
| Decreased hemoglobin | 0 (0.0) | 3 (25.0) | 3 (16.7) |
| Decreased platelet count | 0 (0.0) | 2 (16.7) | 2 (11.1) |
| Increased white blood cell count | 0 (0.0) | 2 (16.7) | 2 (11.1) |
All events that occurred in at least two patients in either treatment group are shown.
DISCUSSION
This trial is the first prospective study to evaluate the plasma levels, safety, and tolerability of single and multiple once-daily doses of caspofungin in neonates/infants (<3 months of age). Due to the plasma volume needed for caspofungin analysis and limitations in the blood volume available for collection in neonates/infants, plasma samples in the current study were limited to peak (end of infusion; C1 h) and trough (C24 h) collections on days 1 and 4. The comparison of peak and trough concentrations for neonates/infants to those for other populations utilized the 90% CI of the GMR estimates compared to predefined clinical significance boundaries of 0.7 and 1.5. Preclinical studies involving in vivo pharmacokinetic/pharmacodynamic analyses suggested that the relevant pharmacokinetic parameter for efficacy may be AUC or trough concentration. Thus, the C24 h comparisons of data from neonates/infants to those for other populations are particularly important for efficacy, while the C1 h comparisons may be more pertinent to safety.
In the current study, caspofungin plasma levels in neonates/infants receiving either single or multiple (once daily) doses at 25 mg/m2 were generally comparable to those in adults receiving caspofungin at 50 mg/day for the treatment of esophageal and/or oropharyngeal candidiasis. Appreciable differences in peak concentrations of caspofungin were not evident on day 1 or 4 relative to the adult data. Slight elevations in trough concentrations of caspofungin were noted for the neonates/infants but were not associated with any concerning safety findings. The caspofungin concentrations achieved in neonates/infants were within the range of clinical experience previously seen for adult patients who received multiple doses of caspofungin of up to 100 mg/day. Collectively, the data suggest that the caspofungin peak-to-trough concentration ratio is smaller in neonates/infants given 25 mg/m2/day than that in adults given 50 mg/day.
Compared to adult patients with invasive candidiasis (who received a loading dose of 70 mg followed by 50 mg/day), modest elevations were found in both the peak and trough caspofungin concentrations on day 4 in the neonates/infants; however, these elevations were within the range of clinical adult experience for caspofungin doses of 70 and 100 mg. Compared with older pediatric patients who received caspofungin at 50 mg/m2/day, caspofungin peak concentrations were somewhat reduced while trough concentrations were somewhat increased, resulting in a decreased peak-to-trough ratio for neonates/infants who received 25 mg/m2/day.
Results from a previous pharmacokinetic study (20) suggested that the caspofungin half-life is shorter in children (2 to 11 years of age) than in adults. On a BSA basis, the caspofungin dose administered to neonates/infants in this study (25 mg/m2 daily) is slightly less than the dose administered to the historical adult group (∼30 mg/m2). Although calculations of plasma exposure in neonates/infants were not possible in this study, the data suggest that the plasma exposure in neonates/infants may be somewhat similar to that in adults, indicating slightly reduced clearance in neonates/infants relative to that in adults (on a BSA basis). The caspofungin doses administered to young children (3 to 24 months of age), older children (2 to 11 years of age), and adolescents (12 to 17 years of age) were twice the caspofungin dose given to the neonates/infants (on a BSA basis), and the data suggest that caspofungin plasma exposure in neonates/infants is similar to that in children, older children, and adolescents. Thus, the data are consistent with reduced clearance in neonates/infants relative to that achieved in the older pediatric populations.
Furthermore, the data suggest that caspofungin clearance increases from infancy to childhood and decreases from childhood to adolescence and subsequently to adulthood. The mechanism(s) for possible changes in plasma clearance with age is unknown. In contrast to many drugs, neither metabolism nor excretion is the rate-controlling step that determines the clearance of caspofungin from plasma in adults (18). Rather, plasma clearance is determined primarily by the rate of distribution of caspofungin from plasma into hepatocytes and possibly other tissue cells. In vitro data suggest that caspofungin tissue distribution may be mediated by uptake transporters and that the OATP1B1 transporter may be involved in the hepatic uptake of caspofungin (13). Thus, one potential explanation is that the expression levels of an uptake transporter or transporters for which caspofungin is a substrate could be altered throughout the course of developmental maturity. Another possibility is that differences in caspofungin clearance with age could be due to differences in physiological factors, such as relative blood flow rates and organ sizes.
Central nervous system involvement is of particular concern in neonates and premature infants with disseminated candidiasis (2, 7). In the current study, only one CSF sample was available for analysis of caspofungin levels. Thus, it is not possible to determine from this study the caspofungin CSF levels achievable in this age group with a dose of 25 mg/m2.
Clinical adverse events were reported for the majority (17/18 patients) of patients, and laboratory adverse events occurred in 8/18 patients. The frequency of adverse events in this study was anticipated, given the fragile nature of this particular patient population, and the adverse events reported are consistent with prematurity and/or other birth complications. Importantly, none of the clinical or laboratory adverse events reported in this study were considered by the investigators to be related to caspofungin therapy.
This was the first prospective study of caspofungin in neonates and very young infants. Although the small number of patients studied precludes making any definitive recommendations about caspofungin dosing in this group, who comprise a broad range of ages and weights, the results suggest that in this population, a caspofungin dose of 25 mg/m2 once daily provides plasma concentrations fairly similar to those observed in adults receiving 50 mg/day. Trough concentrations in the neonatal population receiving caspofungin were slightly elevated relative to those in adult patients and older pediatric patients, but these levels were not associated with any adverse safety outcomes. Caspofungin at 25 mg/m2 daily was generally well tolerated in the neonates/infants in this study.
Acknowledgments
Funding for this study was provided by Merck & Co., Inc. X.S.-L., M.M., P.M., J.P., H.S.J., A.C., G.R., and J.R. received grant support from Merck for conducting this study. The remaining authors are current or former employees of Merck Research Laboratories and may own stock and/or stock options in the company.
In addition to the authors, the following investigators participated in this study: M. T. Moreno, E. Nelson, and M. Jaramillo.
Footnotes
Published ahead of print on 15 December 2008.
REFERENCES
- 1.Arathoon, E. G., E. Gotuzzo, L. M. Noriega, R. S. Berman, M. J. DiNubile, and C. A. Sable. 2002. Randomized, double-blind, multicenter study of caspofungin versus amphotericin B for treatment of oropharyngeal and esophageal candidiases. Antimicrob. Agents Chemother. 46:451-457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Benjamin, D. K., Jr., B. J. Stoll, A. A. Fanaroff, S. A. McDonald, W. Oh, R. D. Higgins, S. Duara, K. Poole, A. Laptook, and R. Goldberg. 2006. Neonatal candidiasis among extremely low birthweight infants: risk factors, mortality rates, and neurodevelopmental outcomes at 18 and 22 months. Pediatrics 117:84-92. [DOI] [PubMed] [Google Scholar]
- 3.Bi, S., M. S. Schwartz, R. B. Desai, A. R. Miller, and B. K. Matuszewski. 2005. A semi-automated procedure for the determination of caspofungin in human plasma using solid-phase extraction and HPLC with fluorescence detection using secondary ionic interactions to obtain a highly purified extract. J. Liq Chromatogr. Relat. Technol. 28:2895-2908. [Google Scholar]
- 4.Bliss, J. M., M. Wellington, and F. Gigliotti. 2003. Antifungal pharmacotherapy for neonatal candidiasis. Semin. Perinatol. 27:365-374. [DOI] [PubMed] [Google Scholar]
- 5.Cornely, O., M. Lasso, R. Betts, N. Klimko, J. Vazquez, G. Dobb, J. Velez, A. Williams-Diaz, J. Lipka, A. Taylor, C. Sable, and N. Kartsonis. 2007. Caspofungin for the treatment of less common forms of invasive candidiasis. J. Antimicrob. Chemother. 60:363-369. [DOI] [PubMed] [Google Scholar]
- 6.DiNubile, M. J., R. J. Lupinacci, R. S. Berman, and C. A. Sable. 2002. Response and relapse rates of candidal esophagitis in HIV-infected patients treated with caspofungin. AIDS Res. Hum. Retrovir. 18:903-908. [DOI] [PubMed] [Google Scholar]
- 7.Fernandez, M., E. H. Moylett, D. E. Noyola, and C. J. Baker. 2000. Candidal meningitis in neonates: a 10-year review. Clin. Infect. Dis. 31:458-463. [DOI] [PubMed] [Google Scholar]
- 8.Mora-Duarte, J., R. Betts, C. Rotstein, A. L. Colombo, L. Thompson-Moya, J. Smietana, R. Lupinacci, C. Sable, N. Kartsonis, and J. Perfect. 2002. Comparison of caspofungin and amphotericin B for invasive candidiasis. N. Engl. J. Med. 347:2020-2029. [DOI] [PubMed] [Google Scholar]
- 9.Mosteller, R. D. 1987. Simplified calculation of body surface area. N. Engl. J. Med. 317:1098. [DOI] [PubMed] [Google Scholar]
- 10.Natarajan, G., M. Lulic-Botica, C. Rongkavilit, A. Pappas, and M. Bedard. 2005. Experience with caspofungin in the treatment of persistent fungemia in neonates. J. Perinatol. 25:770-777. [DOI] [PubMed] [Google Scholar]
- 11.Neely, M., H. S. Jafri, N. Seibel, K. Knapp, P. C. Adamson, S. K. Bradshaw, K. M. Strohmaier, P. Sun, S. Bi, M. F. Dockendorf, J. A. Stone, and N. A. Kartsonis. Pharmacokinetics and safety of caspofungin in older infants and taddlers. Antimicrob. Agents Chemother., in press. [DOI] [PMC free article] [PubMed]
- 12.Odio, C. M., R. Araya, L. E. Pinto, C. I. Castro, S. Vasquez, B. Alfaro, A. Saenz, M. L. Herrera, and T. J. Walsh. 2004. Caspofungin therapy of neonates with invasive candidiasis. Pediatr. Infect. Dis. J. 23:1093-1097. [PubMed] [Google Scholar]
- 13.Sandhu, P., W. Lee, X. Xu, B. F. Leake, M. Yamazaki, J. A. Stone, J. H. Lin, P. G. Pearson, and R. B. Kim. 2005. Hepatic uptake of the novel antifungal agent caspofungin. Drug Metab. Dispos. 33:676-682. [DOI] [PubMed] [Google Scholar]
- 14.Schwartz, M., W. Kline, and B. Matuszewski. 1997. Determination of a cyclic hexapeptide (L-743 872), a novel pneumocandin antifungal agent in human plasma and urine by high-performance liquid chromatography with fluorescence detection. Anal. Chim. Acta 352:299-307. [Google Scholar]
- 15.Smith, P. B., W. J. Steinbach, C. M. Cotten, W. A. Schell, J. A. Perfect, T. J. Walsh, and D. K. Benjamin, Jr. 2007. Caspofungin for the treatment of azole resistant candidemia in a premature infant. J. Perinatol. 27:127-129. [DOI] [PubMed] [Google Scholar]
- 16.Stoll, B. J., and N. Hansen. 2003. Infections in VLBW infants: studies from the NICHD Neonatal Research Network. Semin. Perinatol. 27:293-301. [DOI] [PubMed] [Google Scholar]
- 17.Stone, J. A., S. D. Holland, P. J. Wickersham, A. Sterrett, M. Schwartz, C. Bonfiglio, M. Hesney, G. A. Winchell, P. J. Deutsch, H. Greenberg, T. L. Hunt, and S. A. Waldman. 2002. Single- and multiple-dose pharmacokinetics of caspofungin in healthy men. Antimicrob. Agents Chemother. 46:739-745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stone, J. A., X. Xu, G. A. Winchell, P. J. Deutsch, P. G. Pearson, E. M. Migoya, G. C. Mistry, L. Xi, A. Miller, P. Sandhu, R. Singh, F. deLuna, S. C. Dilzer, and K. C. Lasseter. 2004. Disposition of caspofungin: role of distribution in determining plasma pharmacokinetics. Antimicrob. Agents Chemother. 48:815-823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Villaneuva, A., E. G. Arathoon, E. Gotuzzo, R. S. Berman, M. J. DiNubile, and C. A. Sable. 2001. A randomized double-blind study of caspofungin versus amphotericin for the treatment of candidal esophagitis. Clin. Infect. Dis. 33:1529-1535. [DOI] [PubMed] [Google Scholar]
- 20.Walsh, T. J., P. C. Adamson, N. L. Seibel, P. M. Flynn, M. N. Neely, C. Schwartz, A. Shad, S. L. Kaplan, M. M. Roden, J. A. Stone, A. Miller, S. K. Bradshaw, S. X. Li, C. A. Sable, and N. A. Kartsonis. 2005. Pharmacokinetics, safety, and tolerability of caspofungin in children and adolescents. Antimicrob. Agents Chemother. 49:4536-4545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yalaz, M., M. Akisu, S. Hilmioglu, S. Calkavur, B. Cakmak, and N. Kultursay. 2006. Successful caspofungin treatment of multidrug resistant Candida parapsilosis septicaemia in an extremely low birth weight neonate. Mycoses 49:242-245. [DOI] [PubMed] [Google Scholar]