Abstract
Owing to the lack of oral drugs for human African trypanosomiasis, patients have to be hospitalized for 10 to 30 days to facilitate treatment with parenterally administered medicines. The efficacy of a novel orally administered prodrug, 2,5-bis(4-amidinophenyl)-furan-bis-O-methlylamidoxime (pafuramidine, DB289), was tested in the vervet monkey (Chlorocebus [Cercopithecus] aethiops) model of sleeping sickness. Five groups of three animals each were infected intravenously with 104 Trypanosoma brucei rhodesiense KETRI 2537 cells. On the seventh day postinfection (p.i.) in an early-stage infection, animals in groups 1, 2, and 3 were treated orally with pafuramidine at dose rates of 1, 3, or 10 mg/kg of body weight, respectively, for five consecutive days. The animals in groups 4 and 5 were treated with 10 mg/kg for 10 consecutive days starting on the 14th day p.i. (group 4) or on the 28th day p.i. (group 5), when these animals were in the late stage of the disease. In the groups treated in the early stage, 10 mg/kg of pafuramidine completely cured all three monkeys, whereas lower doses of 3 mg/kg and 1 mg/kg cured only one of three and zero of three monkeys, respectively. Treatment of late-stage infections resulted in cure rates of one of three (group 4) and zero of three (group 5) monkeys. These studies demonstrated that pafuramidine was orally active in monkeys with early-stage T. brucei rhodesiense infections at dose rates above 3 mg/kg for 5 days. It was also evident that the drug attained only minimal efficacy against late-stage infections, indicating the limited ability of the molecule to cross the blood-brain barrier. This study has shown that oral diamidines have potential for the treatment of early-stage sleeping sickness.
Human African trypanosomiasis (HAT) (sleeping sickness) is caused by the flagellated protozoan parasites Trypanosoma brucei gambiense and T. brucei rhodesiense and is inevitably fatal when untreated. The disease is endemic to sub-Saharan Africa, where an estimated 50 million people in more than 20 countries are considered to be at risk (23). WHO statistics show that from 1998 to 2004, the number of HAT cases declined from 37,991 to 17,616 (30), and the estimated prevalence declined in the same period, from 300,000 to 500,000 cases to 50,000 to 70, 000, even though the number of people covered by active screening almost doubled in the same period (30). These declining trends are indicators that the partnerships between individual African governments and the WHO are having a positive impact on the control of HAT. However, these gains could easily be lost through political instability and/or the outbreak of wars, which inevitably lead to a breakdown in surveillance activities (10). At present, HAT remains a major health problem in certain foci in the Democratic Republic of Congo, Angola, and Southern Sudan (10, 30). Since the trypanosomes' remarkable ability to vary their major surface antigen makes successful vaccine development unlikely in the near future (26), the only recourse available is to intensify research and the development of new drugs.
Current drugs that are used to treat HAT include pentamidine, suramin, melarsoprol, and eflornithine (5). These are far from ideal, since they elicit substantial toxic effects and/or require parenteral application, which makes them difficult to administer in the rural African settings where the disease typically occurs. Furthermore, melarsoprol, the only drug available that is effective against the late stages of both T. brucei gambiense and T. brucei rhodesiense disease, has shown incidences of treatment failure of >25% in epidemic areas of northern Uganda and northern Angola (4, 7, 8). Both melarsoprol and eflornithine require long periods of hospitalization and monitoring owing to the complicated treatment schedules needed (1). In addition, melarsoprol may cause reactive encephalopathy; this occurs in 5 to 10% of treated patients and has a fatality rate of ∼50% (5). The provision of an oral drug of low toxicity for sleeping sickness would allow an effective administration of the required doses with minimal supervision from medical personnel. This is an important consideration, since enough qualified people are rarely available in those parts of Africa where the disease is endemic.
A recently synthesized novel prodrug, 2,5-bis(4-amidinophenyl)-furan-bis-O-methlylamidoxime (pafuramidine, DB289) exhibited excellent oral activity and reduced acute toxicity in acute and chronic mouse models of trypanosomiasis (3, 12, 27). The enhanced oral activity of DB289 is attributed to an improved oral absorption of the prodrug in which the cationic functionalities are masked and to its conversion to the active compound 2,5-bis(4-amidinophenyl)furan (furamidine; DB75) in the liver. DB75 strongly binds to the minor groove of DNA at AT-rich sites and weakly binds by intercalation at the GC sites of DNA (25). Consequently, its subsequent mode of action involves interference with the normal function of the pathogen's DNA-dependent enzymes, perhaps topoisomerase II (2). In spite of pafuramidine being an analogue of pentamidine, it appears to possess two properties that would overcome the two disadvantages of pentamidine, namely, parenteral administration and lack of efficacy against central nervous system (CNS) infection.
Recently, following prolonged administration with the drug in a clinical trial for AIDS, abnormal liver enzyme values were found in several volunteers. However, no subject required any treatment or hospitalization for this abnormality. Despite this, pafuramidine is evidently a promising candidate for use as a new drug for oral administration. It was therefore tested in the vervet monkey model of sleeping sickness at the Trypanosomiasis Research Centre of the Kenya Agricultural Research Institute. This nonhuman primate model is known to mimic the human disease closely (21) and is a valuable tool for preclinical chemotherapeutic studies. Experiments were conducted to determine the efficacy of DB289 after multiple oral doses against both early- and late-stage infections with T. brucei rhodesiense.
MATERIALS AND METHODS
Experimental animals.
Fifteen vervet monkeys (Chrolocebus [Cercopithecus] aethiops) of both sexes and weighing between 2.5 and 4.5 kg were obtained from the Institute of Primate Research in Kenya. They were housed in quarantine for a minimum of 90 days and screened for evidence of disease, including zoonotic infections. They were also dewormed using albendazole (Valbazen; Utravetis Ltd., Kenya) and treated for ectoparasites using carbaryl (1-naphthyl methylcarbamate) (Sevin; Bayer Ltd., Kenya). During that period, the monkeys became accustomed to staying in individual squeeze-back stainless steel cages and to being handled. They were fed twice daily on fresh vegetables and commercial monkey cubes (Unga Ltd., Kenya) and given water ad libitum. Before the study, the animals were transferred to experimental wards and acclimatized for a further 2 weeks.
Trypanosomes.
The T. brucei rhodesiense stabilate used was KETRI 2537, a derivative of EATRO 1989, isolated from a patient in Uganda by direct inoculation of blood and lymph node aspirates into a monkey and later cryopreserved (9).
Test drug.
Pafuramidine (DB289) [2,5-bis(4-amidinophenyl)-furan-bis-O-methlylamidoxime; MediChem, Lemont, IL] was provided by Immtech Pharmaceuticals, Inc., as a yellow powder.
Drug preparation.
DB289 was prepared as a 1% suspension in distilled water containing 0.5% (wt/vol) carboxymethylcellulose sodium and 0.1% (wt/vol) Tween 80. The preparation was stored at 4°C and used for up to 3 days before a fresh preparation was made.
Drug administration.
The DB289 suspension was administered orally using a gavage needle once daily for 5 or 10 days depending on the treatment regimen for each group.
Experimental design.
The 15 monkeys were randomized into five treatment groups without sex bias (Tables 1 and 2).
TABLE 1.
Body weight and PCV changes in vervet monkeys infected with T. brucei rhodesiense and treated with different regimens of pafuramidine
| Group (dose [mg/kg]) | Mean preinfection wt (kg) ± SE | % Wt change ata:
|
Mean preinfection % PCV ± SE | % Change in PCV ata:
|
||||
|---|---|---|---|---|---|---|---|---|
| Day 0 p.t.b | Day 28 p.t.c | End point | Day 0 p.t.b | Day 28 p.t.c | End point | |||
| 1 (1) | 3.15 ± 0.45 | −2.20 | −2.1d | 0 | 47.0 ± 4.4 | −4.30 | −17.0d | −23.40 |
| 2 (3) | 3.18 ± 0.33 | −5.00 | −6.60 | −1.60 | 46.0 ± 4.4 | −9.40 | −2.90 | −3.60 |
| 3 (10) | 2.88 ± 0.43 | −6.30 | 0 | 3.50 | 40.3 ± 2.5 | −5.80 | −1.60 | 13.20 |
| 4 (10) | 4.25 ± 0.52 | −4.20 | −5.20 | −0.70 | 51.7 ± 6.1 | −25.80 | −13.50 | −7.70 |
| 5 (10) | 3.32 ± 0.60 | −10.50 | −8.10 | −2.10 | 47.7 ± 1.5 | −41.30 | −9.10 | 1.40 |
Percent change in body weight or PCV from preinfection values. A − indicates a decline.
Values taken before the first treatment dose, i.e., on the 7th day p.i. for groups 1 to 3, 14th day p.i. for group 4, and 28th day p.i. for group 5.
Data for groups 2 to 5 at 28 days p.t.
Percent change in body weight and PCV at 1 day p.t. for group 1.
TABLE 2.
Outcomes of treatment of T. brucei rhodesiense-infected vervet monkeys with different regimens of pafuramidine (DB289)a
| Group | Dose (mg/kg) (length of treatment [days]) | Monkey | Time to detection of trypanosomes in biological fluids (days p.t.)
|
No. of cured monkeys/no. of total monkeys | |||
|---|---|---|---|---|---|---|---|
| Blood
|
CSF
|
||||||
| MIT | WS + HCT | MIT | HC + CT | ||||
| 1 | 1 (5) | 463 | 7 | 9 | NC | NC | 0/3 |
| 484 | NC | 4 | 7 | 14 | |||
| 485 | 3 | 14 | 21 | ||||
| 2 | 3 (5) | 482 | 55 | 79 | 21 | 1/3 | |
| 483 | 69 | 66 | 69 | 55 | |||
| 411 | Cured | ||||||
| 3 | 10 (5) | 454 | Cured | 3/3 | |||
| 468 | Cured | ||||||
| 486 | Cured | ||||||
| 4 | 10 (5) | 471 | 56 | 87 | NC | NC | 1/3 |
| 478 | Cured | ||||||
| 488 | ND | 42 | ND | ||||
| 5 | 10 (10) | 490 | ND | 21 | 42 | 0/3 | |
| 501 | 56 | 73 | NC | ||||
| 520 | ND | 119 | 119 | ||||
ND, not detected for >180 days p.t.; WS, wet smear; HC, hemocytometer counting; CT, concentration techniques for CSF; NC, trypanosome not cleared.
The monkeys were all infected by intravenous injection of 104 trypanosomes diluted from the infected blood of immunosuppressed donor mice using phosphate-buffered saline as previously described for the vervet monkey model (11, 17). On the seventh day postinfection (p.i.) in an early-stage infection, animals in groups 1, 2, and 3 were treated orally with pafuramidine at dose rates of 1, 3, and 10 mg/kg, respectively, for five consecutive days. The animals in the remaining two groups, groups 4 and 5, were treated with 10 mg/kg for 10 consecutive days, starting on the 14th day p.i. (group 4) or on the 28th day p.i. (group 5). The monkeys were monitored for clinical changes and for the presence of trypanosomes in body fluids. Once parasites were detected in any of the body fluids, the monkeys were removed from the study through humane euthanasia.
Monitoring and sample collection.
Physical examinations and sampling of body fluids were carried out weekly for the first 28 days and then once every 2 weeks up to 100 days posttreatment and monthly thereafter until the end of the experiment. Physical examinations and collection of blood and cerebrospinal fluid (CSF) were undertaken on monkeys anesthetized with diazepam (May and Baker, United Kingdom) at 1 mg/kg body weight and with ketamine hydrochloride (Rotexmedica, Trittau, Germany) at 15 mg/kg body weight. Posttreatment monitoring was maintained for a period of 180 days.
At each time point, approximately 1 ml of blood was collected into EDTA by inguinal venipuncture. In addition to blood samples, 1.5 ml of CSF was collected by lumbar puncture of the anesthetized monkeys (11).
Blood collected by inguinal venipuncture was used for determinations of the packed cell volume (PCV) using the microhematocrit method. White cells in the CSF were counted using a hemocytometer chamber.
Determination of parasitemia.
The determination of parasitemia started 3 days p.i. It was done by examination of wet films of ear prick blood according to a method described previously by Herbert and Lumsden (13) (detection limit, 105 trypanosomes/ml of blood) or by the hematocrit centrifugation technique (HCT) according to methods described previously by Woo (31) (detection limit, 103 trypanosomes/ml of blood). Blood collected by inguinal venipuncture was used for parasitemia determinations using both the wet-film and HCT methods.
For estimation of parasites in the CSF, some of the free-flowing CSF was collected into a capillary tube and immediately transferred into a hemocytometer chamber to count the number of trypanosomes and white cells. Negative CSF samples were then centrifuged and reexamined for trypanosomes. In cases where no parasites were detected in either blood or CSF by the above-described methods, Swiss white mice were used to test for the presence of trypanosomes (11). Samples of 0.2 ml of blood or CSF were inoculated intraperitoneally into two mice per sample.
RESULTS
Clinical disease before treatment.
Following infection, trypanosomes were detected in wet smears of peripheral blood with a mean period of 4.2 days (range, 3 to 5 days). Enlargement of peripheral axillar and inguinal lymph nodes was observed by the seventh day p.i., and enlargement of the spleen was observed by the 14th day p.i. The monkeys became dull, had rough hair coats, and showed a transient loss of appetite. The rectal body temperature rose from a preinfection (0 days p.i.) value of 38.2°C ± 0.2°C to 38.9°C ± 0.2°C (mean ± standard error). The rise in temperature was significant (P = 0.01) and coincided with peak parasitemia levels of 7 × 107 trypanosomes/ml of blood. Other clinical signs included loss of body weight as the infection progressed. The decrease in mean body weight was marginal (<6% of preinfection weight) for animals treated while in the first stage of disease at 7 to 11 days p.i. (groups 1 to 3), and a more substantial change (10.5%) was found for animals (group 5) treated during late-stage disease at 28 days p.i. (Table 1). The changes in mean PCV were similarly marginal for animals treated while in the early stage but more pronounced for those treated while in the late stage of disease (Table 1). Animals that were treated in the late stage of disease also had confirmed trypanosomes in CSF (1 to 6 trypanosomes/μl) and elevated white cell counts (mean, 10 cells/μl of CSF; range, 4 to 20 cells/μl of CSF).
Results of treatment with pafuramidine.
Pafuramidine treatment was shown to benefit most of the monkeys. Body weights and PCV improved in all monkeys by the time of termination of the experiment except for those in group 1 (Table 1). In some individuals, there was clearance of both parasitemia and CSF parasitosis (Table 2). However, the onset and durability of the beneficial effects of pafuramidine treatment varied in the different groups.
Group 1 (1 mg/kg for 5 days per os).
In group 1, trypanosomes became undetectable in the blood by direct microscopy or HCT a day after the last (fifth) drug dose and remained so for a mean of 5 days (range, 3 to 9 days). However, even during this period of apparent aparasitemia, viable trypanosomes were isolated from the monkeys' blood by the mouse inoculation test (MIT), indicating that blood parasites were never completely eliminated. In the CSF, trypanosomes were detected first by MIT on 7 to 14 days posttreatment (p.t.) or 14 to 21 days p.t. by microscopy (direct hemocytometer counting or concentration techniques), indicating that the drug treatment delayed the onset of trypanosomes in the CSF. Clinical data, especially the PCV, continued to decline (Table 1), indicating that at the low dose of 1 mg/kg of body weight, pafuramidine treatment did not cure any of the monkey infections. The monkeys were humanely euthanized.
Group 2 (3 mg/kg for 5 days per os).
Animals in group 2 became parasitologically negative by both blood and CSF on the fifth day of dosing. However, two of the three monkeys experienced recrudescence of trypanosomes, first in the CSF and later in the blood (Table 2). In these two animals, CSF trypanosomes were first detected by the MIT at 21 days p.t. and then by direct microscopy (Table 2). In this group, treatment led to improvement in both mean weight and mean PCV (Table 1) although only one of three the monkeys fulfilled the criteria of cure at 180 days p.t. The two monkeys that relapsed were humanely euthanized.
Group 3 (10 mg/kg for 5 days per os).
Animals in group 3 became parasitologically negative by the fifth day of dosing and remained so until the end of the experimental period. Similarly, no trypanosomes were recovered by MIT throughout the 180 days of p.t. monitoring. Meanwhile, weight and PCV returned to preinfection levels by 28 days p.t. (Table 1), and all clinical aberrations resolved, underlining that all three of three monkeys were cured of the infection.
Group 4 (10 mg/kg for 10 days per os starting 14 days p.i.).
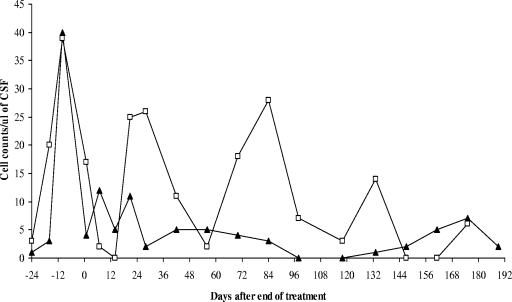
In group 4, following treatment, both weight and PCV improved, although not to preinfection levels (Table 1). All three monkeys in this group became aparasitemic by day 5 of dosing, but only two of three monkeys (monkeys 478 and 488) remained free of blood parasites until 180 days p.t. In the CSF, either trypanosomes were not cleared (monkey 471) or recrudescence occurred within the first 42 days p.t. (monkey 488) (Table 2). Only monkey 478 remained free of trypanosomes in both blood and CSF as determined by HCT and MIT and was considered to be cured (Table 2). The results were confirmed by the pattern of white cell changes in the CSF; these oscillated between 0 and 12 cells/μl of CSF for monkey 478, which was cured, but occasional peaks of above 25 cells/μl of CSF, which declined to lower peaks below 15 cells/μl of CSF, were observed in monkey 488, where there was recrudescence of the infection (Fig. 1).
FIG. 1.
CSF white cell changes in vervet monkeys 478 (▴) and 488 (□) after treatment with pafuramidine at 10 mg/kg for 10 days while in the late stage (14 days p.i.) of disease. Note that in vervet monkey 478, levels of white cells declined after treatment and remained low throughout the observation period, while in vervet monkey 488, the drop in white cell number was transient and showed recrudescences to >25 cells/μl of CSF.
Group 5 (10 mg/kg for 10 days per os starting at 28 days p.i.).
In group 5, all the animals became aparasitemic by day 4 of dosing. However, only two of three of them remained free of blood trypanosomes by the end of the 180 days of p.t. monitoring (Table 2). In the CSF, trypanosomes were not cleared in monkey 501, while in the remaining two monkeys, the trypanosomes disappeared after treatment, and recrudescence occurred (Table 2). The significant improvement in weight and PCV (Table 1) was probably due to the absence of trypanosomes from the hemolymphatic system in spite of the presence of trypanosomes in the CNS.
DISCUSSION
In this study, the oral administration of pafuramidine (DB289) had a profound effect on established T. brucei rhodesiense infections in vervet monkeys. In the acute disease stage, when the monkeys showed high parasitemia levels, treatment with 10 mg/kg orally for five consecutive days cleared three of the three monkeys in group 3 of their trypanosome infections and kept them trypanosome free for the entire monitoring period of 180 days. In addition, the treatment reversed the clinical symptoms of trypanosomiasis, thus fulfilling all criteria for confirmation of cure.
Comparison of this group of monkeys with groups 1 and 2, which also had an early-stage infection with a 5-day treatment schedule (days 7 to 11), shows a clear dose response to pafuramidine. At 3 mg/kg, only one of three animals was cured, and at the lowest dose, 1 mg/kg, all animals relapsed within 1 week after treatment or did not clear parasitemia at all.
The clinical signs described in this report for acute infection mirror those described in previous reports for syringe passage of T. brucei rhodesiense infections in vervet monkeys (15, 21), tsetse-transmitted infections in vervet monkeys (28), and early-stage infections in sleeping sickness patients (16, 18, 22). These clinical manifestations are evidence of hemolymphatic system involvement. The fact that the clinical manifestations were reversed when pafuramidine was administered in sufficient concentrations indicates that the drug was able to access and eliminate trypanosomes from all hemolymphatic sites.
In the two groups of monkeys with a late-stage infection, treatment with 10 mg/kg for 10 days cured only one of three monkeys treated from 14 days p.i. (group 4) and none in the group treated from 28 days p.i. onwards. In group 4, the presence of trypanosomes and white cell counts above the 5-cell/mm3 cutoff for confirmation of late-stage disease (29) was demonstrated in the CSF of the infected monkeys. The fact that trypanosomes were eliminated from the CSF in one animal and the mean PCV in the group was improved (from −25.8% to −13.5%) (Table 1) suggests that orally administered pafuramidine has some ability to cross the blood-brain barrier. Although the levels of pafuramidine (DB289) and its active form, furamidine (DB75), in CSF were not determined in the present study, it is likely that the variation in individual responses resulted from variation in the CSF levels of the drugs. Generally, the activity of a drug may show considerable interanimal variation, which results in different concentrations at the site of action (kinetic differences) or different responses to a given drug concentration (dynamic differences) (19). Studies with melarsoprol, the drug used to treat late-stage sleeping sickness, similarly indicated that the peak levels attained in the CSF of monkeys were about 50 times less than peak levels in plasma and were insufficient in some animals to eliminate all trypanosomes from the CSF compartment (6).
The fact that none of the animals treated with pafuramidine at 10 mg/kg for 10 days starting at 28 days p.i. was cured shows that as the residence time of trypanosomes in the CSF increased, the infections became more difficult to treat. It has been postulated that the invasion of the CSF by trypanosomes progressively leads to parasite invasion of the perivascular spaces (Virchow-Robin spaces) and from there to the parenchyma of the CNS (20). Further evidence of the importance of the residence time of trypanosomes in CSF was provided previously by Schmidt and Sayer (21), who found that one monkey with a disease duration of 107 days had histologically demonstrable meningoencephalitis. Although our study had no component of histology and could therefore not confirm or rule out the presence of meningoencephalitis at 28 days p.i., it is clear that the levels of the active drug (DB75) attained in the CSF were inadequate to eliminate trypanosomes from the sites to which they were sequestered, making the CNS the main source of relapse parasites, as previously observed for mice (14). Even in this group of monkeys, however, the drug cleared bloodstream-form trypanosomes and maintained two of three monkeys trypanosome free throughout the monitoring period, which provides further evidence of the ability of pafuramidine to access hemolymphatic but not CNS sites of the infected monkeys.
To conclude, the study showed that the novel prodrug pafuramidine (DB289) was effective against early T. brucei rhodesiense infection in monkeys. In addition, the drug was well tolerated by all the infected monkeys, confirming previous observations of uninfected vervet monkeys (John Thuita, TRC-KARI, personal communication). The drug was generally not effective against late-stage disease and may therefore not be indicated for this use. However, Sturk et al. (24) previously observed that after intravenous administration in mice, there was a fivefold increase in brain levels of pafuramidine with parenchymal localization of compound fluorescence, which suggested that the unaltered prodrug does penetrate the blood-brain barrier and may be subject to in situ biotransformation. Since the present study showed that pafuramidine was efficacious in clearing trypanosomes from the bloodstream, it would be worth considering the testing of parenteral treatment regimens to improve the bioavailability of the drug in the CNS in nonhuman primates. However, the issue of recently found human toxicity should be taken in consideration in further nonhuman primate studies.
Acknowledgments
We acknowledge the assistance of Tom Adino, Ben Kinyanjui (deceased), Stephen Mbugua, John Oidho, Erastus Amakobe, Simon Karanja, and David Mwangangi for assistance in the study. We are indebted to Jennifer Jenkins for proofreading of the manuscript.
This investigation received financial support from the Bill and Melinda Gates Foundation through the Consortium for Parasitic Drug Development.
This paper is published with the permission of the Director of the Trypanosomiasis Research Centre, Kenya Agricultural Research Institute.
Footnotes
Published ahead of print on 8 December 2008.
REFERENCES
- 1.Blum, J., and C. Burri. 2002. Treatment of late stage sleeping sickness caused by T. b. gambiense: a new approach to the use of an old drug. Swiss Med. Wkly. 132:51-56. [DOI] [PubMed] [Google Scholar]
- 2.Boykin, D. W., A. Kumar, J. Spychala, M. Zhou, R. J. Lombardy, W. D. Wilson, C. C. Dykstra, S. K. Jones, J. E. Hall, R. R. Tidwell, C. Laughton, C. M. Nunn, and S. Neidle. 1995. Dicationic diarylfurans as anti-Pneumocystis carinii agents. J. Med. Chem. 36:912-916. [DOI] [PubMed] [Google Scholar]
- 3.Boykin, D. W., A. Kumar, G. Xiao, W. D. Wilson, B. C. Bender, D. R. McCurdy, J. E. Hall, and R. R. Tidwell. 1998. 2,5-Bis[4-(N-alkylamidino)phenyl)]furans as anti-Pneumocystis carinii agents. J. Med. Chem. 41:124-129. [DOI] [PubMed] [Google Scholar]
- 4.Brun, R., R. Schumacher, C. Schmid, C. Kunz, and C. Burri. 2001. The phenomenon of treatment failures in human African trypanosomosis. Trop. Med. Int. Health 6:906-914. [DOI] [PubMed] [Google Scholar]
- 5.Burri, C., and R. Brun. 2003. Human African trypanosomiasis, p. 1303-1323. In G. C. Cook and A. I. Zumla (ed.), Manson's tropical diseases, 21st ed. Saunders/Elsevier Science, Edinburgh, United Kingdom.
- 6.Burri, C., J. D. Onyango, J. F. Auma, E. M. Burudi, and R. Brun. 1994. Pharmacokinetics of melarsoprol in uninfected vervet monkeys. Acta Trop. 58:35-49. [DOI] [PubMed] [Google Scholar]
- 7.Burri, C., and J. Keiser. 2001. Pharmacokinetic investigations in patients from northern Angola refractory to melasoprol treatment. Trop. Med. Int. Health 6:412-420. [DOI] [PubMed] [Google Scholar]
- 8.Denise, H., K. Mathews, G. Lindergard, S. Croft, and M. P. Barrett. 1999. Trypanosomiasis and leishmaniasis: between the idea and the reality of control. Parasitol. Today 15(2):43-45. [DOI] [PubMed] [Google Scholar]
- 9.Fink, E., and H. Schmidt. 1980. Preclinical testing of potential trypanocidal drugs in primates: preliminary investigation of an experimental diamidine in vervets, p. 173-182. In A. R. Njogu, P. M. Tukei, and J. M. D. Roberts (ed.), Recent developments in medical research in East Africa. KEMRI/KETRI, Nairobi, Kenya.
- 10.Ford, L. B. 2007. Civil conflict and sleeping sickness in Africa in general and Uganda in particular. Conflict Health 1:6. doi: 10.1186/1752-1505-1-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gichuki, C., and R. Brun. 1999. Animal models of CNS (second-stage) sleeping sickness, p. 795-800. In O. Zak and M. Sande (ed.), Handbook of animal models of infection. Academic Press, London, United Kingdom.
- 12.Hall, J. E., J. E. Kerrigan, and K. Ramachandran. 1998. Anti-Pneumocystis activities of aromatic diamidoxime prodrugs. Antimicrob. Agents Chemother. 42:666-674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Herbert, W. J., and W. H. R. Lumsden. 1976. A rapid “matching” method for estimating the host's parasitaemia. Exp. Parasitol. 40:427-431. [DOI] [PubMed] [Google Scholar]
- 14.Jennings, F. W., P. E. McNeil, J. M. Ndung'u, and M. Murray. 1989. Trypanosomiasis and encephalitis: possible aetiology and treatment. Trans. R. Soc. Trop. Med. Hyg. 83:518-519. [DOI] [PubMed] [Google Scholar]
- 15.Kagira, J. M., J. K. Thuita, J. M. Ngotho, R. Mdachi, D. M. Mwangangi, and J. M. Ndung'u. 2006. Haematology of experimental Trypanosoma brucei rhodesiense infection in vervet monkeys. Afr. J. Health Sci. 13:59-65. [Google Scholar]
- 16.Moore D. A., M. Edwards, R. Escombe, D. Agranoff, J. W. Bailey, S. B. Squire, and P. L. Chiodini. 2002. African trypanosomiasis in travellers returning to the United Kingdom. Emerg. Infect. Dis. 8:74-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ndung'u, J. M., R. M. Ngure, J. M. Ngotho, P. D. Sayer, and J. K. Omuse. 1994. Total protein and white cell changes in the cerebrospinal fluid of vervet monkeys infected with Trypanosoma rhodesiense and the post-treatment reaction. J. Protozool. Res. 4:124-135. [Google Scholar]
- 18.Ripamonti, D., M. Massari, C. Arici, E. Gabbi, C. Farina, M. Brini, C. Capatti and F. Suter. 2002. African sleeping sickness in tourists returning from Tanzania: the first 2 Italian cases from a small outbreak among European travellers. Clin. Infect. Dis. 34:18-22. [DOI] [PubMed] [Google Scholar]
- 19.Rowland, M., and T. N. Tozer. 1989. Clinical pharmacokinetics: concepts and applications, 2nd ed. Lea and Febiger, Philadelphia, PA.
- 20.Schmidt, H. 1983. The pathogenesis of trypanosomiasis of the CNS. Studies on parasitological and neurohistological findings in Trypanosoma rhodesiense infected vervet monkeys. Virchows Arch. A Pathol. Anat. Histopathol. 399:333-343. [DOI] [PubMed] [Google Scholar]
- 21.Schmidt, H., and P. Sayer. 1982. Trypanosoma brucei rhodesiense infection in vervet monkeys. II. Provocation of the encephalitic late phase by treatment of infected monkeys. Tropenmed. Parasitol. 33:255-259. [PubMed] [Google Scholar]
- 22.Sinha A., C. Grace, W. K. Alston, F. Westenfeld, and J. H. Maguire. 1999. African trypanosomiasis in two travellers from the United States. Clin. Infect. Dis. 29:840-844. [DOI] [PubMed] [Google Scholar]
- 23.Stuart, K., R. Brun, S. Croft, A. Fairlamb, R. E. Gürtler, J. McKerrow, S. Reed, and R. Tarleton. 2008. Kinetoplastids: related protozoan pathogens, different diseases. J. Clin. Investig. 118:1301-1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sturk, L. M., J. L. Brock, C. R. Bagnell, J. E. Hall, and R. R. Tidwell. 2004. Distribution and quantitation of the anti-trypanosomal diamidine 2,5-bis(4-amidinophenyl)furan (DB75) and its N-methoxy prodrug DB289 in murine brain tissue. Acta Trop. 91:131-143. [DOI] [PubMed] [Google Scholar]
- 25.Tanious, F. A., J. Spychala, A. Kumar, K. Green, D. W. Boykin, and W. D. Wilson. 1994. Different binding mode in AT and GC sequences for unfused-aromatic dications. J. Biomol. Struct. Dyn. 11:1063-1083. [DOI] [PubMed] [Google Scholar]
- 26.Taylor, K. A., and B. Mertens. 1999. Immune response of cattle infected with African trypanosomes. Mem. Inst. Oswaldo Cruz 94:239-244. [DOI] [PubMed] [Google Scholar]
- 27.Thuita, J. K., S. M. Karanja, T. Wenzler, R. E. Mdachi, J. M. Ngotho, J. M. Kagira, R. R. Tidwell, and R. Brun. 2008. Efficacy of the diamidine DB75 and its prodrug DB289, against murine models of human African trypanosomiasis. Acta Trop. 108:6-10. [DOI] [PubMed] [Google Scholar]
- 28.Thuita, J. K., J. M. Kagira, D. M. Mwangangi, E. Matovu, C. M. R. Turner, and D. Masiga. 2008. Trypanosoma brucei rhodesiense transmitted by a single tsetse fly bite in vervet monkeys as a model of human African trypanosomiasis. PLoS NTD 2:e238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.WHO. 1998. The control and surveillance of African trypanosomiasis. WHO Tech. Rep. Ser. 881:1-114. [PubMed] [Google Scholar]
- 30.WHO. 2006. Human African trypanosomiasis (sleeping sickness): epidemiological update. Wkly. Epidemiol. Rec. 81:71-80. [PubMed] [Google Scholar]
- 31.Woo, P. T. 1970. The haematocrit centrifuge technique for the diagnosis of African trypanosomiasis. Acta Trop. 27:384-386. [PubMed] [Google Scholar]