Abstract
The objectives of this study were to evaluate emtricitabine (FTC) pharmacokinetics in pregnant women and their neonates and to determine the optimal prophylactic dose for neonates after birth to prevent mother-to-child transmission of human immunodeficiency virus (HIV). A total of 38 HIV-infected pregnant women were administered tenofovir disoproxyl fumarate (300 mg)-FTC (200 mg) tablets—two tablets at the initiation of labor and one daily for 7 days postpartum. By pair, 11 maternal, one cord blood, and two neonatal FTC concentrations were measured using a high-performance liquid chromatography-tandem mass spectrometry validated method and analyzed by a population approach. Model and mean estimates (interpatient variability) were a two-compartment model for mothers, with an absorption rate constant of 0.54 h−1 (61%), apparent elimination and intercompartmental clearances of 23.2 (17%) and 6.04 liters·h−1, and apparent central and peripheral volumes of 127 and 237 liters, respectively; an effect compartment linked to maternal circulation for cord blood and a neonatal compartment disconnected, after delivery, with a 10.6-h half-life (30%). After the 400-mg FTC administration, the median population area under the concentration-time curve and the minimal and maximal plasma FTC concentrations in pregnant women were 14.3 mg·liter−1·h and 1.68 and 0.076 mg/liter, respectively. At delivery, median (range) predicted maternal and cord blood FTC concentrations were, respectively, 1.16 (0.14 to 1.99) and 0.72 (0.05 to 1.19) mg·liter−1. We concluded that the 400-mg FTC administration in pregnant women produces higher exposition than does the 200-mg administration in other adults, at steady state. FTC was shown to have good placental transfer (80%). Administering 1 mg FTC/kg as soon as possible after birth or 2 mg/kg 12 h after birth should produce neonatal concentrations comparable to the concentrations observed in adults.
To prevent mother-to-child transmission of human immunodeficiency virus (HIV) during delivery, a single-dose administration of nevirapine (NVP) administered at the start of labor is the most common antiretroviral regimen used in resource-limited settings, as recommended by the World Health Organization in the Antiretroviral Drugs for Treating Pregnant Women and Preventing HIV Infection in Infants report (http://www.who.int/hiv/pub/guidelines/pmtctguidelines3.pdf). However, the use of the single-dose administration of NVP results in resistance mutations in 15 to 70% of women, at 4 to 6 weeks postpartum, compromising the success of subsequent treatments with NVP in mother and child (7, 9). A recent clinical study suggests that adding a single dose of tenofovir disoproxyl fumarate (TDF) and emtricitabine (FTC) at delivery may reduce those resistances by half (6).
FTC is a potent, once-daily-administered nucleoside reverse transcriptase inhibitor approved for the treatment of HIV in adults and children older than 3 months in combination with other antiretroviral agents. The physiological changes associated with pregnancy can lead to significant variations in pharmacokinetics (10, 12, 14). However, few pharmacokinetic data on FTC in pregnant women (3) and no data on placental transfer are available. Only one study reports the pharmacokinetics of FTC in neonates exposed to HIV in utero; apparent elimination clearance was 13 ml/min in 5- to 21-day-old neonates and 22 ml/min in 23- to 42-day-old neonates (5). This suggests that the youngest neonates have the lowest elimination clearance. The neonatal pharmacokinetics just after birth is still unknown.
In the present work, a population pharmacokinetic study was performed on maternal, cord, and neonatal plasma samples in order to (i) describe the concentration-time courses of FTC in mothers, the transfer of FTC from maternal plasma to cord plasma, and the neonatal elimination, (ii) study the influence of covariates (such as maternal body weight [BW], gestational age, type of delivery, maternal creatinine, neonatal BW, height, and body surface area) on FTC pharmacokinetics, and (iii) model various dosing strategies to determine the optimal dosing scheme for newborns.
MATERIALS AND METHODS
Patients.
The Tenofovir/Emtricitabine for the Prevention of Mother-to-Child Transmission (PMTCT) in Africa and Asia (TEmAA ANRS 12-109) study was an open, phase I/II trial evaluating the pharmacokinetics and the safety and toxicity of the tenofovir-FTC combination in HIV-infected pregnant women and their neonates. This trial was conducted in Abidjan, Côte d'Ivoire; Phnom Penh, Cambodia; and Soweto, South Africa. Pregnant women (between 28 and 38 weeks of gestation) who are older than 18 years, are infected by HIV-1 or HIV-2, are naïve to all antiretroviral treatment, and had an indication for antiretroviral prophylaxis for PMTCT during pregnancy (in line with international or national recommendations, which are WHO's clinical stage 1 or 2 and CD4 levels of ≥200/mm3 or stage 3 and CD4 levels of ≥350/mm3) were eligible. Neonates with a gestational age greater than 32 weeks and a birth weight greater than 2,000 g were eligible. This study protocol was approved by the national ethics committees of Côte d'Ivoire, South Africa, and Cambodia and by each country's health authorities. The mothers and the fathers of the children to be born provided signed informed-consent forms.
Treatments.
Mothers were administered zidovudine (300 mg twice a day) from the day of enrollment to the delivery date, one tablet of NVP (200 mg) and two tablets of TDF (300 mg)-FTC (200 mg) at the start of labor, and one tablet of TDF (300 mg)-FTC (200 mg) per day for 7 days postpartum. Children were given NVP syrup (2 mg/kg) as a single dose on the first day of life and zidovudine syrup (4 mg/kg) every 12 h for 7 days.
Sampling.
All women received FTC and underwent blood samplings for pharmacokinetic analysis at delivery, at 1, 2, 3, 5, 8, 12, and 24 h after the administration of 400 mg FTC, and before the second, third, and seventh administrations of 200 mg FTC. A cord blood sample was obtained at delivery, and the neonates had samplings on days 1 and 2 of life. Times elapsed between administration and sampling, maternal and fetal BWs, and gestational ages were recorded.
Analytical method.
The FTC assay was performed according to the previously published method (11), with a limit of quantification (LOQ) of 0.01 mg/liter and intra- and interassay precisions of 3.6% and 7.9%, respectively. The bias between observed and theoretical concentration ranged from 0.7 to 14.9%.
Modeling strategy and population pharmacokinetic model.
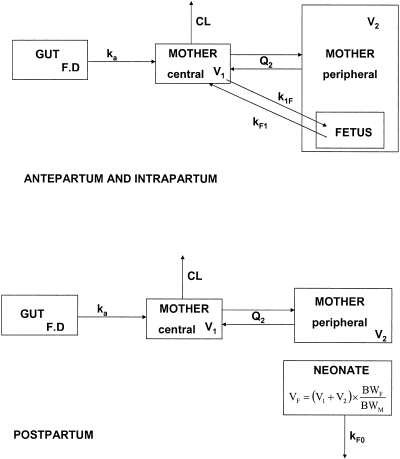
Data were analyzed using the nonlinear mixed-effect modeling software program NONMEM (version VI, level 1.0) with the Digital Fortran compiler (2). The first-order conditional estimation with interaction method was used. A two-compartment model with first-order absorption and elimination best described maternal data. For cord plasma concentrations, an “effect” compartment model of negligible volume and negligible drug accumulation linked to the maternal circulation was used. The effect compartment is modeled as a virtual compartment linked to the maternal plasma compartment by a first-order process which does not modify the compartmental model in the mother. After delivery, this fetal compartment is disconnected, time is reset to zero, and the neonate has his own elimination (Fig. 1). Parameters of the model were the absorption rate constant (ka), maternal elimination clearance from the central compartment (CL), volume of the central maternal compartment (V1), maternal intercompartmental clearance (Q2), volume of the peripheral maternal compartment (V2), mother-to-fetus transfer rate constant (k1F), fetus-to-mother transfer rate constant (kF1), and neonate elimination rate constant (kF0), where the subscripted F stands for fetus. Since FTC was orally administered, only ka, CL/F, V1/F, Q2/F, V2/F, k1F, kF1, and kF0 were identifiable. Analytical equations were used in a $PRED section in NONMEM to estimate these pharmacokinetic parameters. When FTC concentrations were below the LOQ, we set them to half of the LOQ. Several error models were investigated (i.e., multiplicative and additive error models) to describe residual variability. An exponential model was used for intersubject variability (ISV). Only significant ISVs on pharmacokinetics were kept. The effect of each patient covariate was systematically tested via generalized additive modeling on the basic model. Continuous covariates (CO) such as BW, gestational age, creatinine, height, and body surface area were tested according to the following equation, using CL as an example, 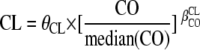 , where θCL is the typical value of clearance for a patient with the median covariate value and
, where θCL is the typical value of clearance for a patient with the median covariate value and 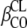 is the estimated influential factor for the continuous covariate. When a covariate was missing, it was set to the median value from all the other women. Categorical covariates (CA; CA is 0 or 1) were tested according to
is the estimated influential factor for the continuous covariate. When a covariate was missing, it was set to the median value from all the other women. Categorical covariates (CA; CA is 0 or 1) were tested according to 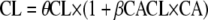 for inducing effect or
for inducing effect or 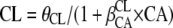 for inhibitory effect. The type of delivery (TD) was tested according to
for inhibitory effect. The type of delivery (TD) was tested according to 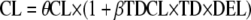 , where DEL is delivery, DEL = 1 before delivery, and DEL = 0 after delivery. A covariate was kept if its effect was biologically plausible; it produced a minimum reduction of 6.63 in the objective function value (OFV) and a reduction in the variability of the pharmacokinetic parameter, assessed by the associated ISV. An intermediate model with all significant covariates was obtained. A backward elimination phase was finally performed by deleting each covariate from the intermediate model to obtain the final model, using a likelihood ratio test.
, where DEL is delivery, DEL = 1 before delivery, and DEL = 0 after delivery. A covariate was kept if its effect was biologically plausible; it produced a minimum reduction of 6.63 in the objective function value (OFV) and a reduction in the variability of the pharmacokinetic parameter, assessed by the associated ISV. An intermediate model with all significant covariates was obtained. A backward elimination phase was finally performed by deleting each covariate from the intermediate model to obtain the final model, using a likelihood ratio test.
FIG. 1.
Population pharmacokinetic model for the simultaneous prediction of FTC concentrations in the mother, the cord blood (top), and the neonate (bottom). A two-compartment model with first-order absorption and elimination best described maternal data. For cord blood FTC concentrations, an “effect” compartment is modeled as a virtual compartment linked to the maternal plasma compartment by a first-order process. After delivery, the fetal compartment is disconnected, and the neonate has his own elimination. F denotes bioavailability, D the maternal FTC dose, ka the absorption rate constant, CL the maternal elimination clearance from the central compartment, V1 the volume of the central maternal compartment, Q2 the maternal intercompartmental clearance, V2 the volume of the peripheral maternal compartment, k1F the mother-to-fetus transfer rate constant, kF1 the fetus-to-mother transfer rate constant, kF0 neonate elimination rate constant, VF the neonate volume of distribution, BWM the maternal BW, and BWF the neonatal BW.
Evaluation and validation.
For evaluation of the goodness of fit, the graphs of the following data were performed: observed and predicted concentrations versus time, observed concentrations versus population predictions, weighted residuals versus time, and weighted residuals versus predictions. Similar graphs using individual predictive post hoc estimation were displayed. The diagnostic graphs were performed using RfN (S. Urien, RFN-831-20070911 [https://sourceforge.net/project/showfiles.php?group_id=29501&package_id=140129&release_id=538680]) with the R program (8).
FTC concentration profiles were simulated and compared with the observed data, thanks to the visual predictive check method, in order to validate the model. More precisely, the vector of pharmacokinetic parameters from 1,000 patients was simulated using the final model. Each parameter vector was drawn in a log-normal distribution, with a variance corresponding to the ISV previously estimated. A simulated residual error was added to each simulated concentration. The simulations were performed using NONMEM. The 5th, 50th, and 95th percentiles of the simulated concentrations at each time were then overlaid on the observed concentration data by using the R program, and a visual inspection was performed.
Maternal FTC concentrations after administration of 400 mg FTC to mothers before delivery and placental transfer.
After the administration of 400 mg FTC to each pregnant woman, minimal (Cmin) and maximal (Cmax) plasma FTC concentrations and areas under the concentration-time curve (AUCs) were derived from the estimated individual pharmacokinetic parameters. Median values and ranges were calculated and compared to data in the literature from other adults. At delivery, cord (i.e., fetal) and maternal plasma FTC concentrations were determined. The ratio of fetal to maternal FTC concentrations was calculated, and its variation as a function of the delay between drug uptake and delivery was followed. In order to better evaluate placental transfer, for the 400-mg dose administered to the mother, maternal and neonatal AUCs were estimated, and the ratio between neonatal and maternal AUC was calculated.
Determination of the optimal dosing scheme for newborns.
The optimal timing for FTC administration to the newborns was determined in order to obtain exposure similar to that observed in adults [i.e., (AUC0→24 h)neonates = 10.4 mg/liter·h) and to guarantee newborn FTC concentration of >0.077 mg/liter (i.e., residual adult FTC concentration), before the administration to the neonate and as long as possible after administration to the neonate. The target minimal concentration of 0.077 mg/liter corresponds to the mean minimal concentration for 200 mg FTC administered once daily in adults in three previous studies (0.071 mg/liter in a Zhong et al. study [18], 0.075 mg/liter in a Blum et al. study [5], and 0.085 mg/liter in a Ramanathan et al. study [15]). The following hypotheses were necessary: (i) neonates have the same bioavailability and absorption rate as their mothers, and (ii) the neonatal volume of distribution VF is proportional to the maternal volume of distribution on a BW basis, i.e., VF = (V1 + V2) × BWneonate/BWMother. Neonatal AUC was calculated taking into account both neonatal administration and mother-to-fetus drug transfer. As adults receive 200-mg doses or 3 mg/kg, a 3-mg/kg administration was simulated, and this dose was modified in order to obtain a neonatal AUC0→24 h close to 10.4 (median AUC for adults after a 200-mg dose). Different administration schemes were simulated in the neonates; 1, 2, or 3 mg/kg was administered 1 h after birth and 2 mg/kg 12 h after birth.
RESULTS
Demographic data.
Data from the 38 enrolled women and 32 of their neonates were available for FTC pharmacokinetic evaluation. Table 1 summarizes the patients' characteristics.
TABLE 1.
Characteristics of the HIV-infected pregnant women (n = 38) enrolled in the pharmacokinetic study of the TEmAA ANRS 12109 trial, step 1
| Covariate | Median (min-max) |
|---|---|
| Maternal BW at delivery (kg) | 58.3 (46.5-88.1) |
| Gestational age (wk) | 39 (33-42) |
| Delivery (vaginal, caesarian section) | |
| (no. of patients) | 24, 14 |
| Maternal creatinine clearance at enrollment | |
| (μmol/liter) | 42.2 (26-88) |
| Neonatal BW at birth (kg) | 2.7 (2.3-3.6) |
| Neonatal ht at birth (cm) | 48.5 (46-53) |
| Body surface area at birth (m2) | 0.20 (0.18-0.23) |
Population pharmacokinetics.
A total of 411 maternal concentrations, 37 cord blood concentrations, and 66 neonatal concentrations were used for pharmacokinetic analysis. Four maternal residual FTC concentrations were excluded because they were 7 to 20 times higher than the three other residual concentrations in the same patient. Seven FTC concentrations were lower than the LOQ, so they were set to half of the LOQ (1). The available data were not sufficient to estimate ISV for V1/F, Q2/F, V2/F, k1F, and kF1, and fixing the variance of these random effects to zero had no influence on the OFVs. Variabilities were thus estimated for ka, CL/F, and kF0. All residual variabilities were best described by a proportional error model. The addition of a correlation between maternal and cord blood residual variabilities, using an L2 item (r = 0.80 [24%]), decreased the OFV by 11.8 units. The effects of maternal BW, serum creatinine, gestational age, and type of delivery were tested on CL/F, and the effects of neonatal BW, height, body surface area, and gestational age were tested on kF0; none of these effects were significant.
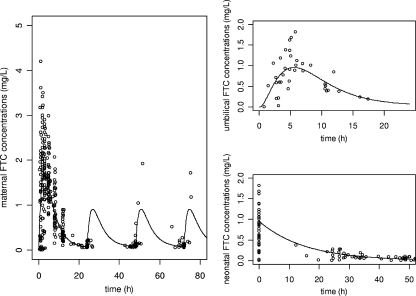
Figure 2 displays FTC observed and predicted plasma concentrations as a function of time for the mother, the cord blood, and the neonate. To better visualize neonatal concentrations, cord blood concentrations were reported on the graph at time zero. Table 2 summarizes the final population pharmacokinetic estimates. Final model performance was appreciated by comparing population-predicted plasma concentrations and individual predicted plasma concentrations to observed plasma concentrations, and population weighted residuals versus predicted concentrations, and versus time for FTC (not shown).
FIG. 2.
(Left) Observed (○) and population-predicted (line) maternal FTC concentrations versus time. (Right) Observed (○) and population-predicted (lines) FTC concentrations in cord blood (top) and neonatal plasma (bottom) versus time.
TABLE 2.
Population pharmacokinetic parameters of FTC from the final model for HIV-infected pregnant women (n = 38) after receiving 400 mg of FTC at the start of the labor and for their neonates (n = 32) enrolled in the TEmAA ANRS 12109 trial, step 1a
| Parameter | Estimate (% RSE) |
|---|---|
| Structural model: | |
| ka (h−1) | 0.54 (11) |
| CL/F (liter/h) | 23.2 (4) |
| V1/F (liter) | 127 (7) |
| Q/F (liter/h) | 6.04 (10) |
| V2/F (liter) | 237 (15) |
| k1F (h−1) | 0.289 (13) |
| kF1 (h−1) | 0.383 (13) |
| kF0 (h−1) | 0.0653 (7) |
| Statistical model: | |
| ωka (%) | 61 (29) |
| ωCL/F (%) | 17 (34) |
| ωkF0 (%) | 30 (35) |
| σMother (%) | 45 (14) |
| σcord (%) | 43 (24) |
| σneonate (%) | 33 (27) |
% RSE, relative standard error (standard error of estimate/estimate × 100); ka, absorption rate constant; CL/F, maternal apparent elimination clearance from the central compartment; V1/F, apparent volume of distribution of the central maternal compartment; Q2/F, apparent maternal intercompartmental clearance; V2/F, apparent volume of distribution of the peripheral maternal compartment; k1F, mother-to-fetus transfer rate constant; kF1, fetus-to-mother transfer rate constant; kF0, neonate elimination rate constant; σ, residual variability estimates (coefficient of variation of residual variability [%]); and ω, interindividual variability estimates (coefficient of variation of intersubject variability [%]).
Validation.
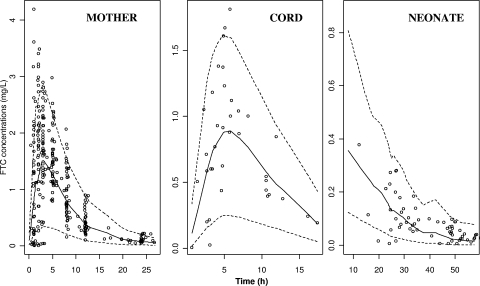
A visual predictive check of the final population pharmacokinetic model (Fig. 3) showed the 5th, 50th and 95th predicted percentiles from the 1,000 simulations and the observed concentrations of FTC. The visual predictive checking confirmed that the average prediction matched the observed concentrations. The variability was reasonably estimated.
FIG. 3.
Evaluation of the final model. Comparison between the 5th (lower dashed line), 50th (full line), and 95th (upper dashed line) percentiles obtained from 1,000 simulations and the observed data (○) for FTC concentrations in mother (left panel), cord blood (middle panel), and neonate (right panel).
Maternal concentrations after 400-mg administrations to mothers before delivery.
Table 3 summarizes the maternal Cmin, Cmax, and AUC values obtained after 400-mg administrations to the pregnant women at the start of labor and the values previously found after 200-mg administrations to adults, at steady state. In the present study, total elimination clearance was 28 liters/h for women at delivery after a 400-mg dose, whereas in previous studies, the mean value was 19.3 liters/h in adults after a 200-mg dose. Total elimination clearance increased by 45% in pregnant women on the day of delivery.
TABLE 3.
Maternal minimal and maximal concentrations (Cmin and Cmax) and AUCs derived from women's individual pharmacokinetic estimates, after a 400-mg FTC dose was administered to each of the HIV-infected pregnant women (n = 38) enrolled in the TEmAA ANRS 12109 trial, step 1, compared to median values for other adults after a 200-mg FTC dose at steady state
Placental transfer.
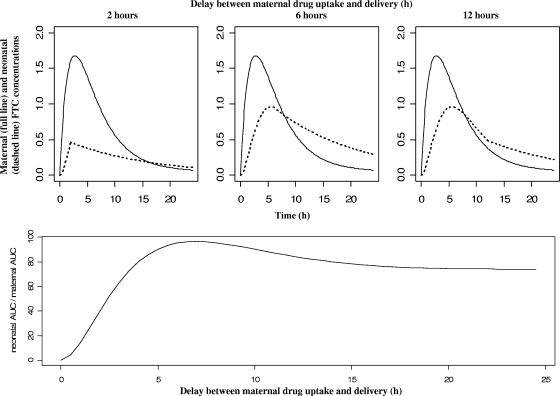
The median delay between samples drawn before the first maternal FTC administration and those taken at delivery was 5.1 h (minimum [min] to maximum [max], 0.6 to 20 h). At delivery, the median predicted neonatal and maternal concentrations were, respectively, 0.72 mg/liter (min to max, 0.05 to 1.19 mg/liter) and 1.16 mg/liter (min to max, 0.14 to 1.99 mg/liter). The median predicted ratio between cord blood and maternal concentrations at delivery was 76% (min to max, 9 to 144), depending on the delay between maternal drug administration and delivery. This range of concentration ratios at delivery suggests that placental transfer depends on the delay between maternal drug intake and delivery and could not be given as a simple percentage. A more representative measure of placental transfer would be the ratio between neonatal and maternal FTC AUCs for 24 h. Figure 4 represented maternal and neonatal concentrations as a function of time when delivery occurred 2, 6, or 12 h after maternal drug intake. This figure (bottom) showed the neonatal-to-maternal AUC ratio as a function of the delay between maternal administration and labor.
FIG. 4.
(Top) Population-predicted FTC concentrations in the mother (full line) and her neonate (dashed line) (cord blood equation before delivery and neonatal equation after) versus time for 2-h (left panel), 6-h (middle panel), or 12-h (right panel) delays between drug administration and delivery time. (Bottom) Neonatal to maternal FTC AUC ratio as a function of the delay between drug administration and delivery time.
Determination of the optimal timing for FTC administration to the newborns.
As the median predicted neonatal concentration was relatively high at delivery (0.72 mg/liter), with a 10.6-h half-life, this remained >0.077 mg/liter (minimal concentrations for adults) for at least three half-lives, i.e., 31.8 h after delivery. Table 4 summarizes neonatal minimal concentrations before administration, AUC0→24 h values, and the time during which neonatal concentrations remained >0.077 mg/liter for 1, 2, or 3 mg/kg at 1 h after birth and 2 mg/kg at 12 h after birth. If FTC was only administered to the mother, thanks to placental transfer, it would produce a neonatal AUC0→24 h of 8.2 mg/liter·h. Administering, as a single dose, 1 mg/kg of FTC 1 h after birth or 2 mg/kg 12 h after birth would allow the neonate to obtain same exposition as adults. These results were obtained assuming a neonatal volume of distribution (VF) proportional to the maternal volume of distribution on a BW basis [mean VF = (V1+V2) × BWneonate/BWMother ≈ (127 + 237) × 2.8/60.3 ≈ 16.9 liters]. However, 1 mg/kg administered 1 h after birth would produce an AUC0→24 h of 9.2 mg/liter·h and a concentration of >0.077 mg/liter during 34 h if VF was in reality two times higher than in the assumption. This dose would produce an AUC0→24 h of 12.0 mg/liter·h and a concentration of >0.077 mg/liter during 40.2 h if the true VF was two times lower than assumed. In both cases, even with a 100% error in the neonatal volume of distribution, the AUC0→24 h was close to 10.4 mg/liter·h.
TABLE 4.
Neonatal parameters estimated for administrations of 0, 1, 2, and 3 mg FTC/kg 1 h after birth and 2 mg/kg 12 h after birth (TEmAA ANRS 12109 trial, step 1)
| Dose (mg/kg)b | Median value
|
||
|---|---|---|---|
| AUC0→24 (mg/liter·h) | Cmin (mg/liter) | Timea (h) | |
| 0 | 8.2 | ||
| 1 (1) | 10.1 | 0.67 | 36.6 |
| 2 (1) | 11.9 | 0.67 | 40.2 |
| 3 (1) | 13.4 | 0.67 | 42.8 |
| 2 (12) | 10.5 | 0.31 | 33.9 |
Time during which neonatal concentrations remained >0.077 mg/liter.
Numbers in parentheses indicate the number of hours after birth at which the dose was administered.
DISCUSSION
In the present work, mother and child FTC pharmacokinetics were satisfactorily described by the proposed compartmental model. The following observations support the validity of this model.
Population-predicted maternal, cord blood, and neonatal concentrations were well correlated with observed concentrations. The population model was validated, thanks to the visual predictive check method.
In pregnant women, the AUC obtained from our population model was decreased (i.e., 14.3 mg/liter·h for a 400-mg dose and 7.15 mg/liter·h for a 200-mg dose) compared to that for nonpregnant adults (10.7 mg/liter·h for a 200-mg dose). This is in agreement with the PATCG/IMPAACT P1026 study which reports, during the third trimester of pregnancy, a median AUC of 8.6 mg/liter·h for a 200-mg dose (3).
As shown in Table 3, despite a higher elimination clearance in pregnant women than in nonpregnant adults, the 400-mg FTC administration before delivery produces higher exposure than does the 200-mg administration in other adults at steady state. Calculating FTC clearance as a dose-to-AUC ratio, we found 28.0 liters/h for pregnant women (our study), compared to 18.7 liters/h (4, 18) and 20.4 liters/h for other adults (15). FTC clearance was increased by 37 or 50%. FTC is primarily excreted by the kidney by both glomerular filtration and tubular secretion, with 86% recovery of the dose achieved in urine, as described in the full prescribing information for Truvada (http://www.gilead.com/pdf/truvada_pi.pdf). During pregnancy, renal plasma flow increases by 25 to 50% and glomerular filtration rate by 50%, which should have enhanced FTC elimination (13). The lowest FTC clearance increase in the PATCG/IMPAACT P1026 study (23.3 liters/h versus 28.0 liters/h in our study) may be due the sampling time during pregnancy (third trimester versus the day of delivery in our study). None of the covariates tested had an effect on maternal absorption or elimination clearance.
No data were reported on FTC placental transfer. In this study, from one sample at delivery (at various times after drug administration) in each mother-cord blood pair, we could draw maternal and cord blood concentration curves as a function of the delay and estimate intersubject and residual variabilities. Placental transfer was estimated as fetal- to maternal-exposure ratio to the drug. We found a relatively constant ratio of 80% for a delivery occurring at least 4 h after maternal drug administration. This transfer seems to be due mainly to a passive diffusion of the drug through the placenta. Data about active transport are missing.
Cord blood concentrations were relatively high (0.72 mg/liter) compared to the adult minimal concentrations previously reported (0.07 mg/liter). This was due to both a good placental transfer of the drug and a higher exposure in mothers; with 400 mg of FTC at delivery time, maternal exposure was higher than the exposure with 200 mg in nonpregnant adults. So, even if women delivered a long time after drug intake, cord blood concentrations should remain over the adult minimal concentration. However, readministering two tablets of Truvada to the mother after 12 h of labor (if she did not deliver yet, as suggested for tenofovir [unpublished data]) would produce reasonable cord blood FTC concentrations (similar to cord blood concentrations of neonates born 5 h after first maternal drug intake).
The FTC median neonatal half-life was 10.6 h, in agreement with the Blum et al. study reporting half-lives of 12.5 h in neonates from birth to 21 days, 11.5 h for 22- to 42-day-old infants, and 11.8 h for 43- to 90-day-old children (5). Moreover, these half-lives are comparable to those for children (9.3 to 11.7 h for 2- to 17-year-old children) (17) and adults (10.5 h in the Blum et al. study, 9.4 h in the Zhong et al. study, and 8.3 h in the Ramanathan et al. study) (4, 15, 18).
Since the model was validated, thanks to the visual predictive check method, it was used to simulate the optimal dosage. For this, it was assumed that the child had the same absorption rate and bioavailability as the mother and that its volume of distribution was proportional to the total maternal volume distribution on a BW basis. Accordingly, in our model, the mean volume of distribution was 16.9 liters for a child weighing 2.7 kg at birth, which is close to the volume of distribution of 14 liters (t1/2 = 12.5 h and CL = 13 ml/min) found in the 18 children from days 0 to 21 of the Blum et al. study (5). Moreover, even with a 100% error in the neonatal volume of distribution, the AUC0→24 h and the time during which the concentration was >0.077 mg/liter showed a <20% change. The optimal single neonatal dose was determined in order to obtain an exposure in neonates similar to the known exposure in adults (i.e., 10.4 mg/liter·h) and concentrations above the residual adult concentration (0.077 mg/liter) before and as long as possible after neonatal administration. Criteria were based on plasma FTC concentrations, although intracellular FTC triphosphate concentrations would have been more appropriate to follow the pharmacologically active part of FTC. It was also supposed that the enzymes of phosphorylation were matured in the neonates (16). For practical reasons, we suggest that FTC should be administered to the neonate at the same time as tenofovir (unpublished data). As previously shown, tenofovir should be administered quickly after birth, i.e., 1 hour after delivery, so we simulated concentrations obtained with 1, 2, or 3 mg FTC/kg given 1 hour after birth to the neonate. A 2-mg/kg FTC administration given 12 h after birth was also simulated. Taking into account the high exposure of the fetus to the drug due to maternal administration (AUC0→24 h = 8.2 mg/liter·h), only 1 mg FTC/kg was needed 1 hour after birth to reach an AUC0→24 h of 10.1 mg/liter·h. However, if the neonate could only be administered FTC 12 h after birth, the dose would increase to 2 mg/kg. This dosage is recommended for a single administration following birth and not for repeated doses as in the Blum et al. study (5).
In conclusion, the maternal 400-mg FTC administration before delivery produces higher exposure than does the 200-mg administration in other adults at steady state. FTC placental transfer, described by the neonatal- to maternal-exposure ratio, was around 80%. Finally, neonates should receive 1 mg FTC/kg as soon as possible after birth or 2 mg/kg 12 h after birth to have concentrations comparable to those observed in adults. The second step of the TEmAA trial will validate these recommendations.
Acknowledgments
We acknowledge the French Agence Nationale de Recherche contre le VIH/SIDA et les hepatitis virales (ANRS) for sponsoring the trial, as well as the European and Developing Countries Clinical Trials Partnership (EDCTP) for their additional financial support.
We greatly thank the local investigators and their staff at the Formations Sanitaires Urbaines de Youpougon and Abobo and the Centre Hospitalier Universitaire de Yopougon in Abidjan, Côte d'Ivoire, the Calmette Hospital and Pasteur Institute in Phnom Penh, Cambodia, and the Perinatal HIV Research Unit and Lesedi Clinic in Soweto, South Africa. We also thank the women who agreed to participate in the trial and their infants. We acknowledge Gilead Sciences for providing the study drugs. D.K.E. was an EDCTP senior fellow from 2005 to 2007.
The TEmAA trial group is constituted as follows. Coinvestigators include Christine Rouzioux, Stéphane Blanche and Jean-Marc Treluyer, Marie-Laure Chaix and Elisabeth Rey (Paris, France), N′dri-Yoman (Abidjan, Côte d'Ivoire), Kruy Leang Sim and Eric Nerrienet (Phnom Penh, Cambodia), and Glenda Gray and James McIntyre (Soweto, South Africa). The trial coordinator is Elise Arrivé (Bordeaux, France). Other members of the TEmAA ANRS 12109 study group (by location and alphabetic order) include the following: Déborah Hirt and Saik Urien (Paris, France); Gérard Allou, Clarisse Amani-Bosse, Divine Avit, Gédéon Bédikou, Kouakou Brou, Patrick Coffié, Patrice Fian, Eulalie Kanga, Broulaye Kone, Suzanne Kouadio, Guy César Kouaho, Jeanne Eliam Kouakou, Sidonie Ngatchou, Touré Pety, Zenica Seoue, and Mamourou Kone (Abidjan, Côte d'Ivoire); Laurence Borand, Pinn Chou, Kearena Chhim, Meng Ly Ek, Viseth Horm Srey, Seng Hout, Sethikar Im, Saroeum Keo, Vannith Lim, Sopheak Ngin, Vara Ouk, Vibol Ung, and the Magna and Maryknoll associations (Phnom Penh, Cambodia); Gail Ashford, Promise Duma, Portia Duma, Sarita Lalsab, Shini Legote, Tshepiso Mabena, Joseph Makhura, Modise Maphutha, Selvan Naidoo, and Mandisa Nyati (Soweto, South Africa). The scientific board includes Bernard Koffi Ngoran (Abidjan, Côte d'Ivoire), Koum Kanal (Phnom Penh, Cambodia), Lynn Morris (Johannesburg, South Africa), Séverine Blesson (ANRS, Paris, France), Camille Aubron-Olivier (Gilead Sciences, Paris, France), Gilles Peytavin (Paris, France), Koen Van Rompay (Davis, CA), and Valériane Leroy (Bordeaux, France). The independent committee includes John Sullivan (Worcester, MA), Philippe Lepage (Brussels, Belgium), Laurent Mandelbrot (Paris, France), Marie-Louise Newell (London, United Kingdom), and Anne-Marie Taburet (Paris, France).
Footnotes
Published ahead of print on 22 December 2008.
REFERENCES
- 1.Beal, S. L. 2001. Ways to fit a PK model with some data below the quantification limit. J. Pharmacokinet. Pharmacodyn. 28:481-504. [DOI] [PubMed] [Google Scholar]
- 2.Beal, S. L., and L. B. Sheiner. 1998. NONMEM user's guide. NONMEM Project Group, University of California—San Francisco, San Francisco, CA.
- 3.Best, B., A. Stek, C. Hu, S. Burchett, S. Rossi, E. Smith, B. Sheeran, J. Read, E. Capparelli, M. Mirochnick, et al. 2008. High-dose lopinavir and standard-dose emtricitabine pharmacokinetics during pregnancy and postpartum, abstr. 629. Abstr. 15th Conf. Retrovir. Opportun. Infect., Boston, MA.
- 4.Blum, M. R., G. E. Chittick, J. A. Begley, and J. Zong. 2007. Steady-state pharmacokinetics of emtricitabine and tenofovir disoproxil fumarate administered alone and in combination in healthy volunteers. J. Clin. Pharmacol. 47:751-759. [DOI] [PubMed] [Google Scholar]
- 5.Blum, M. R., D. Ndiweni, G. Chittick, N. Adda, D. Kargl, and D. Josipovic. 2006. Steady-state pharmacokinetic evaluation of emtricitabine in neonates exposed to HIV in utero, abstr. 568. Abstr. 13th Conf. Retrovir. Opportun. Infect., Denver, CO.
- 6.Chi, B. H., M. Sinkala, F. Mbewe, R. A. Cantrell, G. Kruse, N. Chintu, G. M. Aldrovandi, E. M. Stringer, C. Kankasa, J. T. Safrit, and J. S. Stringer. 2007. Single-dose tenofovir and emtricitabine for reduction of viral resistance to nonnucleoside reverse transcriptase inhibitor drugs in women given intrapartum nevirapine for perinatal HIV prevention: an open-label randomised trial. Lancet 370:1698-1705. [DOI] [PubMed] [Google Scholar]
- 7.Eshleman, S. H., D. R. Hoover, S. Chen, S. E. Hudelson, L. A. Guay, A. Mwatha, S. A. Fiscus, F. Mmiro, P. Musoke, J. B. Jackson, N. Kumwenda, and T. Taha. 2005. Nevirapine (NVP) resistance in women with HIV-1 subtype C, compared with subtypes A and D, after the administration of single-dose NVP. J. Infect. Dis. 192:30-36. [DOI] [PubMed] [Google Scholar]
- 8.Ihaka, R., and R. Gentleman. 1996. R: a language for data analysis and graphics. J. Comput. Graph. Stat. 5:299. [Google Scholar]
- 9.Jackson, J. B., G. Becker-Pergola, L. A. Guay, P. Musoke, M. Mracna, M. G. Fowler, L. M. Mofenson, M. Mirochnick, F. Mmiro, and S. H. Eshleman. 2000. Identification of the K103N resistance mutation in Ugandan women receiving nevirapine to prevent HIV-1 vertical transmission. AIDS 14:F111-F115. [DOI] [PubMed] [Google Scholar]
- 10.Krauer, B., F. Krauer, and F. E. Hytten. 1980. Drug disposition and pharmacokinetics in the maternal-placental-fetal unit. Pharmacol. Ther. 10:301-328. [DOI] [PubMed] [Google Scholar]
- 11.Le Saux, T., S. Chhun, E. Rey, O. Launay, L. Weiss, J. P. Viard, G. Pons, and V. Jullien. 2008. Quantification of seven nucleoside/nucleotide reverse transcriptase inhibitors in human plasma by high-performance liquid chromatography with tandem mass-spectrometry. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 865:81-90. [DOI] [PubMed] [Google Scholar]
- 12.Loebstein, R., A. Lalkin, and G. Koren. 1997. Pharmacokinetic changes during pregnancy and their clinical relevance. Clin. Pharmacokinet. 33:328-343. [DOI] [PubMed] [Google Scholar]
- 13.Mirochnick, M. 2000. Antiretroviral pharmacology in pregnant women and their newborns. Ann. N. Y. Acad. Sci. 918:287-297. [DOI] [PubMed] [Google Scholar]
- 14.Parry, E., R. Shields, and A. C. Turnbull. 1970. Transit time in the small intestine in pregnancy. J. Obstet. Gynaecol. Br. Commonw. 77:900-901. [DOI] [PubMed] [Google Scholar]
- 15.Ramanathan, S., G. Shen, A. Cheng, and B. P. Kearney. 2007. Pharmacokinetics of emtricitabine, tenofovir, and GS-9137 following coadministration of emtricitabine/tenofovir disoproxil fumarate and ritonavir-boosted GS-9137. J. Acquir. Immune Defic. Syndr. 45:274-279. [DOI] [PubMed] [Google Scholar]
- 16.Rodman, J. H., P. M. Flynn, B. Robbins, E. Jimenez, A. D. Bardeguez, J. F. Rodriguez, S. Blanchard, and A. Fridland. 1999. Systemic pharmacokinetics and cellular pharmacology of zidovudine in human immunodeficiency virus type 1-infected women and newborn infants. J. Infect. Dis. 180:1844-1850. [DOI] [PubMed] [Google Scholar]
- 17.Wang, L. H., A. A. Wiznia, M. H. Rathore, G. E. Chittick, S. S. Bakshi, P. J. Emmanuel, and P. M. Flynn. 2004. Pharmacokinetics and safety of single oral doses of emtricitabine in human immunodeficiency virus-infected children. Antimicrob. Agents Chemother. 48:183-191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhong, J., G. E. Chittick, L. H. Wang, J. Hui, J. A. Begley, and M. R. Blum. 2007. Pharmacokinetic evaluation of emtricitabine in combination with other nucleoside antivirals in healthy volunteers. J. Clin. Pharmacol. 47:877-889. [DOI] [PubMed] [Google Scholar]