Abstract
The dose of efavirenz during concomitant rifampin (RMP) administration is a matter of debate. We studied the influence of RMP coadministration on the steady-state pharmacokinetics of efavirenz in human immunodeficiency virus type 1 (HIV-1)-infected patients in South India. Fifty-seven HIV-tuberculosis (TB)-coinfected and 15 HIV-1-infected patients receiving combination antiretroviral therapy (CART) with an efavirenz (600 mg once daily)-containing regimen were recruited. HIV-TB-coinfected patients were receiving treatment with RMP-containing regimens. A complete pharmacokinetic study was conducted with 19 HIV-TB patients on two occasions (with and without RMP). Trough concentrations of efavirenz were measured in the remaining 38 patients during RMP coadministration. The 15 HIV-infected patients underwent complete pharmacokinetic sampling on one occasion. Plasma efavirenz was estimated by high-performance liquid chromatography, and genotyping of CYP2B6 G516T polymorphism was performed by sequencing. Peak and trough concentrations and exposure to efavirenz were significantly higher in TT than in GT and GG genotype patients (P < 0.001). Although RMP coadministration decreased the peak and trough concentrations and exposure to efavirenz by 17.8, 20.4, and 18.6%, respectively, the differences were not statistically significant. The trough concentration of efavirenz was subtherapeutic (less than 1.0 μg/ml) in 6 (8%) of 72 patients. In this South Indian population of HIV-infected patients, CYP2B6 G516T polymorphism but not RMP coadministration significantly influenced the pharmacokinetics of efavirenz; patients with the TT genotype had very high blood levels of efavirenz. While a small proportion of patients had subtherapeutic efavirenz levels, the clinical implications are uncertain, as all had good immunological responses to CART.
Tuberculosis (TB) remains one of the most important opportunistic infections in human immunodeficiency virus (HIV)-infected individuals. The burden of effectively treating HIV-TB-coinfected patients is a well-recognized global public health problem. A decreased risk of death has been observed in patients starting combination antiretroviral therapy (CART) compared with those not receiving CART after the diagnosis of TB (2, 9, 13). In India, there are effective treatments available for both HIV disease and TB through the government program. Concomitant administration of CART and anti-TB medications is often complicated because of drug-drug interactions and the adverse-effect profile (17, 30, 36).
Efavirenz (EFV) is a potent nonnucleoside reverse transcriptase inhibitor for the treatment of HIV type 1 (HIV-1) infection. EFV has been recommended as a first-line option in antiretroviral therapy (ART) and the preferential choice in TB- and HIV-coinfected patients, despite induction of the cytochrome P-450 system by rifampin (RMP). The available pharmacokinetic data provide evidence of a 13 to 25% reduction in EFV levels when it is coadministered with RMP (20), which is lower than those of nevirapine (40%) and protease inhibitors (80 to 95%) (7). Although effective pharmacological, clinical, immunological, and virologic responses have been reported with a 600-mg dose of EFV (11, 22, 27, 28), the adequacy of this dose during concomitant treatment with RMP remains a matter of debate. In fact, after reviewing the current literature, the FDA concluded that the available data are insufficient to support definitive dosing recommendations for the coadministration of EFV and RMP (10). Differences in patients' body weights appear to cause further differences in exposure to EFV, raising the question of whether the EFV dose should be increased in people with higher body weights (20, 26).
Several factors could alter the pharmacokinetics of EFV. Sex is reported to have a modest influence on the pharmacokinetic profiles of certain antiretroviral drugs, including EFV (6, 29). A single nucleotide polymorphism at position 516 on the CYP2B6 gene has been widely reported to influence the pharmacokinetics of EFV (3, 14, 18, 19, 31, 32, 33, 38, 39). India has a large number of HIV-infected individuals, and access to ART is improving. With an increasing number of patients receiving treatment, it is important to study the pharmacokinetics of EFV, which is extensively used, particularly by HIV-TB-coinfected patients. No information is available on the pharmacokinetics of EFV in HIV-infected patients in India, who are genetically different from the other ethnic groups studied so far. Herein we report the influences of sex, body weight, CYP2B6 G516T polymorphism, and RMP coadministration on the steady-state pharmacokinetics of EFV in HIV-infected patients in an ethnic South Indian population.
MATERIALS AND METHODS
Patients.
Seventy-two HIV-infected patients (57 with active TB) who were attending the outpatient clinic at the Government Hospital of Thoracic Medicine, Tambaram, India, during March to July 2006 took part in this study. All of the patients lived in the state of Tamil Nadu in South India, were adults, and weighed more than 30 kg. They were undergoing treatment regularly with EFV (600 mg/day) along with lamivudine (150 mg twice a day) and stavudine (30/40 mg twice a day) or zidovudine (300 mg twice a day) for a minimum period of 1 week. In addition, HIV-TB-coinfected patients were receiving treatment regularly for TB for a minimum period of 2 weeks. Their anti-TB treatment (ATT) regimen consisted of RMP (450 mg for patients with body weights of <60 kg and 600 mg for those with body weights of ≥60 kg), isoniazid (600 mg), pyrazinamide (1,500 mg), and ethambutol (1,200 mg) thrice weekly for 2 months, followed by RMP and isoniazid thrice weekly for 4 months at the same doses. Only those patients who expressed a willingness to participate in the study and agreed to give informed written consent were recruited. Chronic alcoholics and females on hormonal birth control pills were not included in the study. The study commenced after the approval of the Institutional Ethics Committee was obtained.
Design and conduct of the study.
The study was conducted at the Government Hospital of Thoracic Medicine, Chennai, India. Eligible patients were admitted to the hospital at least a day prior to the study. Informed written consent was obtained from all of the patients.
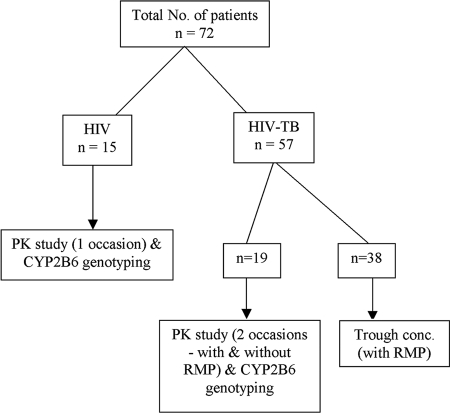
Details of patient recruitment and the tests performed are shown in Fig. 1. A single blood collection at 24 h after EFV administration (trough level) was done for 38 HIV-TB patients while they were receiving treatment for HIV and TB. A complete pharmacokinetic study was conducted with 34 patients. Of these, 19 who had concurrent TB were investigated on two occasions; the first occasion was while the patients were receiving treatment for HIV and TB, and the second occasion was at least 1 month after stopping ATT. A crossover design was employed in which each patient served as his own control. The remaining 15 HIV-infected patients without TB were investigated only on one occasion.
FIG. 1.
Details of patient recruitment to the study.
On the day of the study, blood samples (3 ml) were drawn in heparinized Vacutainer tubes before dosing and serially at 1, 2, 4, 6, 8, 12, and 24 h after dosing. An additional sample (1 ml) was collected in an EDTA Vacutainer tube at any one time point during the pharmacokinetic study from all of the patients. This sample was drawn when the patients were not receiving RMP.
The heparinized blood samples were centrifuged immediately, and plasma was stored at −20°C until estimation of EFV was carried out. The EDTA blood sample was used for DNA extraction and genotyping of CYP2B6 G516T polymorphism.
Estimation of plasma EFV.
Estimation of plasma EFV was performed by high-performance liquid chromatography with UV detection (Shimadzu Corporation, Kyoto, Japan) according to a validated method (32).
Genotyping of CYP2B6 G516T polymorphism.
The genetic characterization of the CYP2B6 gene was performed with genomic DNA extracted from whole blood. A 204-bp fragment in exon 4 of the CYP2B6 gene containing the target site (position 516) was amplified by a single round of PCR with CYP2B6 Forward Primer 5′ CTTGACCTGCTGCTTCTTCC 3′ and CYP2B6 Reverse Primer 5′ TCCCTCTCCGTCTCCCTG 3′ (1).
The amplicon was directly sequenced with a 3100 Avant Genetic analyzer (Applied Biosystems).
Pharmacokinetic analysis.
Pharmacokinetic variables such as the maximum or peak concentration (Cmax), the minimum or trough concentration (Cmin), the time to attain Cmax, exposure or area under the concentration-time curve from 0 to 24 h (AUC0-24), apparent oral clearance (CL), and half-life were calculated by a noncompartmental model with WinNonlin software (version 5.1; Pharsight Corporation, Mountain View, CA).
Statistical evaluation.
Analysis of data was performed with the SPSS (version 14) package. Differences in the pharmacokinetic parameters of EFV between males and females were studied by using the t test. The Wilcoxon signed-rank test (paired test) was used to determine the significance of differences in the pharmacokinetic variables of EFV with and without RMP. Pearson's test was used to determine the correlation between body weight and the pharmacokinetic parameters of EFV. Groupwise comparisons of certain pharmacokinetic variables of EFV among the CYP2B6 G516T genotype patients were done by using Tukey's multiple-comparison test. Deviation of genotype frequencies from Hardy-Weinberg equilibrium expectations was evaluated by using the chi-square test. Allelic frequency was estimated by dividing the total number of copies of individual alleles by all of the alleles in the population. A P value of ≤0.05 was considered statistically significant.
RESULTS
The demographic details of all of the patients are given in Table 1. HIV and HIV-TB patients were similar with respect to age, body weight, pre-CART CD4 cell counts, and duration of ART. There were more males in both groups of patients.
TABLE 1.
Demographic details of study patients
| Characteristic | 72 patients (HIV and HIV-TB) | 57 HIV-TB patients | 15 HIV patients |
|---|---|---|---|
| Age (yr) | 36.8 (26-56)a | 36.0 (26-56) | 35.1 (26-52) |
| No. of males | 63 | 53 | 10 |
| Body wt (kg) | 50.4 (34-75) | 49.2 (34-75) | 50.4 (34-69) |
| Pre-CART CD4 count (cells/mm3) | 98 (1-304) | 111 (1-304) | 91 (13-192) |
| Duration of CART (mo) | 5.1 (0.3-17) | 5.4 (0.3-17) | 4.5 (0.3-14) |
The values shown are means (ranges).
The effect of RMP on the pharmacokinetics of EFV was studied in 19 HIV-TB patients who were investigated on two occasions, that is, during ATT with RMP-containing regimens and at least 1 month after stopping ATT. Pharmacokinetic variables of EFV were compared on the two occasions. Except for four patients, all had higher blood levels of EFV after stopping ATT than during ATT. Although RMP lowered the Cmax, Cmin, and AUC0-24 of EFV and increased the clearance of the drug, the differences were not statistically significant. The mean percent decreases in Cmax, Cmin, and AUC0-24 were 17.8, 20.4, and 18.6%, respectively (Table 2).
TABLE 2.
Steady-state pharmacokinetics of EFV with and without RMPa
| Treatment | Cmax | Cmin | Tmax | AUC(0-24) | CL | t1/2 |
|---|---|---|---|---|---|---|
| EFV | 9.88 ± 9.38 | 5.84 ± 7.54 | 2.53 ± 1.07 | 169.77 ± 205.34 | 7.25 ± 5.10 | 24.98 ± 13.19 |
| EFV + RMP | 8.12 ± 7.10 | 4.65 ± 5.80 | 2.47 ± 0.97 | 138.19 ± 148.28 | 9.27 ± 6.52 | 17.57 ± 9.42b |
The values shown are means ± standard deviations for 19 patients. Tmax, time to attain Cmax; t1/2, half-life.
P < 0.05.
Trough concentrations of EFV were available for 57 patients during concomitant RMP treatment (19 patients who took part in the two-occasion pharmacokinetic study and 38 patients for whom a single blood collection at 24 h after EFV administration was performed) and 34 patients without RMP (19 patients who took part in the two-occasion pharmacokinetic study and 15 patients in the one-occasion pharmacokinetic study). The mean Cmins of EFV in the 57 and 34 patients were 4.39 and 4.37 μg/ml, respectively. Considering all 72 patients (15 HIV and 57 HIV-TB patients) recruited for the study, the trough concentration of EFV was subtherapeutic (less than 1.0 μg/ml) in 6 patients (8%); 2 of them were not on RMP, while 4 were receiving RMP at the time of the study. The mean CD4 cell counts at the start of ART (baseline) in the 72 patients was 98 (range, 1 to 304) cells/mm3. This increased to 304 (range, 32 to 925) cells/mm3 at 6 months in 30 patients and 366 (range, 153 to 582) cells/mm3 in 25 patients, respectively.
There were 25 males and 9 females among the 34 patients who took part in the pharmacokinetic study. Comparisons of pharmacokinetic variables of EFV between males and females were made while they were not receiving ATT. Although males had higher Cmax (7.48 versus 6.02 μg/ml), Cmin (4.1 versus 2.71 μg/ml), and AUC0-24 (120.48 versus 85.13 μg/ml · h) values, the differences were not statistically significant. There was a positive correlation between patients' body weights and EFV clearance; this was statistically significant (r = 0.37, P = 0.034). The pharmacokinetic variables of EFV, such as Cmax, Cmin, AUC0-24, and CL, calculated for 34 patients were highly correlated (r > 0.9, P < 0.001).
Of the 34 patients who underwent complete pharmacokinetic sampling, CYP2B6 G516T genotyping could be performed for 25 patients. Among these, the numbers with the GG, GT, and TT genotypes were 10, 8, and 7, respectively. The G and T allele frequencies were 0.56 and 0.44, respectively. When a χ2 test of observed versus predicted genotype frequencies was conducted, the polymorphism was found to be in Hardy-Weinberg equilibrium. The mean Cmax, Cmin, and AUC0-24 of EFV were significantly higher and the CL was lower in TT genotype patients than in GG and GT genotype patients (P < 0.001). The distribution of EFV AUC0-24 values among patients with the three genotypes is shown in Fig. 2.
FIG. 2.
Plasma exposure to EFV in the different genotypes of CYP2B6 G516T polymorphism. A scatter diagram of the distribution of plasma exposures to EFV in patients with different CYP2B6 G516T genotypes is shown. The numbers of GG, GT, and TT genotype patients were 10, 8, and 7, respectively.
DISCUSSION
Pharmacokinetic variations due to several factors could lead to heterogeneity in the response to ART. In India, patients with TB (with or without HIV coinfection) are treated with a standard 6-month intermittent anti-TB regimen (4). The standard antiretroviral regimen employed for such patients consists of EFV with two nucleoside reverse transcriptase inhibitors. Supportive evidence for a definitive dosing recommendation for EFV coadministered with RMP remains limited. While there have been suggestions to use EFV at a dose of 800 mg/day when giving it along with RMP (15), observational studies have shown that EFV at 600 mg/day is sufficient for most HIV-infected patients (11, 22, 27, 28). There are few clinical studies on the pharmacokinetic effect of RMP on plasma EFV levels (20, 26). These studies have shown that patients with higher body weights have reduced plasma EFV concentrations and suggested that it is advisable to increase the dose of EFV to 800 mg/day when coadministering it with RMP to patients weighing >60 kg. However, the safety and tolerability of the higher dose are a matter of concern (5). This becomes more complicated because of variability in pharmacokinetics, therapeutic response, and side effects in patients belonging to different racial and ethnic groups (33).
This study reports, for the first time, the steady-state pharmacokinetics of EFV in HIV-infected patients in South India. Our findings showed that RMP coadministration did not significantly alter the pharmacokinetics of EFV. Further, the trough concentration of EFV remained within the therapeutic range of the drug (>1.0 μg/ml) in the majority of the patients (66 of 72). Four patients had lower blood EFV levels after stopping ATT than during concomitant ATT. The reasons for this reverse trend are unclear and could be a function of the wide intraindividual variability of this drug; similar findings have been reported by others (11, 20). All of the patients who took part in this study were taking ART regularly, as assessed by the medical officer based on their monthly visit to the hospital to collect their drugs and by pill counting. Among the six patients with subtherapeutic trough levels, four were receiving RMP. Hence, 2 of 15 HIV and 4 of 57 HIV-TB patients had EFV trough levels of <1.0 μg/ml. The numbers of GG, GT, and TT genotype patients in this group were two, two, and one, respectively. The CYP2B6 genotype status of one patient was not known. Five patients showed a good immunological response following ART, while one died. Neither RMP coadministration nor the GG or GT genotype seemed to correlate with subtherapeutic EFV levels, and hence it is not possible to attribute any specific reason for EFV levels to fall below the therapeutic range in these six patients. Further, while an EFV trough level of 1.0 μg/ml is generally accepted as the threshold above which EFV levels must be maintained, the supporting data for this come from the study of Marzolini et al. (25); however, this threshold may be higher than is required for virologic suppression.
Our study findings do not support increasing the dose of EFV when coadministering it with RMP. Although concomitant RMP administration lowered the peak concentration and exposure to EFV by about 18%, this did not seem to have any adverse impact on the immunological outcomes. This is in agreement with that suggested by others, who observed excellent clinical outcomes when giving EFV at 600 mg/day to patients receiving RMP (11, 22, 27, 28). A study conducted with HIV-TB patients receiving EFV at 600 mg/day and RMP reported virological success in about 80% of the patients studied at 48 weeks of treatment (24). In a comparative study of two groups of HIV-infected patients receiving EFV at 600 and 800 mg/day, Manosuthi et al. did not observe any difference in plasma EFV levels and time to virological success (23). A similar viewpoint has been put forth by López-Cortés et al., who did not observe a relationship between the EFV trough concentration and virological efficacy at a dose of 800 mg/day, indicating that a firm recommendation cannot be made to increase the dose of EFV when concomitantly giving it with RMP (21).
It has been reported that patients with higher body weights have lower blood levels of EFV and that such patients would require a higher dose of the drug, particularly during concomitant RMP treatment (20, 26). Our study did not observe any significant correlation between body weight and EFV peak and trough concentrations and exposure. Our study findings also showed that sex did not alter the pharmacokinetic profile of EFV. This is in agreement with those reported by Ribaudo et al. (33) and Rodriguez-Novoa et al. (34) but disagrees with the findings of others (6, 29).
Several polymorphisms in the gene CYP2B6 may influence isoenzyme activity (18) and therefore the hepatic clearance of EFV. The most significant allelic variant that is reported to influence plasma concentrations of EFV is a G-to-T change at codon 516 (that is, a 516 G→T single-nucleotide polymorphism) (3, 14, 18, 19, 33, 34, 35, 38, 39), while other reported polymorphisms include CYP2B6 T983C, CYP3A4 A392G, and CYP3A5 A6986G (14, 38, 40). We studied only the CYP2B6 G516T polymorphism and found that TT genotype patients had significantly elevated blood EFV and drug exposure levels than GG and GT genotype patients. This indicates that pharmacogenetic variations lead to pharmacokinetic differences and that this polymorphism influences the pharmacokinetics of EFV in the Indian population too. Our study was underpowered to observe if changes in EFV pharmacokinetics in response to RMP differed based on genotype. It would be interesting to study the impact of other CYP polymorphisms on EFV pharmacokinetics.
The peak and trough concentrations and exposure to EFV observed in the Indian patients were considerably higher than those reported by López-Cortés et al. in the Spanish population (20) and by Haas et al. in African Americans, Hispanics, and European Americans (14). Similar findings with respect to plasma nevirapine levels were observed in a previous study of ours (31). It is of interest that in spite of the concomitant use of EFV and RMP, the peak concentration of EFV was >4 μg/ml in 11 of 19 patients, and no serious adverse events were reported in any of these patients. In this study, TT genotype patients accounted for 28% of the total (7 of 25 patients), which is similar to that reported by Kwara et al., who observed that 27% of West African patients (7 of 26) had the TT genotype (18). We obtained a T allele frequency of 0.44, which is higher than that reported in Koreans (0.14), Japanese (0.16), Caucasians (0.25), white Americans (0.25), and African Americans (0.28) but similar to that of West Africans (0.42), Chinese (0.43), and Hispanics (0.43) (8, 16, 37). However, the number of patients genotyped for CYP2B6 G516T polymorphism was quite small. Although TT genotype patients were distinct from GG and GT genotype patients with respect to plasma exposure to EFV (Fig. 2), there was a clear split among the TT genotype patients; three patients had very high plasma exposure levels. The possible reason for this dramatic split could be the wide interpatient variability associated with plasma levels of EFV or the interplay of other polymorphisms such as CYP2B6 T983C, which is also reported to influence EFV pharmacokinetics (40). Thus, a high proportion of patients in South India would have high blood levels of EFV. Hence, the time may have come for clinical trials with lower doses of EFV. In fact, the feasibility of genotype-based EFV dose reduction in CYP2B6 G516T allelic carriers was studied and found to relieve patients of EFV-associated central nervous system symptoms (12). Such studies are important, because they would have cost-saving implications for developing countries, apart from the reduction in adverse events for individual patients. In fact, the study of Wyen et al. on CYP2B6 T983C polymorphism and plasma EFV reported that homozygote patients (CC genotype patients) discontinued therapy within 1 week because of central nervous system toxicity (40).
One limitation of this study was that we could not do study patient viral load measurements, which would have enabled us to relate variations in EFV pharmacokinetics to treatment outcomes. Further, we studied the influence of only a single nucleotide polymorphism, 516 G→T, in the CYP2B6 gene; plasma levels of EFV are probably influenced by other polymorphisms, especially CYP2B6 983 T→C (40). In conclusion, this cross-sectional study has shown that CYP2B6 G516T polymorphism influences the pharmacokinetics of EFV. While concomitant RMP treatment reduces plasma levels of EFV by about 18%, this was not statistically significant. Moreover, only a small proportion of patients had subtherapeutic trough concentrations, suggesting that the dose of EFV may not need to be increased. Future studies need to examine more closely the minimum acceptable trough level of EFV for the maintenance of antiretroviral efficacy to clarify the issue of optimal trough levels. Our findings have implications for government programs employing standard ART regimens, as dosage changes for some patients present substantial logistic and clinical challenges. Prospective studies are in progress that would correlate EFV trough concentrations with virological and immunological outcomes in patients undergoing treatment for HIV and TB.
Acknowledgments
We are grateful to P. R. Narayanan and V. Kumaraswami, Tuberculosis Research Centre, Chennai, India, for their encouragement and support. The technical assistance rendered by P. Vennila and statistical assistance by M. Vasantha are acknowledged.
This work received partial support through the NIAID/TRC/ICER program, which also supported the training of Hemanth Kumar. WinNonlin software was a kind gift provided during the above training program at the University of Alabama at Birmingham. The financial support received from the Tamil Nadu State AIDS Control Society, India, is gratefully acknowledged.
Footnotes
Published ahead of print on 5 January 2009.
REFERENCES
- 1.Ariyoshi, N., M. Miyazaki, K. Toide, Y. Sawamura, and T. Kamataki. 2001. A single nucleotide polymorphism of CYP2B6 found in Japanese enhances catalytic activity by autoactivation. Biochem. Biophys. Res. Commun. 281:1256-1260. [DOI] [PubMed] [Google Scholar]
- 2.Badri, M., D. Wilson, and R. Wood. 2002. Effect of HAART on incidence of tuberculosis in South Africa: a cohort study. Lancet 359:2059-2064. [DOI] [PubMed] [Google Scholar]
- 3.Barrett, J. S., A. S. Joshi, M. Chai, T. M. Ludden, W. D. Fiske, and H. J. Pieniaszek, Jr. 2002. Population pharmacokinetic meta-analysis with efavirenz. Int. J. Clin. Pharmacol. Ther. 40:507-519. [DOI] [PubMed] [Google Scholar]
- 4.Blumberg, H. M., W. J. Burman, R. E. Chaisson, C. L. Daley, S. C. Etkind, L. N. Friedman, P. Fujiwara, M. Grzemska, P. C. Hopewell, M. D. Iseman, R. M. Jasmer, V. Koppaka, R. I. Menzies, R. J. O'Brien, R. R. Reves, L. B. Reichman, P. M. Simone, J. R. Starke, and A. A. Vernon. 2003. American Thoracic Society/Centers for Disease Control and Prevention/Infectious Diseases Society of America: treatment of tuberculosis. Am. J. Respir. Crit. Care Med. 167:603-662. [DOI] [PubMed] [Google Scholar]
- 5.Brennan-Benson, P., R. Lyus, T. Harrison, M. Pakianathan, and D. Macallan. 2005. Pharmacokinetic interactions between efavirenz and rifampicin in the treatment of HIV and tuberculosis: one size does not fit all. AIDS 19:1541-1543. [DOI] [PubMed] [Google Scholar]
- 6.Burger, D., I. van der Heiden, C. la Porte, M. van der Ende, P. Groenveld, C. Richter, P. Koopmans, F. Kroon, H. Sprenger, J. Lindemans, P. Schenk, and R. van Schaik. 2006. Interpatient variability in the pharmacokinetics of the HIV non-nucleoside reverse transcriptase inhibitor efavirenz: the effect of gender, race, and CYP2B6 polymorphism. Br. J. Clin. Pharmacol. 61:148-154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Centers for Disease Control and Prevention. 2000. Updated guidelines for the use of rifabutin or rifampicin for the treatment and prevention of tuberculosis among HIV-infected patients taking protease inhibitors or non-nucleoside reverse transcriptase inhibitors. MMWR Morb. Mortal. Wkly. Rep. 49:185-199. [PubMed] [Google Scholar]
- 8.Cho, J. Y., H. S. Lim, J. Y. Chung, K. S. Yu, J. R. Kim, S. G. Shin, and I. J. Jang. 2004. Haplotype structure and allele frequencies of CYP2B6 in a Korean population. Drug Metab. Dispos. 32:1341-1344. [DOI] [PubMed] [Google Scholar]
- 9.Dean, G. L., S. G. Edwards, N. J. Ives, G. Matthews, E. F. Fox, L. Navaratne, M. Fisher, G. P. Taylor, R. Miller, C. B. Taylor, A. de Ruiter, and A. L. Pozniak. 2002. Treatment of tuberculosis in HIV-infected persons in the era of highly active antiretroviral therapy. AIDS 16:75-83. [DOI] [PubMed] [Google Scholar]
- 10.DiGiacinto, J. L., K. M. Chan-Tack, S. M. Robertson, K. S. Reynolds, and K. A. Struble. 2008. Are literature references sufficient for dose recommendations? An FDA case study of efavirenz and rifampin. J. Clin. Pharmacol. 48:518-523. [DOI] [PubMed] [Google Scholar]
- 11.Friedland, G., S. Khoo, C. Jack, and U. Lalloo. 2006. Administration of efavirenz (600 mg/day) with rifampicin results in highly variable levels but excellent clinical outcomes in patients treated for tuberculosis and HIV. J. Antimicrob. Chemother. 58:1299-1302. [DOI] [PubMed] [Google Scholar]
- 12.Gatanaga, H., T. Hayashida, K. Tsuchiya, M. Yoshino, T. Kuwahara, H. Tsukada, K. Fujimoto, I. Sato, M. Ueda, M. Horiba, M. Hamaguchi, M. Yamamoto, N. Takata, A. Kimura, T. Koike, F. Gejyo, S. Matsushita, T. Shirasaka, S. Kimura, and S. Oka. 2007. Successful efavirenz dose reduction in HIV type 1-infected individuals with cytochrome P450 2B6*6 and *26. Clin. Infect. Dis. 45:1230-1237. [DOI] [PubMed] [Google Scholar]
- 13.Girardi, E., F. Palmieri, A. Cingolani, A. Ammassari, N. Petrosillo, L. Gillini, D. Zinzi, A. De Luca, A. Antinori, and G. Ippolito. 2001. Changing clinical presentation and survival in HIV-associated tuberculosis after highly active antiretroviral therapy. J. Acquir. Immune Defic. Syndr. 26:326-331. [DOI] [PubMed] [Google Scholar]
- 14.Haas, D. W., H. J. Ribaudo, R. B. Kim, C. Tierney, G. R. Wilkinson, R. M. Gulick, D. B. Clifford, T. Hulgan, C. Marzolini, and E. P. Acosta. 2004. Pharmacogenetics of efavirenz and central nervous system side effects: an Adult AIDS Clinical Trials Group study. AIDS 18:2391-2400. [PubMed] [Google Scholar]
- 15.Hammer, S., D. Gibb, D. Havlir, L. Mofenson, I. Van Beek, and S. Vella (ed.). 2002. Scaling up antiretroviral therapy in resource-limited settings. Guidelines for a public health approach. Executive summary. World Health Organization, Geneva, Switzerland. http://www.who.int/hiv/topics/arv/scaling_exe_summary.pdf.
- 16.Hiratsuka, M., Y. Takekuma, N. Endo, K. Narahara, S. I. Hamdy, Y. Kishikawa, M. Matsuura, Y. Agatsuma, T. Inoue, and M. Mizugaki. 2002. Allele and genotype frequencies of CYP2B6 and CYP3A5 in the Japanese population. Eur. J. Clin. Pharmacol. 58:417-421. [DOI] [PubMed] [Google Scholar]
- 17.Jones, J. L., D. L. Hanson, M. S. Dworkin, and K. M. DeCock. 2000. HIV-associated tuberculosis in the era of highly active antiretroviral therapy: the adult/adolescent spectrum of HIV disease group. Int. J. Tuberc. Lung Dis. 4:1026-1031. [PubMed] [Google Scholar]
- 18.Kwara, A., M. Lartey, K. W. Sagoe, F. Xexemeku, E. Kenu, J. Oliver-Commey, V. Boima, A. Sagoe, I. Boamah, D. J. Greenblatt, and M. H. Court. 2008. Pharmacokinetics of efavirenz when co-administered with rifampin in TB/HIV co-infected patients: pharmacogenetic effect of CYP2B6 variation. J. Clin. Pharmacol. 48:1032-1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lang, T., K. Klein, J. Fischer, A. K. Nussler, P. Neuhaus, U. Hofmann, M. Eichelbaum, M. Schwab, and U. M. Zanger. 2001. Extensive genetic polymorphism in the human CYP2B6 gene with impact on expression and function in human liver. Pharmacogenetics 11:399-415. [DOI] [PubMed] [Google Scholar]
- 20.López-Cortés, L. F., R. Ruiz-Valderas, P. Viciana, A. Alarcon-Gonzalez, J. Gomez-Mateos, E. Leon-Jimenez, M. Sarasa-Nacenta, Y. Lopez-Pua, and J. Pachon. 2002. Pharmacokinetic interactions between efavirenz and rifampicin in HIV-infected patients with tuberculosis. Clin. Pharmacokinet. 41:681-690. [DOI] [PubMed] [Google Scholar]
- 21.López-Cortés, L. F., R. Ruiz-Valderas, J. Ruiz-Morales, E. Leon, A. V. de Campos, A. Marin-Niebla, M. Marquez-Solero, F. Lozano, and R. Valiente. 2006. Efavirenz trough levels are not associated with virological failure throughout therapy with 800 mg daily and a rifampicin-containing antituberculosis regimen. J. Antimicrob. Chemother. 58:1017-1023. [DOI] [PubMed] [Google Scholar]
- 22.Manosuthi, W., S. Sungkanuparph, A. Thakkinstian, A. Vibhagool, S. Kiertiburanakul, S. Rattanasiri, W. Prasithsirikul, J. Sankote, A. Mahanontharit, and K. Ruxrungtham. 2005. Efavirenz levels and 24-week efficacy in HIV-infected patients with tuberculosis receiving highly active antiretroviral therapy. AIDS 19:1481-1486. [DOI] [PubMed] [Google Scholar]
- 23.Manosuthi, W., S. Kiertiburanakul, S. Sungkanuparph, K. Ruxrungtham, A. Vibhagool, S. Rattanasiri, and A. Thakkinstian. 2006. Efavirenz 600 mg/day versus efavirenz 800 mg/day in HIV-infected patients with tuberculosis receiving rifampicin: 48 weeks results. AIDS 20:131-132. [DOI] [PubMed] [Google Scholar]
- 24.Manosuthi, W., W. Mankatitham, A. Lueangniyomkul, S. Chimsuntorn, and S. Sungkanuparph. 2008. Standard-dose efavirenz vs. standard-dose nevirapine in antiretroviral regimens among HIV-1 and tuberculosis co-infected patients who received rifampicin. HIV Med. 9:294-299. [DOI] [PubMed] [Google Scholar]
- 25.Marzolini, C., A. Telenti, L. A. Decosterd, G. Greub, J. Biollaz, and T. Buclin. 2001. Efavirenz plasma levels can predict treatment failure and central nervous system side effects in HIV-1-infected patients. AIDS 15:71-75. [DOI] [PubMed] [Google Scholar]
- 26.Matteelli, A., M. Regazzi, P. Villani, G. De Iaco, M. Cusato, A. C. Carvalho, S. Caligaris, L. Tomasoni, M. Manfrin, S. Capone, and G. Carosi. 2007. Multiple-dose pharmacokinetics of efavirenz with and without the use of rifampicin in HIV-positive patients. Curr. HIV Res. 5:349-353. [DOI] [PubMed] [Google Scholar]
- 27.Patel, A., K. Patel, J. Patel, N. Shah, B. Patel, and S. Rani. 2004. Safety and antiretroviral effectiveness of concomitant use of rifampicin and efavirenz for antiretroviral-naïve patients in India who are co-infected with tuberculosis and HIV-1. J. Acquir. Immune Defic. Syndr. 37:1166-1169. [DOI] [PubMed] [Google Scholar]
- 28.Pedral-Sampaio, D. B., C. R. Alves, E. M. Netto, C. Brites, A. S. Oliveira, and R. Badaro. 2004. Efficacy and safety of efavirenz in HIV patients on rifampin for tuberculosis. Braz. J. Infect. Dis. 8:211-216. [DOI] [PubMed] [Google Scholar]
- 29.Phair, J. P., and S. L. Becker. 2004. Integrating pharmacokinetics into treatment decisions: strategies for optimal patient care. In iMed Options. Feinberg School of Medicine, Northwestern University, Chicago, IL.
- 30.Pozniak, A. 2002. Mycobacterial diseases and HIV. J. HIV Ther. 7:13-16. [PubMed] [Google Scholar]
- 31.Ramachandran, G., A. K. Hemanth Kumar, S. Rajasekaran, C. Padmapriyadarsini, G. Narendran, S. Anitha, Sudha Subramanyam, V. Kumaraswami, and Soumya Swaminathan. 2007. Steady state pharmacokinetics of nevirapine in HIV-1 infected adults in India. J. Int. Assoc. Physicians AIDS Care 6:251-254. [DOI] [PubMed] [Google Scholar]
- 32.Ramachandran, G., A. K. Hemanth Kumar, S. Swaminathan, V. Kumaraswami, and D. J. Greenblatt. 2006. Simple and rapid liquid chromatography method for determination of EFV in plasma. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 835:131-135. [DOI] [PubMed] [Google Scholar]
- 33.Ribaudo, H. J., D. W. Haas, C. Tierney, R. B. Kim, G. R. Wilkinson, R. M. Gulick, D. B. Clifford, C. Marzolini, C. V. Fletcher, K. T. Tashima, D. R. Kuritzkes, and E. P. Acosta. 2006. Pharmacogenetics of plasma efavirenz exposure after treatment discontinuation: an Adult AIDS Clinical Trials Group Study. Clin. Infect. Dis. 42:401-407. [DOI] [PubMed] [Google Scholar]
- 34.Rodriguez-Novoa, S., P. Barreiro, A. Rendon, I. Jimenez-Nacher, J. Gonzalez-Lahoz, and V. Soriano. 2005. Influence of 516G>T polymorphisms at the gene encoding the CYP450-2B6 isoenzyme on EFV plasma concentrations in HIV-infected subjects. Clin. Infect. Dis. 40:1358-1361. [DOI] [PubMed] [Google Scholar]
- 35.Rotger, M., S. Colombo, H. Furrer, G. Bleiber, T. Buclin, B. L. Lee, O. Keiser, J. Biollaz, L. Decosterd, A. Telenti, and the Swiss HIV Cohort Study. 2005. Influence of CYP2B6 polymorphism on plasma and intracellular concentrations and toxicity of efavirenz and nevirapine in HIV-infected patients. Pharmacogenet. Genomics 15:1-5. [DOI] [PubMed] [Google Scholar]
- 36.Spradling, P., D. Drociuk, S. McLaughlin, L. M. Lee, C. A. Peloquin, K. Gallicano, C. Pozsik I. Onorato, K. G. Castro, and R. Ridzon. 2002. Drug-drug interactions in inmates treated for human immunodeficiency virus and Mycobacterium tuberculosis infection or disease: an institutional tuberculosis outbreak. Clin. Infect. Dis. 35:1106-1112. [DOI] [PubMed] [Google Scholar]
- 37.Tong, K., M. L. He, C. K. Lin, L. Guo, H. F. Kung, J. J. Y. Sung, and S. S. Lee. 2006. The implications of a high allelic frequency of CYP2B6 G516T in ethnic Chinese persons. Clin. Infect. Dis. 43:541-542. [DOI] [PubMed] [Google Scholar]
- 38.Tsuchiya, K., H. Gatanaga, N. Tachikawa, K. Teruya, Y. Kikuchi, M. Yoshino, T. Kuwahara, T. Shirasaka, S. Kimura, and S. Oka. 2004. Homozygous CYP2B6 *6 (Q172H and K262R) correlates with high plasma efavirenz concentrations in HIV-1 patients treated with standard efavirenz-containing regimens. Biochem. Biophys. Res. Commun. 319:1322-1326. [DOI] [PubMed] [Google Scholar]
- 39.Wang, J., A. Sonnerborg, A. Rane, F. Josephson, S. Lundgren, L. Stahle, and M. Ingelman-Sundberg. 2006. Identification of a novel specific CYP2B6 allele in Africans causing impaired metabolism of the HIV drug efavirenz. Pharmacogenet. Genomics 16:191-198. [DOI] [PubMed] [Google Scholar]
- 40.Wyen, C., H. Hendra, M. Vogel, C. Hoffmann, H. Knechten, N. H. Brockmeyer, J. R. Bogner, J. Rockstroh, S. Esser, H. Jaeger, T. Harrer, S. Mauss, J. van Lunzen, N. Skoetz, A. Jetter, C. Groneuer, G. Fatkenheuer, S. H. Khoo, D. Egan, D. J. Back, and A. Owen. 2008. on behalf of the German Competence Network for HIV/AIDS. Impact of CYP2B6 983T>C polymorphism on non-nucleoside reverse transcriptase inhibitor plasma concentrations in HIV-infected patients. J. Antimicrob. Chemother. 61:914-918. [DOI] [PMC free article] [PubMed] [Google Scholar]