Abstract
Hepatitis C virus (HCV) infects an estimated 170 million individuals worldwide and is associated with an increased incidence of liver fibrosis, cirrhosis, and hepatocellular carcinoma. Currently approved therapies to treat HCV infection consist of combinations of pegylated alpha interferon and ribavirin which result in a sustained viral response in 40 to 60% of patients. Efforts to develop improved therapies include the development of direct inhibitors of virally encoded enzymes such as the viral RNA-dependent RNA polymerase. A nucleoside analog, 2′-C-methyl-7-deaza-adenosine (MK-0608), has been shown to inhibit viral RNA replication in the subgenomic HCV genotype 1b replicon, with a 50% effective concentration (EC50) of 0.3 μM (EC90 = 1.3 μM). To determine efficacy in vivo, MK-0608 was administered to HCV-infected chimpanzees, resulting in dose- and time-dependent decreases in plasma viral loads. In separate experiments, chimpanzees dosed for 7 days with MK-0608 at 0.2 and 2 mg per kg of body weight per day by intravenous administration experienced average reductions in viral load of 1.0 and >5 log10 IU/ml, respectively. Two other HCV-infected chimpanzees received daily doses of 1 mg MK-0608 per kg via oral administration. After 37 days of oral dosing, one chimpanzee with a high starting viral load experienced a reduction in viral load of 4.6 log10, and the viral load in the other chimpanzee fell below the limit of quantification (LOQ) of the HCV TaqMan assay (20 IU/ml). Importantly, viral load remained below the LOQ throughout the duration of dosing and for at least 12 days after dosing ended. The results demonstrate a robust antiviral effect on the administration of MK-0608 to HCV-infected chimpanzees.
Hepatitis C virus (HCV) is a small, enveloped, positive-strand RNA virus of the family Flaviviridae, identified as the causative agent of non-A, non-B viral hepatitis (7). Chronic infection with HCV is associated with an increased incidence of fibrosis, cirrhosis, and the development of hepatocellular carcinoma (22). Currently approved therapies for the treatment of HCV infection consist of 12- to 48-week courses of pegylated alpha interferon combined with ribavirin and result in a sustained viral response (SVR), defined as undetectable virus 6 months after the end of treatment, in 40 to 60% of patients (16, 17). Infection with HCV genotype 2 or 3 and lower starting viral loads are associated with a higher probability of attaining SVR (16, 23).
Efforts to develop new treatments with improved efficacy and tolerability compared to those of current therapies have focused on the discovery and development of direct inhibitors of virally encoded enzymes. The positive-strand HCV RNA genome is directly translated via an internal ribosome entry site into a single polyprotein, which is then processed by both cellular and virally encoded proteases into 10 constituent proteins, termed C, E1, E2, p7, and nonstructural protein 2 (NS2), NS3, NS4A, NS4B, NS5A, and NS5B. Drug development efforts have targeted primarily the viral NS3/4A protease and the NS5B RNA-dependent RNA polymerase (RdRp), responsible for viral RNA replication (8).
Both nucleoside and nonnucleoside inhibitors of HCV RdRp have been described previously. A variety of structural classes of nonnucleoside inhibitors of HCV RdRp have also been disclosed previously (2, 5, 9, 11, 15, 20, 24; H. Hashimoto, K. Mizutani, and A. Yoshida, 5 July 2001, international patent application WO 00147883). Certain aspects of the mechanism of action of nonnucleoside inhibitors, including noncompetitive inhibition and apparent allosteric inhibition (25), appear to be in common with each other. Nucleoside inhibitors of HCV RdRp include 2′-C-methyl, 2′-O-methyl, and 4′-substituted nucleoside analogs (4, 13). 2′-C-methyl-adenosine is a potent inhibitor of the bicistronic replicon assay that also inhibits the purified HCV RdRp in vitro as the 5′-triphosphate, apparently by a chain-terminating mechanism (4). Resistance to inhibition by 2′-C-methyl nucleosides is engendered by a single amino acid mutation, S282T, within the RdRp active site (18). Addition of a 7-deaza modification to the nucleobase converting the analog to MK-0608 significantly improves the pharmacokinetic properties in animals (21).
Chimpanzees represent the only nonhuman species that can be naturally and chronically infected with HCV. As with humans, HCV infection in chimpanzees is associated with acute viremia and elevated liver enzyme levels in serum. Acute infections may resolve at a higher rate in chimpanzees than in humans, though data are limited (3). Chronic infections in chimpanzees typically result in milder hepatitis than is seen in humans, suggesting a difference in pathogenesis of infection in the two species. Thus, the chimpanzee model of HCV infection may not recapitulate all aspects of the virus-host interaction of the infection in humans. Additional issues with the chimpanzee model include the low number of infected animals that are available for research, which necessarily translates into a high cost and few animals per study, which adversely affects the statistical power of each study. However, the close genetic relationship between chimpanzee and human and the difficulties inherent in other animal models for HCV infection (reviewed in reference 14) argue strongly that pharmacokinetic/pharmacodynamic data generated with HCV-infected chimpanzees can provide useful guidance for the design of human clinical trials of novel HCV therapies. Nucleoside and nonnucleoside inhibitors of the HCV polymerase have been investigated with the chimpanzee model of chronic HCV infection, and antiviral efficacy has been observed previously (6; D. Standring, presented at the conference of the European Association for the Study of the Liver, Geneva, Switzerland, 2003).
In the present study, MK-0608 was administered to HCV-infected chimpanzees to investigate compound exposure and to determine its efficacy in reducing viral load. A robust antiviral response was observed in HCV-infected chimpanzees dosed with the compound either orally or intravenously (i.v.).
MATERIALS AND METHODS
Chimpanzees.
The housing, maintenance, and care of the chimpanzees (Pan troglodytes) used in the study were in compliance with all relevant guidelines and requirements at Merck Research Laboratories and at New Iberia Research Center (University of Louisiana at Lafayette). The study protocols were reviewed and approved by the Institutional Animal Care and Use Committee at each site. Either chimpanzees included in these studies were originally infected with HCV in the course of studies conducted by other investigators or the origin of the infection is not known. The HCV genotype infecting the chimpanzees was determined by a line probe assay (Versant HCV genotype assay, LiPA; Bayer Diagnostics/Innogenetics) and confirmed by reverse transcription-PCR (RT-PCR) rescue of HCV genetic material and DNA sequencing. Chimpanzee X3 was infected with HCV genotype 3a. Chimps X5, X4, and X6 were infected with HCV genotype 1a (H77 isolate).
Pharmacokinetic analysis.
The concentrations of MK-0608 (structure shown in Fig. 1) in plasma and liver biopsy samples were determined after the compound was administered orally to two chimpanzees (one male, one female) not infected with HCV. Plasma pharmacokinetic analysis could not be carried out with HCV-infected chimps since they are not trained to present their arms for blood sampling, and the extensive sedations that would be required could endanger their health. The compound was dosed at 1 mg/kg of body weight as a solution in Tang once per day for a total of 7 days. Blood samples were collected at various times from 0.5 to 24 h after the first and seventh dose while the animals were alert. Additionally, liver samples were collected by needle aspiration while the chimps were under general anesthesia at 24 h after the first and seventh doses, frozen on dry ice, and stored at −70°C until analysis. Clinical chemistry and hematological data were compiled from serum and blood samples collected prior to the oral administration of the compound for 7 days to the uninfected chimpanzees and again after administration of the last dose. There were no significant changes in the measured parameters.
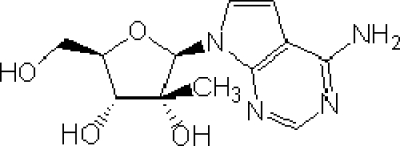
FIG. 1.
2′-C-Methyl-7-deaza-adenosine (MK-0608).
Administration of MK-0608 to HCV-infected chimpanzees.
Doses for i.v. administration of MK-0608 were formulated in sterile saline (0.9%) and stored at 4°C prior to administration. The HCV-infected chimpanzees were sedated using a minimal amount of tiletamine, zolazepam, or ketamine prior to i.v. administration of the compound. Blood samples for viral load determinations were removed just prior to the administration of the next dose of compound. Blood samples for determination of compound concentrations during the i.v. dosing phase were removed just prior to and just after the administration of each dose to represent 24-h and 10-min time points, respectively. Blood was processed into plasma within 1 h of collection. Plasma samples were aliquoted and stored frozen at −70°C.
The compound was also dosed orally as a solution in Tang to alert HCV-infected chimpanzees. Blood samples for determination of viral load and compound concentration were collected while the chimps were under ketamine sedation 4.5 h after oral dosing on selected days. Plasma samples were collected weekly for up to 4 weeks after the last dose was administered for both the i.v.- and oral-dosing phases to monitor the rebound of the viral load.
Concentration of MK-0608 in plasma and liver.
The concentration of MK-0608 in plasma was determined using liquid chromatography-tandem mass spectrometry (LC-MS/MS) in the positive-ion mode using a heated nebulizer (APCI) interface. The nucleoside analog and the internal standard (tubercidin) were isolated from plasma (50 to 100 μl) by solid-phase extraction (SPE) using mixed-phase cation exchange in Oasis 96-well plates (30 mg resin/well). Analytes were eluted from the SPE plate by consecutive elution with two 300-μl volumes of 90:8:2 (vol/vol/vol) acetonitrile-water-ammonium hydroxide (600-μl total volume) added to each well of the plate. An additional 100 μl of acetonitrile-1 M acetic acid (90:10, vol/vol) was added to the eluent from each well, and 5- to 10-μl aliquots were directly injected into the LC-MS/MS system. Chromatography was carried out in hydrophilic-interaction chromatography mode, and quantification was based on selected reaction monitoring of the following precursor/product ion pairs: m/z 281 → 135 (MK-0608) and m/z 267 → 135 (tubercidin). The concentration of the nucleoside analog in liver biopsy samples was determined after the hydrolysis of 2′-C-methyl-7-deaza-adenosine nucleotide anabolites back to their nucleosides upon treatment with acid phosphatase. Samples were suspended in 100 mM ammonium acetate (pH 5) buffer and homogenized using an ultrasonic cell disruptor. Posthomogenization, the internal standard (tubercidin, 25 μl to 1 μg/ml) was added to a 100-μl aliquot of whole-liver homogenate and 200 μl of 100 mM ammonium acetate (pH 5), 50 μl of 100 mM MgCl2, and 20 μl (∼3 to 4 U) of acid phosphatase (acid phosphatase from sweet potato [Sigma]; the stock solution was diluted to ∼160 U/ml with 100 mM ammonium acetate buffer, pH 5). The samples were incubated in a shaking water bath at 37°C for 30 min. Posthydrolysis, MK-0608 and the internal standard (tubercidin) were isolated from incubation mixtures by SPE using mixed-phase cation exchange in Oasis 96-well plates (30 mg resin/well) according to the protocol used for plasma samples and analyzed by LC-MS/MS by using the same procedures described above for plasma.
Viral load determinations.
Viral load determinations were performed on plasma samples either by using the Versant HCV RNA (branched-DNA) assay (version 3.0; Bayer Diagnostics) or the HCV TaqMan assay (analyte-specific reagent version; Roche) for quantitative analysis or by using the Versant HCV qualitative assay (transcription-mediated amplification [TMA]; Bayer Diagnostics) for qualitative detection of HCV.
Viral resistance analysis.
Total RNA was extracted from chimpanzee plasma samples using the Qiagen RNeasy minikit according to the manufacturer's instructions (Qiagen, Inc., Valencia, CA). The RNA was used as a template for the reverse transcriptase reaction mixture (SuperScript III reverse transcriptase; Invitrogen Life Technologies, Carlsbad, CA), which was primed with a 34-nucleotide deoxyribosyladenine (dA) primer. Following RT, the reaction mixtures were heat inactivated at 65°C for 15 min and then digested with RNase H for 20 min at 37°C. Nested PCR was performed using Takara LA Taq hot-start enzyme (Takara Bio Inc., Shiga, Japan) and the following primer pairs: PCR 1 forward (5′ACGGGTCATGGTCRACGGTCAGTAG) and the reverse 34-nucleotide dA primer and PCR 2 forward (5′GAYGTYGTGTGCTGCTCAATGTCTTA) and PCR 2 reverse (5′ATCGGTTGGGGAGGAGGTAGATGC) (where R is a mixture of G and A and Y is a mixture of C and T). For chimp X3, infected with HCV genotype 3a, the following primers were substituted: PCR 1 forward (5′GAGCGTGGTCTGCTGTCTATGTC) and the reverse 34-nucleotide dA primer and PCR 2 forward (5′CTGATAACACCATGTAGTGCTGAGG) and PCR 2 reverse (5′TACCAGCTCACCGWGCTGGCAGG) (where W is a mixture of A and T). PCR products were sequenced on the ABI Prism 3100 genetic analyzer. Low-viral-load samples were genotyped by Bayer Reference Testing Laboratory (Berkeley, CA) with the commercial NS5B genotype assay. This assay generates approximately 200 nucleotides of double-stranded sequence spanning codons 250 to 310. At the low viral loads of these samples (<20 IU/ml), the genotype assay was successful only with select samples.
RESULTS
MK-0608 concentration in plasma and liver after oral dosing.
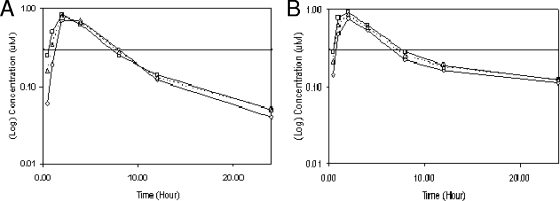
Two uninfected chimpanzees trained to present their forearms voluntarily for blood collection were dosed with 1 mg MK-0608 per kg body weight orally as a solution in Tang once daily for seven consecutive days. This dosing regimen was chosen to determine whether there was any increase in exposure on multiple dosing and to mimic more closely the regimen planned for the longer-term study of HCV-infected chimpanzees. Plasma samples were collected over a 24-h period after the first and seventh doses, and concentrations of the nucleoside analog in the plasma were determined using LC-MS/MS. As shown in Fig. 2, oral administration of the compound resulted in significant levels of compound in plasma over the 24-h dosing period. After the first dose, peak concentrations in plasma averaged 0.78 μM and occurred at 2 h postdose (Table 1). At 24 h postdose, the mean plasma concentration was 0.05 μM, and the mean area under the concentration-time curve from 0 to 24 h (AUC0-24) was 5.6 μM·h. Overall, similar compound concentrations were found in the two chimpanzees. Very minor increases in the AUC0-24 and in the maximum concentration of the drug in serum (Cmax) were evident after 7 days of dosing compared to after the first dose. The compound concentration in plasma 24 h after the seventh dose was significantly higher (∼2.5-fold) than that 24 h after the first dose. The plasma concentrations of MK-0608 exceeded the replicon 50% effective concentration (EC50) (0.3 μM) for approximately 8 h after dosing but did not reach the replicon EC90 (1.3 μM) at any time.
FIG. 2.
Plasma concentrations of MK-0608 in chimpanzees X1 (□) and X2 (⋄) after they received a single dose (A) or seven consecutive daily doses (B) of 1 mg/kg orally. The arithmetic mean is also shown (Δ). The horizontal line depicts the replicon EC50 for MK-0608 (0.3 μM).
TABLE 1.
Compound concentrations after dosing of MK-0608 for 1 and 7 days at 1 mg/kg per day in uninfected chimpanzeesa
| Day | Chimp | AUC0-24 (μM·h) | Cmax (nM) | Tmax (h) | Plasma concn at 24 h (nM) | Liver concn at 24 h (nM) | Ratio of concn in liver to concn in plasma (24 h) |
|---|---|---|---|---|---|---|---|
| 1 | X1 | 5.74 | 830 | 2 | 51 | 2,580 | 51 |
| X2 | 5.51 | 720 | 2 | 43 | 3,940 | 92 | |
| Mean | 5.6 | 780 | 2 | 47 | 3,260 | 69 | |
| 7 | X1 | 7.14 | 920 | 2 | 124 | 6,830 | 55 |
| X2 | 5.86 | 770 | 2 | 113 | 6,930 | 61 | |
| Mean | 6.5 | 850 | 2 | 118 | 6,880 | 58 |
The compound was administered orally, and plasma samples were then collected over a 24-h period after the first and seventh doses. Liver tissue samples were also collected by needle biopsy while the chimps were under general anesthesia. Compound concentrations were determined using LC-MS/MS as described in Materials and Methods. Tmax, time to maximum concentration of the drug in serum.
Concentrations of the nucleoside analog were also determined in liver tissue collected by needle biopsy 24 h after the first and seventh doses (Table 1). Samples were treated with acid phosphatase to convert any phosphorylated species to the nucleoside. The concentration of MK-0608 and its phosphorylated metabolites in liver 24 h after the first dose was on average 69-fold higher than the concentration in plasma at the same time point, suggesting an efficient uptake into liver. A similar ratio of the concentration in liver to the concentration in plasma was determined from samples taken 24 h after the seventh dose. The absolute concentration of the nucleoside analog in liver was 2.1-fold higher after the seventh dose than after the first dose.
Viral load after i.v. administration of MK-0608.
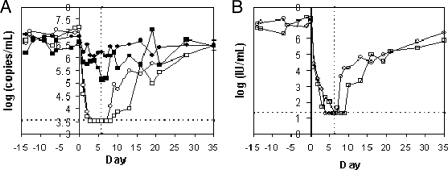
The compound was first dosed to HCV-infected chimpanzees using i.v. administration at 0.2 mg/kg once daily following sedation in order to guarantee that the animals received the intended dose. Administration via oral gavage under sedation was unacceptable due to anticipated effects of the sedation on oral absorption of the compound. Plasma samples were collected on selected days beginning 14 days prior to the first dose to establish a baseline viral load. As shown in Fig. 3A, after 7 days of dosing at 0.2 mg/kg, the viral load in chimpanzee X5 decreased by 0.8 log relative to that at day 0 (just prior to the first dose), and the viral load in chimpanzee X3 decreased by 1.4 logs relative to that at day 0. However, significant variation in the baseline viral loads and in the viral loads after dosing ended obscured the conclusion that the decrease was due solely to administration of the compound. The concentration of the compound in plasma determined at 10 min postdosing was on average 1.27 μM for the two chimps over the 7 days of dosing. At 24 h postdose, the compound concentration was below the limit of quantification (LOQ) (36 nM) in both chimps on all days of dosing.
FIG. 3.
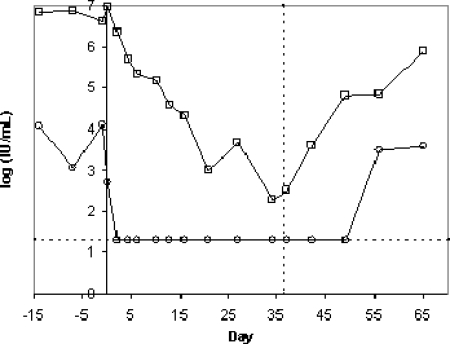
Plasma viral loads during i.v. administration of MK-0608. The compound was dosed in separate experiments at two different dose levels (0.2 mg/kg per day, filled symbols; 2 mg/kg per day, open symbols) to two HCV-infected chimpanzees (X5 [circles] and X3 [squares]). Days are numbered relative to the first day of dosing (day 0). The vertical dashed line indicates the time of administration of the last dose. (A) Plasma samples were periodically collected and viral loads determined using the Versant branched-DNA assay (version 3; Bayer), which has an LOQ of 3,200 copies/ml (3.5 log10, denoted by the horizontal dashed line). Viral loads below the LOQ are graphed at 3,200 copies/ml. (B) Viral loads were also determined from plasma samples from both chimps during dosing at 2 mg/kg using the more sensitive HCV TaqMan assay (Roche), which has an LOQ of 20 IU/ml (1.3 log10 IU/ml, denoted by the horizontal dashed line). Viral loads below the LOQ of the assay are represented at the LOQ. The dashed vertical line represents the time of administration of the last dose.
The chimpanzees were allowed a rest period of about 2 months after the first round of dosing to allow for complete rebound of viral titers. Plasma samples were then taken over a 2-week period without dosing the compound to establish the baseline viral load. Subsequently, the two chimpanzees received 2 mg/kg MK-0608 via i.v. administration under the same dosing and sampling regimen. Plasma viral loads in both chimps decreased rapidly and were below the LOQ of the Versant assay (3,200 copies/ml) after the third dose. Viral loads remained below the LOQ for the remainder of the dosing period and for 24 and 48 h after the last dose in chimps X5 and X3, respectively.
Viral loads from the round of i.v. dosing at 2 mg/kg were also determined using the more sensitive HCV TaqMan assay (Roche) to gain information about the low range of viral loads (Fig. 3B). Viral loads in both chimps decreased to values below the LOQ in the TaqMan assay (20 IU/ml) during the dosing interval and remained below the LOQ for 24 and 72 h after the last dose in chimpanzees X5 and X3, respectively. Thus, the viral loads at the end of dosing represent decreases of 5.8 and 5.9 log10 from day 0 for chimps X5 and X3, respectively. Viral loads rebounded to near starting levels over the 4 weeks that they were monitored after the end of dosing.
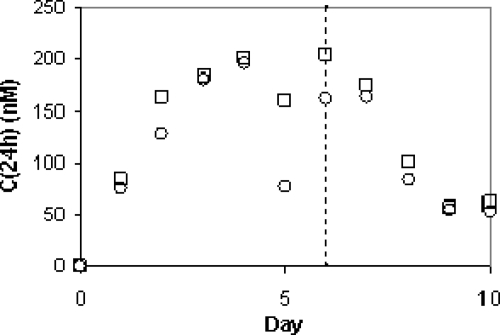
Plasma concentrations of compound were determined immediately prior to and just after the administration of each dose to reflect the 24-h and ∼10-min time points. The plasma concentration of the compound at the ∼10-min time point averaged 9.9 and 11.0 μM for chimps X5 and X3, respectively. The compound concentration at 24 h after each dose ranged from 80 nM after the first dose to about 200 nM after the fourth dose and did not increase thereafter (Fig. 4).
FIG. 4.
Plasma concentrations of the compound 24 h after the 2-mg/kg i.v. dose [C(24h)]. Plasma samples were collected from chimpanzee X5 (○) and chimpanzee X3 (□) 24 h after each dose during the dosing period (days 0 to 6) and for 4 days after dosing ended. Compound concentrations were determined using LC-MS/MS. The dashed line indicates the time of administration of the last dose.
Viral load after oral administration of MK-0608.
To determine the antiviral efficacy of the compound after oral dosing, MK-0608 as a solution in Tang was administered to two chronically HCV-infected chimpanzees at 1 mg/kg once daily for 37 days. Plasma samples were collected approximately 4 h after administration of the compound periodically during the 37 days of dosing for determination of viral load and compound concentration. Chimpanzee X6 had a baseline viral load that varied from 1,110 to 12,900 IU/ml, and chimpanzee X4 had a baseline viral load of 3 × 106 to 9 × 106 IU/ml, as determined using the TaqMan assay. As shown in Fig. 5A, viral loads decreased substantially in both chimps over the duration of dosing. The viral load of chimpanzee X6 decreased after the second dose to a level that was below the LOQ of the TaqMan assay (20 IU/ml). Plasma samples from X6 were also submitted to the more sensitive TMA assay, which has a limit of detection of 10 IU/ml. At day 4, X6 was still HCV positive by TMA assay. However, HCV was not detectable in the plasma of X6 from day 10 through day 42, which was 6 days after the end of dosing. At day 49, X6 was HCV positive by TMA assay, but his viral load was still below the LOQ in the TaqMan assay; by day 56, the viral load had rebounded to near starting levels. The viral load of chimpanzee X4 decreased steadily to reach a minimum of 197 IU/ml at day 33, corresponding to a 4.6-log10 reduction from the initial viral load, and rebounded to 807,000 IU/ml by day 65.
FIG. 5.
Plasma viral loads in two HCV-infected chimps dosed at 1 mg/kg MK-0608 orally once daily for 37 days. Plasma viral loads were assessed by the HCV TaqMan assay (Roche), which has an LOQ of 20 (1.3 log10) IU/ml, for chimpanzees X6 (○) and X4 (□), dosed starting at day 0 and ending at day 36. Values that were below the LOQ are graphed as 1.3 log10 IU/ml. The dashed vertical line represents the time of administration of the last dose. The dashed horizontal line represents the lower LOQ.
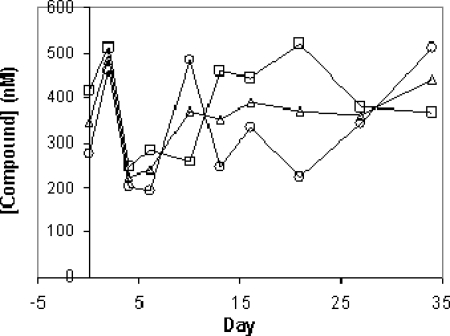
The concentrations of the compound in plasma were determined in samples collected 4.5 h after oral dosing. The 4.5-h time point was chosen to allow maximal absorption of the compound prior to sedation but also to allow for recovery after sedation prior to the chimpanzees' evening meal. As shown in Fig. 6, concentrations at 4.5 h fell in a range of 200 to 500 nM during the duration of dosing. No significant trend in the concentrations across this time interval was apparent. Additionally, at the end of 37 days of dosing, plasma samples were collected 24 h after the last dose. The compound concentrations were determined to be 113 and 122 nM in chimps X4 and X6, respectively, concentrations that were similar to the concentrations in plasma 24 h after 7 days of dosing at 1 mg/kg in the uninfected chimps (Table 1). Assuming that the liver concentration/plasma concentration ratio that was determined during dosing of the HCV-naïve chimps was maintained in the HCV-infected chimps, the concentrations of MK-0608 (and its phosphorylated metabolites) in liver at 24 h after the last dose were ∼6.5 and ∼7.1 μM in chimps X4 and X6, respectively.
FIG. 6.
Compound concentration in plasma 4.5 h after oral dosing at 1 mg/kg MK-0608 once per day. Compound concentrations were determined from plasma samples collected during dosing at 1 mg/kg once daily by oral administration to chimpanzees X6 (○) and X4 (□). The mean is also shown (Δ). The data demonstrate that some variability in the concentrations at 4.5 h is evident, but no systematic increase or decrease is present. Compound concentrations were below the LOQ (36 nM) on days 42, 49, 56, and 65 for both chimps (not shown).
Resistance analysis.
Viral RNA isolated from plasma samples drawn prior to, during, and after the cessation of dosing was genotyped for evidence of sequence variants indicative of resistance. RT-PCR products of NS5B were generated from the time points (indicated in Table 2) of the study using a 2-mg/kg i.v. dose and sequenced as a cDNA population. Heterogeneity was noted among early rebound time points at days 13 and 16 for chimpanzee X3 (a day 10 sample was unavailable for genotyping). Circulating viruses were heterogenous at amino acid 282, encoding either serine or threonine. Clonal analysis of these samples confirmed the presence of both serine- and threonine-encoding sequences. Previous in vitro cell-based resistance selections and enzymatic assays identified the S282T mutation as conferring resistance to MK-0608 and other 2′-methyl-modified nucleosides (18). There were no additional polymorphisms linked to either S or T at residue 282. The S282T mutation was not detectable in a predose sample using an allele-specific detection assay sensitive to 1 in 1,000 copies (S. W. Ludmerer and D. Graham, unpublished observations) and became undetectable in a sample drawn after an additional 3 days postdose on study day 19. It is possible that the first round of dosing of MK-0608 at 0.2 mg/kg selected for and enhanced the proportion of the S282T variant within the total viral population. However, the level of the S282T variant entering the second study (2 mg/kg) was undetectable by a sensitive method. Thus, virus encoding this mutation arose during MK-0608 dosing and rapidly disappeared from the circulating viral population in the absence of continued dosing, consistent with in vitro results that the S282T mutation confers resistance to MK-0608 and that replication is impaired as a result of acquiring this mutation (18). No heterogeneity was noted among any samples drawn from chimpanzee X5, including a day 2 sample where the viral load had been suppressed more than 4 logs.
TABLE 2.
Amino acid identity at position 282 of the product of the HCV NS5B gene rescued from chimpanzees dosed with MK-0608a
| Chimpanzee | Dosing regimenb | Study dayc | Sequencing methodd | Amino acid(s) at position 282e |
|---|---|---|---|---|
| X3 | 2 mg/kg i.v. | −7 | Population | S |
| −1 | Population | S | ||
| 13 | Population, clonal (30) | T (19), S (11) | ||
| 16 | Population, clonal (51) | T (20), S (31) | ||
| 19 | Population, clonal (14) | S (14) | ||
| X5 | 2 mg/kg i.v. | −7 | Population | S |
| −1 | Population | S | ||
| 2 | Population | S | ||
| 9 | Population | S | ||
| 13 | Population | S | ||
| 16 | Population | S | ||
| X6 | 1 mg/kg p.o. | −14 | Populationf | S |
| 4 | Populationf | S | ||
| 49 | Populationf | R, T, I | ||
| 56 | Populationf | S | ||
| X4 | 1 mg/kg p.o. | −14 | Populationf | S |
| 37 | Populationf | S | ||
| 56 | Populationf | S |
Viral RNA was rescued using RT-PCR from plasma samples collected on various days. Corresponding cDNA samples were sequenced across the NS5B gene.
Dose level and route of administration of MK-0608 (either i.v. or per os [p.o.]).
Study day 0 is defined as the first day of dosing. Study day −14 corresponds to 14 days prior to the first day of dosing. For the studies using i.v. administration, all days after day 6 are after the end of dosing. For the studies using oral administration, all days after day 36 are after the end of dosing.
Numbers in parentheses are numbers of individual clones.
Amino acid at position 282 of the product of the HCV NS5B gene rescued from HCV-infected chimpanzees. Numbers in parentheses are numbers of individual clones.
Sequencing was carried out at Bayer Reference Testing Laboratories using the NS5B genotype assay.
Similar observations were made in the study in which MK-0608 was dosed orally for 37 days at 1 mg/kg. The viral load of chimpanzee X6 dropped below the level of quantification within 2 days of dosing, remained below the LOQ for the duration of dosing, and did not rebound until approximately 2 weeks after the end of dosing. Virus initially remained detectable by TMA but, with continued dosing, eventually became undetectable even by TMA. On day 49, approximately 12 days postdosing, virus once again became TMA detectable. Approximately 200 bp of a double-strand sequence spanning codons 250 to 315 was generated from select TMA-positive samples using the highly sensitive commercial NS5B genotyping assay (Bayer Research Testing Laboratories, Berkeley, CA). Whereas the day 2 sample encoded serine at amino acid 282, the nucleotide sequence of the day 49 sample at this codon was varied, with one strand reading CGA (arginine) and the other reading AYC (threonine or isoleucine). One week later, the viral load rebounded to predose levels and only AGC (serine) was detected at amino acid 282. The low viral load (<10 IU/ml) and limited sample availability precluded clonal analysis. Select longitudinal samples collected from chimpanzee X4 were all homogenous for S282 and encoded no other polymorphisms.
DISCUSSION
A detailed understanding of the relationship between the pharmacokinetics and pharmacodynamics of a potential therapeutic agent in a relevant animal model of the disease aids in the design of a strategy for determining compound efficacy in human clinical trials. Thus, the goals of these studies were to determine the efficacy of dosing a nucleoside analog, 2′-C-methyl-7-deaza-adenosine (MK-0608), under different conditions to HCV-infected chimpanzees and to relate the antiviral efficacy to the exposure to the compound.
MK-0608 is a potent inhibitor of HCV replication in vitro. In the subgenomic-replicon assay, MK-0608 inhibits viral RNA replication at an EC50 of 0.3 μM (EC90 = 1.3 μM) in the absence of significant cytotoxicity (50% cytotoxic concentration, >100 μM) (21). The 5′-triphosphate of 2′-C-methyl-7-deaza-adenosine inhibits the purified HCV RdRp from genotype 1b, with an IC50 of 110 nM, and acts as a chain-terminating inhibitor. In preclinical pharmacokinetic experiments with rats, dogs, and rhesus monkeys, MK-0608 demonstrated good to excellent oral bioavailability (50 to 100%) and long plasma half-lives in dogs and rhesus macaques (9 and 14 h, respectively). Additionally, when MK-0608 was administered orally to rats, a high concentration of the active form of the inhibitor, the 5′-triphosphate, was generated in rat liver. When dosed to rats at 2 mg/kg, the 5′-triphosphate of MK-0608 was present at ∼12 μmol/kg liver at 8 h postdose, which represents ∼50% of the total nucleoside (or nucleotide) present, demonstrating a favorable tissue distribution to the liver and an efficient intracellular conversion to the triphosphate. In comparison, about 80% of the total intracellular concentration of nucleoside (or nucleotide) was present as the triphosphate when replicon-containing Huh-7 cells were incubated with MK-0608. Preclinical toxicity data indicated that the compound had a single 50% lethal dose of >2,000 mg/kg in mice (21). The in vitro potency, favorable pharmacokinetic parameters and tissue distribution, and lack of acute toxicity of MK-0608 prompted further investigations into antiviral efficacy.
The plasma and liver concentrations of MK-0608 following oral administration of the compound to two uninfected chimpanzees were determined after single and multiple doses. Maximal plasma concentrations and AUCs increased only slightly after seven doses of compound. Measurable levels of compound remained in plasma 24 h after the first dose (∼50 nM) and increased by about 2.5-fold over the course of seven doses. The plasma trough concentration had likely approached a steady-state level after seven doses, since during the study in which the compound was dosed for 37 days orally at the same dosage (1 mg/kg) to HCV-infected chimps, the plasma trough concentrations were similar (113 and 122 nM in the two chimpanzees).
A high concentration (∼3.2 μM) of the compound was determined in liver tissue samples taken 24 h after the first 1-mg/kg dose, and the liver trough concentration after the seventh dose was ∼6.9 μM, roughly 60 times the plasma concentration at the same time, demonstrating efficient uptake into liver. The ratio of the concentration in liver to the concentration in plasma in chimpanzee was similar to that observed in rat (21). The trough nucleoside analog concentration in liver after the seventh dose was ∼23 times the EC50 (5.3 times the EC90) in the replicon assay. It was not possible to determine accurately the fraction of the nucleoside that existed as the 5′-triphosphate in the chimpanzee liver tissue due to high levels of phosphatase activity. In other preclinical studies with rats, where freeze-clamping techniques can be used on liver tissue in situ to minimize the degradation of the triphosphate, on average about 50% of the total nucleoside existed as the triphosphate. This likely represents a low estimate, owing to residual phosphatase activity (K. Koeplinger, unpublished). However, the efficiency of phosphorylation of MK-0608 in chimpanzee or human liver may not equal that in rat liver. Additionally, HCV infection may alter the concentration of MK-0608 triphosphate in infected liver. When MK-0608 was administered either i.v. or orally to HCV-infected chimpanzees, substantial decreases in viral load were observed. The average decrease in viral load during i.v. administration at 0.2 mg/kg was ∼1 log10, and at 2 mg/kg, it was > 3.5 log10 by the Versant assay and >5 log10 by the TaqMan assay, if the viral loads from day 0 to the end of dosing, day 7, are compared. Notably, though chimpanzee X3 was infected with HCV genotype 3a and chimpanzee X5 was infected with genotype 1a, the decreases in viral load were similar in these chimps, consistent with the broad activity of 2′-C-methyladenosine analogs across genotypes in vitro (S. Carroll, unpublished data). Thus, increasing the dose by 10-fold had the effect of magnifying the viral load drop by >100-fold, much more than can be accounted for by a dose-proportional increase in efficacy. The reasons for an increase in efficacy greater than the dose-proportional increase are not clear, but possibly the higher Cmax after the 2-mg/kg i.v. dose (∼10 μM compared to 1.3 μM after the 0.2-mg/kg i.v. dose) drives greater uptake into liver. Another possible explanation may be related to the compound levels in liver remaining above the replicon EC50 throughout the dosing interval for the higher dose, maintaining suppression of viral replication. A definite basis for the difference in efficacy cannot be ascertained because of the inability to determine accurately the concentration of the triphosphate of MK-0608 in chimpanzee liver under the different dosing conditions.
During 37 days of oral dosing at 1 mg/kg per day, the viral load in chimpanzee X4 declined steadily, reaching a 4.6-log10 decrease at the end of the dosing interval. The viral load in chimpanzee X6 fell below the LOQ after the second oral dose and remained there throughout the duration of dosing. Moreover, the viral load in X6 was undetectable by the most sensitive commercial assay available (TMA) for much of the dosing interval and for 6 days after dosing had ceased. Thus, oral dosing of the compound resulted in a potent antiviral effect, and there was no evidence of viral rebound during the duration of dosing. However, the decrease in viral load during the oral dosing study at 1 mg/kg was not as rapid (in the case of X4 at day 7, viral load had decreased by ∼1.8 log10) as the viral load drop during i.v. dosing at 2 mg/kg (>5 log10 in 7 days in both chimpanzees), even after the difference in dose is accounted for. Though a definitive reason for the difference cannot be determined since different chimpanzees were used in the two studies, one possibility is that the superior efficacy achieved during i.v. dosing may again be related to the higher Cmax, which was ∼12 μM during i.v. dosing at 2 mg/kg and 0.78 μM during oral dosing at 1 mg/kg. Compound concentrations at 24 h after dosing for the two studies differed by at most twofold, and thus the differences in the minimum concentrations of the drug in serum between the two routes of administration are unlikely to account for the difference in the rates of the viral load decline. Possibly, the higher Cmax results in a higher concentration of the nucleoside in liver, where conversion to the 5′-triphosphate leads to retention of the active inhibitor in hepatocytes.
The ability of MK-0608 to clear cells of replicon RNA has been investigated, and a greater-than-dose-proportional effect was observed. At 0.5 μM MK-0608 (1.7 times the EC50), no difference in the numbers of viable cells in the presence of neomycin was observed after 14 days of incubation, indicating that cells were not cured of replicon RNA. However, in the presence of 2.5 μM MK-0608, >99.9% of cells were cured of the replicon (data not shown). The in vitro data are consistent with the greater-than-dose-proportional effects on viral load seen in the chimpanzees in the present study.
Other nucleoside analog inhibitors of HCV RdRp are under investigation as potential therapies for HCV infection. NM283, a 3′-valyl ester prodrug of 2′-C-methyl-cytidine, was administered to HCV-infected chimpanzees and was also under investigation in human clinical trials using HCV-infected patients, and so that investigation offers a clinical correlate to the present study. NM283 was dosed at 8.3 and 16.6 mg/kg per day for 7 days to HCV-infected chimpanzees, resulting in viral load decreases of 0.8 and 1.05 log10, respectively (D. Standring, presented at the conference of the European Association for the Study of the Liver, Geneva, Switzerland, 2003). In a phase II trial of NM283 in HCV-infected patients, subjects receiving an 800-mg dose once daily (∼11 mg/kg) experienced an average viral load decrease of 0.7 log10 after 28 days of dosing (1). The less robust antiviral response observed upon NM283 dosing compared to the response observed in the present study upon dosing of MK-0608 may be a consequence of the lower inhibitory potency of NM-283. The replicon EC50 of NM-107, the nucleoside resulting from processing of NM-283, is ∼1 μM (13), compared to the EC50 of 0.3 μM for MK-0608 in the replicon assay (21). Chimpanzee exposure data were not disclosed, but in humans receiving an 800-mg dose (∼11 mg/kg), the plasma Cmax was 15 μM (X. J. Zhou, N. Afdhal, E. Godofsky, J. Dienstag, V. Rustgi, L. Schick, D. McInery, B. A. Fielman, and N. A. Brown, presented at the 39th Annual Meeting for the European Association for the Study of the Liver, Berlin, Germany, April, 2005). Therefore, the Cmax of NM-107 was comparable to the Cmax of MK-0608 after i.v. dosing at 2 mg/kg to the chimpanzees. The less robust response with NM-283, compared to MK-0608, may be due to decreased exposure of the nucleoside triphosphate in the liver, though data on the liver concentration of NM-107 have not been disclosed.
Dosing of R1626, a prodrug of 4′-azidocytidine, has resulted in decreases in viral load in patients (12). R7128, a prodrug of 2′-F-2′-C-methyl-cytidine (PSI-6130), is also under clinical investigation (M. J. Otto, et al., presented at the 14th International Symposium on Hepatitis C Virus and Related Viruses, Glasgow, Scotland, 2007). HCV-infected patients receiving a 1,500-mg dose of R7128 twice a day experienced mean reductions of 2.7 log10 after 14 days of dosing, with no evidence of viral rebound during dosing (S. Le Pogam, A. Seshaadri, A. Kosaka, S. Hu, A. Beard, J. Symons, N. Cammack, and I. Najera, presented at the conference Resistance to Antiviral Therapies, Paris, France, 2008). The plasma AUC from 0 h to infinity of PSI-6130, the nucleoside resulting from hydrolysis of the prodrug moieties, following a single dose of 1,500 mg of R7128 in healthy subjects averaged 58 μg·h/ml (M. J. Otto, et al., presented at the 14th International Symposium on Hepatitis C Virus and Related Viruses, Glasgow, Scotland, 2007), and plasma AUC0-24s following 14 consecutive daily doses of 1,500 mg R7128 to HCV-infected subjects averaged 92 μg·h/ml (R. Reddy, M. Rodriguez-Torres, E. Gane, R. Robson, J. Lalezari, G. T. Everson, E. DeJesus, J. G. McHutchison, H. E. Vargas, A. Beard1, C. A. Rodriguez1, G. Z. Hill, W. T. Symonds, and M. M. Berrey, presented at the 58th Annual Meeting of the American Association for the Study of Liver Diseases, Boston, MA, 2007). Thus, though a direct comparison cannot be made owing to the different dosing regimens (single versus multiple doses), the plasma exposure of the nucleoside in healthy subjects differed by less than twofold from that in HCV-infected patients under the dosing conditions employed. Data on concentrations of the nucleoside in liver tissue were not disclosed. The robust and stable declines in viral load during dosing with potent nucleoside analogs contrast with the results from clinical evaluation of a nonnucleoside inhibitor of HCV RNA polymerase, with which a viral rebound during dosing has been observed (P. Chandra, D. Raible, D. Harper, J. Speth, S. Villano, and G. Bichier, presented at the Digestive Disease Week conference, Los Angeles, CA, 2006).
Despite the robust antiviral response to dosing of MK-0608, viral loads rebounded after dosing ended in all chimps. The decreases in HCV load during antiviral therapy follow biphasic kinetics, with an initial rapid phase, representing largely the clearance of virus from the plasma, and a second slower phase, whose rate is governed primarily by the clearance of infected hepatocytes (19). It is not surprising that SVR was not achieved during 2-mg/kg i.v. dosing, even though viral loads fell below the LOQ, since the duration of that study was not long enough to allow for complete clearance of infected hepatocytes. While the amplitude of the rapid phase of viral clearance during dosing with the compound was large (>3 logs), suggesting a high degree of inhibition of replication, infected hepatocytes remained after 7 days of dosing.
During oral dosing at 1 mg/kg, the viral load of X6 was not detectable even by the TMA assay for at least 32 days. In clinical trials, a good correlation between achieving a viral load that is undetectable by TMA and achieving SVR has been documented (10). Rescue of viral RNA and sequencing revealed that, at a time when viral load was still very low, the viral sequences from some of the chimpanzees contained mutations at amino acid 282, a position where mutations are known to give rise to resistance to inhibition by 2′-C-methyl nucleosides (18). It is possible that HCV resistant to MK-0608 remained present at very low levels during dosing. When dosing ended and selective pressure was removed, the virus rapidly reverted to wild type while rebounding. The low viral load of virus with apparent mutations at position 282 and the fact that the virus that rebounded after dosing ended contained S282 imply that the mutated virus is deficient in replication compared to the wild type. Clearance of residual, replication-impaired virus resistant to the compound may require either an extended duration of dosing or possibly the addition of one or more other drugs for combination therapy.
Acknowledgments
We gratefully acknowledge the help and expertise of the staff at New Iberia Research Center, where the chimpanzee studies were carried out, in particular, of Jeff Rowell, Jane Fontenot, and Kent Thomassee. We also acknowledge Joseph Leone for the preparative scale synthesis of MK-0608 used in some of these studies.
Footnotes
Published ahead of print on 15 December 2008.
REFERENCES
- 1.Afdahl, N., C. O'Brien, E. Godofsky, M. Rodriguez-Torres, S. C. Pappas, P. Pockros, E. Lwitz, N. Bzowej, V. Rustgi, M. Sulkowski, and K. Sherman. 2006. Valopicitabine (NM283), alone or with peg-interferon, compared to peg-interferon/ribavirin (pegIFN/RBV) re-treatment in hepatitis C patients with prior non-response to pegIFN/RBV: week 24 results. J. Hepatol. 44(Suppl. 2):S19.16356583 [Google Scholar]
- 2.Beaulieu, P. L., M. Bos, Y. Bousquet, G. Fazal, J. Gauthier, J. Gillard, S. Goulet, S. LaPlante, M. A. Poupart, S. Lefebvre, G. McKercher, C. Pellerin, V. Austel, and G. Kukolj. 2004. Non-nucleoside inhibitors of the hepatitis C virus NS5B polymerase: discovery and preliminary SAR of benzimidazole derivatives. Bioorg. Med. Chem. Lett. 14:119-124. [DOI] [PubMed] [Google Scholar]
- 3.Bukh, J., X. Forns, S. U. Emerson, and R. H. Purcell. 2001. Studies of hepatitis C virus in chimpanzees and their importance for vaccine development. Intervirology 44:132-142. [DOI] [PubMed] [Google Scholar]
- 4.Carroll, S. S., J. E. Tomassini, M. Bosserman, K. Getty, M. W. Stahlhut, A. B. Eldrup, B. Bhat, D. Hall, A. L. Simcoe, R. LaFemina, C. A. Rutkowski, B. Wolanski, Z. Yang, G. Migliaccio, R. De Francesco, L. C. Kuo, M. MacCoss, and D. B. Olsen. 2003. Inhibition of hepatitis C virus RNA replication by 2′-modified nucleoside analogs. J. Biol. Chem. 278:11979-11984. [DOI] [PubMed] [Google Scholar]
- 5.Chan, L., O. Pereira, T. J. Reddy, S. K. Das, C. Poisson, M. Courchesne, M. Proulx, A. Siddiqui, C. G. Yannopoulos, N. Nguyen-Ba, C. Roy, D. Nasturica, C. Moinet, R. Bethell, M. Hamel, L. L'Heureux, M. David, O. Nicolas, P. Courtemanche-Asselin, S. Brunette, D. Bilimoria, and J. Bedard. 2004. Discovery of thiophene-2-carboxylic acids as potent inhibitors of HCV NS5B polymerase and HCV subgenomic RNA replication. Part 2: tertiary amides. Bioorg. Med. Chem. Lett. 14:797-800. [DOI] [PubMed] [Google Scholar]
- 6.Chen, C. M., Y. He, L. Lu, H. B. Lim, R. L. Tripathi, T. Middleton, L. E. Hernandez, D. W. Beno, M. A. Long, W. M. Kati, T. D. Bosse, D. P. Larson, R. Wagner, R. E. Lanford, W. E. Kohlbrenner, D. J. Kempf, T. J. Pilot-Matias, and A. Molla. 2007. Activity of a potent hepatitis C virus polymerase inhibitor in the chimpanzee model. Antimicrob. Agents Chemother. 51:4290-4296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Choo, Q. L., G. Kuo, A. J. Weiner, L. R. Overby, D. W. Bradley, and M. Houghton. 1989. Isolation of a cDNA clone derived from a blood-borne non-A, non-B viral hepatitis genome. Science 244:359-362. [DOI] [PubMed] [Google Scholar]
- 8.De Francesco, R., and G. Migliaccio. 2005. Challenges and successes in developing new therapies for hepatitis C. Nature 436:953-960. [DOI] [PubMed] [Google Scholar]
- 9.Dhanak, D., K. J. Duffy, V. K. Johnston, J. Lin-Goerke, M. Darcy, A. N. Shaw, B. Gu, C. Silverman, A. T. Gates, M. R. Nonnemacher, D. L. Earnshaw, D. J. Casper, A. Kaura, A. Baker, C. Greenwood, L. L. Gutshall, D. Maley, A. DelVecchio, R. Macarron, G. A. Hofmann, Z. Alnoah, H. Y. Cheng, G. Chan, S. Khandekar, R. M. Keenan, and R. T. Sarisky. 2002. Identification and biological characterization of heterocyclic inhibitors of the hepatitis C virus RNA-dependent RNA polymerase. J. Biol. Chem. 277:38322-38327. [DOI] [PubMed] [Google Scholar]
- 10.Gerotto, M., F. Dal Pero, G. Bortoletto, A. Ferrari, R. Pistis, G. Sebastiani, S. Fagiuoli, S. Realdon, and A. Alberti. 2006. Hepatitis C minimal residual viremia (MRV) detected by TMA at the end of Peg-IFN plus ribavirin therapy predicts post-treatment relapse. J. Hepatol. 44:83-87. [DOI] [PubMed] [Google Scholar]
- 11.Harper, S., B. Pacini, S. Avolio, M. Di Filippo, G. Migliaccio, R. Laufer, R. De Francesco, M. Rowley, and F. Narjes. 2005. Development and preliminary optimization of indole-N-acetamide inhibitors of hepatitis C virus NS5B polymerase. J. Med. Chem. 48:1314-1317. [DOI] [PubMed] [Google Scholar]
- 12.Klumpp, K., D. Smith, M. Brandl, T. Alfredson, K. Sarma, M. Smith, I. Najera, W.-R. Jiang, S. Le Pogam, V. Leveque, H. Ma, Y. Tu, R. Chan, C.-W. Chen, X. Wu, R. Birudaraj, S. Swallow, J. A. Martin, N. Cammack, H. Berns, S. Fettner, D. Ipe, M. Mannino, E. O'Mara, C. Washington, S. Roberts, G. Cooksley, G. Dore, D. Shaw, D. R. Blue, Jr., F. Zahm, and G. Hill. 2007. Design and characterization of R1626, a prodrug of the HCV replication inhibitor R1479 (4′-azidocytidine) with enhanced oral bioavailability. Antivir. Res. 74:A35. [Google Scholar]
- 13.Klumpp, K., V. Leveque, S. Le Pogam, H. Ma, W. R. Jiang, H. Kang, C. Granycome, M. Singer, C. Laxton, J. Q. Hang, K. Sarma, D. B. Smith, D. Heindl, C. J. Hobbs, J. H. Merrett, J. Symons, N. Cammack, J. A. Martin, R. Devos, and I. Najera. 2006. The novel nucleoside analog R1479 (4′-azidocytidine) is a potent inhibitor of NS5B-dependent RNA synthesis and hepatitis C virus replication in cell culture. J. Biol. Chem. 281:3793-3799. [DOI] [PubMed] [Google Scholar]
- 14.Kremsdorf, D., and N. Brezillon. 2007. New animal models for hepatitis C viral infection and pathogenesis studies. World J. Gastroenterol. 13:2427-2435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Love, R. A., H. E. Parge, X. Yu, M. J. Hickey, W. Diehl, J. Gao, H. Wriggers, A. Ekker, L. Wang, J. A. Thomson, P. S. Dragovich, and S. A. Fuhrman. 2003. Crystallographic identification of a noncompetitive inhibitor binding site on the hepatitis C virus NS5B RNA polymerase enzyme. J. Virol. 77:7575-7581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Manns, M. P., J. G. McHutchison, S. C. Gordon, V. K. Rustgi, M. Shiffman, R. Reindollar, Z. D. Goodman, K. Koury, M. Ling, and J. K. Albrecht. 2001. Peginterferon alfa-2b plus ribavirin compared with interferon alfa-2b plus ribavirin for initial treatment of chronic hepatitis C: a randomised trial. Lancet 358:958-965. [DOI] [PubMed] [Google Scholar]
- 17.McHutchison, J. G., S. C. Gordon, E. R. Schiff, M. L. Shiffman, W. M. Lee, V. K. Rustgi, Z. D. Goodman, M. H. Ling, S. Cort, J. K. Albrecht, et al. 1998. Interferon alfa-2b alone or in combination with ribavirin as initial treatment for chronic hepatitis C. N. Engl. J. Med. 339:1485-1492. [DOI] [PubMed] [Google Scholar]
- 18.Migliaccio, G., J. E. Tomassini, S. S. Carroll, L. Tomei, S. Altamura, B. Bhat, L. Bartholomew, M. R. Bosserman, A. Ceccacci, L. F. Colwell, R. Cortese, R. De Francesco, A. B. Eldrup, K. L. Getty, X. S. Hou, R. L. LaFemina, S. W. Ludmerer, M. MacCoss, D. R. McMasters, M. W. Stahlhut, D. B. Olsen, D. J. Hazuda, and O. A. Flores. 2003. Characterization of resistance to non-obligate chain-terminating ribonucleoside analogs that inhibit hepatitis C virus replication in vitro. J. Biol. Chem. 278:49164-49170. [DOI] [PubMed] [Google Scholar]
- 19.Neumann, A. U., N. P. Lam, H. Dahari, D. R. Gretch, T. E. Wiley, T. J. Layden, and A. S. Perelson. 1998. Hepatitis C viral dynamics in vivo and the antiviral efficacy of interferon-alpha therapy. Science 282:103-107. [DOI] [PubMed] [Google Scholar]
- 20.Nguyen, T. T., A. T. Gates, L. L. Gutshall, V. K. Johnston, B. Gu, K. J. Duffy, and R. T. Sarisky. 2003. Resistance profile of a hepatitis C virus RNA-dependent RNA polymerase benzothiadiazine inhibitor. Antimicrob. Agents Chemother. 47:3525-3530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Olsen, D. B., A. B. Eldrup, L. Bartholomew, B. Bhat, M. R. Bosserman, A. Ceccacci, L. F. Colwell, J. F. Fay, O. A. Flores, K. L. Getty, J. A. Grobler, R. L. LaFemina, E. J. Markel, G. Migliaccio, M. Prhavc, M. W. Stahlhut, J. E. Tomassini, M. MacCoss, D. J. Hazuda, and S. S. Carroll. 2004. A 7-deaza-adenosine analog is a potent and selective inhibitor of hepatitis C virus replication with excellent pharmacokinetic properties. Antimicrob. Agents Chemother. 48:3944-3953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Poynard, T., P. Bedossa, P. Opolon, et al. 1997. Natural history of liver fibrosis progression in patients with chronic hepatitis C. Lancet 349:825-832. [DOI] [PubMed] [Google Scholar]
- 23.Poynard, T., P. Marcellin, S. S. Lee, C. Niederau, G. S. Minuk, G. Ideo, V. Bain, J. Heathcote, S. Zeuzem, C. Trepo, J. Albrecht, et al. 1998. Randomised trial of interferon alpha2b plus ribavirin for 48 weeks or for 24 weeks versus interferon alpha2b plus placebo for 48 weeks for treatment of chronic infection with hepatitis C virus. Lancet 352:1426-1432. [DOI] [PubMed] [Google Scholar]
- 24.Summa, V., A. Petrocchi, V. G. Matassa, M. Taliani, R. Laufer, R. De Francesco, S. Altamura, and P. Pace. 2004. HCV NS5b RNA-dependent RNA polymerase inhibitors: from alpha,gamma-diketoacids to 4,5-dihydroxypyrimidine- or 3-methyl-5-hydroxypyrimidinonecarboxylic acids. Design and synthesis. J. Med. Chem. 47:5336-5339. [DOI] [PubMed] [Google Scholar]
- 25.Tomei, L., S. Altamura, L. Bartholomew, A. Biroccio, A. Ceccacci, L. Pacini, F. Narjes, N. Gennari, M. Bisbocci, I. Incitti, L. Orsatti, S. Harper, I. Stansfield, M. Rowley, R. De Francesco, and G. Migliaccio. 2003. Mechanism of action and antiviral activity of benzimidazole-based allosteric inhibitors of the hepatitis C virus RNA-dependent RNA polymerase. J. Virol. 77:13225-13231. [DOI] [PMC free article] [PubMed] [Google Scholar]