Abstract
Since the eradication of smallpox and the cessation of routine childhood vaccination for smallpox, the proportion of the world's population susceptible to infection with orthopoxviruses, such as variola virus (the causative agent of smallpox) and monkeypox virus, has grown substantially. In the United States, the only vaccines for smallpox licensed by the Food and Drug Administration (FDA) have been live virus vaccines. Unfortunately, a substantial number of people cannot receive live virus vaccines due to contraindications. Furthermore, no antiviral drugs have been fully approved by the FDA for the prevention or treatment of orthopoxvirus infection. Here, we show the inhibitory effect of one new antiviral compound, ST-246, on the in vitro growth properties of six variola virus strains and seven monkeypox virus strains. We performed multiple assays to monitor the cytopathic effect and to evaluate the reduction of viral progeny production and release in the presence of the compound. ST-246 had 50% effective concentrations of ≤0.067 μM against variola virus and <0.04 μM against monkeypox virus. In a dose-dependent manner, plaque size and comet tail formation were markedly reduced in the presence of the drug at low, noncytotoxic concentrations between 0.015 and 0.05 μM. Our in vitro phenotype data suggest that ST-246 inhibits variola and monkeypox viruses similarly by reducing the production and release of enveloped orthopoxvirus and support the development of ST-246 as an antiviral therapeutic compound for the treatment of severe systemic orthopoxvirus infections.
Following the eradication of smallpox (5) and the subsequent cessation of routine childhood vaccinations for smallpox, the number of people susceptible to infection with variola virus (VARV), the etiologic agent that causes smallpox, has dramatically increased worldwide. In addition, encroachment into wildlife habitats, the trade of exotic pets, and the trade of bush meat increase the risk for zoonotic infection with other orthopoxviruses, such as monkeypox virus (MPXV), for which vaccination against smallpox provides some cross protection (7). Given that a large proportion of the worldwide population is susceptible to smallpox, the emergence of MPXV in the United States in 2003, and the continued concern over the intentional release of VARV, there is renewed interest in the development of safer smallpox and other orthopoxvirus vaccines and antiviral therapeutics.
One recently discovered antiviral compound is ST-246, a specific and potent inhibitor of an orthopoxvirus protein critical for virus maturation. ST-246 is a low-molecular-weight compound that was identified by a high-throughput screening assay designed to evaluate compounds for their abilities to inhibit vaccinia virus (VACV)-induced cytopathic effects (CPEs) without a measurable cytotoxic effect (17). Additional CPE assays showed that ST-246 inhibits the replication of multiple species of orthopoxviruses, including VARV (17), MPXV (17), cowpox virus (CPXV) (17), ectromelia virus (12, 17), and camelpox virus (17). Resistance mapping studies with CPXV identified the target protein as the V061 gene product. An understanding of the function of V061 is derived mostly from research on the VACV homolog, F13L. F13L encodes a major envelope protein required for the production of enveloped virions (EVs) (1), which includes intracellular EVs (IEVs), cell-associated EVs (CEVs), and extracellular EVs (EEVs). A phospholipase motif within F13L is necessary for the envelopment of intracellular mature virions (IMVs) within virus-modified membranes to yield egress-competent IEVs (6, 13, 15). Once IEVs obtain the additional membranes, they are transported to the cell surface on microtubules, where their outer membrane fuses with the plasma membrane and exposes CEVs. The CEVs remain attached at the cell surface, propel into neighboring cells from the actin tails which sometimes form beneath them, or release into the extracellular environment as EEVs. Accordingly, the current belief is that spread of the virus from cell to cell is mediated by CEVs and that systemic dissemination of the virus within a host is mediated by EEVs. ST-246 disrupts viral morphogenesis at the stage where IMVs develop into EVs by acquiring additional envelopes. During infection with an orthopoxvirus, preventing the virus from producing EVs during replication would limit the virus to forming IMVs as its only infectious form. In vitro virus yield assays showed that VACV-infected BSC-40 cells produced EEV titers that were 10-fold less in the presence of ST-246 than the titers produced by untreated infected cells (17).
For human orthopoxvirus infection, a treatment regimen that includes an antiviral agent which reduces the number of EV particles by preventing their formation may be therapeutic. In fact, using a ground squirrel model of infection with MPXV, Sbrana et al. showed that ground squirrels challenged with a lethal dose of MPXV and administered ST-246 therapy postexposure were completely protected from clinical disease and death, as long as treatment was started before day 4 postinfection (14). Other in vivo studies have shown protection in mice against lethal challenges with VACV, CPXV, and ectromelia virus (12, 17). In 2007, ST-246 was successfully used in conjunction with cidofovir and intravenous vaccinia immune globulin under an emergency Investigational New Drug approval for the treatment of a severe human case of eczema vaccinatum (16).
The inhibition of a few strains of VARV and MPXV by ST-246 has been previously described by Yang et al. (17). In the study described in this report, we further expanded the evaluation of this in vitro inhibitory activity by testing the activity of ST-246 against a larger number of genetically and phenotypically diverse strains of VARV and MPXV. Our results provide support for the ability of ST-246 to reduce the growth of these viruses by inhibiting the formation of EVs.
MATERIALS AND METHODS
Cells and viruses.
African green monkey kidney cells (BSC-40 cells) were maintained at 35.5°C in 5% CO2 and RPMI (Invitrogen) supplemented with 10% heat-inactivated fetal bovine serum (FBS; Atlas Biologicals), 2 mM l-glutamine (Invitrogen), 10 U/ml penicillin, and 10 μg/ml streptomycin (Invitrogen). Viruses were obtained from crude lysate preparations of infected BSC-40 cells for VARV strains SOM77-ali (Somalia), NEP73-175 (Nepal), BSH74-sol (Bangladesh), SUD47-juba (Sudan), SLN68-258 (Sierra Leone), and BRZ66-39 (Brazil) and MPXV strains V78-I-3945 (Benin), V81-I-179 (Ivory Coast), V77-I-823 (Zaire), V1979-I-005 (Zaire), 2003-RCG-358 (Republic of Congo), and 2003-USA-039 (United States). MPXV V70-I-266 (Sierra Leone) was obtained following purification by sucrose density centrifugation. Strain isolation and propagation were described previously (3, 4, 9, 10). Prior to the performance of each assay, the virus stocks were thawed on ice, vortexed briefly, and sonicated for 1.5 min in an ice bath with a cup-horn sonicator (TMX 500; Tekmar) set at an output amplitude of 40%.
CPE assay.
The CPE assay was performed as described previously, with some modifications (17). Briefly, BSC-40 cells were seeded in 96-well plates and grown to confluence. The cells were infected at a multiplicity of infection (MOI) of 0.1 and were incubated at 35.5°C in 5% CO2 for 1 h. The viral inocula were removed and replaced with growth medium (0.1 ml) containing 2% FBS and ST-246 (SIGA Technologies) at eight concentrations (5, 1.5, 0.5, 0.15, 0.05, 0.015, 0.005, and 0.0015 μM). Each treatment was performed in triplicate. Following incubation for 3 days at 35.5°C in 5% CO2, the cells were stained with 2× crystal violet and the absorbance was measured at 570 nm. Dose-response curves were generated by averaging data from the three data sets for each strain. The effective concentration of compound that protected the monolayers by 50% (EC50) was calculated from the absorbance values by using the XLfit (version 4.1) program (ID Business Solutions, Guilford, United Kingdom).
Plaque size evaluation and IHC.
BSC-40 cells were seeded in six-well plates and grown to confluence. The virus strains were diluted in RPMI containing 2% FBS, and approximately 25 PFU was added to each well. After incubation at 35.5°C in 5% CO2 for 1 h, the cells were washed twice and overlaid with RPMI containing 2% FBS, 1% carboxymethyl cellulose (CMC; Sigma), and ST-246 at five concentrations (0.5, 0.15, 0.05, 0.015, and 0.005 μM). Each treatment was performed in duplicate. The plates were incubated at 35.5°C in 5% CO2 for 3 days (MPXV) or 4 days (VARV), before the cells were fixed with 10% formalin to inactivate the virus. Immunohistochemistry (IHC) was performed as described previously (2, 18). Briefly, the cells were incubated with polyclonal rabbit anti-variola virus antibody and goat anti-rabbit immunoglobulin G-horseradish peroxidase conjugate (KPL 074-1506; Kirkegaard & Perry Laboratories). The plaques were visualized by development with TrueBlue peroxidase substrate (KPL 71-00-64; Kirkegaard & Perry Laboratories). Assays with VARV were performed in a maximum-containment laboratory under biosafety level 4 conditions. Six-well plates containing VARV were double sealed in Kapak/Scotchpak pouches and gamma irradiated at the kill dose of 4.4 × 106 rads (18).
Comet reduction assay and IHC.
The comet reduction assay was performed as described previously, with some modifications (2). The cells, viral dilutions, infection procedures, ST-246 concentrations, gamma irradiation for VARV, and IHC were as described above for the plaque size evaluation assay. During the 3 days (MPXV) or 4 days (VARV) of incubation, the plates were placed at a fixed angle of approximately 5 degrees.
RESULTS
ST-246 protects cell monolayers and inhibits CPEs.
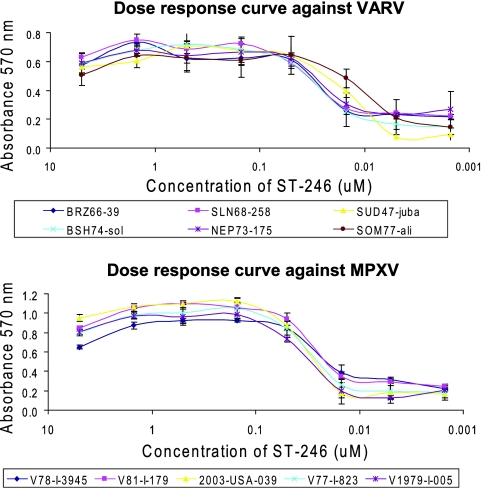
The level of protection of BSC-40 cell monolayers provided by ST-246 from viral CPE was measured by using six VARV strains and five MPXV strains. The strains of MPXV and VARV were selected to represent both phylogenetic clades of each virus species and strains of each virus species with diverse geographic origins, years of isolation, case fatality rates (CFRs), and EEV production and release phenotypes. An MOI of 0.1 was predetermined at a ratio of virus particles/cells required to produce a 90% CPE at 3 days postinfection. After staining of the cell monolayers with crystal violet, three sets of absorbance values were averaged for each strain to generate dose-response curves (Fig. 1). Absorbance values were proportional to the overall health of the cell monolayers, as demonstrated by the reduction in protection afforded by the antiviral compound as the concentration was decreased. Protection of the monolayers from the CPEs induced by all strains of VARV and MPXV tested was observed at ST-246 concentrations from 5 μM to 0.05 μM. A CPE was observed at 0.015 μM, and the monolayers were no longer protected at concentrations of 0.005 μM or lower. The slight reduction in the absorbance values at 5 μM may have been due to the cytotoxicity of the compound, since it was observed against all strains of both orthopoxviruses tested. The EC50s of ST-246 were calculated from inhibition curves and are summarized in Table 1. The ST-246 EC50s were similar among the strains of both orthopoxvirus species tested and varied from 0.019 μM ± 0.0046 μM to 0.067 μM ± 0.0282 μM for VARV strains and from 0.023 μM ± 0.0026 μM to 0.039 μM ± 0.0016 μM for MPXV strains.
FIG. 1.
ST-246 dose-response curves. BSC-40 cells were infected with VARV (top panel) or MPXV (bottom panel) at an MOI of 0.1. The CPE was evaluated by plotting the absorbance at 570 nm of crystal violet-stained cell monolayers versus the concentration of ST-246. The results represent the means ± the standard deviations of three independent measurements.
TABLE 1.
EC50s of ST-246 against VARV and MPXV
| Virus | Strain | EC50 (μM)a |
|---|---|---|
| Variola | BRZ66-39 | 0.067 ± 0.0282 |
| SLN68-258 | 0.037 ± 0.0063 | |
| SUD47-juba | 0.019 ± 0.0046 | |
| BSH74-sol | 0.028 ± 0.0124 | |
| NEP73-175 | 0.021 ± 0.0139 | |
| SOM77-ali | 0.028 ± 0.0303 | |
| Monkeypox | V78-I-3945 | 0.023 ± 0.0026 |
| V81-I-179 | 0.032 ± 0.0061 | |
| 2003-USA-039 | 0.036 ± 0.0045 | |
| V77-I-823 | 0.030 ± 0.0114 | |
| V1979-I-005 | 0.039 ± 0.0016 |
The results represent mean values ± standard deviations from three independent data sets.
ST-246 reduces viral plaque size.
The effect of ST-246 on viral plaque size was evaluated with VARV- and MPXV-infected cell monolayers incubated under a semisolid overlay of CMC. Plaque visualization by IHC provided specificity due to the detection of viral antigen with pooled rabbit sera raised against VARV strain Bangladesh. The activity of ST-246 was tested at several concentrations against six VARV strains and seven MPXV strains in independent, duplicate wells (Fig. 2). The plaque size phenotypes were similar among the MPXV strains, whereas the plaque sizes varied among the VARV strains, with strain SLN68-258 producing the largest plaques and strain SOM77-ali producing the smallest plaques. Consistent with the results from the CPE assay, the concentrations of ST-246 which reduced the viral plaque size were similar for both VARV and MPXV (Fig. 2). Considerable plaque size reductions were observed against all VARV and MPXV strains tested at ST-246 concentrations of 0.5, 0.15, and 0.05 μM. With 0.015 μM ST-246, the VARV and MPXV plaque sizes were reduced by approximately 50%, and with 0.005 μM ST-246, the VARV and MPXV plaque sizes were equivalent to those in the untreated wells. Therefore, the concentration of ST-246 that effectively reduced the plaque sizes of both VARV and MPXV was between 0.05 and 0.015 μM.
FIG. 2.
Plaque size reduction assays. Confluent monolayers of BSC-40 cells were infected with 25 PFU of VARV (top panel) or MPXV (bottom panel). Infected cells were incubated with various concentrations of ST-246 (none, 0.5, 0.15, 0.05, 0.015, or 0.005 μM) in RPMI (2 ml) containing 2% FBS and 1% CMC. Plaque sizes were visualized by IHC staining.
ST-246 inhibits comet tail formation.
Comet tail reduction assays were performed to evaluate the ability of ST-246 to inhibit EEV production and release, as measured by the formation of comet tails. Comet tails are formed as EEVs are released from infected cell surfaces, migrate through the growth medium, and establish secondary infections in neighboring cells. The comet tail formation phenotypes were similar among the MPXV strains, whereas the comet tail sizes varied among the VARV strains, with strain SLN68-258 forming the most prominent comet tails and strain SOM77-ali forming the smallest comet tails. IHC staining of viral antigen demonstrated that comet tail formation was drastically inhibited by ST-246 at concentrations of 0.5 to 0.05 μM for all VARV and MPXV strains tested (Fig. 3). Only minimal inhibition was observed with 0.015 μM ST-246 and no inhibition was observed with 0.005 μM ST-246 compared to the levels of inhibition noted for untreated cells. Consistent with the results of the plaque size reduction assay, the concentration of ST-246 that substantially reduced EEV particle production and release and comet tail formation by all VARV and MPXV strains tested was between 0.05 and 0.015 μM.
FIG. 3.
Comet tail formation reduction assays. Confluent monolayers of BSC-40 cells were infected with 25 PFU of VARV (top panel) or MPXV (bottom panel). Infected cells were incubated at a fixed angle with various concentrations of ST-246 (none, 0.5, 0.15, 0.05, 0.015, or 0.005 μM) in RPMI (2 ml) containing 2% FBS. Comet tails were visualized by IHC staining.
DISCUSSION
In an effort to further characterize the inhibitory ability of ST-246 against the two foremost orthopoxviruses that have been characterized to cause serious systemic disease in humans, we performed three different in vitro assays with numerous strains of MPXV and VARV. MPXV forms two phylogenetic clades, the Congo Basin isolates, which have reported CFRs of ∼10%, and the West African and U.S. isolates, which have lower CFRs (11). Strains of MPXV were selected for this study to represent both phylogenetic clades: West African and U.S. isolates V78-I-3945 (Benin) (3), V81-I-179 (Ivory Coast) (10), V70-266 (Sierra Leone) (10), and 2003-USA-039 (United States) (10) and Congo Basin isolates V77-I-823 (Zaire) (10), V1979-I-005 (Zaire) (11), and 2003-RCG-358 (Republic of Congo) (11). These strains have similar biologic phenotypes of plaque size and comet tail formation. Similarly, VARV also forms two primary clades: clade I includes isolates with the highest reported CFRs (up to 30%), and clade II includes isolates considered to cause either intermediate or mild disease on the basis of clinical outcomes and age-adjusted CFRs (4, 9). The strains of VARV were selected to represent strains with diverse geographic origins, years of isolation, CFRs, and levels of EEV production and release. Comparison of the levels of EEV production and release by 25 different strains of VARV revealed an extensive range of levels of EEV production, and strains were classified into three groups consisting of those with minor, intermediate, and prominent levels of EEV production (11a). On the basis of those findings, we selected six VARV strains for comparison in this study. SLN68-258 (Sierra Leone) and BRZ66-39 (Brazil) are clade II isolates with prominent EEV and comet tail production, BSH74-sol (Bangladesh) and SUD47-juba (Sudan) are clade I isolates with intermediate EEV and comet tail production, and SOM77-ali (Somalia) and NEP73-175 (Nepal) are clade I isolates with minor EEV and comet tail production.
Yang et al. reported the isolation of an ST-246-resistant variant of CPXV (17). Sequence analysis of the variant revealed a single base change in the V061 gene that resulted in the substitution of a cysteine for a glycine at amino acid position 277. In order to ensure that our isolates did not contain this substitution, we aligned amino acid sequences from VARV (C17L, VARV 040) and MPXV (C19L, MPXV 048) homologues to the ST-246 target protein, CPXV V061 (data not shown). None of the isolates for which sequences are available (MPXV strains V70-266, 2003-USA-039, V1979-I-005, and 2003-RCG-358 and VARV strains SOM77-ali, NEP73-175, BSH74-sol, SUD47-juba, SLN68-258, and BRZ66-39) contained the substitution that conferred drug resistance in CPXV. Sequences are not available for MPXV isolates V78-I-3945, V81-I-179, and V77-I-823; however, we can infer from the results of each assay that showed that ST-246 had equivalent inhibitory activity against all strains that these strains are not variants resistant to ST-246.
Our results from three independent assays provide evidence that ST-246 inhibits the in vitro production of the orthopoxviruses of primary concern for severe human infection, VARV and MPXV; our data are supportive of the fact that the mechanism of action is similar to that described for other orthopoxviruses: it blocks the formation and release of EVs. Our CPE assay showed that cell monolayers were well protected from virus-induced damage at ST-246 concentrations of 0.1 μM or greater for all strains tested. Although the drug may have a slight toxic effect at 5.0 μM, as seen by lower absorbance values, antiviral EC50s were achieved at concentrations approximately 100-fold less than that concentration. EC50s overlapped between VARV (0.019 to 0.067 μM) and MPXV (0.023 to 0.039 μM). Overall, the drug effectively inhibited EV production and release at concentrations below 0.07 μM for all VARV and MPXV strains tested without any observed cytotoxic effects on the cell monolayers. These results are consistent with those of Yang et al., who determined that EC50s against VARV Butler, VARV Bangladesh, and MPXV Zaire were 0.02, 0.05, and 0.01 μM, respectively (17).
In addition, we have shown that ST-246 blocks the production and release of EVs in our comet tail reduction and plaque size reduction assays. These two assays incorporate the specific staining of orthopoxvirus antigen by IHC, thereby providing a direct, sensitive, specific, and simple means of assessing EV production and release and evaluating the activity of the drug. In the plaque size evaluation assay, a semisolid overlay of CMC was used to prevent EEV particles from diffusing through the growth medium and producing satellite plaques or comets; thus, the majority of plaques were uniform in size and did not form comets. A reduction in plaque size signifies a reduction in the production and release of CEV and EEV particles. We demonstrated a remarkable decrease in plaque size with ST-246 at concentrations of 0.05 μM or greater against all VARV and MPXV strains tested, regardless of the strain's plaque size phenotype. The reduction observed at 0.015 μM was less, and the reduction was absent at 0.005 μM. These results corresponded to those observed in the comet tail reduction assay. In our comet tail reduction assay, we exploited the buoyant density of EEV particles to optimize the visualization of comets by incubating the plates on an incline. Incubation on a slight incline allows partial control of the direction of EEV migration and subsequent comet tail formation (8). Directionality enhances comet tail visualization, especially when antiviral compounds are evaluated. Thus, observation of the length and the width of a comet tail provides a direct and simple visual indication of the production and release of EEV particles. The slight discoloration that was observed in the wells of the VARV plates that was not seen in the wells of the MPXV plates was due to the gamma irradiation step required for the VARV plates.
Current data suggest that ST-246 inhibits IMV particles from acquiring a second and third envelope during orthopoxvirus maturations, which results in the reduction of EVs, one of the two infectious forms of orthopoxvirus. These phenotypic assays suggest that the decrease in the levels of EV production and release observed in VARV- or MPXV-infected cells treated with ST-246 was equivalent for all VARV and MPXV isolates. While the relationship between EEV production and pathogenicity is complex and most likely involves numerous host-virus interactions, these observations are important because inhibition of all VARV strains tested was observed: strains which produce comet tails ranging in size from minor to prominent, as well as strains associated with both high and low CFRs. Thus, ST-246 may be valuable for the treatment of smallpox, regardless of the VARV strain encountered. As well, equal inhibition was demonstrated against MPXV strains from the West African and U.S. clade and the Congo Basin clade, regardless of their plaque phenotypes, comet tail formation phenotypes, or associated CFRs. Our studies demonstrate that ST-246 has activity at nanomolar levels against a variety of genetically and phenotypically distinct VARV and MPXV strains. These findings support future investigations for the use of ST-246 as an antiviral agent for the treatment and prevention of VARV and MPXV infections.
Acknowledgments
We thank James Gathany, Division of Creative Services, Centers for Disease Control and Prevention, for all photography.
For disclosure of competing financial interests, Robert Jordan and Dennis E. Hruby are employees of SIGA Technologies, Inc., the company that produced ST-246.
The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the Centers for Disease Control and Prevention.
S.K.S., V.A.O., and K.L.K. performed the comet tail formation reduction and plaque size reduction assays. S.K.S. performed the CPE assays and drafted the manuscript. R.J. analyzed the data from the CPE assays and calculated the EC50s. All authors contributed to the study design and review of the manuscript.
Footnotes
Published ahead of print on 15 December 2008.
REFERENCES
- 1.Blasco, R., and B. Moss. 1991. Extracellular vaccinia virus formation and cell-to-cell virus transmission are prevented by deletion of the gene encoding the 37,000-dalton outer envelope protein. J. Virol. 65:5910-5920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chen, Z., P. Earl, J. Americo, I. Damon, S. K. Smith, Y. H. Zhou, F. Yu, A. Sebrell, S. Emerson, G. Cohen, R. J. Eisenberg, J. Svitel, P. Schuck, W. Satterfield, B. Moss, and R. Purcell. 2006. Chimpanzee/human mAbs to vaccinia virus B5 protein neutralize vaccinia and smallpox viruses and protect mice against vaccinia virus. Proc. Natl. Acad. Sci. USA 103:1882-1887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Douglass, N. J., M. Richardson, and K. R. Dumbell. 1994. Evidence for recent genetic variation in monkeypox viruses. J. Gen. Virol. 75(Pt 6):1303-1309. [DOI] [PubMed] [Google Scholar]
- 4.Esposito, J. J., S. A. Sammons, A. M. Frace, J. D. Osborne, M. Olsen-Rasmussen, M. Zhang, D. Govil, I. K. Damon, R. Kline, M. Laker, Y. Li, G. L. Smith, H. Meyer, J. W. Leduc, and R. M. Wohlhueter. 2006. Genome sequence diversity and clues to the evolution of variola (smallpox) virus. Science 313:807-812. [DOI] [PubMed] [Google Scholar]
- 5.Fenner, F., D. A. Henderson, I. Arita, Z. Jezek, and I. D. Ladnyi. 1988. Smallpox and its eradication. World Health Organization, Geneva, Switzerland.
- 6.Husain, M., and B. Moss. 2002. Similarities in the induction of post-Golgi vesicles by the vaccinia virus F13L protein and phospholipase D. J. Virol. 76:7777-7789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jezek, Z., B. Grab, M. V. Szczeniowski, K. M. Paluku, and M. Mutombo. 1988. Human monkeypox: secondary attack rates. Bull. W. H. O. 66:465-470. [PMC free article] [PubMed] [Google Scholar]
- 8.Law, M., R. Hollinshead, and G. L. Smith. 2002. Antibody-sensitive and antibody-resistant cell-to-cell spread by vaccinia virus: role of the A33R protein in antibody-resistant spread. J. Gen. Virol. 83:209-222. [DOI] [PubMed] [Google Scholar]
- 9.Li, Y., D. S. Carroll, S. N. Gardner, M. C. Walsh, E. A. Vitalis, and I. K. Damon. 2007. On the origin of smallpox: correlating variola phylogenics with historical smallpox records. Proc. Natl. Acad. Sci. USA 104:15787-15792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li, Y., V. A. Olson, T. Laue, M. T. Laker, and I. K. Damon. 2006. Detection of monkeypox virus with real-time PCR assays. J. Clin. Virol. 36:194-203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Likos, A. M., S. A. Sammons, V. A. Olson, A. M. Frace, Y. Li, M. Olsen-Rasmussen, W. Davidson, R. Galloway, M. L. Khristova, M. G. Reynolds, H. Zhao, D. S. Carroll, A. Curns, P. Formenty, J. J. Esposito, R. L. Regnery, and I. K. Damon. 2005. A tale of two clades: monkeypox viruses. J. Gen. Virol. 86:2661-2672. [DOI] [PubMed] [Google Scholar]
- 11a.Olson, V. A., K. L. Karem, S. K. Smith, C. M. Hughes, and I. K. Damon. J. Gen. Virol., in press. [DOI] [PubMed]
- 12.Quenelle, D. C., R. M. Buller, S. Parker, K. A. Keith, D. E. Hruby, R. Jordan, and E. R. Kern. 2007. Efficacy of delayed treatment with ST-246 given orally against systemic orthopoxvirus infections in mice. Antimicrob. Agents Chemother. 51:689-695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Roper, R. L., and B. Moss. 1999. Envelope formation is blocked by mutation of a sequence related to the HKD phospholipid metabolism motif in the vaccinia virus F13L protein. J. Virol. 73:1108-1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sbrana, E., R. Jordan, D. E. Hruby, R. I. Mateo, S. Y. Xiao, M. Siirin, P. C. Newman, A. P. da Rosa, and R. B. Tesh. 2007. Efficacy of the antipoxvirus compound ST-246 for treatment of severe orthopoxvirus infection. Am. J. Trop. Med. Hyg. 76:768-773. [PubMed] [Google Scholar]
- 15.Sung, T. C., R. L. Roper, Y. Zhang, S. A. Rudge, R. Temel, S. M. Hammond, A. J. Morris, B. Moss, J. Engebrecht, and M. A. Frohman. 1997. Mutagenesis of phospholipase D defines a superfamily including a trans-Golgi viral protein required for poxvirus pathogenicity. EMBO J. 16:4519-4530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vora, S., I. K. Damon, V. Fulginiti, S. G. Weber, M. Kahana, S. L. Stein, S. I. Gerber, S. Garcia-Houchin, E. R. Lederman, D. Hruby, L. Collins, D. Scott, K. Thompson, J. V. Barson, R. L. Regnery, C. Hughes, R. S. Daum, Y. Li, H. Zhao, S. Smith, Z. Braden, K. Karem, V. A. Olson, W. B. Davidson, G. Trindade, T. Bolken, R. Jordan, D. Tien, and J. Marcinak. 2008. Severe eczema vaccinatum in a household contact of a smallpox vaccinee. Clin. Infect. Dis. 46:1555-1561. [DOI] [PubMed] [Google Scholar]
- 17.Yang, G., D. C. Pevear, M. H. Davies, M. S. Collett, T. Bailey, S. Rippen, L. Barone, C. Burns, G. Rhodes, S. Tohan, J. W. Huggins, R. O. Baker, R. L. Buller, E. Touchette, K. Waller, J. Schriewer, J. Neyts, E. DeClercq, K. Jones, D. Hruby, and R. Jordan. 2005. An orally bioavailable antipoxvirus compound (ST-246) inhibits extracellular virus formation and protects mice from lethal orthopoxvirus challenge. J. Virol. 79:13139-13149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yang, H., S. K. Kim, M. Kim, P. A. Reche, T. J. Morehead, I. K. Damon, R. M. Welsh, and E. L. Reinherz. 2005. Antiviral chemotherapy facilitates control of poxvirus infections through inhibition of cellular signal transduction. J. Clin. Investig. 115:379-387. [DOI] [PMC free article] [PubMed] [Google Scholar]