Abstract
Clinical isolates of Escherichia coli collected from 1991 through 2005 at a tertiary-care center were studied for qnr and aac(6′)-Ib-cr genes. Isolates bearing aac(6′)-Ib-cr emerged in 1998, coinciding with an increase in ciprofloxacin resistance. The presence of aac(6′)-Ib-cr was multiclonal and was associated with the presence of extended-spectrum β-lactamases.
Plasmid-mediated qnr and aac(6′)-Ib-cr genes confer reduced quinolone susceptibility, facilitating the selection of chromosomal mutations that confer high-level resistance (7, 8, 15). Qnr and Aac(6′)-Ib-cr have therefore been hypothesized as potential contributors to the increase in prevalence of quinolone resistance among gram-negative bacteria. Epidemiological surveys have found qnr genes in various Enterobacteriaceae (14). Findings from a recent study suggest that qnrA and qnrB may have contributed to the emergence of fluoroquinolone resistance among Klebsiella pneumoniae and Enterobacter spp. (17). This survey also observed an association between ceftazidime (CAZ) resistance and the presence of qnr genes, suggesting that in some species, the frequent clinical association of cephalosporin resistance with fluoroquinolone resistance might be due to the genetic linkage of these two elements on plasmids.
The cr variant of aac(6′)-Ib encodes an aminoglycoside acetyltransferase that confers reduced susceptibility to ciprofloxacin by the N-acetylation of its piperazinyl amine (15). Aac(6′)-Ib-cr has two amino acid changes, Trp102Arg and Asp179Tyr, which together are necessary and sufficient for the enzyme's ability to acetylate ciprofloxacin. Several prior surveys of aac(6′)-Ib-cr prevalence (5, 10, 12) were limited to cephalosporin- and/or ciprofloxacin-resistant isolates, which were collected over relatively short time frames. Hence, little is known about the epidemiological patterns of aac(6′)-Ib-cr in population-representative clinical isolates over time. Interestingly, in two of these earlier studies aac(6′)-Ib-cr was found predominantly in Escherichia coli. We therefore surveyed bloodstream isolates of E. coli collected over a 15-year period for aac(6′)-Ib-cr and qnr genes in order to more broadly characterize the changes over time in the prevalence of these resistance elements in a large collection of clinical isolates. Additionally, we examined the isolates for evidence of an association between the genes of interest and CAZ resistance.
(This work was presented in part at the 48th Interscience Conference on Antimicrobial Agents and Chemotherapy/46th Annual Meeting of the Infectious Diseases Society of America, Washington, DC, 2008.)
Bacterial strains.
Since 1990 all patient-unique bloodstream isolates at Hadassah Ein-Kerem Hospital in Jerusalem, Israel, have been preserved at −70°C. For this study, we screened all available nosocomial E. coli isolates (i.e., isolated from cultures taken >3 days after admission) from 1991 through 2005. Certain isolates were unavailable due to nonsystematic clerical errors.
Susceptibility testing.
Disk susceptibility testing and clavulanic acid enhancement of cephalosporin susceptibility for the detection of extended-spectrum ß-lactamases (ESBLs) followed the recommendations of the CLSI by using unsupplemented Mueller-Hinton agar and incubation at 37°C for 16 to 20 h (3).
Screening for plasmid-mediated quinolone resistance.
Screening for qnr was carried out by multiplex PCR amplification of qnrA, qnrB, and qnrS as previously described (16, 17).
Because Aac(6′)-Ib-cr confers resistance to kanamycin, albeit at a level lower than that of Aac(6′)-Ib (15), screening for aac(6′)-Ib-cr was carried out in two steps; strains were screened for kanamycin susceptibility (Sigma Chemical Co., Rehovot, Israel) at a concentration of 32 μg/ml, followed by direct screening by gap-ligase chain reaction (LCR) for G535T, one of the two defining mutations of aac(6′)-Ib-cr (15). Gap-LCR is a powerful method for detecting single base changes (1). For this technique, two same-directional primers, separated by a gap of several nucleotides, are chosen. These hybridize to complementary strands of target DNA and will be extended by DNA polymerase and subsequently ligated into a single long oligonucleotide when the mutation of interest, which corresponds to the 3′ end of the first primer, is present. This oligonucleotide can then be amplified. We used as forward primers aac-glcr-F1 (5′-AGGTACCGTAACCACCCCAT) and aac-glcr-F2-P (5′/5Phos/GTCCAGCCGTGTACATGG), matching positions 516 to 535 and 539 to 556, respectively, with respect to the Aac(6′)-Ib-cr translational starting point. Our reverse primers were aac-glcr-R3 (5′-CCATGTACACGGCTGGACC) and aac-glcr-R4-P (5′/5Phos/TGGGGTGGTTACGGTACCT) and were designed to amplify a 41-bp fragment corresponding to the oligonucleotide formed by the ligation of the forward primers. Bacterial DNA was prepared with a DNA isolation kit (Genekam Biotechnology AG, Duisburg, Germany). The four primers, at a concentration of 0.033 μM each, were added to the template DNA. Gap-LCR amplification was performed with AmpliTaq DNA polymerase Stoffel fragment (Applied Biosystems, Foster City, CA) at 0.033 U/μl, Taq DNA ligase (New England Biolabs, Beverly, MA) at 0.4 U/μl, and dATP, dTTP, and dGTP, each at 200 μM, in a final volume of 30 μl containing 1× Taq DNA ligase reaction buffer. The template concentration can play a major role in the specificity of the gap-LCR amplification procedures. By optimizing the assay conditions, we were able to use unquantified extracts of whole-cell DNA and maintain specificity. The gap-LCR conditions were 94°C for 30 s, 50°C for 30 s, 72°C for 5 s, and 60°C for 4 min, with a cycle number of 20. The amplification products were provisionally identified from their sizes in ethidium bromide-stained 16% polyacrylamide gels (Fig. 1). All strains that tested positive by gap-LCR were sequenced to confirm the presence of the G535T mutation and the T304C mutation. The gap-LCR assay was validated by assaying 10 strains known by sequencing to carry the aac(6′)-Ib-cr gene, as well as 8 negative isolates, among which were isolates carrying the wild-type aac(6′)-Ib gene. Gap-LCR gave correct results for all 18 isolates.
FIG. 1.
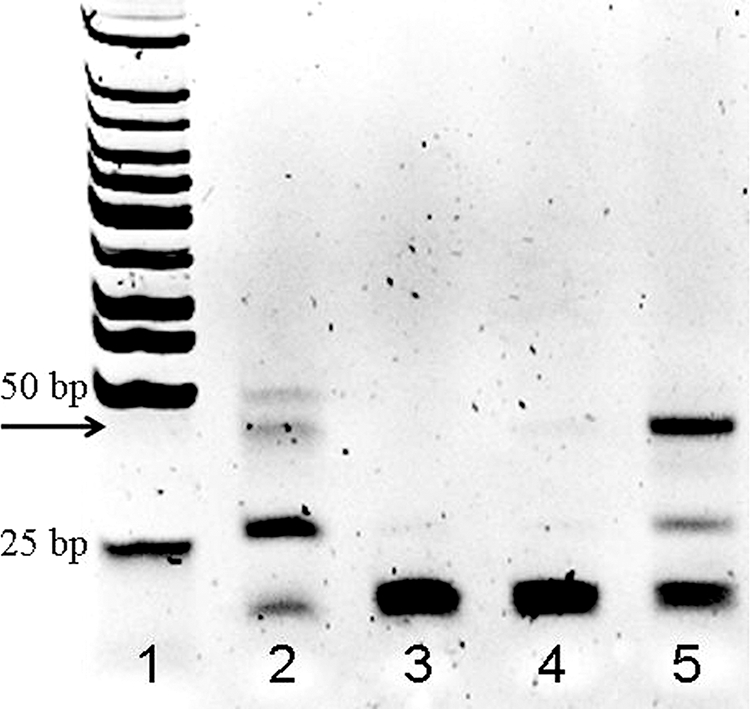
Polyacrylamide gel analysis of gap-LCR assay. Lane 1, low-molecular-weight DNA ladder (New England Biolabs, Beverly, MA); lane 2, pBAD24-aac(6′)-Ib-cr; lane 3, pBAD24-aac(6′)-Ib; lane 4, aac(6′)-Ib-cr-negative E. coli; lane 5, aac(6′)-Ib-cr-positive E. coli. Arrow points to the putative reaction product.
PFGE.
Pulsed-field gel electrophoresis (PFGE) after restriction with XbaI was performed according to a standardized protocol (13) on all aac(6′)-Ib-cr+ isolates. The band patterns were analyzed according to accepted criteria (18).
Detection of ESBL genes by PCR.
Amplification of the major ESBL resistance determinants was performed for all strains exhibiting an ESBL phenotype using previously validated primers for the detection of blaTEM (9), blaSHV (6), and two sets of primers for blaCTX-M genes (11).
Statistical methods.
Fisher's exact test (http://www.langsrud.com/fisher.htm) was used to compare the prevalence of ciprofloxacin resistance (defined as a MIC of ≥2 μg/ml) (3), aac(6′)-Ib-cr, and qnr before and after 1 January 1998. The confidence intervals for the risk ratios were calculated at http://www.cebm.utoronto.ca/practise/ca/statscal/.
Results.
From 1991 through 2005, there were 904 episodes of nosocomial E. coli bloodstream infection and 718 isolates were available for analysis. From 1991 through 1997, 27 of 265 E. coli (11.3%) isolates were ciprofloxacin intermediate or resistant, compared with 144 of 453 (46.6%) from 1998 through 2005 (P < 0.001) (Fig. 2). From 1991 through 1997, no isolate had aac(6′)-Ib-cr, whereas from 1998 onwards, 32 of 453 (7.1%) of the isolates had aac(6′)-Ib-cr (P < 0.001) (Fig. 2). Among the aac(6′)-Ib-cr+ isolates, 30 of 32 (93.8%) were not susceptible to ciprofloxacin. PFGE of all the aac(6′)-Ib-cr+ strains demonstrated a cluster of five indistinguishable isolates and an identical pair with a different pattern, while the remaining 25 isolates were unrelated (Fig. 3). A single isolate from 2002 harbored qnrA. The genes qnrB and qnrS were not found. Thus, aac(6′)-Ib-cr was the predominant plasmid-mediated quinolone resistance gene in E. coli, and its polyclonal emergence coincided with the rise in ciprofloxacin resistance. Unlike qnr genes in other Enterobacteriaceae (17), the presence of the aac(6′)-Ib-cr gene was strongly associated with ciprofloxacin resistance defined by CLSI criteria.
FIG. 2.
Percentage of clinical E. coli isolates (n = 718) harboring resistance to ciprofloxacin (MIC ≥ 2 μg/ml) and harboring aac(6′)-Ib-cr. No aac(6′)-Ib-cr genes were found before 1998.
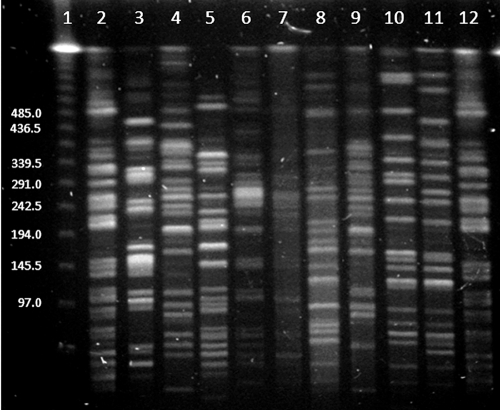
FIG. 3.
PFGE of 10 E. coli isolates from 2004 and E. coli KL-16 (serving as the control strain) after restriction with XbaI. Lane 1, phage lambda ladder (size marks in Kb); lanes 2 and 12, E. coli KL-16; lanes 3 to 11, E. coli isolates from 2004 (in lane 4, one of the five PFGE-identical isolates).
The presence of aac(6′)-Ib-cr was also associated with CAZ resistance (risk ratio, 5.8 [95% confidence interval, 3.9 to 8.4]). To adjust for the potential confounding effect of an association between fluoroquinolone resistance and CAZ resistance independent of the aac(6′)-Ib-cr gene, isolates were stratified by quinolone susceptibility (Table 1). The association between CAZ resistance and aac(6′)-Ib-cr was present in both fluoroquinolone-susceptible and -resistant E. coli.
TABLE 1.
Distribution of ciprofloxacin and ceftazidime susceptibilities among aac(6′)-Ib-cr+ and aac(6′)-Ib-cr E. coli isolatesa
| Ciprofloxacin susceptibility | No. of isolates with indicated CAZ susceptibility
|
Risk ratio | 95% CI | |||
|---|---|---|---|---|---|---|
|
aac(6′)-Ib-cr positive
|
aac(6′)-Ib-cr deficient
|
|||||
| CAZ-R | CAZ-S | CAZ-R | CAZ-S | |||
| S + I/R | 18 | 14 | 67 | 619 | 5.8 | 3.9-8.4 |
| S | 1 | 2 | 28 | 517 | 6.5 | 1.2-33.4 |
| I/R | 17 | 12 | 39 | 102 | 2.1 | 1.4-3.2 |
S, susceptible; R, resistant; I, intermediate; CI, confidence interval.
A total of 25 of 32 (78%) aac(6′)-Ib-cr+ E. coli manifested an ESBL phenotype. Of these, one isolate harbored both blaSHV-12 and blaCTX-M-15 genes. Two additional isolates contained blaSHV-12. Two groups of blaCTX-M genes were identified in 22 strains. One group consisted of two strains identified as CTX-M-25; sequence analysis of five nonrelated isolates (by PFGE) that belonged to the second group revealed that all were CTX-M-15. Seventeen isolates also harbored blaTEM that was further identified in two strains as TEM-1. Thus, although aac(6′)-Ib-cr appeared in different clones it was associated with a limited number of ESBL-encoding genes.
Discussion.
The prevalence of quinolone resistance among nosocomial Enterobacteriaceae increased, at a different rate for each genus, following the introduction of ciprofloxacin into the Hadassah hospitals at the end of 1989. We recently showed that this increase coincided with the entry of the qnr genes into the K. pneumoniae and Enterobacter spp. populations (17). Using gap-LCR, an inexpensive technique better suited to large-scale epidemiologic surveys than previous surveillance methods (2, 12), we demonstrated a similar pattern of penetration of aac(6′)-Ib-cr into multiple clinical E. coli clones, coincident with a rise in fluoroquinolone resistance from 11% in 1997 to 37% in 2005. A caveat regarding any observational study of this kind is that correlation does not prove causation. Also, further studies are needed to determine whether these observations hold up globally.
Of note, we also demonstrated an epidemiologic link between aac(6′)-Ib-cr and CAZ resistance and found that a majority of aac(6′)-Ib-cr+ isolates (22 out of 32 [68.8%]) harbored a CTX-M ESBL. To ascertain that these genes in our collection are located on the same plasmid was beyond the scope of this work. However, our findings support earlier suggestions of a linkage between aac(6′)-Ib-cr and CTX-M ESBLs (4, 12) and raise the possibility that the use of ciprofloxacin—a widely prescribed fluoroquinolone in the world—is a driver of both fluoroquinolone resistance and the emergence of CTX-M ESBLs.
Acknowledgments
This work was supported by grant Morasha 1833/07 from the Israel Science Foundation to J.S.
Footnotes
Published ahead of print on 22 December 2008.
REFERENCES
- 1.Abravaya, K., J. J. Carrino, S. Muldoon, and H. H. Lee. 1995. Detection of point mutations with a modified ligase chain reaction (Gap-LCR). Nucleic Acids Res. 23:675-682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ambrozic Avgustin, J., R. Keber, K. Zerjavic, T. Orazem, and M. Grabnar. 2007. Emergence of the quinolone resistance-mediating gene aac(6′)-ib-cr in extended-spectrum-β-lactamase-producing Klebsiella isolates collected in Slovenia between 2000 and 2005. Antimicrob. Agents Chemother. 51:4171-4173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Clinical and Laboratory Standards Institute. 2007. Performance standards for antimicrobial susceptibility testing; 15th informational supplement. M100-S17. Clinical and Laboratory Standards Institute, Wayne, PA.
- 4.Coque, T. M., A. Novais, A. Carattoli, L. Poirel, J. Pitout, L. Peixe, F. Baquero, R. Canton, and P. Nordmann. 2008. Dissemination of clonally related Escherichia coli strains expressing extended-spectrum beta-lactamase CTX-M-15. Emerg. Infect. Dis. 14:195-200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Endimiani, A., L. L. Carias, A. M. Hujer, C. R. Bethel, K. M. Hujer, F. Perez, R. A. Hutton, W. R. Fox, G. S. Hall, M. R. Jacobs, D. L. Paterson, L. B. Rice, S. G. Jenkins, F. C. Tenover, and R. A. Bonomo. 2008. Presence of plasmid-mediated quinolone resistance in Klebsiella pneumoniae isolates possessing blaKPC in the United States. Antimicrob. Agents Chemother. 52:2680-2682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hoffmann, H., E. Sturenburg, J. Heesemann, and A. Roggenkamp. 2006. Prevalence of extended-spectrum beta-lactamases in isolates of the Enterobacter cloacae complex from German hospitals. Clin. Microbiol. Infect. 12:322-330. [DOI] [PubMed] [Google Scholar]
- 7.Jacoby, G. A., K. E. Walsh, D. M. Mills, V. J. Walker, H. Oh, A. Robicsek, and D. C. Hooper. 2006. qnrB, another plasmid-mediated gene for quinolone resistance. Antimicrob. Agents Chemother. 50:1178-1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Martinez-Martinez, L., A. Pascual, and G. A. Jacoby. 1998. Quinolone resistance from a transferable plasmid. Lancet 351:797. [DOI] [PubMed] [Google Scholar]
- 9.Monstein, H. J., A. Ostholm-Balkhed, M. V. Nilsson, M. Nilsson, K. Dornbusch, and L. E. Nilsson. 2007. Multiplex PCR amplification assay for the detection of blaSHV, blaTEM and blaCTX-M genes in Enterobacteriaceae. APMIS 115:1400-1408. [DOI] [PubMed] [Google Scholar]
- 10.Park, C. H., A. Robicsek, G. A. Jacoby, D. Sahm, and D. C. Hooper. 2006. Prevalence in the United States of aac(6′)-Ib-cr encoding a ciprofloxacin-modifying enzyme. Antimicrob. Agents Chemother. 50:3953-3955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pitout, J. D., N. Hamilton, D. L. Church, P. Nordmann, and L. Poirel. 2007. Development and clinical validation of a molecular diagnostic assay to detect CTX-M-type beta-lactamases in Enterobacteriaceae. Clin. Microbiol. Infect. 13:291-297. [DOI] [PubMed] [Google Scholar]
- 12.Pitout, J. D. D., Y. Wei, D. L. Church, and D. B. Gregson. 2008. Surveillance for plasmid-mediated quinolone resistance determinants in Enterobacteriaceae within the Calgary Health Region, Canada: the emergence of aac(6′)-Ib-cr. J. Antimicrob. Chemother. 61:999-1002. [DOI] [PubMed] [Google Scholar]
- 13.Ribot, E. M., M. A. Fair, R. Gautom, D. N. Cameron, S. B. Hunter, B. Swaminathan, and T. J. Barrett. 2006. Standardization of pulsed-field gel electrophoresis protocols for the subtyping of Escherichia coli O157:H7, Salmonella, and Shigella for PulseNet. Foodborne Pathog. Dis. 3:59-67. [DOI] [PubMed] [Google Scholar]
- 14.Robicsek, A., G. A. Jacoby, and D. C. Hooper. 2006. The worldwide emergence of plasmid-mediated quinolone resistance. Lancet Infect. Dis. 6:629-640. [DOI] [PubMed] [Google Scholar]
- 15.Robicsek, A., J. Strahilevitz, G. A. Jacoby, M. Macielag, D. Abbanat, P. C. Hye, K. Bush, and D. C. Hooper. 2006. Fluoroquinolone-modifying enzyme: a new adaptation of a common aminoglycoside acetyltransferase. Nat. Med. 12:83. [DOI] [PubMed] [Google Scholar]
- 16.Robicsek, A., J. Strahilevitz, D. F. Sahm, G. A. Jacoby, and D. C. Hooper. 2006. qnr prevalence in ceftazidime-resistant Enterobacteriaceae isolates from the United States. Antimicrob. Agents Chemother. 50:2872-2874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Strahilevitz, J., D. Engelstein, A. Adler, V. Temper, A. E. Moses, C. Block, and A. Robicsek. 2007. Changes in qnr prevalence and fluoroquinolone resistance in clinical isolates of Klebsiella pneumoniae and Enterobacter spp. collected from 1990 to 2005. Antimicrob. Agents Chemother. 51:3001-3003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tenover, F. C., R. D. Arbeit, R. V. Goering, P. A. Mickelsen, B. E. Murray, D. H. Persing, and B. Swaminathan. 1995. Interpreting chromosomal DNA restriction patterns produced by pulsed-field gel electrophoresis: criteria for bacterial strain typing. J. Clin. Microbiol. 33:2233-2239. [DOI] [PMC free article] [PubMed] [Google Scholar]