Abstract
Malaria is one of the most significant causes of infectious disease in the world. The search for new antimalarial chemotherapies has become increasingly urgent due to the parasites’ resistance to current drugs. Ellagic acid is a polyphenol found in various plant products. In this study, antimalarial properties of ellagic acid were explored. The results obtained have shown high activity in vitro against all Plasmodium falciparum strains whatever their levels of chloroquine and mefloquine resistance (50% inhibitory concentrations ranging from 105 to 330 nM). Ellagic acid was also active in vivo against Plamodium vinckei petteri in suppressive, curative, and prophylactic murine tests, without any toxicity (50% effective dose by the intraperitoneal route inferior to 1 mg/kg/day). The study of the point of action of its antimalarial activity in the erythrocytic cycle of Plasmodium falciparum demonstrated that it occurred at the mature trophozoite and young schizont stages. Moreover, ellagic acid has been shown to potentiate the activity of current antimalarial drugs such as chloroquine, mefloquine, artesunate, and atovaquone. This study also proved the antioxidant activity of ellagic acid and, in contrast, the inhibitory effect of the antioxidant compound N-acetyl-l-cysteine on its antimalarial efficacy. The possible mechanisms of action of ellagic acid on P. falciparum are discussed in light of the results. Ellagic acid has in vivo activity against plasmodia, but modification of the compound could lead to improved pharmacological properties, principally for the oral route.
New drugs against malaria are urgently needed, and traditional methods of malaria treatment could be a promising source of new antimalarial compounds (5). We have recently initiated several collaborative programs with West African countries aimed at selecting, by ethnopharmacological methods, plants largely used by traditional healers and the local populations for malaria treatment. The in vitro antiplasmodial activities of the crude extracts and fractions (from bioguided fractionation of the promising crude extracts of plants) enabled us to identified ellagic acid as one of the active ingredients. The in vitro antimalarial activity of this molecule was previously reported by other researchers (3, 10). In this study, we clarify the high level of in vitro and in vivo antiplasmodial properties of ellagic acid, its antioxidant activity, and its potential pro-oxidant effect and gain a deeper understanding of its mechanisms of action.
MATERIALS AND METHODS
Compounds.
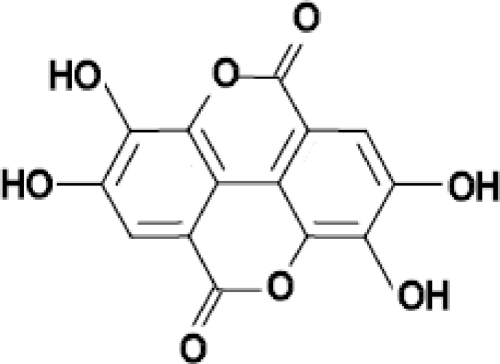
Ellagic acid hydrate (Fig. 1) was obtained from Acros Organics (Belgium), with a molecular weight 302.19 g/mol. The following other reagents were also obtained: chloroquine diphosphate salt (Sigma, France), mefloquine hydrochloride (Hoffmann-La Roche, Switzerland), artesunate (Sanofi-Synthelabo, France), artemisinin (Sigma, Germany), and atovaquone (GlaxoSmithKline, United Kingdom).
FIG. 1.
Ellagic acid. The chemical formula is C14H6O8; the molecular weight is 302.197 g/mol.
In vitro antiplasmodial activity.
Five strains (W2-Indochina, FcM29-Cameroon, FcB1-Colombia, F32-Tanzania, and Dd2) of Plasmodium falciparum were used to evaluate in vitro antiplasmodial activity. These strains were continuously cultured using standard methods (27). The in vitro antiplasmodial activity was evaluated by the radioactive micromethod as previously described (4, 11). Each 50% inhibitory concentration (IC50) was calculated as the concentration inhibiting 50% of parasitic growth.
In vitro cytotoxicity tests.
The cytotoxicity of ellagic acid was tested against MRC5 (human diploid embryonic lung), KB (human epidermoid carcinoma), and Vero (African green monkey kidney) cells in culture. The cells were grown in Dulbecco's modified Eagle's medium supplemented with 25 mM glucose, 10% (vol/vol) fetal calf serum, 100 IU penicillin, 100 μg/ml streptomycin, and 1.5 μg/ml amphotericin B and kept under 5% CO2 at 37°C. Ninety-six-well plates were seeded with 600 cells per well in 200 μl of medium. Twenty-four hours later, ellagic acid, first dissolved in dimethyl sulfoxide (DMSO) and then diluted in RPMI medium, was added (the final concentration of DMSO was 1%), and the cells were incubated for a further 72 h. Controls received an equal volume of DMSO. The number of viable cells was then measured at 490 nm with MTS reagent (Promega), and the IC50 was calculated as the concentration of compounds eliciting a 50% inhibition of cell proliferation.
In vitro potentiation tests.
The synergy between ellagic acid and chloroquine, artemisinin, artesunate, mefloquine, and atovaquone was assessed by potentiation experiments as previously described (7). Chloroquine-resistant Plasmodium falciparum strain W2-Indochina (IC50 for chloroquine of 300 nM), was used. Several combinations of ellagic acid and the corresponding other molecules were incubated in 96-well plates, and the inhibition was evaluated as described above. The ellagic acid 50% fractional inhibition concentrations (FIC50s) were calculated by dividing the IC50 of the combination by the IC50 of ellagic acid alone. The corresponding other molecule FIC50 was also calculated. Potentiation results are the fractional inhibition concentrations indicating the type of the combined effect of drugs. The final value of the FIC50 indicated if the interaction was an additive (FIC50 equal to 1), antagonistic (FIC50 was >1), or synergistic (FIC50 was <1) effect.
Chloroquine- and mefloquine-resistant reversion assays.
To know if the synergistic association of ellagic acid with chloroquine and mefloquine was not due to a chloroquine- and mefloquine-resistant reversion effect, a chloroquine-resistant reversion test was carried out as a positive control using verapamil hydrochloride (Sigma, France) on chloroquine-resistant strain W2-Indochina (IC50 for chloroquine of 300 nM) and on chloroquine-sensitive strain F32-Tanzania (IC50 for chloroquine of 30 ± 2 nM) according to the experimental procedures described previously by Martin et al. (16). For a mefloquine resistance reversion assay, the positive control consisted of using penfluridol (Sigma, France) (17, 18), a piperidine analogue, and Plasmodium falciparum strains FcM29-Cameroon, sensitive to mefloquine (IC50 of 7 ± 4 nM), and Dd2, which showed a reduced susceptibility to mefloquine in vitro (IC50 of 21 ± 3 nM). Amplification assays of the P. falciparum MDR1 gene on strains Dd2 and FcM29 confirmed that Dd2 showed a reduced susceptibility to mefloquine by expressing an average of three copies, compared with reference strain FcM29, which expressed only one copy (data not shown). This agrees with previous reports showing the same results (20).
The 50% reversant concentration (CR50) was determined in three independent experiments.
Stage of ellagic acid action in the erythrocytic life cycle.
Strain FcB1-Colombia was synchronized to a 4-h period. The method consisted of alternatively synchronizing young forms with 5% d-sorbitol and late forms with Plasmion (15). Ellagic acid was tested in 24-well plates with cultures at 0.5 to 1% parasitemia (hematocrit, 2%). Cultures were subjected to 8-h pulses (corresponding to one-sixth of the erythrocytic cycle time). The ellagic acid concentrations used on strain FcB1 were 25 ng/ml, 100 ng/ml (IC50 against FcB1), and 250 ng/ml. After being pulsed, the cultures were washed three times with RPMI 1640 medium (Gibco Invitrogen, France) and then returned to normal conditions. At the end of the experiment (the ring stage of the next erythrocytic cycle), parasitemia was calculated by microscopic examination and counting of Giemsa-stained smears (6). The results were expressed as a percentage of inhibition of parasitic growth.
Test of antiplasmodial activity in vivo.
In vivo assays of ellagic acid were performed on a rodent malaria strain of the parasite (Plasmodium vinckei petteri), with the classical 4-day suppressive test after inoculation of 2 × 107 parasites/mouse (19). Artesunate (5 mg/kg/day) was used as a control. Three doses of ellagic acid were tested (1, 50, and 100 mg/kg/day). Groups of five mice (Swiss female mice, 8 weeks old) were used for each dose. Control mice were treated with the vehicle either orally or intraperitoneally. Another five mice were kept as untreated controls (same batch, no parasite, and no treatment), and finally, five mice were inoculated but not treated.
Female Swiss mice (Janvier, Fance) weighing around 30 g were inoculated intraperitoneally or per os daily for 4 consecutive days. The first treatment started 3 h after parasite inoculation, and the others were given at the same time on the following days. Parasitemia levels (parasitized erythrocytes/total erythrocytes) were determined on day 4 with thin blood smears.
The ED50 was assessed as the dose leading to a 50% inhibition of parasite growth compared with growth in the control (treated with an equal volume of vehicle: 100 μl of a 50:50 mixture of physiological serum and DMSO).
The mice were monitored after day 4 until day 60, and every week, parasitemia was determined. Surviving mice treated intraperitoneally or per os were monitored every day until day 60.
In vivo prophylaxis-curative test.
Groups of five mice (Swiss female mice, 8 weeks old) were used, and 1 million parasites (P. vinckei petteri) were injected intraperitoneally into mice at day 0. Mice were treated with ellagic acid in the vehicle either orally (100 mg/kg/day) or intraperitoneally (10 mg/kg/day) for 4 days. For the first group, mice were treated for 4 days before parasite inoculation (day −4 to day 0), and for the second group, mice were treated for 4 days after parasite inoculation (day 0 to day 4). The third group was treated for 8 days, from 4 days before parasite inoculation until 4 days after (day −4 to day 4). Parasitemia levels were determined on day 6 and day 8 with thin blood smears and compared with controls (mice treated with equal volumes of vehicle either orally or intraperitoneally). Surviving mice were monitored every day until day 60.
In vivo toxicity test.
Toxicity was evaluated via the oral and intraperitoneal routes. Healthy 8-week-old female Swiss mice (Janvier, France) were treated with concentrations of ellagic acid between 100 mg/kg/day and 1 g/kg/day once a day for four consecutive days and observed for 30 days, and mortality and any signs of toxicity were recorded.
Any procedures involving animals fully conformed to Europeans regulations (EEC directive 86/609, dated 24 November 1986). The experiments involving animals were carried out in the animal room of the Parasitology Department of University Hospital (Toulouse, France), which is under the control of the National Veterinary Services. All in vivo studies were approved by the French Institutional Animal Experimentation Ethics Committee (approval numbers MP/R/04/31/11/07 [antiplasmodial activity and prophylaxis-curative test] and MP/05/05/01/08 [toxicity tests]).
Modulation of antimalarial activity by NAC and antioxidant effects of ellagic acid.
The modulating effects of N-acetyl-l-cysteine (NAC) (Sigma, France) on the antimalarial activity of ellagic acid were assessed using strain FcM29. The experimental procedures were adapted from the protocol described previously by Arreesrisom et al. (2). The final concentration of NAC used without an effect on parasitic growth was 0.87 μM. The final concentration in the culture medium of ellagic acid was 0.18 μM (IC50 of ellagic acid for strain FcM29). The antiplasmodial activity was evaluated by the radioactive micromethod.
The oxygen-dependent respiratory burst in treated human monocytes was measured by chemiluminescence in the presence of 5-amino-2,3-dihydro-1,4-phthalazinedione (luminol) using a thermostatically (37°C) controlled luminometer (Wallac 1420; Victor2, Finland). The generation of chemiluminescence was monitored continuously for 1 h after incubation of the cells with luminol (66 μM), and cells were treated with phorbol myristate acetate (PMA) (30 μM) and ellagic acid (3.3 μM). The measurements were performed in duplicate, and statistical analyses were done using the area under the curve expressed in counts × seconds.
RESULTS
Ellagic acid inhibits Plasmodium growth in vitro without cytotoxicity.
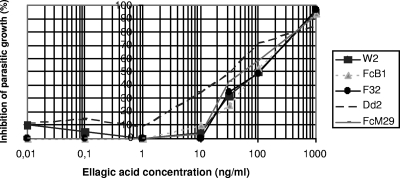
The data for the antimalarial activity of ellagic acid in vitro on five P. falciparum strains are shown in Fig. 2 and Table 1. The IC50s for ellagic acid (range, 105 to 330 nM) were approximately in the same range for all the P. falciparum strains tested whatever their chemosensitivities. Cytotoxicity tests in vitro showed no cytotoxicity on Vero, KB, and MRC5 cells for the dose of 100 μM (data not shown). The security index (cytotoxicity/activity ratio) therefore appeared to be up to 495 (range, 303 to 952).
FIG. 2.
Means of ellagic acid antiplasmodial activities in vitro against five Plasmodium falciparum strains according to data from at least three independent experiments for each strain.
TABLE 1.
In vitro antiplasmodial activity of ellagic acid on five Plasmodium falciparum strains
| Compound | Mean IC50 (nM) ± SD for straina:
|
||||
|---|---|---|---|---|---|
| F32 | Dd2 | FcB1 | W2 | FcM29 | |
| Ellagic acid | 330 ± 27 | 105 ± 27 | 300 ± 17 | 330 ± 24 | 180 ± 20 |
| Chloroquine | 29 ± 5 | 114 ± 29 | 120 ± 28 | 299 ± 22 | 400 ± 12 |
| Artemisinin | 12.5 ± 6 | 13.5 ± 5 | 12.2 ± 5 | 13.30 ± 4 | 12.2 ± 5 |
Each IC50 corresponds to the mean of data from at least three independent experiments.
Ellagic acid potentiates the in vitro antiplasmodial activity of the major antimalarial drugs.
Ellagic acid showed synergistic activity with chloroquine, atovaquone, mefloquine, and artesunate but was slightly antagonistic with artemisinin (Table 2).
TABLE 2.
Effects of antimalarial agents in combination with ellagic acid on strain W2a
| Molecule tested with ellagic acid | Mean FIC50 ± SD | Type of combination |
|---|---|---|
| Chloroquine | 0.63 ± 0.05 | Synergistic |
| Mefloquine | 0.64 ± 0.09 | Synergistic |
| Artemisinin | 1.26 ± 0.13 | Antagonistic |
| Artesunate | 0.57 ± 0.13 | Synergistic |
| Atovaquone | 0.53 ± 0.21 | Synergistic |
Values are the means of the FIC50 (which is an interaction coefficient indicating whether the combined effect of drugs is synergistic, additive, or antagonistic) and standard deviations for assays run in triplicate on different days. The combination was considered to be synergistic if the FIC50 was <1, additive if the FIC50 was equal to 1, and antagonistic if the FIC50 was >1.
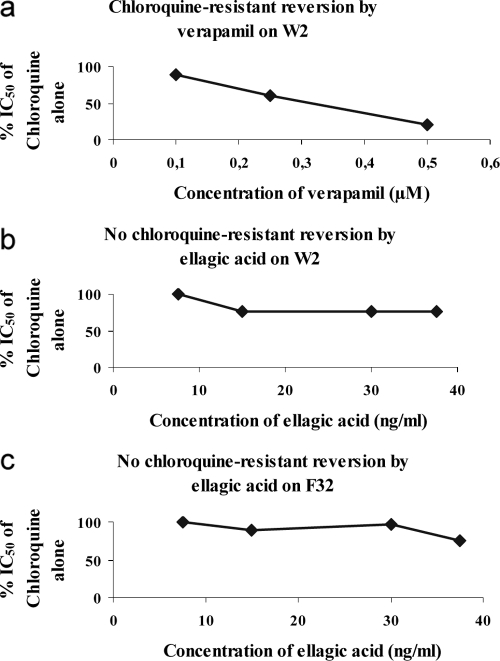
To determine if the synergism of ellagic acid with chloroquine was not due to a chloroquine-resistant reversion effect, the chloroquine-resistant reversion by ellagic acid was compared with the effect of the known chloroquine-resistance-reversing compound verapamil on strain W2. The results in Fig. 3 show that ellagic acid was not a reversal agent of chloroquine resistance as was verapamil, which had a CR50 of 0.31 ± 0.07 μM. This reversion effect was not found on chloroquine-sensitive strain F32, which was used as a negative control for our experiments.
FIG. 3.
Chloroquine resistance reversal by ellagic acid. Values correspond to the means of data from three independent experiments. (a) Mean of reversal concentration of verapamil (positive control) for chloroquine-resistant strain W2 (CR50 equal to 0.31 ± 0.07 μM). (b) Absence of reversion by ellagic acid for chloroquine-resistant strain W2. (c) Absence of reversion by ellagic acid for chloroquine-sensitive strain F32 (negative control).
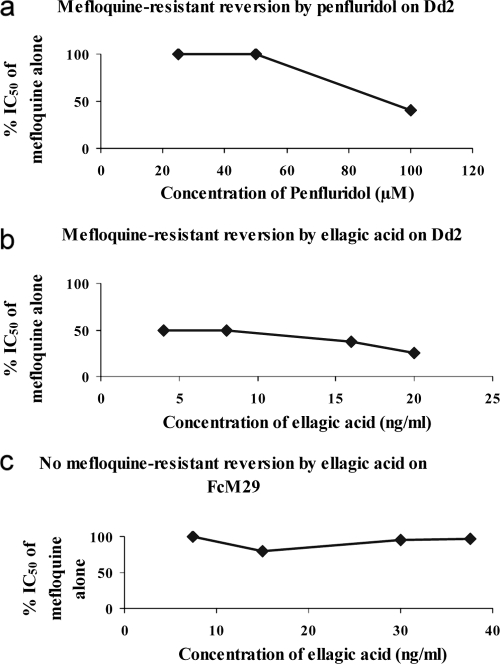
Ellagic acid shows reversion on strain Dd2, with reduced susceptibility to mefloquine.
The mefloquine-resistant reversion effect of ellagic acid was carried out in comparison with the effect of penfluridol on strain Dd2. The results in Fig. 4 show the reversal effect of ellagic acid on reduced mefloquine susceptibility strain Dd2, with a CR50 of 5 ± 4 ng/ml. This reversal effect is more important than that of the control with penfluridol, which reverses only at a CR50 of 96 ± 7 ng/ml. We did not find this reversal effect with mefloquine-susceptible strain FcM29, which was used as a negative control.
FIG. 4.
Mefloquine resistance reversal by ellagic acid. Values are the means of three independent experiments. (a) Means of reversal concentrations of penfluridol (positive control) for mefloquine-resistant strain Dd2 (CR50 for penfluridol of 96 ± 7 ng/ml). (b) Means of reversal concentrations of ellagic acid for mefloquine-resistant strain Dd2 (CR50 for ellagic acid of 5 ± 4 ng/ml). (c) Absence of reversion by ellagic acid for mefloquine-sensitive strain FcM29 (negative control).
Ellagic acid acts on late stages of the erythrocytic Plasmodium life cycle.
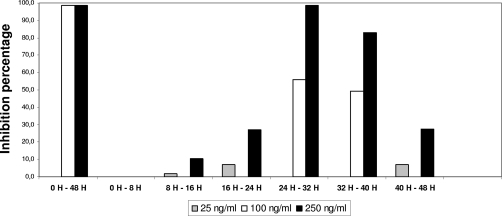
During the erythrocytic life cycle, the period of activity of ellagic acid at pharmacological doses was between the 24th and the 40th hours (Fig. 5). This period in the erythrocytic life cycle corresponds to the trophozoite and early schizont forms of the parasites. This stage of the malaria life cycle is the most metabolically active phase, with protein, RNA, and DNA synthesis taking place (1).
FIG. 5.
Point of action of ellagic acid on the erythrocytic life cycle.
Intraperitoneal ellagic acid shows high curative antiplasmodial activity in vivo and also prophylactic effects without any toxicity.
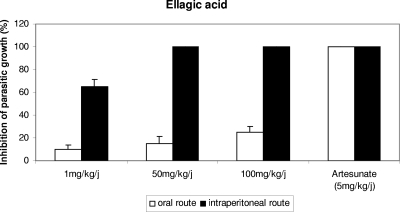
Mice infected with Plasmodium vinckei petteri were treated with 1, 50, and 100 mg/kg/day of ellagic acid by the oral and intraperitoneal routes. The results showed that with the 4-day suppressive test, the ED50 of ellagic acid administered by the intraperitoneal route was inferior to 1 mg/kg/day (Fig. 6), and at doses of 50 and 100 mg/kg/day, 100% inhibition of parasite growth was obtained. On the other hand, mice treated orally showed very little inhibition of parasite growth.
FIG. 6.
Percentage of inhibition of parasite growth in vivo compared with that in untreated control mice versus dose of ellagic acid (or artesunate) at day 4 of treatment by the oral and intraperitoneal routes assessed by the 4-day suppressive test.
The parasitemia of surviving mice treated orally and intraperitoneally was monitored from days 5 to 60. No recrudescence of malaria was observed in mice treated intraperitoneally with 50 and 100 mg/kg/day of ellagic acid and 5 mg/kg/day of artesunate until day 60.
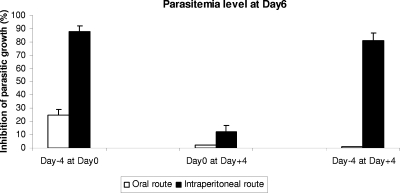
To assess the prophylactic effects of ellagic acid, mice were also treated either by the oral (100 mg/kg/day) or by the intraperitoneal (10 mg/kg/day) route with ellagic acid 4 days before and/or after parasite inoculation. The parasitemia levels at day 6 showed that there was a high protective effect on mice treated intraperitoneally with ellagic acid before parasite inoculation (Fig. 7). Mice treated orally, the controls, and those that received treatment after inoculation died from a high level of parasitemia 7 days after parasite inoculation, whereas mice treated with ellagic acid by the intraperitoneal route before parasite inoculation were still alive at day 9.
FIG. 7.
Percentages of inhibition of parasite growth in vivo and standard deviations at day 6 in all groups of mice infected with P. vinckei petteri and either orally (100 mg/kg/day) or intraperitoneally (10 mg/kg/day) treated by doses of ellagic acid compared with untreated controls. Day zero corresponds to the day of Plasmodium infection.
Because of the interesting antimalarial properties in vivo, toxicity tests were carried out with mice, using doses of 100, 250, 500, 750, and 1,000 mg/kg/day by the oral and intraperitoneal routes during four consecutive days. The mice were monitored until day 30. No mortality was observed whatever the dose of ellagic acid and the route used (data not shown). We concluded that there was no toxicity of ellagic acid by the oral or intraperitoneal route in mice given a 50% lethal dose of up to 1 × 4 g/kg/day, and thus, the therapeutic index by the intraperitoneal route was up to 1,000 for the suppressive test (or superior to 4,000 with cumulative doses).
Ellagic acid has antioxidant properties, but its level of antimalarial activity is decreased by NAC.
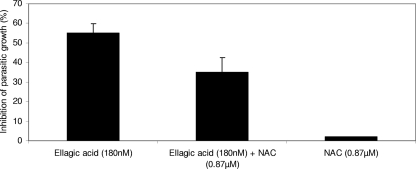
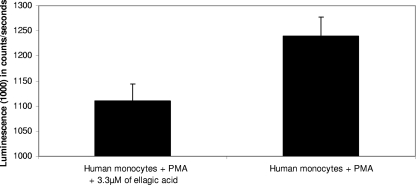
The modulating effects of NAC (an antioxidant compound) on the inhibition of parasite growth by ellagic acid were assessed using Plasmodium falciparum strain FcM29 with an NAC concentration of 0.87 μM. This concentration of NAC used in the incubation medium was in accordance with the dosing scheme described in the literature for the use of NAC as an adjunctive treatment (2). In the presence of NAC, the inhibition of parasite maturation by ellagic acid was reduced, while alone, NAC was without effect on the parasitic growth of strain FcM29 (Fig. 8). The antioxidant effect of ellagic acid was measured by chemiluminescence in the presence of PMA, which is a powerful inducer of the production of reactive oxygen species (ROS) in human monocytes. As can be seen in Fig. 8, the antioxidant properties of ellagic acid reduced the production of ROS.
FIG. 8.
Inhibition effect of ellagic acid antiplasmodial activity by the antioxidant compound NAC on strain FcM29 of P. falciparum.
DISCUSSION
Ellagic acid is a polyphenol found in numerous fruits and vegetables, and this molecule seems to be a primary component of several tannin-bearing antimalarial plants found in African flora (28).
The first report of the inhibition of the growth of Plasmodium falciparum by ellagic acid extracted from plants (Tristaniopsis calobuxus of New Caledonia) was done by Verotta et al. (29), who obtained an IC50 of ellagic acid, whatever the chloroquine resistance of strains used, of between 103 and 145 ng/ml (331 and 480 nM). Similar results were found by Banzouzi et al. (3) with ellagic acid from Alchornea cordifolia (West African plant). These results are in accordance with ours, with our IC50s being between 105 and 330 nM. In contrast, no antimalarial activity of ellagic acid (extracted from Punica granatum L) was found by Reddy et al. (21). In all our experiments, ellagic acid hydrate was bought from Acros Organics in order to obtain pure (97%), cheap, and reproducible batches.
Synergism was found between ellagic acid and current antimalarial drugs (chloroquine, artesunate, mefloquine, and atovaquone), and this is the first report of this potentiation in vitro. This synergy with current antimalarial drugs could enable the quantity of each drug to be reduced during the treatment and thus limit the side effects. Such synergy is interesting in the fight against the emergence of resistance, which is one of the major problems in eradicating malaria. Although ellagic acid showed no reversant activity on chloroquine resistance, mefloquine reduced susceptibility in strain Dd2. In zones of endemicity, P. falciparum mdr1 gene amplification has been implicated in the reduced susceptibility of P. falciparum isolates to several drugs such as quinine, halofantrine, and mefloquine (9).
Reddy et al. (21) and Verotta et al. (29) previously suggested that ellagic acid had negligible cytotoxicity. In our case, the ratio between cytotoxicity and activity showed an in vitro selectivity index of more than 495.
In addition, ellagic acid showed high levels of activity and prophylactic effects in vivo in a murine model when administered by the intraperitoneal route and without any toxicity. In a curative test, the ED50 of ellagic acid for Plasmodium vinckei petteri was around 1 mg/kg/day by the intraperitoneal route. In comparison, artesunate, the most potent artemisinin derivative, under the same conditions (similar vehicle and same murine Plasmodium strain), showed an ED50 inferior to 5 mg/kg/day. In prophylactic-curative tests, mice treated with ellagic acid by the intraperitoneal route before parasite inoculation had a high-level reduction (between 79 and 93%) of parasitemia compared with the controls at day 6, suggesting a prophylactic effect of ellagic acid. Poor activity of ellagic acid was found when administered orally, probably due to lower bioavailability. It was previously shown in rats that ellagic acid is partially absorbed, metabolized by intestinal flora, and excreted in bile and urine as glucuronide and glutathione conjugates (25). A more recent study using rats indicated that only 9.6% of ellagic acid was detected in stomach 1 h after oral ingestion and that after 2 h, only traces were detected. Moreover, no ellagic acid was detected in any of the rat organs/tissues or fluids collected over 24 h after consumption (8). In mice, ellagic acid presents the highest concentrations in blood only 30 min after oral administration, with absorption occurring mostly within 2 h (26).
Weak levels of ellagic acid in blood after oral administration could explain the absence of in vivo efficacy by this way in our study. Even with a high concentration (1 g/kg/day), no antimalarial efficacy was reported, explaining rather chemical modifications of ellagic acid in stomach or in the gastrointestinal tract, with a loss of its antimalarial activity.
This loss of the antiplasmodial activity of ellagic acid after oral administration found in our study is correlated with the limited bioavailability of ellagic acid reported after pomegranate juice ingestion (24). These results are thus not limited to the anti-Plasmodium activity of ellagic acid but could be transposed to potential chemopreventive, antioxidant, and anti-inflammatory bioactivities of ellagitannins as used by health food consumers (especially in the United States), who appreciate pomegranate juice because it is one of the richest sources of ellagic acid.
Protection of ellagic acid from stomach acidity or the synthesis of a prodrug that is able to release efficient amounts of ellagic acid in the plasma could be considered. It may be possible to enhance the oral absorption of ellagic acid by using metabolites. For example, oral treatment with ellagitannins, which, after hydrolysis, release ellagic acid in the jejunum (12), could also be explored. Indeed, the intestinal flora then metabolizes ellagic acid to metabolites, which were absorbed preferentially, with their lipophilicity being increased (12).
Additional absorption, distribution, metabolism, and excretion studies might be used in lead optimization to profile compound derivatives or metabolites or even other salt forms (13).
The point of action of ellagic acid in the parasite life cycle corresponded with protein and nucleic acid synthesis. The pharmacological targets of ellagic acid and its mechanism of action on P. falciparum are not well known. However, Dell'Agli et al. (10) previously investigated the activity of recombinant plasmepsin II, one of the hemoglobin proteases, and the in vitro detoxification of hematin into β-hematin. They showed that ellagic acid inhibited the formation of β-hematin like some quinoline antimalarial drugs because of its ability to form a π-π complex and had an IC50 three times higher than that of chloroquine. Currently, plasmepsin II is not considered to be an antimalarial target.
Previous reports of ellagic acid showed its effective antioxidant properties and biological effects including antimutagenic and tumor chemoprotective activities (21, 23).
In the present study, the antioxidant activity of ellagic acid was confirmed by using PMA on human monocytes (Fig. 9). At the same time, ellagic acid, in the presence of the known antioxidant NAC, showed reduced in vitro antimalarial activity (range, 15 to 40%) (Fig. 8). The concentration of NAC used was 0.87 μM, and at this concentration, it had no antiplasmodial effect. This last result could suggest pro-oxidant properties of ellagic acid in its antimalarial action. Ellagic acid could act as both a pro- and antioxidant in the same way as several polyphenols such as curcumin (14, 22). It could be useful to carry out experiments to verify the pro-oxidant effects of ellagic acid, given that another antimalarial molecule (artesunate) seems to act by oxidative effects (2). A clinical report has shown that a molecule with the dual activities of antioxidant and antimalarial was interesting in the case of severe malaria (30).
FIG. 9.
Antioxidant effect of ellagic acid on human monocytes using PMA, which induced a strong generation of ROS.
In conclusion, ellagic acid is a natural compound with very good activity against malaria parasites and high synergy with current antimalarial drugs in vitro. In vivo activity tests showed a high therapeutic index by the intraperitoneal route. Moreover, the dual antioxidant and pro-oxidant properties of ellagic acid are interesting. However, an improvement of the oral antimalarial efficacy of ellagic acid could lead this molecule to being a new future antimalarial drug.
Acknowledgments
We gratefully acknowledge J. Y. Lallemand and J. F. Magnaval for their constant support. We also thank A. Abdoulaye, I. Moussa (Abdou Moumouni University of Niamey), and R. Nongonierma (Cheikh Anta Diop University of Dakar) for their participation in the chemical studies; John Woodley for editing the English of the manuscript; G. Aubert of the Institut de Chimie des Substances Naturelles (ICSN-CNRS) for the cytotoxicity studies; and Marie Delpech for fruitful discussions. We are grateful to Matthias Frank and Francine Ntoumi from the Department of Parasitology, Institute for Tropical Medicine, University of Tübingen, Tübingen, Germany, for generously providing strain Dd2.
We gratefully acknowledge financial support from the ICSN (CNRS).
Footnotes
Published ahead of print on 17 November 2008.
REFERENCES
- 1.Arnot, D. E., and K. Gull. 1998. The Plasmodium cell-cycle: facts and questions. Ann. Trop. Med. Parasitol. 92:361-365. [DOI] [PubMed] [Google Scholar]
- 2.Arreesrisom, P., A. M. Dondorp, S. Looareesuwan, and R. Udomsangpetch. 2007. Suppressive effects of the anti-oxidant N-acetylcysteine on the anti-malarial activity of artesunate. Parasitol. Int. 56:221-226. [DOI] [PubMed] [Google Scholar]
- 3.Banzouzi, J. T., R. Prado, H. Menan, A. Valentin, C. Roumestan, M. Mallie, Y. Pelissier, and Y. Blache. 2002. In vitro antiplasmodial activity of extracts of Alchornea cordifolia and identification of an active constituent: ellagic acid. J. Ethnopharmacol. 81:399-401. [DOI] [PubMed] [Google Scholar]
- 4.Benoit, F., A. Valentin, Y. Pelissier, F. Diafouka, C. Marion, D. Kone-Bamba, M. Kone, M. Mallie, A. Yapo, and J. M. Bastide. 1996. In vitro antimalarial activity of vegetal extracts used in West African traditional medicine. Am. J. Trop. Med. Hyg. 54:67-71. [DOI] [PubMed] [Google Scholar]
- 5.Benoit-Vical, F. 2005. Ethnomedicine in malaria treatment. IDrugs 8:45-52. [PubMed] [Google Scholar]
- 6.Benoit-Vical, F., J. Lelievre, A. Berry, C. Deymier, O. Dechy-Cabaret, J. Cazelles, C. Loup, A. Robert, J. Magnaval, and B. Meunier. 2007. Trioxaquines: new antimalarial agents active on all erythrocytic forms including gametocytes. Antimicrob. Agents Chemother. 51:1463-1472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Benoit-Vical, F., A. Robert, and B. Meunier. 2000. In vitro and in vivo potentiation of artemisinin and synthetic endoperoxide antimalarial drugs by metalloporphyrins. Antimicrob. Agents Chemother. 44:2836-2841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Borges, G., S. Roowi, J. M. Rouanet, G. G. Duthie, M. E. Lean, and A. Crozier. 2007. The bioavailability of raspberry anthocyanins and ellagitannins in rats. Mol. Nutr. Food Res. 51:714-725. [DOI] [PubMed] [Google Scholar]
- 9.Cowman, A. F., D. Galatis, and J. K. Thompson. 1994. Selection for mefloquine resistance in Plasmodium falciparum is linked to amplification of the pfmdr1 gene and cross-resistance to halofantrine and quinine. Proc. Natl. Acad. Sci. USA 91:1143-1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dell'Agli, M., S. Parapini, N. Basilico, L. Verotta, D. Taramelli, C. Berry, and E. Bosisio. 2003. In vitro studies on the mechanism of action of two compounds with antiplasmodial activity. Planta Med. 69:162-164. [DOI] [PubMed] [Google Scholar]
- 11.Desjardins, R. E., C. J. Canfield, J. D. Haynes, and J. D. Chulay. 1979. Quantitative assessment of antimalarial activity in vitro by a semiautomated microdilution technique. Antimicrob. Agents Chemother. 16:710-718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Espin, J. C., R. Gonzalez-Barrio, B. Cerda, C. Lopez-Bote, A. I. Rey, and F. A. Tomas-Barberan. 2007. Iberian pig as a model to clarify obscure points in the bioavailability and metabolism of ellagitannins in humans. J. Agric. Food Chem. 55:10476-10485. [DOI] [PubMed] [Google Scholar]
- 13.Food and Drug Administration. 2007. Guidance for industry. Malaria: developing drug and nonvaccine biological products for treatment and prophylaxis. Center for Drug Evaluation and Research, U.S. Department of Health and Human Services, Rockville, MD.
- 14.Kang, J., J. Chen, Y. Shi, J. Jia, and Y. Zhang. 2005. Curcumin-induced histone hypoacetylation: the role of reactive oxygen species. Biochem. Pharmacol. 69:1205-1213. [DOI] [PubMed] [Google Scholar]
- 15.Lelievre, J., A. Berry, and F. Benoit-Vical. 2005. An alternative method for Plasmodium culture synchronization. Exp. Parasitol. 109:195-197. [DOI] [PubMed] [Google Scholar]
- 16.Martin, S. K., A. J. Oduola, and W. K. Milhous. 1987. Reversal of chloroquine resistance in Plasmodium falciparum by verapamil. Science 235:899-901. [DOI] [PubMed] [Google Scholar]
- 17.Mungthin, M., P. G. Bray, and S. A. Ward. 1999. Phenotypic and genotypic characteristics of recently adapted isolates of Plasmodium falciparum from Thailand. Am. J. Trop. Med. Hyg. 60:469-474. [DOI] [PubMed] [Google Scholar]
- 18.Oduola, A. M., G. O. Omitowoju, L. Gerena, D. E. Kyle, W. K. Milhous, A. Sowunmi, and L. A. Salako. 1993. Reversal of mefloquine resistance with penfluridol in isolates of Plasmodium falciparum from south-west Nigeria. Trans. R. Soc. Trop. Med. Hyg. 87:81-83. [DOI] [PubMed] [Google Scholar]
- 19.Peters, W. 1970. The chemotherapy of rodent malaria. XII. Substituted tetrahydrofurans, a new chemical family of antimalarials. The action of 2-(p-chlorophenyl)-2-(4-piperidyl)-tetrahydrofuran against Plasmodium berghei and Plasmodium chabaudi. Ann. Trop. Med. Parasitol. 64:189-202. [PubMed] [Google Scholar]
- 20.Price, R. N., A. C. Uhlemann, A. Brockman, R. McGready, E. Ashley, L. Phaipun, R. Patel, K. Laing, S. Looareesuwan, N. J. White, F. Nosten, and S. Krishna. 2004. Mefloquine resistance in Plasmodium falciparum and increased pfmdr1 gene copy number. Lancet 364:438-447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Reddy, M. K., S. K. Gupta, M. R. Jacob, S. I. Khan, and D. Ferreira. 2007. Antioxidant, antimalarial and antimicrobial activities of tannin-rich fractions, ellagitannins and phenolic acids from Punica granatum L. Planta Med. 73:461-467. [DOI] [PubMed] [Google Scholar]
- 22.Sandur, S. K., H. Ichikawa, M. K. Pandey, A. B. Kunnumakkara, B. Sung, G. Sethi, and B. B. Aggarwal. 2007. Role of pro-oxidants and antioxidants in the anti-inflammatory and apoptotic effects of curcumin (diferuloylmethane). Free Radic. Biol. Med. 43:568-580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Seeram, N. P., L. S. Adams, S. M. Henning, Y. Niu, Y. Zhang, M. G. Nair, and D. Heber. 2005. In vitro antiproliferative, apoptotic and antioxidant activities of punicalagin, ellagic acid and a total pomegranate tannin extract are enhanced in combination with other polyphenols as found in pomegranate juice. J. Nutr. Biochem. 16:360-367. [DOI] [PubMed] [Google Scholar]
- 24.Seeram, N. P., Y. Zhang, R. McKeever, S. M. Henning, R. P. Lee, M. A. Suchard, Z. Li, S. Chen, G. Thames, A. Zerlin, M. Nguyen, D. Wang, M. Dreher, and D. Heber. 2008. Pomegranate juice and extracts provide similar levels of plasma and urinary ellagitannin metabolites in human subjects. J. Med. Food 11:390-394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Teel, R. W. 1987. Distribution and metabolism of ellagic acid in the mouse following intraperitoneal administration. Cancer Lett. 34:165-171. [DOI] [PubMed] [Google Scholar]
- 26.Teel, R. W., and R. M. Martin. 1988. Disposition of the plant phenol ellagic acid in the mouse following oral administration by gavage. Xenobiotica 18:397-405. [DOI] [PubMed] [Google Scholar]
- 27.Trager, W., and J. B. Jensen. 1976. Human malaria parasites in continuous culture. Science 193:673-675. [DOI] [PubMed] [Google Scholar]
- 28.Vattem, D. A., R. Ghaedian, and K. Shetty. 2005. Enhancing health benefits of berries through phenolic antioxidant enrichment: focus on cranberry. Asia Pac. J. Clin. Nutr. 14:120-130. [PubMed] [Google Scholar]
- 29.Verotta, L., M. Dell'Agli, A. Giolito, M. Guerrini, P. Cabalion, and E. Bosisio. 2001. In vitro antiplasmodial activity of extracts of Tristaniopsis species and identification of the active constituents: ellagic acid and 3,4,5-trimethoxyphenyl-(6′-O-galloyl)-O-beta-D-glucopyranoside. J. Nat. Prod. 64:603-607. [DOI] [PubMed] [Google Scholar]
- 30.Watt, G., K. Jongsakul, and R. Ruangvirayuth. 2002. A pilot study of N-acetylcysteine as adjunctive therapy for severe malaria. QJM 95:285-290. [DOI] [PubMed] [Google Scholar]