Abstract
Peptides derived from the α-helical domains of human immunodeficiency virus (HIV) type 1 (HIV-1) gp41 inhibit HIV-1 fusion to the cell membrane. Enfuvirtide (T-20) is a peptide-based drug that targets the step of HIV fusion, and as such, it effectively suppresses the replication of HIV-1 strains that are either wild type or resistant to multiple reverse transcriptase and/or protease inhibitors. However, HIV-1 variants with T-20 resistance have emerged; therefore, the development of new and potent inhibitors is urgently needed. We have developed a novel HIV fusion inhibitor, SC34EK, which is a gp41-derived 34-amino-acid peptide with glutamate (E) and lysine (K) substitutions on its solvent-accessible site that stabilize its α-helicity. Importantly, SC34EK effectively inhibits the replication of T-20-resistant HIV-1 strains as well as wild-type HIV-1. In this report, we introduce SC29EK, a 29-amino-acid peptide that is a shorter variant of SC34EK. SC29EK blocked the replication of T-20-resistant HIV-1 strains and maintained antiviral activity even in the presence of high serum concentrations (up to 50%). Circular dichroism analysis revealed that the α-helicity of SC29EK was well maintained, while that of the parental peptide, C29, which showed moderate and reduced inhibition of wild-type and T-20-resistant HIV-1 strains, was lower. Our results show that the α-helicity in a peptide-based fusion inhibitor is a key factor for activity and enables the design of short peptide inhibitors with improved pharmacological properties.
The envelope proteins of human immunodeficiency virus (HIV) type 1 (HIV-1) exist as functional trimeric complexes of gp120-gp41 heterodimers and play an important role in viral entry into host cells. Interactions of gp120 with CD4 molecules expressed on the cell surface cause structural changes that allow further interactions with the CXCR4 or CCR5 coreceptor. These interactions also induce a conformational change in gp120 that initiates gp41-mediated membrane fusion that leads to viral entry (4). In the process of fusion, the amino-terminal heptad repeat (N-HR) of gp41 trimer interacts with the carboxyl-terminal heptad repeat (C-HR) of gp41 trimer to form a six-helix bundle that makes viral and cell membranes accessible (3).
Peptides derived from N-HR or C-HR, such as N36 (3, 18) and enfuvirtide (T-20) (30), suppress the six-helix bundle formation, resulting in the inhibition of membrane fusion. T-20 blocks the entry of various HIV-1 strains, even those resistant to inhibitors of reverse transcriptase and/or protease (15, 16). However, T-20-resistant HIV-1 variants, which frequently show mutations in gp41, such as V38A and N43D, have emerged (14, 25, 26, 28, 32). Therefore, novel fusion inhibitors that suppress the replication of T-20-resistant variants are urgently needed.
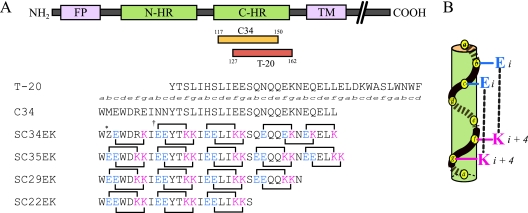
C34, a C-HR-derived peptide (Fig. 1A), also inhibits fusion in vitro and does so much more efficiently than T-20 (3, 18, 22). Previously, we remodeled C34 by introducing amino acid substitutions that resulted in highly soluble and active derivatives (24). We replaced amino acids at the solvent-accessible site of the helical bundle with glutamate (E) and lysine (K) and maintained those at the interactive site, as these are critical for the interaction with N-HR. In an α-helical heptad repeat, residues separated by three positions (position i versus position i + 4) are located on the same side of the helix and are closely positioned in space (Fig. 1B). Hence, we introduced consecutive EK motifs separated by three residues (E at positions i and i + 1 and K at positions i + 4 and i + 5) of the solvent-accessible site of C34, which resulted in a repeat of the following type: X-EE-XX-KK (where X indicates the original amino acid in HIV-1). A C34 derivative, SC34EK, which has two complete and three incomplete X-EE-XX-KK motifs (Fig. 1), showed enhanced anti-HIV-1 activity compared with that of the parental peptide, C34 (24). A similar result was obtained with T-20EK, the peptide derived by introducing this motif into T-20 (23). Circular dichroism (CD) analysis revealed that both the α-helicity of SC34EK and the thermal stability of the N36-SC34EK complex were enhanced. Interestingly, the antiviral activity of SC35EK, which has five complete X-EE-XX-KK motifs, was comparable to that of SC34EK (24), indicating that five complete X-EE-XX-KK motif repeats are not required for strong anti-HIV-1 activity. To address how many complete X-EE-XX-KK motifs are involved in the potent antiviral activity of SC34EK, we synthesized SC29EK and SC22EK (Fig. 1), which contain four and three complete repeats of X-EE-XX-KK, respectively, and evaluated them for their activities against T-20-resistant viruses.
FIG. 1.
(A) Schematic diagram of HIV-1 gp41 and sequences of C-HR-derived peptides. FP, fusion peptide; TM, transmembrane domain. The residues at each position in the helical turns are denoted in italics. *Z, an artificial amino acid, norleucine, instead of methionine, to avoid oxidation of the side chain of methionine; †, possible electrostatic interactions indicated by lines and correlating amino acids. (B) One heptad helical turn.
MATERIALS AND METHODS
Cells.
HeLa CD4/LTR-β-galactosidase cells obtained from M. Emerman through the AIDS Research and Reference Reagent Program (Germantown, MD) and 293T cells were grown in Dulbecco's modified Eagle medium (DMEM) supplemented with 10% fetal calf serum (FCS), 100 U/ml penicillin G, and 50 μg/ml streptomycin.
Viruses.
An HIV-1 infectious clone, pNL4-3 (1), was used for the construction and production of HIV-1 clones. Clones with a certain resistance mutation(s) were introduced by site-directed mutagenesis (29) into the pNL4-3 construct. Although the vast majority of HIV-1 strains have a glycine (G) at position 36 in gp41, the NL4-3 strain used in this study has an aspartic acid (D) residue, which results in impairment of the fusion kinetics of HIV-1 (13, 17). Therefore, in this study, we first constructed a D36G clone, pNL4-3D36G, and used this as a template for the introduction of T-20-resistant mutations, as described previously (21, 31). We constructed three T-20-resistant clones, HIV-1D36G/V38A, HIV-1D36G/N43D, and HIV-1D36G/N43D/S138A (8), and two C34-resistant clones, HIV-1D36G/N126K and HIV-1ΔV4/D36G/I37K/N126K/L204I (ΔV4 indicates a five-amino acid [FNSTW] deletion in the V4 region of gp120) (22). Infectious HIV-1 clones were generated by transfection of plasmid clones into 293T cells.
Antiviral agents.
Peptide-based fusion inhibitors, including T-20, were synthesized by standard 9-fluorenylmethoxy carbonyl-based solid-phase techniques (24). High-pressure liquid chromatography purification of crude materials on a preparative Cosmosil 5C18 AR-II column with a linear gradient of acetonitrile containing 0.1% trifluoroacetic acid gave the desired peptide samples for biological tests. 2′,3′-Dideoxycytidine (ddC) was purchased from Sigma-Aldrich (St. Louis, MO).
Determination of efficacies of antiviral agents.
The efficacies of the antiviral agents were determined by multinuclear activation of galactosidase indicator (MAGI) assays (12, 22). Briefly, 104 HeLa CD4/LTR-β-galactosidase cells per well were plated in flat 96-well culture plates. On the following day, the cells were inoculated with the HIV-1 clones (60 MAGI U/well, which gave 60 blue cells after 48 h of incubation) and were cultured in the presence of various concentrations of drugs in fresh medium. After incubation for 48 h after virus inoculation, all of the blue cells that were stained with 5-bromo-4-chloro-3-indolyl-β-d-galactoside in each well were counted. The activities of the test compounds were determined as the concentration that blocked HIV-1 replication by 50% (the 50% effective concentration [EC50]).
Effect of sera on anti-HIV activity.
The effect of the FCS concentration on antiviral activity was measured by MAGI assays with FCS at several concentrations (5, 10, 20, and 50%). The effect of serum components on antiviral activity was assessed by MAGI assays. Briefly, T-20 or SC29EK was dissolved in phosphate-buffered saline (PBS), FCS, or serum freshly prepared from HIV-seronegative healthy volunteers at 4 μM; and the mixtures were incubated for 2 h at 37°C. The mixture was diluted to a concentrations of about 1× or 5× the EC50 by using a DMEM-based complete medium supplemented with 10% FCS and was subjected to the MAGI assay.
CD analysis.
N36- and C-HR-derived peptide complexes were incubated at 37°C for 30 min (final peptide concentration, 10 μM in PBS). The CD spectra were acquired on a spectropolarimeter (model J-710; Jasco Inc., Tokyo, Japan) at 25°C as the average of eight scans. Thermal stability was assessed by monitoring the change in the CD signal at 222 nm as a function of temperature. Thermal unfolding at intervals of 0.5°C was performed after a 15-min equilibration at the desired temperature and an integration time of 1.0 s. The midpoint of the thermal unfolding transition (melting temperature [Tm]) of each complex was determined from the maximum of the first derivative, with respect to the reciprocal of the temperature, of the [θ]222 values.
RESULTS
Antiviral activities of peptides into which EK is introduced.
Because W117, W120, and I124, which are crucial for binding to N-HR (2, 3), are located in the N terminus of C34, we deleted the C-terminal region of SC35EK to produce short peptides. SC29EK, which has four complete X-EE-XX-KK motifs, inhibited HIV-1NL4-3 infection at a level comparable to that at which SC34EK did (Table 1). As was observed with SC34EK, SC29EK also maintained an inhibitory effect toward T-20-resistant clones. Although SC34EK blocked the replication of C34-resistant clone HIV-1ΔV4/D36G/I37K/N126K/L204I, SC29EK failed to do so. On the other hand, C29, with a 5-amino-acid deletion from the C terminus of C34, exerted drastically reduced antiviral activity. SC22EK, which consisted of three X-EE-XX-KK motifs, also showed much reduced antiviral activity compared with the activities of SC29EK and SC34EK. A native peptide corresponding to SC22EK, C22, exhibited no activity against HIV-1NL4-3 at concentrations up to 10 μM (data not shown). Thus, to inhibit the physiological interaction of N-HR and C-HR, a peptide 22 amino acids in length, without modification, may be insufficient. The D36G substitution enhanced the susceptibility of HIV-1 to T-20 (28) but not to C34 or its derivatives (Table 1). These results suggest that four X-EE-XX-KK motifs are required to maintain the inhibitory effect of the peptides on the membrane fusion of HIV-1 strains resistant to T-20, as well as HIV-1NL4-3.
TABLE 1.
Activities of HIV-1 gp41-derived peptides against T-20-resistant mutants
| Virus | EC50a (nM)
|
|||||
|---|---|---|---|---|---|---|
| T-20 | SC22EK | C29 | SC29EK | C34 | SC34EK | |
| HIV-1NL4-3 | 15 ± 1 (6.3) | 217 ± 41 (0.3) | 245 ± 42 (4.7) | 2.4 ± 0.1 (1.3) | 2.3 ± 0.1 (1.0) | 1.6 ± 0.2 (0.7) |
| HIV-1D36G | 2.4 ± 0.6 | 686 ± 94 | 52 ± 18 | 1.9 ± 0.0 | 2.3 ± 0.6 | 2.4 ± 1.0 |
| HIV-1D36G/V38A | 23 ± 8 (9.6) | 289 ± 84 (0.4) | 504 ± 193 (9.7) | 3.0 ± 0.6 (1.6) | 4.4 ± 1.4 (1.9) | 2.2 ± 0.4 (0.9) |
| HIV-1D36G/N43D | 49 ± 10 (20) | 114 ± 36 (0.2) | >1,000 (>19) | 4.1 ± 0.6 (2.2) | 7.9 ± 0.9 (3.4) | 1.6 ± 0.4 (0.7) |
| HIV-1D36G/N43D/S138A | 84 ± 16 (35) | >1,000 (>1.5) | >1,000 (>19) | 3.4 ± 0.9 (1.8) | 15 ± 2 (6.4) | 1.5 ± 0.3 (0.6) |
| HIV-1D36G/N126K | 3.4 ± 0.6 (1.4) | >1,000 (>1.5) | 192 ± 22 (3.7) | 2.7 ± 0.1 (1.4) | 7.0 ± 2.0 (3.0) | 12 ± 1 (5.0) |
| HIV-1ΔV4/D36G/I37K/N126K/L204Ib | 390 ± 155 (163) | 252 ± 71 (0.4) | >1,000 (>19) | 50 ± 11 (26) | 171 ± 15 (74) | 3.0 ± 0.2 (1.3) |
Antiviral activity, shown as the EC50, was determined by the MAGI assay. Each EC50 represents the mean ± standard deviation obtained from at least three independent experiments. The values in parentheses indicate relative changes (n-fold) in the EC50 compared with the EC50 in the presence of the D36G substitution.
ΔV4 indicates a 5-amino-acid deletion (FNSTW) in the V4 region of gp120.
α-Helicity of the six-helix bundle.
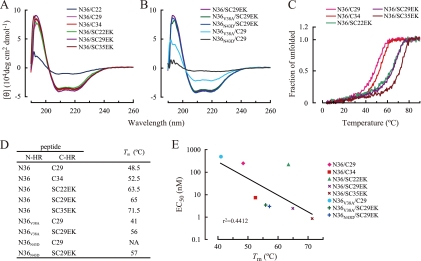
To elucidate the mechanism by which SC29EK exerts strong anti-HIV activity, we performed CD analysis of the N36-SC29EK complex. The CD spectrum for the N36-SC29EK complex revealed an α-helix conformation with a characteristic double minimum at 208 nm and 222 nm, similar to the conformations of the N36-C34 and N36-SC35EK complexes. The N36-C29 complex showed an α-helical conformation, while a complex of N36 with C22 showed decreased α-helical spectra (Fig. 2A), in direct correlation to moderately and severely decreased antiviral activities of C29 and C22, respectively. The CD spectra of complexes of N36 peptides containing T-20 resistance-associated mutations with SC29EK, N36V38A-SC29EK and N36N43D-SC29EK, were almost identical to the CD spectrum of N36 with SC29EK, indicating that SC29EK retains binding affinity for the mutated N36 peptides (Fig. 2B). On the other hand, the mutated N36 peptides and C29 complexes showed little α-helical conformation. These results indicate that introduction of the X-EE-XX-KK motif increases the binding affinity of SC29EK for the mutated N36 peptides.
FIG. 2.
Analysis of N36 and various C-HR-derived peptides complexes by CD spectroscopy. CD spectra for N36- and C-HR-derived peptide complexes (A) and mutated N36-C29 or SC29EK complexes (B). (C) Temperature-dependent transitions of the dissociation degree of N36 and various C-HR-derived peptide complexes. (D) Tms of complexes of various N-HR peptides and C-HR peptides. NA, not available. (E) Relation between EC50s of C-HR-derived peptides and Tms of N36 and various C-HR-derived peptide complexes. The strength of the correlation between EC50s and Tms is increased (r2 = 0.8002) when the data for SC22EK are excluded.
The thermal stabilities of these complexes were assessed by monitoring the shift in [θ]222 (Fig. 2C). A relatively low Tm (48.5°C) (Fig. 2D) and approximately 80% α-helicity at 37°C (Fig. 2C) were observed with the N36-C29 complex, consistent with its moderate antiviral activity (Table 1). The Tms of N36- and C-HR-derived peptides into which a X-EE-XX-KK motif was introduced were higher than the Tm of the N36-C34 complex (Fig. 2D), while the Tms of peptides with the native sequence but without the introduced motif were lower. The relationships between the EC50s of C-HR-derived peptides and their Tms are shown in Fig. 2E. The correlation between the EC50 and the Tms was weak (r2 = 0.4412); however, with the exclusion of the data for SC22EK, which showed weak antiviral activity, despite its high Tm, the strength of this correlation was increased (r2 = 0.8002), suggesting that other factors, including solubility and intrapeptide interactions, may be involved in the enhanced antiviral activity of EK-containing peptides.
Effect of serum on antiviral activity.
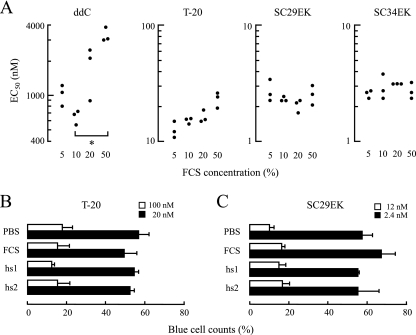
Finally, we assessed the anti-HIV activity of SC29EK in the presence of a high concentration of FCS and in fresh human serum. The activities against HIV-1NL4-3 in the presence of various concentrations of FCS (5, 10, 20, and 50%) were determined. A reverse transcriptase inhibitor, ddC, was used as a control. The antiviral activity of ddC was decreased in a concentration-dependent manner (Fig. 3A). In the presence of 50% FCS, the reduction in the EC50 of ddC was statistically significant (P = 0.01). Similarly, but to a much lesser extent, the EC50 of T-20 appeared to be reduced with the FCS concentration in a concentration-dependent manner. Even in the presence of 50% FCS, the mean EC50 was comparable to the EC50s in 5% and 10% FCS (P = 0.082 and 0.075, respectively). However, the effects of SC29EK and SC34EK were less affected by increased FCS concentrations.
FIG. 3.
Effect of serum components on antiviral activity. Antiviral activities in the presence of serum at various concentrations (5, 10, 20, and 50%) were determined by the MAGI assay. (A) Black dots indicate EC50s (nM), each of which was determined three times independently (*, P = 0.01 by Student's t test). Antiviral activities of T-20 (B) and SC29EK (C) in human HIV-seronegative sera (hs1 and hs2) were assessed by counting the number of blue cells. Bars indicate the percentages of blue cell counts in PBS, FCS, and human serum compared with the count obtained with no antiviral agents (control, for which the value was 100%). Error bars represent the standard deviation of each mean.
For further evaluation, sera freshly isolated from two HIV-seronegative healthy volunteers were prepared. T-20 and SC29EK incubated for 2 h at 37°C in fresh human serum, FCS, or PBS were diluted with a DMEM-based complete medium supplemented with 10% FCS and were subjected to the MAGI assay. The final FCS concentrations in the various sera that included FCS in these diluted mixtures ranged from 9.75 to 12.2%. Because the antiviral activities of T-20 and SC29EK were not significantly influenced by the FCS concentration (Fig. 3A), it is unlikely that the differences in the FCS concentrations in this experiment had any effect on their antiviral activities. Compared with the inhibitory effects of the PBS-treated peptides, small changes in the inhibitory effects of both T-20 and SC29EK treated with FCS and human sera were observed (Fig. 3B and C). Taken together, these findings suggest that SC29EK stably exerts its strong anti-HIV-1 activity in vivo in the same manner that T-20 does.
DISCUSSION
We show here that SC29EK inhibits the membrane fusion of T-20-resistant HIV-1 strains, suggesting that four X-EE-XX-KK motifs are sufficient to inhibit the fusion of T-20-resistant variants. As revealed by the EC50s and Tms (Table 1 and Fig. 2), resistance-associated mutations in the N-HR region, such as V38A and N43D, seem to decrease the binding affinity of C-HR-derived peptides for N-HR. Therefore, HIV-1 strains with V38A or N43D show resistance to T-20. However, the anti-HIV-1 activity of SC29EK was less affected by these mutations, because at the physiological temperature for HIV-1 replication, SC29EK showed a stable interaction with N36 peptides containing mutations conferring resistance to T-20. The activity of SC29EK against the C34-resistant clone HIV-1ΔV4/D36G/I37K/N126K/L204I was decreased, while SC34EK maintained its activity. One of the primary mutations underlying C34 resistance, I37K, is located close to but outside of the putative binding site of SC29EK. Previously, we reported that an N126K substitution in C-HR enhances the intra-gp41 binding of N-HR and C-HR (22); therefore, we hypothesized that the activity of SC29EK might be decreased by competition with C-HR with the N126K mutation. However, SC29EK also inhibits the entry of HIV-1D36G/N126K. Although no structural analysis of the mutated six-helix bundle was performed, it is possible that mutations conferring C34 resistance might induce some structural changes at or adjacent to the SC29EK binding site, because a peptide shortened by a further 7 amino acids, SC22EK, suppressed the entry of the C34-resistant clone.
C34 itself did not have an α-helical spectrum, while SC29EK did (data not shown). SC29EK may achieve its strong antiviral activity by forming an α-helix as a result of E/K substitutions on the solvent-accessible site (Fig. 1). CD analysis shows that HIV-1 builds up resistance to T-20 by introducing certain mutations in N-HR, such as V38A and N43D, which reduce the binding affinity between N-HR and C-HR. SC29EK can efficiently inhibit the fusion of these mutant HIV-1 strains, suggesting that the ability of SC29EK to bind to mutated N-HR and its weak affinity for C-HR are maintained. On the other hand, the D36G, N126K, and S138A mutations increase viral fusion activity (13) by enhancement of the binding affinity of C-HR for N-HR (22, 31). SC29EK effectively suppresses the replication of viruses that have these mutations, such as HIV-1D36G, HIV-1D36G/N43D/S138A, and HIV-1D36G/N126K. This indicates that the binding capacity of SC29EK is stronger than that of mutated C-HR containing the N126K or the S138A mutation. Therefore, the monomeric α-helical form may inhibit the interactions of N-HR and C-HR with mutations that affect their binding affinity and thus the formation of the six-helix bundle.
Although SC22EK has enhanced α-helicity and a high Tm, it has less antiviral activity than SC29EK. In the interaction between N-HR- and C-HR-derived peptides, while the cavity-forming region (from L54 to Q66) of the C terminus of N-HR (the “pocket”) and the cavity-binding region (side chains of W117, W120, and I124) of the N terminus of C-HR (the “knob”) play an important role (2, 3, 10), another region of C-HR may also be required. A constrained 14-residue peptide (C14linkmid), which corresponds to the knob region, shows chemical cross-linking and contains amino acid substitutions (27), and it is about 15,000-fold less active than SC29EK, which contains proximal regions in addition to the knob region. These findings also suggest that the knob region of C-HR is important but not sufficient for the formation of a stable complex. Another possible explanation of the weak activity of C14linkmid is that because not only the binding of N-HR and C-HR but also dynamic structural changes are easily anticipated during fusion, it would be difficult to maintain tight binding to the target N-HR due to its rigid constrained form. To maintain the binding of C-HR to N-HR despite such drastic conformational changes during fusion, there may be some unknown interaction, besides the interaction between the pocket and the knob regions, that is necessary for membrane fusion. At present, we cannot conclude whether (i) the length of the peptide itself is crucial, (ii) some other domain has a role, or (iii) a combination of both is important. Further experiments will be needed to clarify the mechanism of inhibition. Such information will be valuable for the generation of effective short peptide inhibitors or small molecules. To generate effective small-molecule inhibitors, if the second possibility is correct, a combination of two agents, one of which interacts with the pocket and the other of which interacts with an unidentified domain, should provide enhanced efficacy. To date, only a limited number of small-molecule compounds that inhibit the six-helix bundle formation with marginal activities have been reported (5, 9, 11), although among the peptide-based inhibitors, several effective peptides have been developed, including T1249 (7), SC34EK (24), T2635 (6), and T-20EK (23).
The Tm of the N36-SC29EK complex was higher than that of the N36-C29 complex, suggesting that EK substitutions reinforced the affinity of binding to N-HR through enhanced α-helicity. It has been considered that the enhanced α-helical structure is maintained by intrahelical salt bridges formed by the introduction of EK substitutions (19). We recently revealed that an electrostatic interaction formed by the EK alignment is involved in enhanced α-helicity (22a), indicating that the strong α-helical stability of SC29EK is probably provided by a mechanism identical to that for SC34EK. Similar peptides with substitutions of glutamate and arginine provided to increase α-helicity have been reported (6). These peptides also increase the stability of the helix and have activity against T-20-resistant HIV-1. Moreover, these peptides were relatively stable in an in vivo model. It is possible that enhanced binding affinity confers nonspecific binding to other α-helical regions of cellular proteins, for example, human serum albumin, which contains 31 α-helical regions (20). However, this effect will be minimal, because the antiviral activity of SC29EK was highly stable in the presence of higher concentrations of FCS and was less affected by human serum.
In this study, we demonstrated that a 29-amino-acid short peptide, SC29EK, suppresses the replication of T-20-resistant variants. SC29EK maintained its activity in the presence of high concentrations of sera, indicating that SC29EK is a candidate short peptide fusion inhibitor.
Acknowledgments
This work was supported in part by a grant for Research for Health Sciences Focusing on Drug Innovation from the Japan Health Sciences Foundation (E.K., S.O., N.F., M.M.) and a grant for the Promotion of AIDS Research from the Ministry of Health and Welfare of Japan (M.M.). T.N., K.I., and H.N. are supported by the 21st Century COE Program of the Ministry of Education, Culture, Sports, Science, and Technology. S.G.S. was supported by the National Institutes of Health (grants R01AI076119 and 1R21AI079801).
Footnotes
Published ahead of print on 29 December 2008.
REFERENCES
- 1.Adachi, A., H. E. Gendelman, S. Koenig, T. Folks, R. Willey, A. Rabson, and M. A. Martin. 1986. Production of acquired immunodeficiency syndrome-associated retrovirus in human and nonhuman cells transfected with an infectious molecular clone. J. Virol. 59:284-291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chan, D. C., C. T. Chutkowski, and P. S. Kim. 1998. Evidence that a prominent cavity in the coiled coil of HIV type 1 gp41 is an attractive drug target. Proc. Natl. Acad. Sci. USA 95:15613-15617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chan, D. C., D. Fass, J. M. Berger, and P. S. Kim. 1997. Core structure of gp41 from the HIV envelope glycoprotein. Cell 89:263-273. [DOI] [PubMed] [Google Scholar]
- 4.Chan, D. C., and P. S. Kim. 1998. HIV entry and its inhibition. Cell 93:681-684. [DOI] [PubMed] [Google Scholar]
- 5.Cianci, C., D. R. Langley, D. D. Dischino, Y. Sun, K. L. Yu, A. Stanley, J. Roach, Z. Li, R. Dalterio, R. Colonno, N. A. Meanwell, and M. Krystal. 2004. Targeting a binding pocket within the trimer-of-hairpins: small-molecule inhibition of viral fusion. Proc. Natl. Acad. Sci. USA 101:15046-15051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dwyer, J. J., K. L. Wilson, D. K. Davison, S. A. Freel, J. E. Seedorff, S. A. Wring, N. A. Tvermoes, T. J. Matthews, M. L. Greenberg, and M. K. Delmedico. 2007. Design of helical, oligomeric HIV-1 fusion inhibitor peptides with potent activity against enfuvirtide-resistant virus. Proc. Natl. Acad. Sci. USA 104:12772-12777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Eron, J. J., R. M. Gulick, J. A. Bartlett, T. Merigan, R. Arduino, J. M. Kilby, B. Yangco, A. Diers, C. Drobnes, R. DeMasi, M. Greenberg, T. Melby, C. Raskino, P. Rusnak, Y. Zhang, R. Spence, and G. D. Miralles. 2004. Short-term safety and antiretroviral activity of T-1249, a second-generation fusion inhibitor of HIV. J. Infect. Dis. 189:1075-1083. [DOI] [PubMed] [Google Scholar]
- 8.Fikkert, V., P. Cherepanov, K. Van Laethem, A. Hantson, B. Van Remoortel, C. Pannecouque, E. De Clercq, Z. Debyser, A. M. Vandamme, and M. Witvrouw. 2002. env chimeric virus technology for evaluating human immunodeficiency virus susceptibility to entry inhibitors. Antimicrob. Agents Chemother. 46:3954-3962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Frey, G., S. Rits-Volloch, X. Q. Zhang, R. T. Schooley, B. Chen, and S. C. Harrison. 2006. Small molecules that bind the inner core of gp41 and inhibit HIV envelope-mediated fusion. Proc. Natl. Acad. Sci. USA 103:13938-13943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ji, H., W. Shu, F. T. Burling, S. Jiang, and M. Lu. 1999. Inhibition of human immunodeficiency virus type 1 infectivity by the gp41 core: role of a conserved hydrophobic cavity in membrane fusion. J. Virol. 73:8578-8586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jiang, S., H. Lu, S. Liu, Q. Zhao, Y. He, and A. K. Debnath. 2004. N-substituted pyrrole derivatives as novel human immunodeficiency virus type 1 entry inhibitors that interfere with the gp41 six-helix bundle formation and block virus fusion. Antimicrob. Agents Chemother. 48:4349-4359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kimpton, J., and M. Emerman. 1992. Detection of replication-competent and pseudotyped human immunodeficiency virus with a sensitive cell line on the basis of activation of an integrated beta-galactosidase gene. J. Virol. 66:2232-2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kinomoto, M., M. Yokoyama, H. Sato, A. Kojima, T. Kurata, K. Ikuta, T. Sata, and K. Tokunaga. 2005. Amino acid 36 in the human immunodeficiency virus type 1 gp41 ectodomain controls fusogenic activity: implications for the molecular mechanism of viral escape from a fusion inhibitor. J. Virol. 79:5996-6004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Labrosse, B., L. Morand-Joubert, A. Goubard, S. Rochas, J. L. Labernardiere, J. Pacanowski, J. L. Meynard, A. J. Hance, F. Clavel, and F. Mammano. 2006. Role of the envelope genetic context in the development of enfuvirtide resistance in human immunodeficiency virus type 1-infected patients. J. Virol. 80:8807-8819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lalezari, J. P., K. Henry, M. O'Hearn, J. S. Montaner, P. J. Piliero, B. Trottier, S. Walmsley, C. Cohen, D. R. Kuritzkes, J. J. Eron, Jr., J. Chung, R. DeMasi, L. Donatacci, C. Drobnes, J. Delehanty, and M. Salgo. 2003. Enfuvirtide, an HIV-1 fusion inhibitor, for drug-resistant HIV infection in North and South America. N. Engl. J. Med. 348:2175-2185. [DOI] [PubMed] [Google Scholar]
- 16.Lazzarin, A., B. Clotet, D. Cooper, J. Reynes, K. Arasteh, M. Nelson, C. Katlama, H. J. Stellbrink, J. F. Delfraissy, J. Lange, L. Huson, R. DeMasi, C. Wat, J. Delehanty, C. Drobnes, and M. Salgo. 2003. Efficacy of enfuvirtide in patients infected with drug-resistant HIV-1 in Europe and Australia. N. Engl. J. Med. 348:2186-2195. [DOI] [PubMed] [Google Scholar]
- 17.Lu, J., P. Sista, F. Giguel, M. Greenberg, and D. R. Kuritzkes. 2004. Relative replicative fitness of human immunodeficiency virus type 1 mutants resistant to enfuvirtide (T-20). J. Virol. 78:4628-4637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lu, M., and P. S. Kim. 1997. A trimeric structural subdomain of the HIV-1 transmembrane glycoprotein. J. Biomol. Struct. Dyn. 15:465-471. [DOI] [PubMed] [Google Scholar]
- 19.Marqusee, S., and R. L. Baldwin. 1987. Helix stabilization by Glu−… Lys+ salt bridges in short peptides of de novo design. Proc. Natl. Acad. Sci. USA 84:8898-8902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Matsuo, K., R. Yonehara, and K. Gekko. 2004. Secondary-structure analysis of proteins by vacuum-ultraviolet circular dichroism spectroscopy. J. Biochem. 135:405-411. [DOI] [PubMed] [Google Scholar]
- 21.Mink, M., S. M. Mosier, S. Janumpalli, D. Davison, L. Jin, T. Melby, P. Sista, J. Erickson, D. Lambert, S. A. Stanfield-Oakley, M. Salgo, N. Cammack, T. Matthews, and M. L. Greenberg. 2005. Impact of human immunodeficiency virus type 1 gp41 amino acid substitutions selected during enfuvirtide treatment on gp41 binding and antiviral potency of enfuvirtide in vitro. J. Virol. 79:12447-12454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nameki, D., E. Kodama, M. Ikeuchi, N. Mabuchi, A. Otaka, H. Tamamura, M. Ohno, N. Fujii, and M. Matsuoka. 2005. Mutations conferring resistance to human immunodeficiency virus type 1 fusion inhibitors are restricted by gp41 and Rev-responsive element functions. J. Virol. 79:764-770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22a.Nishikawa, H., S. Nakamura, E. Kodama, S. Ito, K. Kajiwara, K. Izumi, Y. Sakagami, S. Oishi, T. Ohkubo, Y. Kobayashi, A. Otaka, N. Fujii, and M. Matsuoka. Electrostatically constrained alpha-helical peptide inhibits replication of HIV-1 resistant to enfuvirtide. Int. J. Biochem. Cell Biol., in press. [DOI] [PubMed]
- 23.Oishi, S., S. Ito, H. Nishikawa, K. Watanabe, M. Tanaka, H. Ohno, K. Izumi, Y. Sakagami, E. Kodama, M. Matsuoka, and N. Fujii. 2008. Design of a novel HIV-1 fusion inhibitor that displays a minimal interface for binding affinity. J. Med. Chem. 51:388-391. [DOI] [PubMed] [Google Scholar]
- 24.Otaka, A., M. Nakamura, D. Nameki, E. Kodama, S. Uchiyama, S. Nakamura, H. Nakano, H. Tamamura, Y. Kobayashi, M. Matsuoka, and N. Fujii. 2002. Remodeling of gp41-C34 peptide leads to highly effective inhibitors of the fusion of HIV-1 with target cells. Angew. Chem. Int. Ed. Engl. 41:2937-2940. [DOI] [PubMed] [Google Scholar]
- 25.Poveda, E., B. Rodes, C. Toro, L. Martin-Carbonero, J. Gonzalez-Lahoz, and V. Soriano. 2002. Evolution of the gp41 env region in HIV-infected patients receiving T-20, a fusion inhibitor. AIDS 16:1959-1961. [DOI] [PubMed] [Google Scholar]
- 26.Rimsky, L. T., D. C. Shugars, and T. J. Matthews. 1998. Determinants of human immunodeficiency virus type 1 resistance to gp41-derived inhibitory peptides. J. Virol. 72:986-993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sia, S. K., P. A. Carr, A. G. Cochran, V. N. Malashkevich, and P. S. Kim. 2002. Short constrained peptides that inhibit HIV-1 entry. Proc. Natl. Acad. Sci. USA 99:14664-14669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wei, X., J. M. Decker, H. Liu, Z. Zhang, R. B. Arani, J. M. Kilby, M. S. Saag, X. Wu, G. M. Shaw, and J. C. Kappes. 2002. Emergence of resistant human immunodeficiency virus type 1 in patients receiving fusion inhibitor (T-20) monotherapy. Antimicrob. Agents Chemother. 46:1896-1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Weiner, M. P., G. L. Costa, W. Schoettlin, J. Cline, E. Mathur, and J. C. Bauer. 1994. Site-directed mutagenesis of double-stranded DNA by the polymerase chain reaction. Gene 151:119-123. [DOI] [PubMed] [Google Scholar]
- 30.Wild, C., T. Greenwell, and T. Matthews. 1993. A synthetic peptide from HIV-1 gp41 is a potent inhibitor of virus-mediated cell-cell fusion. AIDS Res. Hum. Retrovir. 9:1051-1053. [DOI] [PubMed] [Google Scholar]
- 31.Xu, L., A. Pozniak, A. Wildfire, S. A. Stanfield-Oakley, S. M. Mosier, D. Ratcliffe, J. Workman, A. Joall, R. Myers, E. Smit, P. A. Cane, M. L. Greenberg, and D. Pillay. 2005. Emergence and evolution of enfuvirtide resistance following long-term therapy involves heptad repeat 2 mutations within gp41. Antimicrob. Agents Chemother. 49:1113-1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zollner, B., H. H. Feucht, M. Schroter, P. Schafer, A. Plettenberg, A. Stoehr, and R. Laufs. 2001. Primary genotypic resistance of HIV-1 to the fusion inhibitor T-20 in long-term infected patients. AIDS 15:935-936. [DOI] [PubMed] [Google Scholar]