Abstract
Invasive otitis externa (IOE) due to Aspergillus is a rare, potentially life-threatening, invasive fungal infection affecting immunocompromised patients. The invasive process may lead to skull base osteomyelitis with progressive cranial nerve palsies and can result in irreversible hearing and neurological impairment. We report two cases of Aspergillus IOE treated with voriconazole alone and a literature review of antifungal therapy of Aspergillus IOE. Twenty-five patients, including the two described in the present report, were analyzed. Eighteen patients were treated with amphotericin B, and nine of them received itraconazole as an additional agent. Three patients received initial therapy with itraconazole, and one patient was treated with both voriconazole and caspofungin therapy. The two patients in the present report received voriconazole therapy alone with good clinical and biological tolerance despite prolonged treatment. The last patient did not receive antifungal therapy, as the diagnosis was made postmortem. Eighteen patients underwent an initial extensive surgical debridement. The majority of the patients had a favorable outcome, 17 patients experienced a complete recovery, and 6 showed a partial improvement. Both of the patients reported on here had favorable outcomes, and no aggressive surgical debridement was required. Although voriconazole has been shown to be effective for the treatment of invasive aspergillosis, its precise role in the management of Aspergillus IOE had not been documented. These observations demonstrate that voriconazole could be an effective and well-tolerated therapeutic option for the management of Aspergillus IOE.
Invasive otitis externa (IOE) is a particular entity among ear infections (6). Its main feature is its spreading from the external auditory canal to adjacent anatomical structures including soft tissues, cartilage, and bone. The invasive process can lead to skull base osteomyelitis, progressive cranial nerve palsies, and even death if IOE is not recognized and treated early. Invasive external otitis typically occurs in elderly diabetic patients, and Pseudomonas aeruginosa is the most common causative microbial pathogen (11, 35).
Fungal pathogens, mostly Aspergillus spp., are a rare cause of IOE (5). As for other localizations of invasive aspergillosis, Aspergillus IOE occurs in immunocompromised patients, usually with profound and long-lasting neutropenia or under long-term steroid therapy (21), as well as in patients with uncontrolled diabetes mellitus (14).
The treatment of Aspergillus sp. IOE classically includes extensive surgical debridement and intensive long-term antifungal therapy including amphotericin B and/or itraconazole. Despite this management, this pathology is associated with substantial morbidity and mortality, mostly due to late diagnosis and patient comorbidities (2, 37). Treatment failure could also be a result of suboptimal therapeutic management as a consequence of antifungal agent toxicity. In particular, the side effects of amphotericin B, especially renal failure, may require interruption of antifungal agents or a decrease in dosage.
Voriconazole might become a therapeutic option for the management of Aspergillus IOE. This broad-spectrum azole exhibits high anti-Aspergillus activity and good long-term tolerance. We report herein the first two cases of Aspergillus sp. IOE successfully treated with voriconazole alone and provide a literature review of Aspergillus sp. IOE.
CASE REPORTS
Case 1.
A 48-year-old man with a history of relapsing polychondritis was diagnosed with right external otitis characterized by acute ear pain and a foreign-body sensation. Immunosuppressive therapy consisted of oral methotrexate (12.5 mg/week) and prednisone (10 mg/day). He was initially given oral amoxicillin-clavulanic acid, but as his pain increased, he was switched to oral ofloxacin.
Two weeks after the initial diagnosis, he was admitted to our hospital because of a failure to improve. The patient noticed severe ear pain and relative hearing loss on the right side. Physical examination revealed an erythematous and edematous right ear canal with discharge and a tympanic membrane perforation. Magnetic resonance imaging showed soft-tissue filling of the right mastoid air cells and the middle ear and thickening of the roof of the right external ear canal.
A biopsy specimen from the external ear canal revealed nonspecific chronic inflammation with associated calcium oxalate crystal deposition. Mycological microscopy examination of the discharge showed acute-angle branching septate fungal hyphae, and culture yielded Aspergillus niger. Gram staining did not reveal any other microorganism, and bacterial culture was negative. The patient was treated with oral voriconazole (200 mg orally twice a day [b.i.d.]), and no extensive surgical debridement had to be performed. A computed tomography (CT) scan of the petrous temporal bone performed 2 months following treatment initiation demonstrated progressive pneumatization of the mastoid bone. After 3 months on voriconazole therapy, the patient had fully recovered, with normal clinical and otoscopic examinations and no more hearing loss. The patient showed good clinical and biological tolerance of long-term antifungal therapy. He received a total of 5 months of subsequent voriconazole therapy without a relapse after a 1-year follow-up.
Case 2.
A 40-year-old diabetic woman was admitted to our hospital in 2006 with a 4-month history of left-side otalgia and otorrhea. She had a history of insulin-dependent diabetes complicated by blindness (secondary to diabetic retinopathy and bilateral endophthalmitis) and end-stage nephropathy. She had undergone renal transplantation 1 year earlier, and chronic allograft rejection had led to chronic renal failure (creatinine clearance, 40 ml/min). Her immunosuppressive therapy consisted of prednisone (10 mg/day), mycophenolate mofetil (500 mg three times a day) and tacrolimus (0.5 mg b.i.d.).
Initially, a diagnosis of otitis externa was made. She was treated with two successive courses of oral antibiotic therapy and topical ofloxacin, with partial improvement. Four months after the first evaluation, she was admitted because of worsening pain. On clinical examination, the left external ear canal was markedly painful, congested, and filled with black necrotic tissue debris. A CT scan of the temporal bone demonstrated left-side mastoiditis and a soft-tissue mass filling the external auditory canal (Fig. 1). Gallium scintigraphy showed high uptake in the left external ear canal and in the occipital region and homolateral skull base. A biopsy of the external ear canal revealed chronic inflammation and fungal hyphae within necrotic granulation tissue; the culture yielded A. niger. Voriconazole therapy was initiated, first at 200 mg orally b.i.d. and then at 300 mg b.i.d. to obtain a trough concentration of more than 1 mg/liter (22). Anti-Pseudomonas intravenous antibiotics initiated upon admission (ceftazidime and ciprofloxacin) were discontinued.
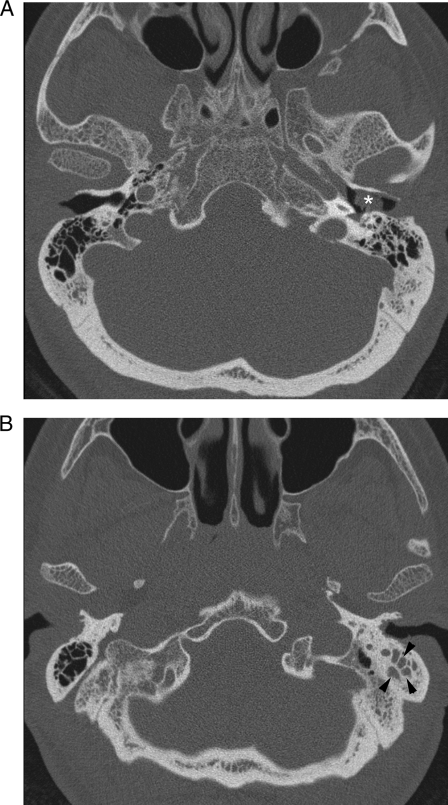
FIG. 1.
Axial CT scans of the temporal bone (image B was obtained inferior to image A) show soft tissue (*) filling the left external auditory canal and abnormal soft-tissue attenuation (arrowheads) in the left inferior mastoid air cells (compare with the opposite side).
Because of hearing loss due to a left tympanic membrane perforation, the patient underwent a left tympanoplasty with a mastoidectomy 2 months after voriconazole initiation. Surgical specimen cultures were negative for fungi and bacteria. Histology only disclosed nonspecific inflammation without hyphae, thereby verifying complete sterilization.
After an 8-month course of oral voriconazole, the patient experienced complete resolution of her otalgia and a clinical examination was normal except for moderate left-ear hearing loss. The follow-up CT scan had also improved (Fig. 2), with a normal auditory canal. Voriconazole was continued for a further 4 months. In total, the patient received 12 months of voriconazole therapy with good clinical and biological tolerance, except for moderate liver cholestasis. She had experienced no relapse within 10 months after antifungal therapy discontinuation.
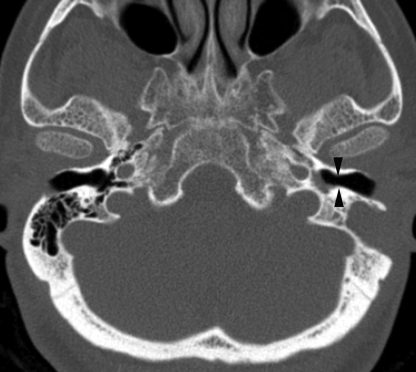
FIG. 2.
Axial CT scan of the temporal bone. After therapy, the left external canal is air filled (arrowheads).
MATERIALS AND METHODS
We searched the MEDLINE database for English-language reports of Aspergillus IOE published up to August 2008. The key words used were invasive otitis externa, malignant external otitis, mastoiditis, skull base osteomyelitis, and Aspergillus. In addition, a secondary search was conducted by reviewing references cited in these papers. All Aspergillus IOE cases, defined as a locally invasive Aspergillus infection beginning in the external auditory canal, penetrating the epithelial barrier, and manifesting signs of local subcutaneous tissue invasion (11), were included. We specifically selected cases presenting with an ear-invasive infectious process in association with symptoms of external ear canal infection (granulations or purulent discharge from the external auditory canal with an intact tympanic membrane, an abnormal external auditory canal on clinical examination, or an external auditory canal biopsy revealing Aspergillus spp.) and cases considered by the authors as IOE. Mastoiditis due to hematogenous spread (20) or secondary to contiguous spreading from the sinuses or tympanic cavity (4, 15, 41, 42, 44) were excluded. Furthermore, cases with unspecified treatment could not be included in our analysis (24, 28). Reports were reviewed, and clinical information was extracted.
RESULTS
Patient demographic characteristics.
Twenty-three patients were included in the retrospective literature review. Their demographics, underlying conditions, treatment, and outcome data are listed in Table 1. Eighteen patients were males, and five were females; most of the patients were adults (n = 21), and the median age was 46 years (range, 7 to 85 years). Nineteen patients had an identified cause of acquired immunodeficiency, the most common underlying condition being AIDS (n = 7) (7, 27, 33, 34, 45). Five patients suffered from acute leukemia (17, 25, 30, 32, 38), and five had diabetes mellitus as the sole underlying condition (2, 13, 16, 19, 23). One patient had myelodysplasia (3), one had neuroblastoma (12), one suffered from chronic otitis externa (10), and three patients had no identified underlying diseases (8, 13, 37).
TABLE 1.
Characteristics and clinical courses of patients with IOE caused by Aspergillus species
| Referencea | Age (yr)/genderb | Underlying diseasec | Aspergillus species isolated | Treatmentd | Adjuvant treatmente | Outcome | Response assessment |
|---|---|---|---|---|---|---|---|
| 30 | 68/M | AML | A. fumigatus | AmB (2 g) | SD | Recovery | Clinical |
| 8 | 85/M | None | A. fumigatus | AmB (1.5 g) and then AmB (1 g) and rifampin for 3 wk | SD | Relapse, improvement, unrelated death | Clinical |
| 3 | 80/M | Myelodysplasia | Unspecified | AmB (1.5 g) | SD | Improvement, unrelated death | Clinical |
| 10 | 38/M | Chronic otitis externa | A. fumigatus | Itz (400 mg/day, unspecified duration) | SD | Recovery | Clinical, histological |
| 32 | 64/F | AML, diabetes mellitus | Unspecified | AmB (2 g), Itz 6 wk (400 and then 200 mg/day) | SD | Relapse, improvement with Itz, unrelated death | Clinical, histological, gallium scan |
| 25 | 46/F | AML | A. flavus | AmB | SD | Recovery | Clinical |
| 33 | 42/M | AIDS | A. fumigatus | AmB (4 wk) and then Itz (5 wk) | SD | Recovery | Clinical, microbiological |
| 33 | 30/M | AIDS | A. fumigatus | AmB (4 wk) and then Itz (17 wk at 200 mg/day) | SD | Improvement | Clinical |
| 16 | 61/M | Diabetes mellitus | A. flavus | AmB (3 g) | SD | Recovery | Clinical |
| 13 | 67/F | Diabetes mellitus | A. flavus | AmB (1.25 g), Itz 17 wk (400 mg/day) | SD, HBOT | Recovery | Clinical, CT scan |
| 13 | 82/M | None | A. flavus | AmB (0.8 g), Itz 38 wk (800 and then 400 mg/day) | SD, HBOT | Recovery | Clinical, gallium scan |
| 17 | 20/M | ALL | A. flavus | AmB | SD | Death due to invasive aspergillosis | No response |
| 34 | 41/M | AIDS | A. fumigatus | Timentin-gentamicin | Unspecified | Death | No response |
| 19 | 65/M | Diabetes mellitus | A. flavus | AmB (2 g) | SD | Recovery | Clinical, gallium scan |
| 45 | 18/M | AIDS | A. fumigatus | Itz (unspecified duration) | SD | Improvement, unrelated death | Clinical |
| 27 | 27/F | AIDS | A. fumigatus | Itz (400 mg/day) and then AmB | No | Relapse, improvement, death due to pneumonia | Clinical |
| 7 | 41/M | AIDS | A. fumigatus | AmB (2 g) and then Itz (unspecified duration) | SD | Recovery | Clinical |
| 7 | 36/M | AIDS | Unspecified | Surgery alone and then AmB | SD | Relapse 2 mo after first surgery, recovery | Clinical, CT scan |
| 38 | 14/F | ALL | A. flavus, A. niger, A. fumigatus | AmB and then Itz (400 mg/day, unspecified duration) | SD | Recovery, unrelated death | Clinical |
| 37 | 58/M | None | A. niger | Itz (6 wk) | None | Recovery | Clinical, gallium scan |
| 12 | 7/M | Neuroblastoma | A. flavus | AmB (6 days) and then Itz (77 wk at 200 mg/day) | None | Recovery | Clinical, histological, CT scan |
| 2 | 73/M | Diabetes mellitus | A. niger | AmB (2 g in 3 wk) and then Itz (13 wk at 600 mg/day) | None | Recovery | Clinical, CT scan |
| 23 | 77/M | Diabetes mellitus | A. flavus | Voriconazole (14 wk at 400 mg/day) and caspofungin | SD, HBOT | Recovery | Clinical, gallium scan |
| PR | 48/M | Relapsing polychondritis | A. niger | Voriconazole (19 wk at 400 mg/day) | None | Recovery | Clinical, CT scan |
| PR | 40/F | Diabetes mellitus, renal transplantation | A. niger | Voriconazole (52 wk at 600 mg/day) | None | Recovery | Clinical, histological, CT scan |
PR, present report.
F, female; M, male.
ALL, acute lymphoblastic leukemia; AML, acute myeloid leukemia.
AmB, amphotericin B; Itz, itraconazole.
HBOT, hyperbaric oxygen therapy; SD, surgical debridement.
Nature of infection.
All cases but one were monomicrobial, and the species isolated included Aspergillus fumigatus (n = 9), Aspergillus flavus (n = 8), and A. niger (n = 2). In one case, the infection was polymicrobial and included A. niger, A. flavus, and A. fumigatus (38). In three reports, the Aspergillus species was not identified (3, 7, 32).
Treatment and tolerance.
All patients but one received long-term antifungal therapy. One patient received no treatment since the diagnosis was made postmortem (34). Seventeen patients were initially treated with amphotericin B, and nine of them also received itraconazole as an additional agent. One patient first received itraconazole and then was subsequently switched to amphotericin B because of a relapse (27). In three cases, itraconazole was used as the only antifungal drug (36). One patient received therapy with both voriconazole and caspofungin (23).
The median total dose of amphotericin B administered was 2 g (range, 0.8 to 2.5 g). Four of 18 patients who had received amphotericin B experienced an adverse event which justified discontinuation or a decreased dosage. Three patients experienced acute renal failure, and one had severe and persistent hypokalemia. No adverse effects were observed with long-term itraconazole or voriconazole therapy. Eighteen patients underwent prompt and extensive surgical debridement, whereas only three received hyperbaric oxygen therapy as adjuvant treatment (12, 22).
Response at the end of therapy.
Most of the 23 patients had favorable outcomes; 15 patients experienced a complete recovery, and 6 had a partial response to treatment. The last two died of invasive aspergillosis (17, 34); one of them did not receive any antifungal therapy because the diagnosis was postmortem, and the other had received a reduced dosage of amphotericin B because of acute renal failure. Five patients with initial clinical improvement died of other causes generally related to their underlying conditions (8, 27, 32, 38).
DISCUSSION
Invasive aspergillosis in immunocompromised hosts is characterized by a wide spectrum of clinical presentations, ranging from local to disseminated infections (36). Aspergillus is an uncommon cause of IOE. The first case was described in 1985 in a 68-year-old man with relapsing acute myelogenous leukemia (30). Since then, only 24 additional cases have been reported in the English-language literature, including our 2 cases.
During IOE, invasion is related to the spread of Aspergillus from the external auditory canal. The fungal infection slowly invades adjacent soft tissues and the mastoid air cells. Diagnosis is often delayed because Aspergillus is very rarely involved in IOE, compared with P. aeruginosa (5). As a result, if not recognized early, the infection can lead to extensive skull base osteomyelitis (40), with multiple cranial nerve palsies and sometimes a fatal outcome.
According to the literature review, treatment of Aspergillus IOE requires long-term antifungal therapy in association with appropriate management of the underlying condition, mostly diabetes mellitus. In addition, as in the management of Aspergillus osteomyelitis (43), prompt surgical debridement consisting of radical mastoidectomy is required in the majority of cases. Only three patients were exposed to hyperbaric oxygen therapy. This treatment is usually considered as an adjunctive therapy for refractory cases, although its efficacy remains debated (28, 31).
In the reviewed reports, the most commonly used antifungal agent was amphotericin B. This drug has been shown to be effective in the treatment of Aspergillus IOE, but its substantial toxicity profile must be taken into account, especially for patients with serious underlying comorbidities.
The two immunocompromised patients in the present report were successfully treated with long-term voriconazole therapy alone. Amphotericin B was not considered the optimal therapeutic option because the first patient had already received a potentially nephrotoxic agent (methotrexate) and the second suffered from chronic renal transplant rejection with severely impaired renal function. Both patients received long-term voriconazole therapy (5 and 12 months, respectively) with good clinical and biological tolerance, except for moderate liver cholestasis in the second patient. It is noteworthy that the initial voriconazole therapeutic plasma range was not optimal for the second patient whereas she received a standard dosage. This result may be explained by the high interindividual variability in voriconazole pharmacokinetics. In both cases, clinical and radiological improvement was observed after 2 months of voriconazole therapy and no initial aggressive surgical debridement was required. Of note, the surgery performed for local complications in case 2 did not demonstrate any evolutive infection when histological and mycological examinations were combined.
There is only one other reported case of Aspergillus IOE partly treated with voriconazole (23). A 77-year-old man with non-insulin-dependent diabetes mellitus was successfully treated with both voriconazole and caspofungin for A. flavus IOE. In addition, surgical debridement was performed, as well as intensive hyperbaric oxygen treatment. The patient experienced complete recovery, and antifungal therapy was discontinued after 14 weeks.
Furthermore, another case of Aspergillus-related ear infection was successfully treated with voriconazole (1). The patient was a 19-year-old female with systemic lupus erythematosus and tympanogenic mastoiditis (in this form of mastoiditis, infection is due to contiguous spreading from the middle ear, whereas in IOE the infectious process starts in the external auditory canal). She underwent a wide cortical mastoidectomy and was treated with intravenous voriconazole for 7 weeks with a successful outcome.
Voriconazole is currently considered the first-line therapeutic option for invasive aspergillosis (43), based on its high intrinsic anti-Aspergillus activity and its superiority against intravenous amphotericin B in a large randomized trial (18). In addition, this broad-spectrum azole is distributed throughout the body, including soft tissues and bone, where its good diffusion has been recently documented (9). Furthermore, long-term voriconazole therapy has been demonstrated to be effective in several patients with Aspergillus bone infections (26). This antifungal agent is well tolerated despite prolonged treatment and available intravenously and orally. However, the present report underlines the high pharmacokinetic interindividual variability of voriconazole due primarily to drug-drug interactions, liver dysfunction, or cytochrome CYP2C19 polymorphisms. Therapeutic drug monitoring of voriconazole is now recommended to improve its safety and efficacy, especially in immunocompromised patients (29, 39).
In conclusion, based on its favorable bone penetration, its tolerance, and its efficacy as demonstrated in these reported cases, voriconazole may be considered an attractive first-line therapeutic option for Aspergillus IOE.
Footnotes
Published ahead of print on 22 December 2008.
REFERENCES
- 1.Amonoo-Kuofi, K., P. Tostevin, and J. R. Knight. 2005. Aspergillus mastoiditis in a patient with systemic lupus erythematosus: a case report. Skull Base 15:109-112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bellini, C., P. Antonini, S. Ermanni, M. Dolina, E. Passega, and E. Bernasconi. 2003. Malignant otitis externa due to Aspergillus niger. Scand. J. Infect. Dis. 35:284-288. [DOI] [PubMed] [Google Scholar]
- 3.Bickley, L. S., R. F. Betts, and C. W. Parkins. 1988. Atypical invasive external otitis from Aspergillus. Arch. Otolaryngol. Head Neck Surg. 114:1024-1028. [DOI] [PubMed] [Google Scholar]
- 4.Bryce, G. E., P. Phillips, M. Lepawsky, and M. J. Gribble. 1997. Invasive Aspergillus tympanomastoiditis in an immunocompetent patient. J. Otolaryngol. 26:266-269. [PubMed] [Google Scholar]
- 5.Carfrae, M. J., and B. W. Kesser. 2008. Malignant otitis externa. Otolaryngol. Clin. N. Am. 41:537-549. [DOI] [PubMed] [Google Scholar]
- 6.Chandler, J. R. 1968. Malignant external otitis. Laryngoscope 78:1257-1294.4970362 [Google Scholar]
- 7.Chen, D., A. K. Lalwani, J. W. House, and D. Choo. 1999. Aspergillus mastoiditis in acquired immunodeficiency syndrome. Am. J. Otol. 20:561-567. [PubMed] [Google Scholar]
- 8.Cunningham, M., V. L. Yu, J. Turner, and H. Curtin. 1988. Necrotizing otitis externa due to Aspergillus in an immunocompetent patient. Arch. Otolaryngol. Head Neck Surg. 114:554-556. [DOI] [PubMed] [Google Scholar]
- 9.Denes, E., A. Boumediene, H. Durox, A. Oksman, F. Saint-Marcoux, M. L. Darde, and J. M. Gaulier. 2007. Voriconazole concentrations in synovial fluid and bone tissues. J. Antimicrob. Chemother. 59:818-819. [DOI] [PubMed] [Google Scholar]
- 10.Denning, D. W., R. M. Tucker, L. H. Hanson, and D. A. Stevens. 1989. Treatment of invasive aspergillosis with itraconazole. Am. J. Med. 86:791-800. [DOI] [PubMed] [Google Scholar]
- 11.Doroghazi, R. M., J. B. Nadol, Jr., N. E. Hyslop, Jr., A. S. Baker, and L. Axelrod. 1981. Invasive external otitis. Report of 21 cases and review of the literature. Am. J. Med. 71:603-614. [DOI] [PubMed] [Google Scholar]
- 12.Finer, G., D. Greenberg, E. Leibovitz, A. Leiberman, I. Shelef, and J. Kapelushnik. 2002. Conservative treatment of malignant (invasive) external otitis caused by Aspergillus flavus with oral itraconazole solution in a neutropenic patient. Scand. J. Infect. Dis. 34:227-229. [DOI] [PubMed] [Google Scholar]
- 13.Gordon, G., and N. A. Giddings. 1994. Invasive otitis externa due to Aspergillus species: case report and review. Clin. Infect. Dis. 19:866-870. [DOI] [PubMed] [Google Scholar]
- 14.Gupta, S., J. Koirala, R. Khardori, and N. Khardori. 2007. Infections in diabetes mellitus and hyperglycemia. Infect. Dis. Clin. N. Am. 21:617-638. [DOI] [PubMed] [Google Scholar]
- 15.Hall, P. J., and J. B. Farrior. 1993. Aspergillus mastoiditis. Otolaryngol. Head Neck Surg. 108:167-170. [DOI] [PubMed] [Google Scholar]
- 16.Hanna, E., G. Hughes, I. Eliachar, J. Wanamaker, and W. Tomford. 1993. Fungal osteomyelitis of the temporal bone: a review of reported cases. Ear Nose Throat J. 72:532, 537-541. [PubMed] [Google Scholar]
- 17.Harley, W. B., J. S. Dummer, T. L. Anderson, and S. Goodman. 1995. Malignant external otitis due to Aspergillus flavus with fulminant dissemination to the lungs. Clin. Infect. Dis. 20:1052-1054. [DOI] [PubMed] [Google Scholar]
- 18.Herbrecht, R., D. W. Denning, T. F. Patterson, J. E. Bennett, R. E. Greene, J. W. Oestmann, W. V. Kern, K. A. Marr, P. Ribaud, O. Lortholary, R. Sylvester, R. H. Rubin, J. R. Wingard, P. Stark, C. Durand, D. Caillot, E. Thiel, P. H. Chandrasekar, M. R. Hodges, H. T. Schlamm, P. F. Troke, and B. de Pauw. 2002. Voriconazole versus amphotericin B for primary therapy of invasive aspergillosis. N. Engl. J. Med. 347:408-415. [DOI] [PubMed] [Google Scholar]
- 19.Kountakis, S. E., J. V. Kemper, Jr., C. Y. Chang, D. J. DiMaio, and C. M. Stiernberg. 1997. Osteomyelitis of the base of the skull secondary to Aspergillus. Am. J. Otolaryngol. 18:19-22. [DOI] [PubMed] [Google Scholar]
- 20.Lamprecht, J., A. G. Kuhn, and S. Sauer. 1990. Aspergillus mastoiditis in infected granulomatosis—a case report. Laryngorhinootologie 69:341-344. [DOI] [PubMed] [Google Scholar]
- 21.Latgé, J. P. 1999. Aspergillus fumigatus and aspergillosis. Clin. Microbiol. Rev. 12:310-350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lewis, R. E. 2008. What is the “therapeutic range” for voriconazole? Clin. Infect. Dis. 46:212-214. [DOI] [PubMed] [Google Scholar]
- 23.Ling, S. S., and C. Sader. 2008. Fungal malignant otitis externa treated with hyperbaric oxygen. Int. J. Infect. Dis. 12:550-552. [DOI] [PubMed] [Google Scholar]
- 24.Mardinger, O., D. Rosen, B. Minkow, Z. Tulzinsky, D. Ophir, and A. Hirshberg. 2003. Temporomandibular joint involvement in malignant external otitis. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 96:398-403. [DOI] [PubMed] [Google Scholar]
- 25.Menachof, M. R., and R. K. Jackler. 1990. Otogenic skull base osteomyelitis caused by invasive fungal infection. Otolaryngol. Head Neck Surg. 102:285-289. [DOI] [PubMed] [Google Scholar]
- 26.Mouas, H., I. Lutsar, B. Dupont, O. Fain, R. Herbrecht, F. X. Lescure, and O. Lortholary. 2005. Voriconazole for invasive bone aspergillosis: a worldwide experience of 20 cases. Clin. Infect. Dis. 40:1141-1147. [DOI] [PubMed] [Google Scholar]
- 27.Muñoz, A., and E. Martinez-Chamorro. 1998. Necrotizing external otitis caused by Aspergillus fumigatus: computed tomography and high resolution magnetic resonance imaging in an AIDS patient. J. Laryngol Otol. 112:98-102. [DOI] [PubMed] [Google Scholar]
- 28.Narozny, W., J. Kuczkowski, C. Stankiewicz, J. Kot, B. Mikaszewski, and T. Przewozny. 2006. Value of hyperbaric oxygen in bacterial and fungal malignant external otitis treatment. Eur. Arch. Otorhinolaryngol. 263:680-684. [DOI] [PubMed] [Google Scholar]
- 29.Pascual, A., T. Calandra, S. Bolay, T. Buclin, J. Bille, and O. Marchetti. 2008. Voriconazole therapeutic drug monitoring in patients with invasive mycoses improves efficacy and safety outcomes. Clin. Infect. Dis. 46:201-211. [DOI] [PubMed] [Google Scholar]
- 30.Petrak, R., J. Pottage, and S. Levin. 1985. Invasive external otitis caused by Aspergillus fumigatus in an immunocompetent patient. J. Infect. Dis. 151:196. [Google Scholar]
- 31.Phillips, J., and S. Jones. 2005. Hyperbaric oxygen as an adjuvant treatment for malignant otitis externa. Cochrane Database Syst. Rev. 18:CD004617. doi: 10.1002/14651858.CD004617.pub2. [DOI] [PubMed] [Google Scholar]
- 32.Phillips, P., G. Bryce, J. Shepherd, and D. Mintz. 1990. Invasive external otitis caused by Aspergillus. Rev. Infect. Dis. 12:277-281. [DOI] [PubMed] [Google Scholar]
- 33.Reiss, P., R. Hadderingh, L. J. Schot, and S. A. Danner. 1991. Invasive external otitis caused by Aspergillus fumigatus in two patients with AIDS. AIDS 5:605-606. [DOI] [PubMed] [Google Scholar]
- 34.Ress, B. D., M. Luntz, F. F. Telischi, T. J. Balkany, and M. L. Whiteman. 1997. Necrotizing external otitis in patients with AIDS. Laryngoscope 107:456-460. [DOI] [PubMed] [Google Scholar]
- 35.Rubin Grandis, J., B. F. Branstetter IV, and V. L. Yu. 2004. The changing face of malignant (necrotising) external otitis: clinical, radiological, and anatomic correlations. Lancet Infect. Dis. 4:34-39. [DOI] [PubMed] [Google Scholar]
- 36.Schwartz, S., and E. Thiel. 1997. Clinical presentation of invasive aspergillosis. Mycoses 40(Suppl. 2):21-24. [DOI] [PubMed] [Google Scholar]
- 37.Shelton, J. C., P. J. Antonelli, and R. Hackett. 2002. Skull base fungal osteomyelitis in an immunocompetent host. Otolaryngol. Head Neck Surg. 126:76-78. [DOI] [PubMed] [Google Scholar]
- 38.Slack, C. L., D. W. Watson, M. J. Abzug, C. Shaw, and K. H. Chan. 1999. Fungal mastoiditis in immunocompromised children. Arch. Otolaryngol. Head Neck Surg. 125:73-75. [DOI] [PubMed] [Google Scholar]
- 39.Smith, J., N. Safdar, V. Knasinski, W. Simmons, S. M. Bhavnani, P. G. Ambrose, and D. Andes. 2006. Voriconazole therapeutic drug monitoring. Antimicrob. Agents Chemother. 50:1570-1572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sreepada, G. S., and J. A. Kwartler. 2003. Skull base osteomyelitis secondary to malignant otitis externa. Curr. Opin. Otolaryngol. Head Neck Surg. 11:316-323. [DOI] [PubMed] [Google Scholar]
- 41.Stanley, R. J., T. V. McCaffrey, and L. H. Weiland. 1988. Fungal mastoiditis in the immunocompromised host. Arch. Otolaryngol. Head Neck Surg. 114:198-199. [DOI] [PubMed] [Google Scholar]
- 42.Strauss, M., and E. Fine. 1991. Aspergillus otomastoiditis in acquired immunodeficiency syndrome. Am. J. Otol. 12:49-53. [PubMed] [Google Scholar]
- 43.Walsh, T. J., E. J. Anaissie, D. W. Denning, R. Herbrecht, D. P. Kontoyiannis, K. A. Marr, V. A. Morrison, B. H. Segal, W. J. Steinbach, D. A. Stevens, J. A. van Burik, J. R. Wingard, and T. F. Patterson. 2008. Treatment of aspergillosis: clinical practice guidelines of the Infectious Diseases Society of America. Clin. Infect. Dis. 46:327-360. [DOI] [PubMed] [Google Scholar]
- 44.Winslow, C. P., A. Dichard, and K. A. McGuire. 2001. Osteomyelitis of the temporomandibular joint. Am. J. Otolaryngol. 22:142-145. [DOI] [PubMed] [Google Scholar]
- 45.Yates, P. D., T. Upile, P. R. Axon, and J. de Carpentier. 1997. Aspergillus mastoiditis in a patient with acquired immunodeficiency syndrome. J. Laryngol. Otol. 111:560-561. [DOI] [PubMed] [Google Scholar]