Abstract
Patients taking quinolones as inpatients (n = 55) or outpatients (n = 40) and newly hospitalized patients who were not on quinolone therapy (n = 41) were assessed for fecal carriage of quinolone-resistant Escherichia coli (QREC) strains before and after therapy/hospitalization. Fluoroquinolone use in the previous 6 months was found to be a risk factor for QREC carriage before therapy/hospitalization. The prevalence of QREC strains in fecal flora increased steadily with the duration of quinolone therapy.
Quinolones, with their enhanced systemic activity against many gram-negative aerobes, have been widely used since the first introduction of ciprofloxacin in 1987 (3). It was suggested that the rapid bactericidal activity of quinolones would be advantageous for minimizing resistance. However, as the use of fluoroquinolones has increased, the occurrence of strains with decreased susceptibility or with resistance has also increased (10, 12). Formerly, the quinolone-resistant microorganisms were mainly recovered from hospital or intensive care unit infections. Recently, though, we have seen quinolone-resistant community-acquired infections, of which urinary tract infections are the most challenging (2, 6). Studies revealing the effects of quinolones on the intestinal flora have shown that quinolones might select quinolone-resistant Escherichia coli (QREC) strains especially when used for prophylaxis in neutropenic patients or for the treatment of urinary tract infections (4, 8, 11). We have determined the prevalence of and risk factors for the fecal carriage of QREC strains and its relationship with the use and duration of quinolone therapy on an inpatient and outpatient basis.
A total of 150 patients were enrolled in the study. Fourteen patients were excluded because they did not complete follow-up visits. Quinolone therapy was prescribed for 95 patients (59 [62%] female) for either 7 or 10 days. Fifty-five patients were treated as inpatients (group A) and 40 as outpatients (group B). The mean age ± standard deviation was 52 ± 15 years. Fifty-five patients (58%) received ciprofloxacin, 30 (31.6%) received moxifloxacin, and 10 (10.5%) received levofloxacin. A third group (group C) consisting of 41 patients who were newly hospitalized but did not receive quinolone therapy was also included. For the majority of the patients (70.5%), quinolone was prescribed for the treatment of urinary tract infections and/or lower respiratory tract infections. A standard questionnaire was given to all patients, and the data related to age, sex, underlying disease, and receipt of quinolone or other antimicrobials or hospitalization in the previous 6 months were collected. Written informed consent was obtained from all patients.
We collected fecal samples from all patients at day zero, as the baseline to detect the colonization rate of QREC strains before therapy (groups A and B) or before hospitalization (group C), and then on days 3 and 6 for patients (n = 48) receiving a 7-day course of treatment and on days 3, 6, and 9 for patients (n = 47) receiving a 10-day course of treatment. Group A patients were divided into two subgroups (A1 and A2) for day zero analysis only. Thirty-three were patients hospitalized for ≥72 h (A1), and 22 were hospitalized for <72 h (A2). In detecting the baseline colonization rate of QREC at day zero, we analyzed group B and group A2 together, representing the outpatient colonization rate. The fecal specimens of group C patients were also cultured before hospitalization (day zero) and on hospital days 3, 6, and 9 in order to detect the effect of hospitalization alone on QREC carriage.
Fecal samples were transferred to the laboratory in a transport medium and inoculated within 12 h of collection onto eosin methylene blue agar (Becton Dickinson, TX) supplemented with 1 μg/ml ciprofloxacin. The susceptibility breakpoint against E. coli bacteria was determined as 1 μg/ml according to the CLSI criteria (5). Isolates were identified to the species level by using conventional methods. Lactose-fermenting, indole-positive colonies were identified as E. coli bacteria. MICs were determined by the agar dilution method according to the CLSI criteria. E. coli ATCC 25922 was used for quality assurance.
Categoric variables were compared by using the chi-square test. Logistic regression analysis was performed to determine the risk factors for harboring QREC on day zero. The differences between groups A, B, and C in the rates of acquisition of QREC strains during quinolone therapy or hospitalization were determined by Kaplan-Meier analysis and the log rank test. Data were analyzed with the help of STATA 9.0 software (StataCorp, College Station, TX).
In total, fecal samples from 32 patients (23.5%) (14 patients [42.4%] in group A1, 14 patients [22.5%] in group B plus group A2, and 4 patients [10%] in group C) grew QREC strains on day zero. The risk factors for harboring QREC strains in fecal flora on day zero were older age (>50) (P = 0.042) and hospitalization (P = 0.012) or quinolone use (P = 0.002) in the previous 6 months (Table 1). The results of multivariate analysis, including independent variables of older age, quinolone use in the previous 6 months, hospitalization in the previous 6 months, having a comorbidity(ies), and being an inpatient, revealed that quinolone use in the previous 6 months was the only risk factor for QREC carriage (odds ratio, 3.45; confidence interval, 1.27 to 9.36; P = 0.015).
TABLE 1.
Risk factors for harboring QREC on day zeroa
| Characteristic | No. (%) on day 0
|
P value for QREC on day 0 | |
|---|---|---|---|
| Patients with QREC (n = 32) | Patients without QREC (n = 104) | ||
| Female | 20 (62) | 63 (60) | 0.845 |
| Age (yr) | 51 ± 13c | 57 ± 16c | 0.061 (Student's t test) |
| Age of >50 yr | 24 (75) | 57 (55) | 0.042 |
| Comorbidityb | 28 (87) | 82 (79) | 0.276 |
| Hospitalized (inpatient) | 18 (56) | 56 (54) | 0.811 |
| Fluoroquinolone use in the previous 6 mo | 12 (38) | 14 (13) | 0.002 |
| Hospitalization in the previous 6 mo | 17 (53) | 30 (29) | 0.012 |
Results obtained with univariate analysis.
Cormorbities included diabetes mellitus, chronic obstructive pulmonary diseases, and chronic renal failure.
Values are means ± standard deviations.
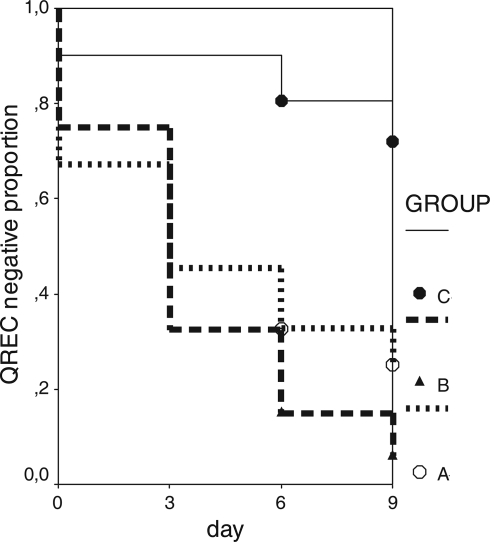
There was a gradual increase in the rate of QREC carriage during therapy in groups A and B, but the increase was not different in these two groups (P = 0.09; log rank test). The effects of different quinolones on QREC selection could not be compared because the number of patients receiving levofloxacin was limited. Group C consisted of 41 patients who had no history of quinolone use in the previous 6 months and who did not take quinolone or any other antimicrobial therapy during their hospital stay. Seventeen (41%) of the group C patients had a history of hospitalization in the previous 6 months. On day zero, QREC growth was detected in samples from four patients (10%). The prevalence of QREC strains in group C was 10%, 22%, and 37% on hospital day 3, 6, and 9, respectively. A survival analysis (Kaplan-Meier) was performed to compare the QREC acquisition rates of the three groups (Fig. 1). When group C patients were compared with group A and group B patients, there was a significant difference in the QREC acquisition rates (A versus C, P < 0.001, and B versus C, P < 0.001). The MIC of ciprofloxacin for all QREC strains, irrespective of the sampling day, was ≥32 μg/ml.
FIG. 1.
Kaplan-Meier curves for groups A, B, and C.
There is an association between the use of quinolones and the isolation of QREC from urinary tract infections particularly, as recently reported from various countries (2, 7, 10). In all our study groups, there were patients harboring QREC strains in fecal flora before quinolone therapy or hospitalization (day zero), and previous quinolone use was the major risk factor for harboring QREC. However, 12 of these patients (42.8%) had neither previous quinolone use nor a history of hospitalization. This finding might reflect the dissemination of resistant E. coli strains among the population or might be the result of some other source, such as quinolone-receiving poultry, although we could not obtain published data on the prevalence of QREC strains in food products in our country (4, 6).
The QREC colonization rate was higher in group A1 (42.4%). This high rate may be explained by facilitated colonization of the digestive tract with QREC strains under circumstances of longer (≥72 h) hospitalization and a history of quinolone use. A steady increase in the rate of QREC prevalence in group A and B patients suggested that quinolone therapy might induce fecal QREC carriage in the presence of risk factors. In group C patients, the initial QREC colonization rate (10%) on day zero was lower than that in groups A and B. Group C patients had no history of quinolone use. They had only a history of hospitalization, which might explain the difference. The increase afterwards in group C might be due to the effect of hospitalization, which was also found, with univariate analysis, to be a risk for QREC carriage. The median numbers of QREC-free days of groups A, B, and C were 5.4, 4.8, and 9.0, respectively (P < 0.001; log rank test). These data show that quinolone use induces QREC carriage more effectively than hospitalization alone. Hillier et al. suggested high-dose, shorter-duration antibiotic use for the treatment of community-acquired infections in order to minimize antimicrobial resistance (9). The patients in our study received approved usual doses of ciprofloxacin, moxifloxacin, or levofloxacin. Although a higher dosing regimen was said to be more effective in the prevention of resistance selection (mutant prevention concentration), all our strains had high levels of resistance, which made this suggestion impractical for our patients. Higher MICs strongly indicated the preexistence of strains with at least some initial mutations which “easily” gain second-step mutations in the presence of quinolones (6). This might be the result of widespread exposure to quinolones in the community. In our country, the use of parenteral forms of many antibiotics is restricted, which has prompted physicians to prescribe oral quinolones more than in the past (1). Moreover, fecal-oral infections, especially bacterial gastroenteritis, are still prevalent and the empirical use of quinolones remains attractive when indicated. We have not studied the quinolone resistance mechanisms and clonal distribution of the QREC strains, which is an important shortcoming of the study. In conclusion, it will be better to reevaluate the indications for and the duration of quinolone therapy in the treatment of community-acquired infections in view of the growing rate of quinolone resistance.
Acknowledgments
We gratefully thank Kenan Kose and Atilla Elhan from the Ankara University Medical Statistics Department for their great contributions to the statistical analysis of the data.
There are no conflicts of interest to declare.
Footnotes
Published ahead of print on 8 December 2008.
REFERENCES
- 1.Altunsoy, A., Ö. Ergönül, A. Azap, and İ. Balik. 2006. Antibiotic use and resistance: outcome of a nationwide governmental antibiotic restriction policy, abstr. K-1415. 46th Intersci. Conf. Antimicrob. Agents Chemother., San Francisco, CA, 27 to 30 September 2006.
- 2.Arslan, H., O. K. Azap, O. Ergonul, and F. Timurkaynak. 2005. Risk factors for ciprofloxacin resistance among Escherichia coli strains isolated from community-acquired urinary tract infections in Turkey. J. Antimicrob. Chemother. 56:914-918. [DOI] [PubMed] [Google Scholar]
- 3.Bearden, D. T., and L. H. Danziger. 2001. Mechanism of action of and resistance to quinolones. Pharmacotherapy 21:224-232. [DOI] [PubMed] [Google Scholar]
- 4.Carratala, J., A. Fernandez-Sevilla, F. Tubau, M. A. Dominguez, and F. Gudiol. 1996. Emergence of fluoroquinolone-resistant Escherichia coli in fecal flora of cancer patients receiving norfloxacin prophylaxis. Antimicrob. Agents Chemother. 40:503-505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.CLSI. 2008. Performance standards for antimicrobial susceptibility testing; 18th informational supplement. M100-S18. Clinical and Laboratory Standards Institute, Wayne, PA.
- 6.Garau, J., M. Xercavins, M. Rodriguez-Carballeira, J. R. Gomez-Vera, I. Coll, D. Vidal, T. Llovet, and R. Bremon. 1999. Emergence and dissemination of quinolone-resistant Escherichia coli in the community. Antimicrob. Agents Chemother. 43:2736-2741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Goettsch, W., W. Van Pelt, N. Nagelkerke, M. G. R. Hendrix, A. G. M. Buiting, P. L. Petit, L. J. M. Sabbe, A. J. A. Griethuysen, and A. J. Neeling. 2000. Increasing resistance to fluoroquinolones in Escherichia coli from urinary tract infections in the Netherlands. J. Antimicrob. Chemother. 46:223-228. [DOI] [PubMed] [Google Scholar]
- 8.Gupta, K., T. M. Hooton, and W. E. Stamm. 2005. Isolation of fluoroquinolone-resistant rectal Escherichia coli after treatment of acute uncomplicated cystitis. J. Antimicrob. Chemother. 56:243-246. [DOI] [PubMed] [Google Scholar]
- 9.Hillier, S., Z. Roberts, F. Dunstan, C. Butler, A. Howard, and S. Palmer. 2007. Prior antibiotics and risk of antibiotic-resistant community-acquired urinary tract infection: a case-control study. J. Antimicrob. Chemother. 60:92-99. [DOI] [PubMed] [Google Scholar]
- 10.Kahlmeter, G., P. Menday, and O. Cars. 2003. Non-hospital antimicrobial usage and resistance in community-acquired Escherichia coli urinary tract infection. J. Antimicrob. Chemother. 52:1005-1010. [DOI] [PubMed] [Google Scholar]
- 11.Perea, S., M. Hidalgo, A. Arcediano, M. J. Ramos, C. Gomez, J. Hornedo, C. Lumbreras, D. Folgueira, H. Cortes-Funes, and A. Rodriguez-Noriega. 1999. Incidence and clinical impact of fluoroquinolone-resistant Escherichia coli in the fecal flora of cancer patients treated with high dose chemotherapy and ciprofloxacin prophylaxis. J. Antimicrob. Chemother. 44:117-120. [DOI] [PubMed] [Google Scholar]
- 12.Zervos, M. J., E. Hershberger, D. P. Nicolau, D. J. Ritchie, L. K. Blackner, E. A. Coyle, A. J. Donnelly, S. F. Eckel, R. H. K. Eng, A. Hiltz, A. G. Kuyumjian, W. Krebs, A. McDaniel, P. Hogan, and T. J. Lubowski. 2003. Relationship between fluoroquinolone use and changes in susceptibility to fluoroquinolones of selected pathogens in 10 United States teaching hospitals. Clin. Infect. Dis. 37:1643-1648. [DOI] [PubMed] [Google Scholar]