Abstract
Anidulafungin is a novel semisynthetic echinocandin with potent activity against Candida (including azole-resistant isolates) and Aspergillus spp. and is used for serious systemic fungal infections. The purpose of these studies was to characterize the clearance mechanism and potential for drug interactions of anidulafungin. Experiments included in vitro degradation of anidulafungin in buffer and human plasma, a bioassay for antifungal activity, in vitro human cytochrome P450 inhibition studies, in vitro incubation with rat and human hepatocytes, and mass balance studies in rats and humans. Clearance of anidulafungin appeared to be primarily due to slow chemical degradation, with no evidence of hepatic-mediated metabolism (phase 1 or 2). Under physiological conditions, further degradation of the primary degradant appears to take place. The primary degradation product does not retain antifungal activity. Anidulafungin was not an inhibitor of cytochrome P450 enzymes commonly involved in drug metabolism. Mass balance studies showed that anidulafungin was eliminated in the feces predominantly as degradation products, with only a small fraction (10%) eliminated as unchanged drug; fecal elimination likely occurred via biliary excretion. Only negligible renal involvement in the drug's elimination was observed. In conclusion, the primary biotransformation of anidulafungin is mediated by slow chemical degradation, with no evidence for hepatic enzymatic metabolism or renal elimination.
The echinocandins are a relatively new class of parenterally administered antifungal agents that have been developed for the treatment of serious systemic fungal infections. Members of this class include caspofungin, micafungin, and anidulafungin; the latter is a novel semisynthetic echinocandin with potent in vitro and in vivo activities against Candida spp. and Aspergillus spp., the major causes of deep-seated mycoses (4, 5, 24). Anidulafungin is approved in the United States and Europe for the treatment of candidemia in nonneutropenic patients and other forms of invasive Candida infections (11, 25). Anidulafungin (Fig. 1) has distinct pharmacokinetic characteristics, activity against azole-resistant Candida spp., and a favorable safety profile, all of which support its use for systemic fungal infections (26).
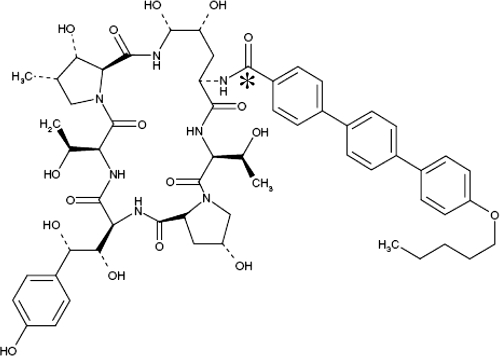
FIG. 1.
Chemical structure of anidulafungin. *, position of the 14C radiolabel.
Echinocandins mediate their antifungal activity by noncompetitive inhibition of (1,3)-β-d-glucan synthase, a fungus-specific enzyme essential for the synthesis of cell wall glucan (5). While this mode of action is common to all members of the class, there appear to be some differences among echinocandins with respect to their pharmacokinetic properties, including drug disposition (3, 5, 26). Some of these differences may have implications for the concomitant use of these agents with other drugs and for their use in special patient populations, particularly those under intensive care.
To this end, the studies described here were designed to characterize the clearance mechanism and disposition of anidulafungin in rats and humans, as well as to elucidate any potential interactions with key metabolic enzymes commonly involved in drug-drug interactions.
MATERIALS AND METHODS
Bioanalyses.
A high-performance liquid chromatography-tandem mass spectrometry (HPLC-MS/MS) assay was developed to quantitate anidulafungin and its primary degradant. For analysis, either fresh samples or frozen samples thawed in an ice bath were vortexed. A 100-μl aliquot of each sample and 300 μl of internal standard working solution (efavirenz; 20 ng/ml in methanol) were vortexed and centrifuged at 13,000 rpm for 5 min. A 200-μl aliquot of supernatant was injected into a BDS Hypersil C18 column (50 by 4.6 mm; 3 μm; mobile phase, 57% acetonitrile and 43% 25 mM ammonium acetate, pH 4.0, solution) coupled to an API 3000 mass spectrometer utilizing a turbo ion spray source. Ions were detected in multiple reaction monitoring scan mode with an acquisition time of 3.0 min and precursor→product ion pairs of 1,140.8→1,122.8 for anidulafungin and 316.1→244.1 for the internal standard. For all samples, the peak areas of both anidulafungin and degradant, with the same mass-to-charge ratio, were recorded. For the assay validated using human plasma with heparin, extraction recoveries of drug and internal standards were 88 to 89% and 98%, respectively. The lower limit of quantification for the assay was 0.1 μg/ml. The intraday and interday accuracies (percentage of target concentration) and precision (percent coefficient of variation around the mean of determined values) were calculated for the lower limit of quantification sample concentration and for each of the quality control sample concentrations (0.3, 4, and 16 μg/ml) used during sample analysis. Intraday accuracy ranged from 96.2 to 114%, and precision ranged from 4.9 to 11.4%. Interday accuracy ranged from 97.6 to 104%, and precision ranged from 3.2 to 13.4%. Performance of the assay using rat plasma with heparin was similar (data not presented).
The primary degradant of anidulafungin was not available as a pure substance, which precluded use of an analytical standard to quantify this moiety. In order to estimate concentrations of the degradant in vitro and in vivo, a study was conducted to measure the disappearance of anidulafungin (initial concentration of 8.0 μg/ml) and formation of the degradation product under conditions where both compounds were expected to be quantifiable (i.e., in pH 8.0 phosphate-buffered saline [PBS] at room temperature). Samples were obtained at 0, 24, 48, 72, and 96 h and assayed by HPLC-MS/MS as described above. In these studies, chromatographic retention times were approximately 1.5, 1.0, and 2.1 min, respectively, for anidulafungin, primary degradant, and internal standard. The rates of anidulafungin disappearance and degradant formation (based on mean peak areas) were calculated, and the difference in slopes was taken as the difference in MS response sensitivity between the two compounds. This approach relied on the assumption that the primary degradant was the only degradation product formed and that it remained stable under the incubation conditions.
In vitro degradation studies.
The in vitro degradation studies were designed to determine the rate of degradation of anidulafungin as well as the rate of formation of the primary degradant. Anidulafungin (8.0 μg/ml) was incubated in PBS at pH 7.4 for 96 h at 37°C. In addition, anidulafungin at concentrations of 0.1, 1.0, and 8.0 μg/ml was incubated in heparinized human plasma at 37°C for 144 h. Aliquots of anidulafungin in PBS were sampled at 0, 24, 48, 72, and 96 h and in human plasma at 0, 2, 4, 6, 12, 24, 48, 72, 96, and 144 h. Three separate aliquots were sampled for each time point, and all samples were stored at −80°C and subsequently assayed by HPLC-MS/MS.
In vitro cytochrome P450 inhibition studies.
In vitro cytochrome P450 studies were designed to assess the ability of anidulafungin to inhibit the in vitro metabolism mediated by the hepatic cytochrome P450 isoenzymes CYP1A2, CYP2B6, CYP2C8, CYP2C9, CYP2C19, CYP2D6, and CYP3A, which are responsible for the metabolism of the majority of drugs (12, 37-39).
In vitro experiments were carried out using human hepatic microsomes and selective substrates for each isoenzyme activity, based on previously described methods, as follows: for CYP1A2, phenacetin O-deethylase (32); for CYP2B6, bupropion hydroxylase (36); for CYP2C8, amodiaquine N-deethylase (36); for heterologously expressed CYP2C8 (rhCYP2C8), rosiglitazone N-desmethylase (35); for CYP2C9, diclofenac 4′-hydroxylase (20); for CYP2C19, S-mephenytoin 4′-hydroxylase (36); for CYP2D6, bufuralol 1′-hydroxylase (19); for CYP3A, midazolam 1′-hydroxylase (18).
For the CYP1A2, CYP2C9, CYP2D6, and CYP3A assays, four human liver samples were obtained from the liver transplant unit at the Medical College of Wisconsin (Milwaukee, WI) and Indiana University School of Medicine (Indianapolis) under protocols approved by the appropriate committee for the conduct of human research. A mixture of equal protein concentrations of microsomes from each sample was prepared by differential centrifugation using the method described by van der Hoeven and Coon (34). These particular assays were carried out in duplicate incubations at concentrations of 0, 2.20, 4.40, 8.80, and 17.5 μM anidulafungin. Either UV detection at 220 nm (CYP3A), 254 nm (CYP1A2), or 282 nm (CYP2C9) or fluorescence detection with excitation at 252 nm and emission at 302 nm (CYP2D6) was used to detect the remaining amount of respective metabolic end product after assay termination. All chromatographic data were collected through a model 941 analog/digital interface (PE Nelson Systems Inc., Cupertino, CA) and analyzed with ACCESS*CHROM, version 1.8 (PE Nelson Systems Inc., Cupertino, CA). The CYP2B6, CYP2C8, and CYP2C19 assays used microsomes from a pool of 53 individual human livers and were carried out in duplicate in the presence of anidulafungin at 0, 0.056, 0.18, 0.56, 1.8, 5.5, or 17.5 μM (delivered to the incubation mixtures in 2 μl of a 50/50 solution of CH3CN-H2O).
Additional studies were carried out in human hepatic microsomal fractions to investigate the effect of anidulafungin in vitro on the metabolism of cyclosporine by using radiolabeled [3H]cyclosporine A. A pilot study was first undertaken to establish the optimal concentration of human hepatic microsomal protein for metabolism of cyclosporine A. Based on these results, incubations were carried out with 1.0 mg/ml human hepatic microsomal protein and anidulafungin at concentrations of 0, 1.5, 4.5, 9, 15, and 30 μg/ml (0 to 26 μM). Ketoconazole (10 μM), a known inhibitor of CYP3A-mediated metabolism of cyclosporine A (23), was used as the positive control. Incubations were initiated by adding [3H]cyclosporine A (approximately 5,000,000 dpm) to methanol to achieve a final incubation concentration of 2 μg/ml. Reactions were terminated at 0, 15, 30, and 45 min by addition of acetonitrile, after which samples (one sample per concentration at each time point) were analyzed by HPLC for the presence of cyclosporine A and metabolites. HPLC was carried out using a Hewlett Packard 1100 system, with UV detection at 210 nm. Radioactivity was detected using a Packard Radiomatic Flo-One A525 500TR, with a Packard Ultima Flo M scintillation cocktail at a flow rate of 3 ml/min. The HPLC system utilized a Develosil TMS-UG-5 chromatography column, based on the method described by Omar et al. (23).
Incubation with rat and human hepatocytes.
The in vitro incubation studies were designed to characterize and compare the degradation of anidulafungin in rat and human hepatocytes. Optimal experimental conditions, i.e., concentrations of anidulafungin and the container type for incubation, were first established by conducting a series of pilot experiments. Using silanized glass tubes in a water-jacketed incubator with 95% air-5% CO2, suspensions of rat and human hepatocytes (approximately 200,000 viable cells per tube) were incubated with anidulafungin at 1.0 and 10.0 μM at 37°C. Incubations (triplicate determinations at each time point) were stopped at 0, 0.5, 1, 2, and 4 h by adding 0.5 ml of ice-cold methanol. Hepatocyte incubations containing bufuralol, which undergoes phase 1, phase 2, and sequential phase 1-to-2 biotransformations, were performed in parallel as positive controls. Incubation samples were analyzed by HPLC-MS/MS for concentrations of the respective parent drugs and metabolites. The percentage of parent drug remaining relative to its initial amount was calculated, as was the relative amount of each metabolite, based on the HPLC-MS/MS chromatogram peak areas relative to the reference parent compound.
Bioassay for active moiety.
The bioassays for active moieties were designed to determine whether the loss of parent compound over time corresponded to a loss in biological activity of anidulafungin. Solutions of 8, 20, and 100 μg/ml anidulafungin in PBS (pH 7.4) were incubated at either room temperature or 37°C in 25-ml aliquots per sterile Erlenmeyer flask. Samples were taken at baseline and at 24-h intervals over a 10-day period and frozen immediately. Using a modification of the procedure described by Marchetti et al. (21), aliquots of each sample from this 10-day period were added to agar plates cultured with Candida albicans strain VCAL1005 (ATCC 90027). Plates were incubated overnight at 30°C and zones of inhibition measured. A standard curve of known anidulafungin concentrations versus zone size was obtained by graphing zone size (x axis) versus log10 concentration (y axis). Using linear regression analysis, a line equation was determined, based on which the drug concentration in each experimental sample was calculated.
Mass balance and bile excretion in rats.
The purpose of the mass balance and bile excretion study was to determine the pharmacokinetics, mass balance, and biliary excretion of drug-derived radioactivity in rats following administration of a single intravenous (i.v.) bolus dose of [14C]anidulafungin at 5 mg/kg of body weight.
[14C]anidulafungin in rats.
This study was conducted under a protocol approved by the local ethical review committee. The position of the 14C radiolabel is indicated in Fig. 1. The study used 30 male Sprague-Dawley rats (including one control rat that received vehicle only). Five of the drug-treated rats were bile duct cannulated. Whole blood and plasma samples were collected from three noncannulated rats sacrificed at each of the following time points: 0.25, 0.5, 1, 2, 6, 12, 24, and 168 h postdose. Blood samples were collected via cardiac puncture, and an aliquot was used to separate plasma. Bile samples were collected from five bile duct-cannulated rats at intervals of 0 to 4, 4 to 8, and 8 to 24 h postdosing, and at each 24-h interval until sacrifice at 168 h postdose. Urine, feces, carcass, and cage washings were collected from the three noncannulated rats sacrificed at 168 h postdosing, from all cannulated rats, and from the control rat. Urine and fecal samples were collected at period intervals until sacrifice, while carcass and cage washings were collected at the time of sacrifice. All samples were analyzed for drug-derived radioactivity by liquid scintillation counting.
Mass balance in humans.
The purpose of this phase 1 clinical study was to determine the pharmacokinetics and mass balance following administration of a single dose of [14C]anidulafungin to healthy adult male subjects (n = 9). This study was conducted in compliance with the Declaration of Helsinki and the International Conference on Harmonization Good Clinical Practices Guidelines. All subjects provided written informed consent prior to study initiation. The protocol and informed consent form were approved by the MDS Pharma Services Institutional Review Board (Lincoln, NE).
A dose of 100 mg/100 μCi of [14C]anidulafungin, infused intravenously over 100 min at a rate of 1 mg/min, was planned for the study; the mean dose actually administered was 88.3 mg (range, 87.6 to 88.7 mg) and 95 μCi. Subjects were confined to the study unit for 10 days after dosing for pharmacokinetic sampling and were asked to return for follow-up between 6 and 8 weeks postdose.
A total of 21 blood samples were collected in heparinized tubes at 0 (predose), 1, 1.66 (end of infusion), 1.75, 2, 2.4, 3, 4, 6, 12, and 18 h after the start of infusion and thereafter every 24 h on days 2 to 10 relative to the start of infusion and at follow-up. A total of 14 urine samples were collected as follows: predose, at the intervals 0 to 2, 2 to 4, 4 to 8, and 8 to 24 h, every 24 h from days 2 to 10, and at follow-up. In addition, 11 fecal samples were collected as follows: predose, every 24 h until day 10, and at follow-up. Expired air samples were collected at predose and at 1, 2, 3, 4, 5, and 6 h after the start of the infusion.
Concentrations of anidulafungin (i.e., parent drug) were determined in plasma using a validated LC-MS/MS assay (7) and in fecal samples using an LC radiochemical detection method. Drug-derived radioactivity was determined in plasma, whole blood, urine, expired air samples, and fecal sample extracts by liquid scintillation counting. Pharmacokinetic parameters were determined by noncompartmental analyses using WinNonlin version 4.0 (Pharsight, Mountain View, CA).
RESULTS
In vitro chemical degradation studies.
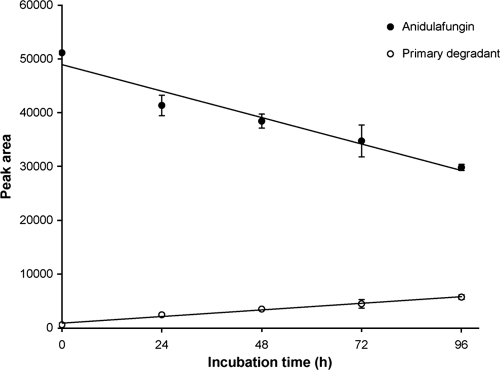
In the study to determine the electrospray ionization (ESI)-MS response factor for detecting the primary anidulafungin degradation product relative to anidulafungin, the concentration of anidulafungin (as measured by mean peak area) decreased linearly over 96 h and corresponded to a linear increase in concentration of the degradant (Fig. 2). The slope of the anidulafungin degradation curve at pH 8.0 and room temperature (−205 peak area units/h) was approximately fourfold higher than the slope of the degradation product formation curve (51.5 peak area units/h), indicating that the ESI-MS response for the primary anidulafungin degradant was approximately fourfold lower than for anidulafungin. Therefore, in the subsequent in vitro study, the concentration of the degradant was calculated using the calibration curves of anidulafungin and by applying a fourfold correction factor.
FIG. 2.
Changes in HPLC-MS/MS peak areas for anidulafungin and its primary degradation product over time in pH 8.0 phosphate-buffered saline at room temperature. Each data point is the mean of three determinations. Linear regression lines are shown for each curve.
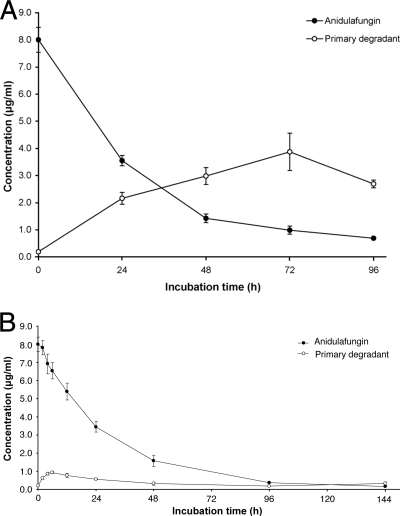
When anidulafungin was incubated in PBS at pH 7.4 at 37°C, parent drug concentration decreased steadily, with subsequent appearance of the degradation product (Fig. 3A). However, the appearance of degradant did not account for all of the disappearance of anidulafungin, and after 72 h the degradation rate of the primary degradant exceeded the rate of its formation.
FIG. 3.
In vitro concentration-time profiles of anidulafungin and its primary degradation product following incubation of 8 μg/ml anidulafungin in pH 7.4 phosphate-buffered saline (A) and human plasma (B), both at 37°C. Each data point is the mean of three determinations. The concentrations indicated for the degradation product were estimated from chromatographic peak areas, relative to anidulafungin peak areas, by applying a mass spectrometer sensitivity factor of 4 derived from the results presented in Fig. 2.
In human plasma at 37°C, anidulafungin degradation appeared to take place at a similar rate as in buffer (Fig. 3B). After 96 h under these conditions, anidulafungin had decreased by 95.4% from an initial concentration of 8.0 μg/ml. Figure 3B also demonstrates that the anidulafungin primary degradant was relatively unstable in plasma. Although the initial formation rate of the degradant appeared to be similar to the initial rate of disappearance of anidulafungin, after 6 h of incubation the rate of degradation of the degradant was higher than that for anidulafungin. Incubation with anidulafungin at 1.0 μg/ml yielded similar results (data not shown). For the incubation at 0.1 μg/ml, the concentration of anidulafungin decreased to approximately 0.04 μg/ml by 24 h and was not quantifiable thereafter. The primary degradant was not quantifiable at any time point in the 0.1-μg/ml incubation.
In vitro cytochrome P450 inhibition studies.
Anidulafungin at concentrations ranging from 0 to 17.5 μM had no relevant inhibitory effect on the catalytic activities of CYP1A2, CYP2B6, CYP2C9, CYP2C19, CYP2D6, or CYP3A in human microsomal preparations; half-maximal (50%) inhibitory concentrations (IC50) of anidulafungin for these cytochrome P450 isozymes were >17.5 μM (Table 1). It should be noted that a concentration of 17.5 μM was determined to be the maximal concentration of compound that was soluble under the incubation conditions examined.
TABLE 1.
IC50 values and activities observed with anidulafungin at 17.5 μM for clinically important cytochrome P450 isozymesa
| Isozyme | IC50 (μM) | % of control activity |
|---|---|---|
| CYP1A2 | >17.5 | 90.0 |
| CYP2B6 | >17.5 | 56.7 |
| CYP2C8b | 12.0 | 42.5 |
| rhCYP2C8c | >17.5 | 65.7 |
| CYP2C9 | >17.5 | 82.4 |
| CYP2C19 | >17.5 | 90.0 |
| CYP2D6 | >17.5 | 100 |
| CYP3A | >17.5 | 100 |
IC50 and percent control values are based on the results of duplicate determinations for four to six different concentrations of anidulafungin (see text for details).
Primary CYP2C8 assay with amodiaquine as substrate.
Secondary CYP2C8 assay using heterologously expressed enzyme, with rosiglitazone as substrate.
Anidulafungin demonstrated minor inhibition of CYP2C8, with an IC50 of 12 μM; however, anidulafungin caused a small interference in this particular assay, which could have resulted in a slight underestimation of inhibitory potency. To address this, anidulafungin inhibition of CYP2C8 was also examined in rhCYP2C8 using a second substrate, rosiglitazone. This secondary assay showed 34.3% inhibition at 17.5 μM anidulafungin, a similar result to that obtained when using amodiaquine as the marker substrate for CYP2C8 activity. The observed remaining percentage of control substrate at 17.5 μM anidulafungin for each CYP450 isozyme examined is shown in Table 1.
At clinically relevant concentrations, anidulafungin had no inhibitory effect on CYP3A-mediated metabolism of cyclosporine. Results of HPLC analysis showed that the formation of the primary metabolites of cyclosporine in the presence of anidulafungin was similar to that in controls without anidulafungin. Furthermore, concentrations of [3H]cyclosporine were not affected by the presence of anidulafungin at any of the time points, as demonstrated by the mean distribution of radioactivity in the incubation samples. In contrast, there was no metabolism of cyclosporine in the presence of the CYP3A inhibitor ketoconazole (positive control).
Incubation with rat and human hepatocytes.
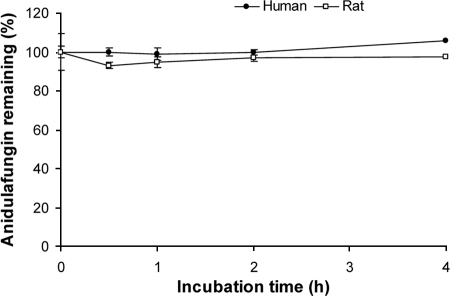
In control incubations with human or rat hepatocytes, sequential phase 1-to-2 biotransformation of bufuralol was detected in both species, indicating that the hepatocytes used in this study were metabolically active. When anidulafungin was present as substrate, there was no loss of parent compound at either of the substrate concentrations used (1 or 10 μM). This is illustrated in Fig. 4 for the 10 μM concentration. In addition, the primary degradant of anidulafungin was not detected.
FIG. 4.
Lack of hepatic metabolism of anidulafungin in rat and human hepatocytes as shown by the percentage of 10 μM anidulafungin remaining over time. Values are the means of three determinations. Error bars have been omitted for clarity, but the coefficient of variation ranged from 1.7 to 3.1% for rat hepatocytes and 1.3 to 9.4% for human hepatocytes.
Bioassay for active moiety.
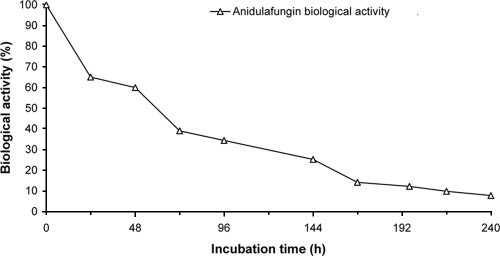
During incubation of plasma samples containing anidulafungin at 8, 20, and 100 μg/ml under physiological conditions (pH 7.4 and 37°C), the biological activity of anidulafungin (assessed by zones of inhibition) decreased over time (Fig. 5). The decrease in activity was less pronounced in the samples maintained at room temperature (data not presented) than in those kept at 37°C. Increase in temperature, therefore, appeared to accelerate the chemical degradation of anidulafungin.
FIG. 5.
Loss of anidulafungin activity over time, as determined by bioassay, following incubation of 8 μg/ml of anidulafungin in phosphate-buffered saline, pH 7.4, at 37°C. Each point represents a single determination. The percentage of biological activity at each time point was expressed as 100 times the concentration at that time point divided by the starting concentration (8.7 μg/ml).
Mass balance and bile excretion in rats.
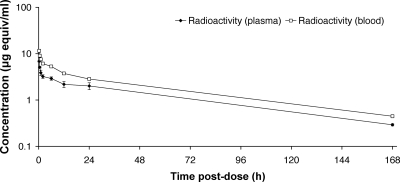
Following i.v. dosing in rats, the disposition of drug-derived radioactivity in plasma and blood appeared to be multiexponential, characterized by a rapid initial decrease followed by a slower terminal phase (Fig. 6). The terminal phase half-lives for drug-derived radioactivity in the blood and plasma were 52.1 h and 52.9 h, respectively (Table 2). Whole blood was found to have higher concentrations of drug-derived radioactivity than plasma, and the blood/plasma area under the time-concentration curve (AUC) ratio was 1.5.
FIG. 6.
Concentration-time profile for drug-derived radioactivity in plasma and blood following i.v. administration of [14C]anidulafungin (5 mg/kg) to male Sprague-Dawley rats (n = 3 per time point). Standard deviation bars have been omitted from the blood values for clarity. The coefficient of variation ranged from 4 to 17% for plasma and from 1 to 8% for blood.
TABLE 2.
Pharmacokinetic parameters of drug-derived radioactivity in plasma and blood following i.v. bolus administration of [14C]anidulafungin (5 mg/kg) to male Sprague-Dawley rats
| Parameter (units)a | Drug-derived radioactivityb
|
|
|---|---|---|
| Plasma | Whole blood | |
| Cmax (μg eq/ml)c | 6.84 | 11.5 |
| Tmax (h) | 0.25 | 0.25 |
| AUC0-t (μg eq·h/ml) | 227 | 340 |
| AUC0-∞ (μg eq·h/ml) | 249 | 374 |
| t1/2 (h) | 52.9 | 52.1 |
| Vdss (ml/kg) | 1,056 | 696 |
| CL (ml/h·kg) | 20.1 | 13.4 |
Abbreviations: Cmax, highest observed concentration; Tmax, time to Cmax; AUC0-t, AUC from time zero to the last quantifiable time point; t1/2, terminal-phase half-life; Vdss, volume of distribution at steady state; CL, total body clearance.
Values represent the composite pharmacokinetic profile derived from the mean values of plasma concentrations from three animals sacrificed at each time point.
Assumes 1 g of blood or plasma is equivalent to 1 ml.
Drug-derived radioactivity appeared to be widely distributed, since the volume of distribution was roughly equivalent to the total body volume. In noncannulated rats, the entire radioactive dose was accounted for through 168 h. Drug-derived radioactivity was primarily recovered in the feces (77.1%), and the remaining radioactivity was recovered in the carcass (23.1%). Negligible amounts (<1% of the dose) of radioactivity were recovered in the urine and in cage washings. In the bile duct-cannulated rats, 94.8% of the radioactive dose was accounted for through 168 h. Drug-derived radioactivity was primarily recovered in the carcasses, bile, and feces, accounting for 40.4%, 33.9%, and 17.1% of the dose administered, respectively. The remaining radioactivity (2.94%) was recovered in the urine. Negligible amounts (<1% of the dose) of radioactivity were recovered in cage washings.
Mass balance study in humans.
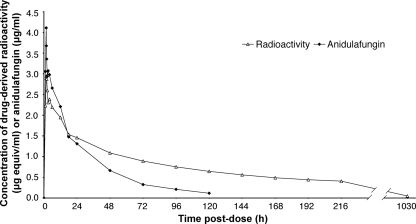
Following administration of a single i.v. dose of [14C]anidulafungin, the plasma concentrations of the parent drug were quantifiable in all subjects until day 5. Drug-derived radioactivity remained quantifiable until the subjects were discharged on day 10; only a trace amount of radioactivity was detectable at the final sampling time at 6 to 8 weeks postdosing. Mean plasma concentrations of drug-derived radioactivity were generally similar to parent drug concentrations during the initial hours after dosing but were markedly higher after the first day throughout the observation period (Fig. 7). Accordingly, the mean plasma AUC for total radioactivity was higher and clearance was lower compared with parent drug (Table 3). The mean terminal elimination half-lives for anidulafungin and total radioactivity were 27.7 and 119 h, respectively (Table 3). The pharmacokinetics of drug-derived radioactivity were similar between blood and plasma (Table 3). The blood-to-plasma ratios for mean Cmax and AUC from 0 h to ∞ (AUC0-∞) values were 112% and 100%, respectively, suggesting no preferential accumulation of drug-derived radioactivity into erythrocytes.
FIG. 7.
Mean (and standard deviation) plasma concentration-time profiles of anidulafungin (i.e., parent drug) and drug-derived radioactivity following a single i.v. infusion of [14C]anidulafungin to adult healthy male subjects in a phase 1 clinical study (n = 9). Standard deviation bars have been omitted for clarity. The coefficient of variation ranged from 11 to 32% for radioactivity and from 14 to 39% for anidulafungin.
TABLE 3.
Pharmacokinetic parameters of anidulafungin (parent drug) and drug-derived radioactivity following i.v. infusion of [14C]anidulafungin to healthy male subjects in a phase I clinical studya
| Pharmacokinetic parameter | Mean (% CV) (n = 9)
|
||
|---|---|---|---|
| Anidulafungin (plasma) | Drug-derived radioactivityb
|
||
| Plasma | Whole blood | ||
| Cmax (mg/liter) | 3.63 (15.6) | 3.02 (12.2) | 3.38 (9.07) |
| Tmax (h) | 1.78 (6.65) | 1.78 (6.62) | 1.75 (6.32) |
| AUC0-t (mg·h/liter) | 92.5 (14.4) | 182 (12.3) | 175 (8.11) |
| AUC0-∞ (mg·h/liter) | 98.0 (13.6) | 250 (11.1) | 249 (9.27) |
| t1/2 (h) | 27.7 (8.43) | 119 (11.0) | 139 (9.11) |
| MRT (h) | 35.6 (7.09) | 162 (10.7) | 174 (9.60) |
| Vdss (liters) | 32.6 (17.7) | 57.6 (16.1) | 61.6 (8.94) |
| CL (liters/h) | 0.913 (13.0) | 0.356 (10.9) | 0.356 (9.85) |
Abbreviations: Cmax, highest observed concentration; Tmax, time to Cmax; AUC0-t, AUC curve from time zero to the last quantifiable time point; t1/2, terminal-phase half-life; MRT, mean residence time; Vdss, volume of distribution at steady state; CL, total body clearance; CV, coefficient of variation.
Radioactivity results are presented as milligram equivalents of anidulafungin.
After more than 216 h postdosing, the mean cumulative amount of radioactivity recovered in the urine was 0.56% (range, 0.37% to 0.75%) and that in the feces was 29.5% (range, 6.99% to 52.0%). Only 10% of the radioactive dose was recovered as intact drug in the feces through 216 h postdosing. No radioactivity was found in expired air. Overall, an average of 30.0% (range, 7.4% to 52.8%) of the administered radioactive dose was recovered in the urine and feces through 216 h postdose. Pharmacokinetic samples obtained at 6 to 8 weeks after dosing showed no anidulafungin in plasma or feces, no radioactivity in urine, and only trace amounts of radioactivity in plasma (0.051 ± 0.013 μg equiv/ml) and feces (<0.2% of the injected dose/sample).
DISCUSSION
Based on the studies described, it can be concluded that anidulafungin does not undergo hepatic metabolism and does not interact with cytochrome P450 isozymes commonly involved in drug-drug interactions. Furthermore, intact parent drug, as well as metabolites, appear to be eliminated via biliary excretion, without involvement of the kidneys. The conclusion that anidulafungin is not a substrate for phase 1 and phase 2 metabolic pathways is strongly supported by the results of our in vitro metabolism studies and is further substantiated by previous reports indicating that hepatic impairment, regardless of severity, does not increase the systemic exposure to anidulafungin (6).
While a slight inhibitory effect of anidulafungin on CYP2C8 activity was observed in vitro, the unbound systemic concentrations of the drug in patients following the clinical dosing regimen (14, 25) are much lower than its inhibitory potency. These observations are consistent with results from a population pharmacokinetic analysis, which indicate that drugs that are substrates, inhibitors, or generalized inducers of cytochrome P450 enzymes do not alter the clearance of anidulafungin (10). Due to the lack of interaction between anidulafungin and the key cytochrome P450 enzymes, no requirement for dosage adjustments is anticipated when anidulafungin is given in conjunction with drugs that are either inhibitors or inducers of hepatic enzymes. This assumption has been corroborated by drug interaction studies of anidulafungin and concomitant administration of cyclosporine, tacrolimus, voriconazole, liposomal amphotericin B, or rifampin (7-10, 25).
Instead of hepatic metabolism, the primary degradation of parent drug appears to occur via slow, nonenzymatic, chemical degradation to an inactive metabolite. Under physiological conditions, further enzymatic degradation of the primary degradant by plasma peptidases presumably takes place. This conclusion is indicated by the fact that the degradant is observed in both plasma and buffer matrices but is more quickly removed in the former. Data obtained from degradation studies using different initial concentrations of anidulafungin suggest that the rate of parent drug degradation is independent of its concentration. The disappearance kinetics of primary degradant in plasma seem to be formation rate dependent. The primary degradant and, presumably, any secondary metabolites appear to be pharmacologically inactive, since the antifungal activity of anidulafungin was shown to decrease over time, corresponding to the drug's degradation.
The in vitro degradation studies were complicated by the lack of analytical standards to quantify this primary degradation product. Under conditions where the degradant was assumed to be stable (pH 8 buffer, room temperature), there was a strong linear correlation between anidulafungin loss and degradant formation, which implies negligible subsequent degradation of the primary degradation product. Based on the results of this initial experiment, an anidulafungin/degradant ESI-MS sensitivity factor of 4 was established, therefore allowing estimation of degradant concentrations in both buffer and plasma samples for subsequent studies. It should be noted that this factor relies on the assumptions that (i) there is only one primary degradation product of anidulafungin and (ii) that it was stable under the initial experimental conditions. If these assumptions were not entirely borne out, then the rate of degradant formation in the latter degradation studies may have been slightly underestimated.
The pathway for elimination of anidulafungin in vivo is delineated by the results of the mass balance studies in rats and humans. Given the position of the radiolabel used in these studies (Fig. 1), anidulafungin, the primary degradant, and any subsequent peptidic product still retaining the terphenyl side chain would all be detectable by the method used. However, any amino acid or peptide not containing the radioactively labeled side chain would remain undetectable. In humans, the slower clearance and longer half-life of drug-derived radioactivity compared with parent drug suggest that the primary degradant or other degradation products persisted in the body for a longer time period. The human mass balance study was somewhat limited by the long half-life of radioactivity in the study, as only about 29.5% of the administered radioactivity could be recovered during the in-house observation period (through days 1 to 10 postdose). Within that time frame, most of the radioactivity was observed in the feces, with negligible urinary recovery and no recovery in expired air. When the subjects returned for week 6 to 8 sampling, only trace amounts of radioactivity were recovered in the plasma and feces. These observations suggest that most of the radioactive degradation products of anidulafungin were eliminated in the feces within several weeks postdosing, after the subjects had left the study center.
Elimination in the feces presumably occurred via biliary excretion in humans, an assumption further justified by the results of the rat study using bile duct-cannulated animals. In this respect it should be noted that anidulafungin is not a substrate for organic anion transporting protein or P-glycoprotein (15), which are key transport proteins currently identified as being involved in the biliary elimination of drugs. Therefore, it would be reasonable to assume that the elimination of the small fraction (about 10%) of parent drug in the feces occurred by passive diffusion, with only minimal involvement of drug transporters. Due to the difficulty in obtaining a stable degradation product(s) of anidulafungin, similar experiments assessing the potential action of drug transport proteins on these metabolites have not been conducted to date, and the exact nature of the biliary excretion of the degradation products, therefore, remains unclear. The assumed lack of renal and hepatic involvement in the elimination of anidulafungin is also supported by pharmacokinetic studies in renal or hepatic impairment subjects (6). These studies indicated that the pharmacokinetics of anidulafungin were not significantly affected by impaired renal or hepatic function, regardless of severity, and suggested that dose adjustment of anidulafungin is not necessary in these patient populations.
As the human and rat pharmacokinetic studies illustrate, anidulafungin is widely distributed throughout the body, with a volume of distribution (Vd) that approximates total body fluid. Anidulafungin concentration-time profiles observed in the in vitro chemical degradation studies described here show a long t1/2 of parent drug in both buffer and human plasma. This is consistent with the findings of the human mass balance study and with previously published pharmacokinetic studies in human volunteers, which showed a t1/2 of 18 to 27 h (10, 33).
Similar to caspofungin and micafungin, anidulafungin is thought to undergo spontaneous chemical degradation to a ring-opened peptide, which would be analogous to the primary degradant described in our studies. In addition, it has been suggested that caspofungin is slowly metabolized by hydrolysis to its constitutive amino acids and via N-acetylation (2, 28, 30, 31) and that hepatic enzymes are involved in secondary degradations pathways of micafungin (1, 16, 17). While the relative contributions of these pathways to caspofungin and micafungin metabolism have not been elucidated, there appears to be some involvement of the liver in their elimination (27). This is supported by data indicating that moderate impairment of liver function increases the systemic exposure to caspofungin and requires a reduction in daily caspofungin dose from 50 to 35 mg. Furthermore, coadministration of rifampin, which is known to induce hepatic metabolic enzymes, results in a reduction in the systemic levels of caspofungin, thus requiring an increase in its daily dose from 50 to 70 mg (22, 29). It is also likely that rifampin may affect the clearance of caspofungin by interaction with transport proteins such as organic anion transporting protein (28, 30). Coadministration of other drugs that are general inducers of drug-metabolizing enzymes, such as efavirenz, nevirapine, phenytoin, dexamethasone, or carbamazepine, could result in clinically meaningful reductions in caspofungin levels in patients requiring dose adjustments (22). Micafungin, in addition to undergoing ring opening, is metabolized by arylsulfatase, with further metabolism by catechol-O-methyltransferase (13). It also undergoes hydroxylation at the side chain, catalyzed by hepatic cytochrome P450 isozymes (1). Micafungin is a weak inhibitor of CYP3A in vitro and it is hence recommended that patients receiving concomitant sirolimus, nifedipine, or itraconazole be monitored for toxicity and that the dosage of these medications be reduced if necessary (1). While all three of the currently approved echinocandins are thought to share a similar nonenzymatic ring opening as their primary clearance mechanism, anidulafungin appears to have less interaction with cytochrome P450 enzymes than observed for either caspofungin or micafungin.
In conclusion, under physiological conditions anidulafungin undergoes slow chemical degradation to a primary degradation product, which is likely further metabolized by plasma peptidases. Cytochrome P450 enzymes are not involved in the clearance of anidulafungin. The primary degradation product, and presumably subsequent metabolites, lacks antifungal activity. Anidulafungin, as well as its degradation products, is thought to be exclusively eliminated via biliary excretion. The elimination profile and lack of cytochrome P450 interaction of anidulafungin explain why no dosage adjustments are required for patients with hepatic or renal insufficiency and for patients receiving concomitant medications.
Acknowledgments
These studies were sponsored by Pfizer Inc. and Vicuron Pharmaceuticals (formerly Versicor Inc.), a subsidiary of Pfizer Inc.
Editorial support was provided by D. Wolf of PAREXEL and was funded by Pfizer Inc.
Footnotes
Published ahead of print on 24 November 2008.
REFERENCES
- 1.Astellas Pharma US, Inc. January 2008, last revision. Mycamine (micafungin sodium) for injection. U.S. prescribing information. http://www.astellas.us/docs/mycamine.pdf.
- 2.Balani, S. K., X. Xu, B. H. Arison, M. V. Silva, A. Gries, F. A. DeLuna, D. Cui, P. H. Kari, T. Ly, C. E. Hop, R. Singh, M. A. Wallace, D. C. Dean, J. H. Lin, P. G. Pearson, and T. A. Baillie. 2000. Metabolites of caspofungin acetate, a potent antifungal agent, in human plasma and urine. Drug Metab. Dispos. 28:1274-1278. [PubMed] [Google Scholar]
- 3.Damle, B., M. Stogniew, and J. Dowell. 2008. Pharmacokinetics and tissue distribution of anidulafungin in rats. Antimicrob. Agents Chemother. 52:2673-2676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Del Carmen Serrano, M., A. Valerde Conde, M. Chavez, S. Bernal, R. M. Claro, J. Pemán, M. Ramirez, and E. Martín-Mazuelos. 2003. In vitro activity of voriconazole, itraconazole, caspofungin, anidulafungin (VER002, LY303366) and amphotericin B against Aspergillus spp. Diagn. Microbiol. Infect. Dis. 45:131-135. [DOI] [PubMed] [Google Scholar]
- 5.Denning, D. W. 2003. Echinocandin antifungal drugs. Lancet 362:1142-1151. [DOI] [PubMed] [Google Scholar]
- 6.Dowell, J. A., M. Stogniew, D. Krause, and B. Damle. 2007. Anidulafungin does not require dosage adjustment in subjects with varying degrees of hepatic or renal impairment. J. Clin. Pharmacol. 47:461-470. [DOI] [PubMed] [Google Scholar]
- 7.Dowell, J. A., M. Stogniew, D. Krause, T. Henkel, and B. Damle. 2007. Lack of pharmacokinetic interaction between anidulafungin and tacrolimus. J. Clin. Pharmacol. 47:305-314. [DOI] [PubMed] [Google Scholar]
- 8.Dowell, J. A., J. Schranz, A. Baruch, and G. Foster. 2005. Safety and pharmacokinetics of coadministered voriconazole and anidulafungin. J. Clin. Pharmacol. 45:1373-1382. [DOI] [PubMed] [Google Scholar]
- 9.Dowell, J. A., M. Stogniew, D. Krause, T. Henkel, and I. E. Weston. 2005. Assessment of the safety and pharmacokinetics of anidulafungin when administered with cyclosporine. J. Clin. Pharmacol. 45:227-233. [DOI] [PubMed] [Google Scholar]
- 10.Dowell, J. A., W. Knebel, T. Ludden, M. Stogniew, D. Krause, and T. Henkel. 2004. Population pharmacokinetic analysis of anidulafungin, an echinocandin antifungal. J. Clin. Pharmacol. 44:590-598. [DOI] [PubMed] [Google Scholar]
- 11.European Medicines Agency. 2007. ECALTA: summary of product characteristics. EMEA, London, England. http://www.emea.euprimary.eu/humandocs/PDFs/EPAR/ecalta/H-788-PI-en1.pdf.
- 12.Guengerich, F. P. 1995. Human cytochrome P450 enzymes, p. 473-535. In P. R. Ortiz de Montellano (ed.), Cytochrome P450: structure, mechanism, and biochemistry, 2nd ed. Plenum Press, New York, NY.
- 13.Hebert, M. F., H. E. Smith, T. C. Marbury, S. K. Swan, W. B. Smith, R. W. Townsend, D. Buell, J. Keirns, and I. Bekersky. 2005. Pharmacokinetics of micafungin in healthy volunteers, volunteers with moderate liver disease, and volunteers with renal dysfunction. J. Clin. Pharmacol. 45:1145-1152. [DOI] [PubMed] [Google Scholar]
- 14.Inskeep, P., and J. Lin. 2008. Plasma protein binding of anidulafungin is similar to other major echinocandins, abstr. P1048. 18th Eur. Congr. Clin. Microbiol. Infect. Dis., 19 to 22 April 2008, Barcelona, Spain. European Society of Clinical Microbiology and Infectious Diseases, Basel, Switzerland.
- 15.Inskeep, P., R. Walsky, B. Feng, and S. Campbell. 2008. Lack of anidulafungin interactions with CYP enzymes and transporters in in vivo and in vitro systems, abstr. P1049. 18th Eur. Congr. Clin. Microbiol. Infect. Dis., 19 to 22 April 2008, Barcelona, Spain. European Society of Clinical Microbiology and Infectious Diseases, Basel, Switzerland.
- 16.Kaneko, H., Y. Yamato, T. Hashimoto, I. Ishii, T. Shiragami, A. Kawamura, M. Terakawa, and A. Kagayama. 2002. Drug interactions of micafungin in vitro. Jpn. J. Chemother. 50(Suppl. 1):94-103. [In Japanese.] [Google Scholar]
- 17.Kohno, S., and H. Yamaguchi. 2002. Overview of micafungin (MCFG). Jpn. J. Chemother. 50(Suppl. 1):1-7. [In Japanese.] [Google Scholar]
- 18.Kronbach, T., D. Mathys, M. Umeno, F. J. Gonzalez, and U. A. Meyer. 1989. Oxidation of midazolam and triazolam by human liver cytochrome P450IIIA4. Mol. Pharmacol. 36:89-96. [PubMed] [Google Scholar]
- 19.Kronbach, T., D. Mathys, G. Gut, T. Catin, and U. A. Meyer. 1987. High-performance liquid chromatographic assays for bufuralol l1-hydroxylase debrisquine 4-hydroxylase, and dextromethorphan O-demethylase in microsomes and purified cytochrome P-450 isozymes of human liver. Anal. Biochem. 162:24-32. [DOI] [PubMed] [Google Scholar]
- 20.Leemann, T., C. Transon, and P. Dayer. 1992. Cytochrome P450TB (CYP2C): a major monooxygenase catalyzing diclofenac 4′hydroxylation in human liver. Life Sci. 52:29-34. [DOI] [PubMed] [Google Scholar]
- 21.Marchetti, O., P. A. Majcherczyk, M. P. Glauser, J. Bille, P. Moreillon, and D. Sanglard. 2001. Sensitive bioassay for determination of fluconazole concentrations in plasma using a Candida albicans mutant hypersusceptible to azoles. Antimicrob. Agent Chemother. 45:696-700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Merck and Co., Inc. July 2008, last revised. Cancidas (caspofungin acetate) for injection. U.S. prescribing information. Merck, Whitehouse Station, NJ. http://www.merck.com/product/usa/pi_circulars/c/cancidas/cancidas_pi.pdf.
- 23.Omar, G., P. H. Whiting, G. M. Hawksworth, M. J. Humphrey, and M. D. Burke. 1997. Ketoconazole and fluconazole inhibition of the metabolism of cyclosporine A by human liver in vitro. Ther. Drug Monit. 19:436-445. [DOI] [PubMed] [Google Scholar]
- 24.Ostrosky-Zeichner, L., J. Rex, P. G. Pappas, R. J. Hamill, R. A. Larsen, H. W. Horowitz, W. G. Powderly, N. Hyslop, C. A. Kauffman, J. Cleary, J. E. Mangino, and J. Lee. 2003. Antifungal susceptibility survey of 2,000 bloodstream Candida isolates in the United States. Antimicrob. Agents Chemother. 47:3149-3154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pfizer Inc. May 2007, last revised. Eraxis (anidulafungin) for injection. U.S. prescribing information. Pfizer Inc., New York, NY. http://www.pfizer.com/pfizer/download/uspi_eraxis.pdf.
- 26.Raasch, R. H. 2004. Anidulafungin: review of a new echinocandin antifungal agent. Expert Rev. Anti. Infect. Ther. 2:499-508. [DOI] [PubMed] [Google Scholar]
- 27.Sandhu, P., W. Lee, X. Xu, B. F. Leake, M. Yamazaki, J. A. Stone, J. H. Lin, P. G. Pearson, and R. B. Kim. 2005. Hepatic uptake of the novel antifungal agent caspofungin. Drug Metab. Dispos. 33:676-682. [DOI] [PubMed] [Google Scholar]
- 28.Sandhu, P., X. Xu, P. J. Bondiskey, S. K. Balani, M. L. Morris, Y. S. Tang, A. R. Miller, and P. G. Pearson. 2004. Disposition of caspofungin, a novel antifungal agent, in mice, rats, rabbits, and monkeys. Antimicrob. Agents Chemother. 48:1272-1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stone, J. A., E. M. Migoya, L. Hickey, G. A. Winchell, P. J. Deutsch, K. Ghosh, A. Freeman, S. Bi, R. Desai, S. C. Dilzer, K. C. Lasseter, W. K. Kraft, H. Greenberg, and S. A. Waldman. 2004. Potential for interactions between caspofungin and nelfinavir or rifampin. Antimicrob. Agents Chemother. 48:4306-4314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stone, J. A., X. Xu, G. A. Winchell, P. J. Deutsch, P. G. Pearson, E. M. Migoya, G. C. Mistry, L. Xi, A. Miller, P. Sandhu, R. Singh, F. deLuna, S. C. Dilzer, and K. C. Lasseter. 2004. Disposition of caspofungin: role of distribution in determining pharmacokinetics in plasma. Antimicrob. Agents Chemother. 48:815-823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stone, E. A., H. B. Fung, and H. L. Kirschenbaum. 2002. Caspofungin: an echinocandin antifungal agent. Clin. Ther. 24:351-377. [DOI] [PubMed] [Google Scholar]
- 32.Tassaneeyakul, W., D. J. Birkett, M. E. Veronese, M. E. Mcmanus, R. H. Tukey, L. C. Quattrochi, H. V. Gelboin, and J. O. Minors. 1993. Specificity of substrate and inhibitor probes for human cytochromes P450 1A1 and 1A2. J. Pharmacol. Exp. Ther. 265:401-407. [PubMed] [Google Scholar]
- 33.Thye, D., B. Shepherd, R. J. White, H. E. Weston, and I. Henkel. 2001. Anidulafungin: a phase I study to identify the maximum tolerated dose in healthy volunteers, abstr. A36. Abstr. 41st Int. Conf. Antimicrob. Agents Chemother. American Society for Microbiology, Washington, DC.
- 34.Van der Hoeven, T. A., and M. J. Coon. 1974. Preparation and properties of partially purified cytochrome P 450 and reduced nicotinamide-adenine dinucleotide phosphate-cytochrome P 450 reductase from rabbit liver microsomes. J. Biol. Chem. 249:6302-6310. [PubMed] [Google Scholar]
- 35.Walsky, R. L., R. S. Obach, E. A. Gaman, J.-P. R. Gleeson, and W. R. Proctor. 2005. Selective inhibition of human cytochrome P4502C8 by montelukast. Drug Metab. Dispos. 33:413-418. [DOI] [PubMed] [Google Scholar]
- 36.Walsky, R. L., and R. S. Obach. 2004. Validated assays for human cytochrome P450 activities. Drug Metab. Dispos. 32:647-660. [DOI] [PubMed] [Google Scholar]
- 37.Wrighton, S. A., M. VandenBranden, and B. J. Ring. 1996. The human drug metabolizing cytochromes P450. J. Pharmacokinet. Biopharm. 24:461-473. [DOI] [PubMed] [Google Scholar]
- 38.Wrighton, S. A., B. J. Ring, and M. VandenBranden. 1995. The use of in vitro metabolism techniques in the planning and interpretation of drug safety studies. Toxicol. Pathol. 23:199-208. [DOI] [PubMed] [Google Scholar]
- 39.Wrighton, S. A., and J. C. Stevens. 1992. The human hepatic cytochrome P450 involved in drug metabolism. Crit. Rev. Toxicol. 22:1-21. [DOI] [PubMed] [Google Scholar]