Abstract
Amodiaquine retains efficacy against infection by chloroquine-resistant Plasmodium falciparum; however, little information is available on its efficacy against infection by chloroquine-resistant Plasmodium vivax. Patients presenting to a rural clinic with a pure P. vivax infection that recurred after recent antimalarial treatment were retreated, this time with amodiaquine monotherapy, and the risk of further recurrence within 4 weeks was assessed. Of the 87 patients with pure P. vivax infection, 15 patients did not complete a full course of treatment, 4 of whom were intolerant to treatment. In the 72 patients completing treatment, 91% (63 of 69) had cleared their parasitemia within 48 h with no early treatment failure. Follow-up to day 28 or recurrent parasitemia was achieved for 56 patients (78%). The cumulative incidence of treatment failure by day 28 was 22.8% (95% confidence interval, 7.3 to 38%). The in vitro sensitivity profile was determined for a separate set of isolates from outpatients with pure P. vivax infection. The median 50% inhibitory concentration of amodiaquine was 11.3 nM (range, 0.37 to 95.8) and was correlated significantly with that of chloroquine (Spearman rank correlation coefficient, 0.602; P < 0.001) Although amodiaquine results in a rapid clinical response, the risk of recurrence by day 28 is unacceptably high, reducing its suitability as an alternative treatment of infection by chloroquine-resistant P. vivax in this region.
Whereas chloroquine-resistant strains of Plasmodium falciparum were documented in Cambodia and Brazil over 50 years ago (8, 44), chloroquine resistant (CQR) Plasmodium vivax has taken longer to emerge, with the first cases being described for patients in Papua New Guinea and Papua, Indonesia, in 1989 (3, 34). Subsequent clinical studies have reported the presence of CQR P. vivax across Asia (6, 21, 24, 26, 40) as well as South America (12, 38). Despite this apparent threat to chloroquine-based treatment practices, few studies have addressed suitable alternative treatment regimens (2).
Amodiaquine is a 4-aminoquinoline which has been used extensively to treat and prevent P. falciparum malaria since the 1940s. In 1990, amodiaquine prophylaxis was found to be associated with fatal hepatitis and agranulocytosis, which resulted in the WHO recommendation that malaria control programs discontinue use of the drug (27, 43). However, a subsequent systematic review found the drug to be safe and well tolerated as a treatment (25), and although it is still contraindicated as a prophylactic agent, it is now widely used for the treatment of infection by CQR P. falciparum, more recently in combination with artesunate (1).
Studies of amodiaquine monotherapy for infection by P. vivax are limited mostly to case reports (36), early prophylaxis studies (41), and animal studies (11); there are also reports in which the drug was used in combination with other antimalarials (15, 23). In southern Papua, Indonesia, where high-grade drug resistance has emerged to infection by both P. falciparum and P. vivax (33, 37), we have conducted a series of drug trials to optimize treatment strategies (15, 32). In 2004 and 2005, patients who failed treatment for recurrent P. vivax infections were treated again, this time with amodiaquine monotherapy, and their clinical responses were documented. In the present study, we have pooled the clinical responses following amodiaquine monotherapy and have correlated them with the in vitro responses of P. vivax to amodiaquine, including cross-sensitivity to other drugs.
MATERIALS AND METHODS
Study site and design.
This prospective open-label drug efficacy study of amodiaquine monotherapy for P. vivax monoinfection was carried out at two rural clinics west of Timika in southern Papua, Indonesia, between March 2004 and August 2005. This lowland region is partly forested, and Anopheles koliensis, Anopheles farauti, and Anopheles punctulatus are responsible for unstable malaria transmission (22, 28). The annual incidence of malaria in the region is 876 per 1,000 people per year, divided 60:40 between P. falciparum and P. vivax infections (19). Local protocols recommend that all patients with patent parasitemia at any level be given antimalarial therapy.
The present study is based on an in vivo antimalarial drug sensitivity protocol described previously (7). The results for 11 patients were presented previously as part of a pilot study (33).
Patients.
Patients who were previously enrolled in therapeutic trials taking place at either clinic (32, 33) and who had any peripheral parasitemia with pure P. vivax infection during follow-up were eligible for enrollment, irrespective of parasite density or the presence of fever. Pregnant or lactating women and children weighing less than 10 kg were excluded, as were patients with WHO danger signs or signs of severity (42), a parasitemia level of >4%, or concomitant disease requiring hospital admission.
Clinical laboratory examinations.
A standardized data sheet, with a record of demographic information, details of symptoms and their duration, and a history of previous antimalarial medication, was completed for all patients enrolled in the study. Clinical examination findings were documented, including the axillary temperature. Venous blood was taken for blood film examination, hematocrit determination, and white blood cell (WBC) count. Parasite counts were determined as the number of parasites per 200 WBCs by using Giemsa-stained thick films, and peripheral parasitemia was assumed to be present with WBC counts of 7,300 μl−1. A thick smear was considered negative upon initial review if no parasites were seen in 100 high-power fields. A thin smear was also examined to confirm parasite species and was used for quantification if parasitemia was greater than 200 per 200 WBCs. For cross-checking, 200 high-power fields were examined before slides were considered negative. All slides were cross-checked by a second experienced microscopist, and if readings were discordant, the slides were read by a third microscopist and a consensus was reached.
Patients were examined daily thereafter until they became aparasitemic. At each visit, a blood smear was taken and a symptom questionnaire was completed. Patients were then seen weekly for 4 weeks. At each of these clinic appointments, a full physical examination was performed, the symptom questionnaire was completed, and blood was taken to check the parasite count and hemoglobin level by using a battery-operated portable photometer (HemoCue Hb201+; Angelholm, Sweden). Blood spots on chromatography paper (Whatman BFC 180; Maidstone, United Kingdom) were also collected on day 0 and the day of treatment failure.
Treatment.
Amodiaquine was given at a total dose of 30 mg of base/kg of body weight, divided into three daily doses, and participants were observed for 60 min to exclude those with adverse reactions and to ensure that the medication was not vomited. If vomiting occurred within 60 min, the whole dose was repeated once. If vomiting occurred again within 60 min, the patient was withdrawn from the study. For children, the tablet was crushed and placed in 5 ml of water, and the correct dose was administered as a suspension.
Primaquine (15 mg of base/kg of body weight for 14 days) was administered on day 28 of participation in the study if there was no evidence of glucose-6-phosphate dehydrogenase deficiency. Patients with P. falciparum parasitemia during follow-up were treated with unsupervised quinine (10 mg of salt/kg of body weight for each dose, which was taken orally three times a day for 7 days) plus doxycycline (100 mg twice daily) if they were ≥8 years of age and not pregnant. When there were treatment failures for patients with P. vivax parasitemia, the patients were retreated using unsupervised quinine (10 mg of salt/kg of body weight for each dose, which was taken orally three times a day for 7 days).
In vitro drug susceptibility assay.
Previous studies have demonstrated that P. vivax in vitro drug susceptibility cannot be ascertained reliably from patients with a parasitemia level of less than 5,000 μl−1. Since most of the patients enrolled in the in vivo study had low parasitemia levels or were young children who often refused venipuncture, blood for in vitro drug susceptibility assays was collected from hospital clinic outpatients who had parasitemia levels greater than 5,000 μl−1. Parasitemia levels of isolates tested ranged between ∼0.005% and 0.01%, a level at which the inoculum size does not significantly affect the results of the assay (14). Susceptibilities to chloroquine and amodiaquine were measured using an identical protocol modified from the WHO microtest as described previously (35). The in vitro activities of artesunate, mefloquine, and piperaquine were tested for all isolates and that of lumefantrine was tested for a subset of 27 isolates. Incubation was stopped when parasites had matured to at least 40% schizonts in the drug-free control well. A thick blood smear from each well was Giemsa stained and examined microscopically. The number of schizonts per 200 asexual stage parasites was determined, and the result for each drug concentration was normalized to the control well. The dose response data were analyzed using nonlinear regression analysis (WinNonlin 4.1; Pharsight Corporation) to obtain the IC50s.
The susceptibilities of 53 samples were published in a previous study of the confounding factors of assay conditions on the in vitro response (35). That study documented a significant stage specificity of drug activity, with isolates predominantly at the trophozoite stage being less susceptible to drug activity; for this reason, the analysis of in vitro drug susceptibilities in the present study was restricted to those isolates that had predominantly ring stages initially (ring-to-trophozoite ratio greater than 1).
Molecular analysis.
In patients with recurrent P. vivax infections, either alone or mixed, genomic DNA from blood spots was extracted using a QIAamp DNA mini kit (Qiagen, Doncaster, Australia). Genotypes in paired isolates were determined by restriction fragment analyses of the pvmsp3α locus and size polymorphisms in pvmsp1 fragments F1 and F3, as described previously (9, 17).
Statistical analysis.
Data were double entered and validated using EpiData 3.02 software (EpiData Association, Odense, Denmark), and analyses were performed using SPSS for Windows version 14 (SPSS, Inc., Chicago, IL). The Mann-Whitney U test was used for nonparametric comparisons, and Student's t test or one-way analysis of variance was used for parametric comparisons. Proportions were examined using χ2 with Yates' correction or by Fisher's exact test.
Efficacy endpoints were assessed by survival analysis, in which the cumulative risk of failure was calculated by the Kaplan Meier product limit formula. Data for patients who were lost to follow-up or who presented with a peripheral parasitemia without P. vivax were censored from analysis. Those patients with recurrent vomiting or adverse drug effects which required early termination of treatment and the administration of rescue therapy were excluded from the primary analysis but included in a secondary conservative analysis in which they were regarded as therapeutic failures.
Ethics.
The study was approved by the Ethics Committee of the National Institute of Health Research and Development, Indonesian Ministry of Health (Jakarta, Indonesia), and the Ethics Committee of Menzies School of Health Research (Darwin, Australia). Written informed consent was obtained from adult patients and parents of enrolled children. The trial was registered at the NIH clinical trials website (http://www.clinicaltrials.gov/ct) under identifier code NCT00157859.
RESULTS
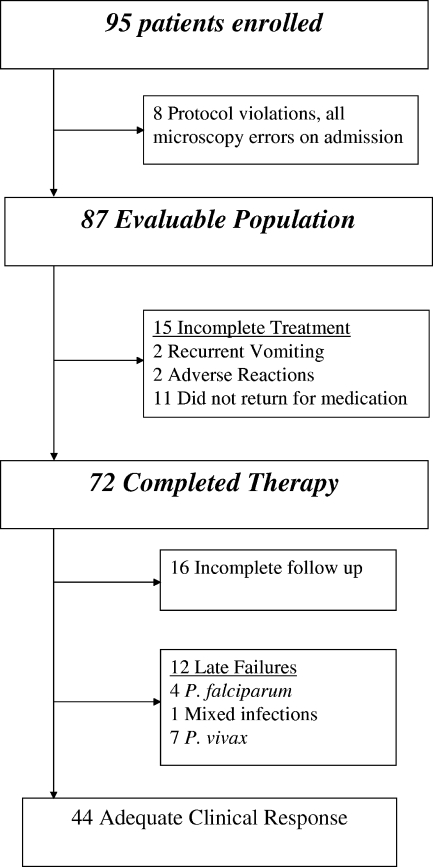
Between April 2004 and July 2005, 95 patients with P. vivax infection were enrolled in the in vivo study. The day after enrollment, the blood films were rechecked, and slides from eight patients were found not to contain pure P. vivax; these patients were excluded from further analysis (Fig. 1). The baseline characteristics of the remaining 87 patients in the evaluable population are presented in Table 1.
FIG. 1.
Trial profile of patients treated with amodiaquine.
TABLE 1.
Baseline characteristics of patients in the evaluable population
| Patient parameter | Result (no. of patients with indicated characteristic/total no. of patients) |
|---|---|
| No. in evaluable population | 87 |
| Parasitemia levela [95% CI] | 672 [455-993] |
| Gametocyte carriage (%) | 25 (20/81) |
| Male (%) | 55 (48/87) |
| Age (yr) | |
| Median [range] | 13 [2-53] |
| <5 (%) | 21 (18/87) |
| 5-14 (%) | 37 (32/87) |
| >14 (%) | 43 (37/87) |
| Temp >37.5°C (%) | 8.5 (7/82) |
| Symptomatic (%) | 51 (42/83) |
| Hemoglobinb [SD] | 10.5 [1.7] |
| Anemiac (%) | 41 (30/74) |
| Splenomegaly (%) | 64 (52/81) |
Geometric mean; μl−1.
Mean; g/dl.
Hemoglobin <10 g/dl.
The active case detection was reflected in the low parasite density upon admission (geometric mean, 672 μl−1; 95% confidence interval [CI], 455 to 993) with only 51% (42 of 82) being symptomatic (fever or history of fever in the preceding 24 h).
Overall 63% of patients (54 of 87) had been treated previously for P. vivax infection, either alone (39 patients) or mixed with P. falciparum (15 patients) with the remaining patients previously infected with pure P. falciparum (25 patients), Plasmodium malariae (6 patients), or Plasmodium ovale (2 patients). The prior treatment was administered for a median of 29 days (range, 4 to 47 days), with 54 patients receiving treatment with artemether-lumefantrine, 19 with chloroquine, 10 with DHA-piperaquine, and 4 with quinine.
Tolerability.
In total, 4.6% of patients (4 of 87) vomited their medication within 30 min of administration. Although this proportion was higher for children under 5 years old (11%; 2 of 18) than for older children and adults (2.9%; 2 of 69), it did not reach statistical significance (P = 0.19). Two patients (a child and an adult) were unable to tolerate their medication because of recurrent vomiting. A further two patients were withdrawn by the attending physician due to possible drug reactions: a 2-year-old child experienced urticaria after the first dose, and a 4-year-old child developed acute asthma on day 2. These four patients were withdrawn from the study and treated with oral quinine; they made a full recovery. A further 11 patients failed to return to the clinic to complete their course of medication.
Of the 72 patients who received a full treatment course, the mean dose of amodiaquine given was 31.0 mg/kg (95% CI, 29.7 to 32.2; range, 17.7 to 65.6). Follow-up to day 28 or to the day of treatment failure in these patients was achieved for 78% of patients (56 of 72).
Therapeutic response.
Overall, 52% of patients (36 of 69) cleared their peripheral parasitemia within 24 h, with a median parasite reduction ratio of 8.7 (range, 0.3 to 330). By 48 h, 91% (63 of 69) were aparasitemic, and 73% of those patients initially presenting with symptoms (19 of 26) were asymptomatic. There were no early parasitological failures. In total, 25% of patients (20 of 81) had P. vivax gametocytes present on the admission blood film. By day 7, none of the patients with P. vivax infections had gametocytes present upon blood film examination, and no gametocytes were recorded on subsequent blood films during follow-up. In total, 41% of patients (30 of 74) were anemic on admission (hemoglobin <10 g/dl). By day 7, this proportion had fallen to 30% (6 of 20 patients), and by day 28, none of the 9 patients tested were anemic.
Twelve patients had a recurrence of malaria during follow-up, seven with P. vivax, four with P. falciparum, and one with a mixed infection of both species. At the time of recurrence, 42% of these patients (5 of 12) were symptomatic, reporting malaise, fever, or a history of fever. Of those patients completing treatment, the cumulative risk of recurrence of P. vivax by day 28 (either alone or mixed) was 22.8% (95% CI, 7.3 to 38), with all recurrences occurring on or after day 27. In the evaluable population, patients who had incomplete treatment courses or who required early rescue treatment were assumed to be treatment failures; thus, the cumulative risk of treatment failure rose to 39% (95% CI, 24 to 53).
Molecular analysis of recurrent infections.
DNA was available from both pre- and posttreatment isolates from five of the eight patients with P. vivax infection who failed treatment. PCR amplification of these paired isolates was successful for four of these pairs, with recurrence of the same genotype present in three cases.
In vitro susceptibility.
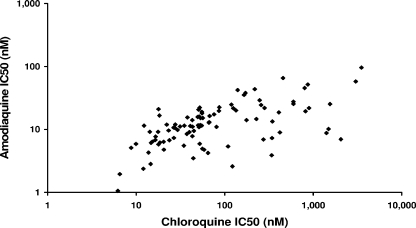
Between March 2004 and August 2008, 137 isolates with the initial majority at the ring stage (ring-to-trophozoite ratio greater than 1) were subjected to in vitro susceptibility testing, from which reliable data could be derived for 103 (75%). None of these isolates came from patients for whom the in vivo response was also documented. The median 50% inhibitory concentration (IC50) for amodiaquine was 11.3 nM (range, 0.37 to 95.8), compared to 52.7 nM (range, 4.6 to 3,506) for chloroquine, 1.29 nM (range, 0.043 to 13.6) for artesunate, 16.0 nM (range, 4.8 to 228.6) for lumefantrine, 8.2 nM (range, 0.81 to 137.9) for mefloquine, and 17.5 nM (range, 1.8 to 119.9) for piperaquine. The IC50s of amodiaquine were correlated with those for chloroquine (Spearman rank correlation coefficient [rs] = 0.602, P < 0.001) (Fig. 2), artesunate (rs = 0.577, P < 0.001), mefloquine (rs = 0.636, P < 0.001), and piperaquine (rs = 0.658, P < 0.001) but not with that of lumefantrine (n = 27). In total, 34% of the isolates (33 of 98) had chloroquine IC50s greater than 100 nM, whereas the maximum reported IC50 of amodiaquine was 95.8 nM.
FIG. 2.
In vitro correlation of isolates tested for both amodiaquine and chloroquine (n = 98, rs = 602; P < 0.001).
DISCUSSION
In Asia and South America, P. vivax is a major and underappreciated cause of morbidity (29). Chloroquine treatment remains the mainstay for vivax malaria in countries where it is endemic, but it is being increasingly undermined by the emergence and spread of CQR. The evidence on which to base alternative treatment strategies is limited (2). Amodiaquine, a 4-aminoquinoline, has been shown to retain activity against CQR strains of P. falciparum, although with various levels of efficacy (25), and it has been incorporated into national control programs, usually in combination with artesunate (1). The efficacy of amodiaquine against CQR strains of P. vivax has been less studied. Two clinical trials of amodiaquine in combination with artesunate (in Papua, Indonesia) or sulfadoxine-pyrimethamine (in Papua New Guinea) revealed recurrence rates of 12 to 26% by day 28 (15, 23). Both studies were conducted in areas with highly CQR P. vivax. CQR P. vivax was first documented in Papua, Indonesia, in 1991 (3), but over the ensuing decade, recurrence rates in the north of the province have risen to almost 95% by day 28 (39). We have reported high-grade resistance in patients in Timika, southern Papua, Indonesia, with 29% of patients failing treatment within 1 week of chloroquine monotherapy and 16% showing signs of early treatment failure (33).
In the present study, we have pooled our experience of patients treated with amodiaquine monotherapy to assess its in vitro and in vivo efficacy against infection by CQR P. vivax. Amodiaquine was reasonably well tolerated, although almost one-fifth of patients failed to complete treatment, either because of nausea and vomiting (4.5%) or noncompliance (13%). In contrast to the high risk of early treatment failure following chloroquine monotherapy, there were no high-grade failures following amodiaquine monotherapy, and by 48 h, the peripheral parasitemia in 91% of patients had cleared. Despite this apparent early treatment success, it is noteworthy that at the start of treatment many of the enrolled patients had relatively low parasitemia levels, which had been detected actively, and half of whom were asymptomatic. In those patients completing treatment, the cumulative incidence of recurrence of P. vivax infection at day 28 was 23%. Clinical trials with P. vivax are confounded by both the occurrence of reinfections and relapses from liver stages (hypnozoites) (5). Whereas parasite genotyping has proved useful for the interpretation of clinical trials in P. falciparum, its utility in discriminating relapse, reinfection, and recrudescence in P. vivax is limited (10, 18).
In our study, four recurrent isolates could be genotyped at three loci; three of the isolates were the same as the isolate present upon admission of the patient. Assuming that these three isolates and the four indeterminate isolates were recrudescent infection, the cumulative risk of failure risk could be adjusted to 21%. An alternative and equally plausible explanation is that these recurrent infections represent relapses from hypnozoites from the same genotype (10, 18). In Papua, P. vivax relapses occur 3 to 6 weeks apart in up to 70% of patients (4). The long terminal elimination of chloroquine ensures that recurrent infections occurring within 28 days of supervised administration will grow in the presence of chloroquine concentrations normally inhibitory to sensitive parasites and thus can be considered resistant (2). Desethylamodiaquine, the active metabolite of amodiaquine, has a terminal elimination half-life of approximately 9 to 18 days (16, 31), significantly shorter than the 20 to 60 days reported for chloroquine (13). Its ability to suppress the first relapse is therefore likely to be correspondingly diminished compared to that of chloroquine. We observed no recurrences of infection by P. vivax before day 27. Interestingly, the cumulative risk of recurrence by day 28 was similar to that observed in a subsequent study of pure P. vivax infection treated with amodiaquine plus 3 days with artesunate (23% and 26%, respectively) (15), suggesting that the recurrent P. vivax infections represent predictable relapses rather than recrudescence of resistant isolates. Venous sampling of drug concentrations at the time of recurrence would have helped to define resistant isolates but, unfortunately, was not available for our study.
Currently, the only available strategy for preventing the relapse of infection by P. vivax is a prolonged course of primaquine (4), although shorter courses with high doses hold promise (20). Local guidelines advocate the administration of 14 days of primaquine, although in the present study, this was delayed until the end of the study. Earlier administration might have decreased the observed recurrence rate; however, in practice, long courses of unsupervised therapy are rarely adhered to and thus are likely to be of limited benefit. Treatment regimens which lead to the retention of a prolonged antimalarial suppression, such as piperaquine, mefloquine, and, in sensitive areas, chloroquine, provide the only practical means currently available for delaying the timing of clinical illness from relapses in areas of endemicity (32). Importantly, there were no early failures following amodiaquine treatment, as were frequently observed with chloroquine monotherapy (33). Within 24 h, the median parasite reduction ratio was 8, similar to that observed following mefloquine or halofantrine treatment of infection by P. vivax in Thailand, but significantly less than that of chloroquine-sensitive isolates, which had a median of 36 (30). The in vitro susceptibility profiles of the Papuan isolates highlight the low IC50s for amodiaquine but showed appreciable cross-resistance with chloroquine, mefloquine, artesunate, and piperaquine. Hence, although amodiaquine retains substantial efficacy against infection by CQR strains of P. vivax, it is vulnerable to cross-resistance in this region. Despite the strong correlation between amodiaquine and chloroquine susceptibilities, none of the isolates tested had an IC50 greater than 100 nM, even though one-third of the same isolates had chloroquine IC50s above this level.
In summary, amodiaquine monotherapy retained significant clinical and in vitro efficacy against P. vivax, although recurrence rates within 28 days were unacceptably high. Since combination therapy with artesunate does not improve the clinical outcome at day 28, and practical strategies for reducing the relapses are not immediately forthcoming, the utility of amodiaquine as an alternative to chloroquine in areas of emerging antimalarial drug resistance remains tenuous.
Acknowledgments
We are grateful to the staff of PT Freeport Indonesia Public Health & Malaria Control Department, International SOS, and Lembaga Pengembangan Masyarakat Amungme Kamoro for support and technical assistance. We thank Mauritz Okeseray, Rosmini, Buhari, and Budi Prasetyorini for their support and technical assistance. We are also grateful to Morrison Bethea and PT Freeport Indonesia for their ongoing support. Pascal Ringwald (WHO) kindly provided the amodiaquine tablets, and the Australian Red Cross Blood Service supplied human sera.
The study was funded by the Wellcome Trust-NHRMC (Wellcome Trust ICRG GR071614MA-NHMRC ICRG ID 283321). NA is supported by an NHMRC Practitioner Fellowship. R.N.P. is funded by a Wellcome Trust Career Development Award (074637).
We declare no conflicts of interest.
Footnotes
Published ahead of print on 22 December 2008.
REFERENCES
- 1.Adjuik, M., P. Agnamey, A. Babiker, S. Borrmann, P. Brasseur, M. Cisse, F. Cobelens, S. Diallo, J. F. Faucher, P. Garner, S. Gikunda, P. G. Kremsner, S. Krishna, B. Lell, M. Loolpapit, P. B. Matsiegui, M. A. Missinou, J. Mwanza, F. Ntoumi, P. Olliaro, P. Osimbo, P. Rezbach, E. Some, and W. R. Taylor. 2002. Amodiaquine-artesunate versus amodiaquine for uncomplicated Plasmodium falciparum malaria in African children: a randomised, multicentre trial. Lancet 359:1365-1372. [DOI] [PubMed] [Google Scholar]
- 2.Baird, J. K. 2004. Chloroquine resistance in Plasmodium vivax. Antimicrob. Agents Chemother. 48:4075-4083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baird, J. K., H. Basri, Purnomo, M. J. Bangs, B. Subianto, L. C. Patchen, and S. L. Hoffman. 1991. Resistance to chloroquine by Plasmodium vivax in Irian Jaya, Indonesia. Am. J. Trop. Med. Hyg. 44:547-552. [DOI] [PubMed] [Google Scholar]
- 4.Baird, J. K., and S. L. Hoffman. 2004. Primaquine therapy for malaria. Clin. Infect. Dis. 39:1336-1345. [DOI] [PubMed] [Google Scholar]
- 5.Baird, J. K., B. Leksana, S. Masbar, D. J. Fryauff, M. A. Sutanihardja, Suradi, F. S. Wignall, and S. L. Hoffman. 1997. Diagnosis of resistance to chloroquine by Plasmodium vivax: timing of recurrence and whole blood chloroquine levels. Am. J. Trop. Med. Hyg. 56:621-626. [DOI] [PubMed] [Google Scholar]
- 6.Baird, J. K., M. F. Sustriayu Nalim, H. Basri, S. Masbar, B. Leksana, E. Tjitra, R. M. Dewi, M. Khairani, and F. S. Wignall. 1996. Survey of resistance to chloroquine by Plasmodium vivax in Indonesia. Trans. R. Soc. Trop. Med. Hyg. 90:409-411. [DOI] [PubMed] [Google Scholar]
- 7.Baird, J. K., I. Wiady, D. J. Fryauff, M. A. Sutanihardja, B. Leksana, H. Widjaya, Kysdarmanto, and B. Subianto. 1997. In vivo resistance to chloroquine by Plasmodium vivax and Plasmodium falciparum at Nabire, Irian Jaya, Indonesia. Am. J. Trop. Med. Hyg. 56:627-631. [DOI] [PubMed] [Google Scholar]
- 8.Box, E. D., Q. T. Box, and M. D. Young. 1963. Chloroquine-resistant Plasmodium falciparum from Porto Velho, Brazil. Am. J. Trop. Med. Hyg. 12:300-304. [DOI] [PubMed] [Google Scholar]
- 9.Bruce, M. C., M. R. Galinski, J. W. Barnwell, G. Snounou, and K. P. Day. 1999. Polymorphism at the merozoite surface protein-3α locus of Plasmodium vivax: global and local diversity. Am. J. Trop. Med. Hyg. 61:518-525. [DOI] [PubMed] [Google Scholar]
- 10.Chen, N., A. Auliff, K. Rieckmann, M. Gatton, and Q. Cheng. 2007. Relapses of Plasmodium vivax infection result from clonal hypnozoites activated at predetermined intervals. J. Infect. Dis. 195:934-941. [DOI] [PubMed] [Google Scholar]
- 11.Collins, W. E., J. S. Sullivan, D. J. Fryauff, J. Kendall, V. Jennings, G. G. Galland, and C. L. Morris. 2000. Adaptation of a chloroquine-resistant strain of Plasmodium vivax from Indonesia to New World monkeys. Am. J. Trop. Med. Hyg. 62:491-495. [DOI] [PubMed] [Google Scholar]
- 12.de Santana Filho, F. S., A. R. Arcanjo, Y. M. Chehuan, M. R. Costa, F. E. Martinez-Espinosa, J. L. Vieira, M. G. Barbosa, W. D. Alecrim, and M. G. Alecrim. 2007. Chloroquine-resistant Plasmodium vivax, Brazilian Amazon. Emerg. Infect. Dis. 13:1125-1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ducharme, J., and R. Farinotti. 1996. Clinical pharmacokinetics and metabolism of chloroquine. Focus on recent advancements. Clin. Pharmacokinet. 31:257-274. [DOI] [PubMed] [Google Scholar]
- 14.Gluzman, I. Y., P. H. Schlesinger, and D. J. Krogstad. 1987. Inoculum effect with chloroquine and Plasmodium falciparum. Antimicrob. Agents Chemother. 31:32-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hasugian, A. R., H. L. Purba, E. Kenangalem, R. M. Wuwung, E. P. Ebsworth, R. Maristela, P. M. Penttinen, F. Laihad, N. M. Anstey, E. Tjitra, and R. N. Price. 2007. Dihydroartemisinin-piperaquine versus artesunate-amodiaquine: superior efficacy and posttreatment prophylaxis against multidrug-resistant Plasmodium falciparum and Plasmodium vivax malaria. Clin. Infect. Dis. 44:1067-1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hietala, S. F., A. Bhattarai, M. Msellem, D. Roshammar, A. S. Ali, J. Stromberg, F. W. Hombhanje, A. Kaneko, A. Bjorkman, and M. Ashton. 2007. Population pharmacokinetics of amodiaquine and desethylamodiaquine in pediatric patients with uncomplicated falciparum malaria. J. Pharmacokinet. Pharmacodyn. 34:669-686. [DOI] [PubMed] [Google Scholar]
- 17.Imwong, M., S. Pukrittayakamee, A. C. Gruner, L. Renia, F. Letourneur, S. Looareesuwan, N. J. White, and G. Snounou. 2005. Practical PCR genotyping protocols for Plasmodium vivax using Pvcs and Pvmsp1. Malar. J. 4:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Imwong, M., G. Snounou, S. Pukrittayakamee, N. Tanomsing, J. R. Kim, A. Nandy, J. P. Guthmann, F. Nosten, J. Carlton, S. Looareesuwan, S. Nair, D. Sudimack, N. P. Day, T. J. Anderson, and N. J. White. 2007. Relapses of Plasmodium vivax infection usually result from activation of heterologous hypnozoites. J. Infect. Dis. 195:927-933. [DOI] [PubMed] [Google Scholar]
- 19.Karyana, M., L. Burdarm, S. Yeung, E. Kenangalem, N. Wariker, R. Maristela, K. G. Umana, R. Vemuri, M. J. Okoseray, P. M. Penttinen, P. Ebsworth, P. Sugiarto, N. M. Anstey, E. Tjitra, and R. N. Price. 2008. Malaria morbidity in Papua Indonesia, an area with multidrug resistant Plasmodium vivax and Plasmodium falciparum. Malar. J. 7:148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Krudsood, S., N. Tangpukdee, P. Wilairatana, N. Phophak, J. K. Baird, G. M. Brittenham, and S. Looareesuwan. 2008. High-dose primaquine regimens against relapse of Plasmodium vivax malaria. Am. J. Trop. Med. Hyg. 78:736-740. [PMC free article] [PubMed] [Google Scholar]
- 21.Kurcer, M. A., Z. Simsek, F. Y. Zeyrek, S. Atay, H. Celik, I. Kat, and S. Topluoglu. 2004. Efficacy of chloroquine in the treatment of Plasmodium vivax malaria in Turkey. Ann. Trop. Med. Parasitol. 98:447-451. [DOI] [PubMed] [Google Scholar]
- 22.Lee, V. H., S. Atmosoedjono, S. Aep, and C. D. Swaine. 1980. Vector studies and epidemiology of malaria in Irian Jaya, Indonesia. Southeast Asian J. Trop. Med. Public Health 11:341-347. [PubMed] [Google Scholar]
- 23.Marfurt, J., I. Mueller, A. Sie, P. Maku, M. Goroti, J. C. Reeder, H. P. Beck, and B. Genton. 2007. Low efficacy of amodiaquine or chloroquine plus sulfadoxine-pyrimethamine against Plasmodium falciparum and P. vivax malaria in Papua New Guinea. Am. J. Trop. Med. Hyg. 77:947-954. [PubMed] [Google Scholar]
- 24.Marlar-Than, Myat-Phone-Kyaw, Aye-Yu-Soe, Khaing-Khaing-Gyi, Ma-Sabai, and Myint-Oo. 1995. Development of resistance to chloroquine by Plasmodium vivax in Myanmar. Trans. R. Soc. Trop. Med. Hyg. 89:307-308. [DOI] [PubMed] [Google Scholar]
- 25.Olliaro, P., C. Nevill, J. LeBras, P. Ringwald, P. Mussano, P. Garner, and P. Brasseur. 1996. Systematic review of amodiaquine treatment in uncomplicated malaria. Lancet 348:1196-1201. [DOI] [PubMed] [Google Scholar]
- 26.Phan, G. T., P. J. de Vries, B. Q. Tran, H. Q. Le, N. V. Nguyen, T. V. Nguyen, S. H. Heisterkamp, and P. A. Kager. 2002. Artemisinin or chloroquine for blood stage Plasmodium vivax malaria in Vietnam. Trop. Med. Int. Health 7:858-864. [DOI] [PubMed] [Google Scholar]
- 27.Phillips-Howard, P. A., and A. B. Bjorkman. 1990. Ascertainment of risk of serious adverse reactions associated with chemoprophylactic antimalarial drugs. Bull. W. H. O. 68:493-504. [PMC free article] [PubMed] [Google Scholar]
- 28.Pribadi, W., I. Sutanto, S. Atmosoedjono, R. Rasidi, L. K. Surya, and L. Susanto. 1998. Malaria situation in several villages around Timika, south central Irian Jaya, Indonesia. Southeast Asian J. Trop. Med. Public Health 29:228-235. [PubMed] [Google Scholar]
- 29.Price, R. N., E. Tjitra, C. A. Guerra, S. Yeung, N. J. White, and N. M. Anstey. 2007. Vivax malaria: neglected and not benign. Am. J. Trop. Med. Hyg. 77:79-87. [PMC free article] [PubMed] [Google Scholar]
- 30.Pukrittayakamee, S., A. Chantra, J. A. Simpson, S. Vanijanonta, R. Clemens, S. Looareesuwan, and N. J. White. 2000. Therapeutic responses to different antimalarial drugs in vivax malaria. Antimicrob. Agents Chemother. 44:1680-1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pussard, E., F. Verdier, F. Faurisson, J. M. Scherrmann, J. Le Bras, and M. C. Blayo. 1987. Disposition of monodesethylamodiaquine after a single oral dose of amodiaquine and three regimens for prophylaxis against Plasmodium falciparum malaria. Eur. J. Clin. Pharmacol. 33:409-414. [DOI] [PubMed] [Google Scholar]
- 32.Ratcliff, A., H. Siswantoro, E. Kenangalem, R. Maristela, R. M. Wuwung, F. Laihad, E. P. Ebsworth, N. M. Anstey, E. Tjitra, and R. N. Price. 2007. Two fixed-dose artemisinin combinations for drug-resistant falciparum and vivax malaria in Papua, Indonesia: an open-label randomised comparison. Lancet 369:757-765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ratcliff, A., H. Siswantoro, E. Kenangalem, M. Wuwung, A. Brockman, M. D. Edstein, F. Laihad, E. P. Ebsworth, N. M. Anstey, E. Tjitra, and R. N. Price. 2007. Therapeutic response of multidrug-resistant Plasmodium falciparum and P. vivax to chloroquine and sulfadoxine-pyrimethamine in southern Papua, Indonesia. Trans. R. Soc. Trop. Med. Hyg. 101:351-359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rieckmann, K. H., D. R. Davis, and D. C. Hutton. 1989. Plasmodium vivax resistance to chloroquine? Lancet ii:1183-1184. [DOI] [PubMed] [Google Scholar]
- 35.Russell, B., F. Chalfein, B. Prasetyorini, E. Kenangalem, K. Piera, R. Suwanarusk, A. Brockman, P. Prayoga, P. Sugiarto, Q. Cheng, E. Tjitra, N. M. Anstey, and R. N. Price. 2008. Determinants of in vitro drug susceptibility testing of Plasmodium vivax. Antimicrob. Agents Chemother. 52:1040-1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schuurkamp, G. J., P. E. Spicer, R. K. Kereu, and P. K. Bulungol. 1989. A mixed infection of vivax and falciparum malaria apparently resistant to 4-aminoquinoline: a case report. Trans. R. Soc. Trop. Med. Hyg. 83:607-608. [DOI] [PubMed] [Google Scholar]
- 37.Siswantoro, H., A. Ratcliff, E. Kenangalem, M. Wuwung, R. Maristela, R. Rumaseuw, F. Laihad, E. P. Ebsworth, N. M. Anstey, R. N. Price, and E. Tjitra. 2006. Efficacy of existing antimalarial drugs for uncomplicated malaria in Timika, Papua, Indonesia. Indonesian Med. J. 15:221-258. [Google Scholar]
- 38.Soto, J., J. Toledo, P. Gutierrez, M. Luzz, N. Llinas, N. Cedeno, M. Dunne, and J. Berman. 2001. Plasmodium vivax clinically resistant to chloroquine in Colombia. Am. J. Trop. Med. Hyg. 65:90-93. [DOI] [PubMed] [Google Scholar]
- 39.Sumawinata, I. W., Bernadeta, B. Leksana, A. Sutamihardja, Purnomo, B. Subianto, Sekartuti, D. J. Fryauff, and J. K. Baird. 2003. Very high risk of therapeutic failure with chloroquine for uncomplicated Plasmodium falciparum and P. vivax malaria in Indonesian Papua. Am. J. Trop. Med. Hyg. 68:416-420. [PubMed] [Google Scholar]
- 40.Tjitra, E., J. Baker, S. Suprianto, Q. Cheng, and N. M. Anstey. 2002. Therapeutic efficacies of artesunate-sulfadoxine-pyrimethamine and chloroquine-sulfadoxine-pyrimethamine in vivax malaria pilot studies: relationship to Plasmodium vivax dhfr mutations. Antimicrob. Agents Chemother. 46:3947-3953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vrbova, H., S. Gibney, F. D. Gibson, D. Jolley, P. F. Heywood, J. Stace, K. R. Trenholme, and M. P. Alpers. 1992. Chemoprophylaxis against malaria in Papua New Guinea: a trial of amodiaquine and a combination of dapsone and pyrimethamine. P. N. G. Med. J. 35:275-284. [PubMed] [Google Scholar]
- 42.World Health Organization. 2000. Severe falciparum malaria. Trans. R. Soc. Trop. Med. Hyg. 94(Suppl. 1):S1-S90. [PubMed] [Google Scholar]
- 43.World Health Organization. 1990. Practical chemotherapy of malaria: report of a WHO scientific group. Technical Report Series, no. 805. World Health Organization, Geneva, Switzerland. [PubMed]
- 44.Young, M. D. 1961. Amodiaquine and hydroxychloroquine resistance in Plasmodium falciparum. Am. J. Trop. Med. Hyg. 10:689-693. [DOI] [PubMed] [Google Scholar]