Abstract
A four-part, randomized, crossover study with healthy subjects evaluated the effects of gastric pH, the dosing frequency and prandial state, food consumption timing, and gastric motility on the absorption of posaconazole. In part 1, a single dose (SD) of posaconazole (400 mg) was administered alone or with an acidic beverage or a proton pump inhibitor (PPI), or both. In part 2, posaconazole (400 mg twice daily and 200 mg four times daily) was administered for 7 days with and without a nutritional supplement (Boost). In part 3, an SD of posaconazole (400 mg) was administered while the subjects were fasting and before, during, and after a high-fat meal. In part 4, an SD of posaconazole (400 mg) and the nutritional supplement were administered alone, with metoclopramide, and with loperamide. Compared to the results obtained with posaconazole alone, administration with an acidic beverage increased the posaconazole maximum concentration in plasma (Cmax) and the area under the concentration-time curve (AUC) by 92% and 70%, respectively, whereas a higher gastric pH decreased the posaconazole Cmax and AUC by 46% and 32%, respectively. Compared to the results obtained with posaconazole alone, posaconazole at 400 mg or at 200 mg plus the nutritional supplement increased the posaconazole Cmax and AUC by 65% and 66%, respectively, and by up to 137% and 161%, respectively. Administration before a high-fat meal increased the Cmax and the AUC by 96% and 111%, respectively, while administration during and after the meal increased the Cmax and the AUC by up to 339% and 387%, respectively. Increased gastric motility decreased the Cmax and the AUC by 21% and 19%, respectively. Strategies to maximize posaconazole exposure in patients with absorption difficulties include administration with or after a high-fat meal, with any meal or nutritional supplement, with an acidic beverage, or in divided doses and the avoidance of proton pump inhibitors.
Posaconazole is an extended-spectrum triazole antifungal with potent in vitro and in vivo activities against many clinically important yeasts and molds (16, 27). The agent has demonstrated efficacy as treatment for patients with refractory invasive fungal infections (IFIs), including aspergillosis (12, 26, 36, 39), and as antifungal prophylaxis for neutropenic patients and hematopoietic stem cell transplant (HSCT) recipients with graft-versus-host disease (GVHD) (5, 35). Posaconazole is approved for use in the United States for the prophylaxis of invasive Aspergillus and Candida infections in immunocompromised patients and the treatment of oropharyngeal candidiasis, including infections refractory to itraconazole or fluconazole, or both (31). In the European Union, the agent is approved for use as treatment for refractory IFIs, as first-line treatment for oropharyngeal candidiasis in immunocompromised patients or patients with severe disease, and as prophylaxis for IFIs in patients receiving remission-induction chemotherapy for acute myelogenous leukemia or myelodysplastic syndromes and HSCT recipients (33).
The pharmacokinetics of posaconazole have been studied in healthy volunteers (6), patients with refractory IFIs or febrile neutropenia (34), patients with acute myelogenous leukemia and neutropenia (19), and HSCT recipients with GVHD (17) or neutropenia with or without mucositis (13). Posaconazole is well absorbed after oral administration and has a large volume of distribution (V), suggesting extensive tissue distribution (6). Posaconazole exhibits dose-proportional pharmacokinetics for doses of up to 800 mg/day (6). Posaconazole has a half-life (t1/2) of approximately 35 h, its maximum concentration in plasma (Cmax) is achieved about 3 to 5 h postadministration, and steady state is attained by 7 to 10 days (6, 14; G. Krishna and A. Sansone-Parsons, presented at the 41st American Society of Health-System Pharmacists Midyear Clinical Meeting and Exhibition, Anaheim, CA, 3 to 7 December 2006). In healthy volunteers, age, gender, and race or ethnicity do not have a clinically relevant effect on the pharmacokinetics of posaconazole (30). Similar plasma posaconazole concentrations have been observed in adult and pediatric patients with IFIs (18).
In healthy volunteers, the administration of posaconazole oral suspension with a high-fat meal, a low-fat meal, or a liquid nutritional supplement (Boost Plus) increases the mean area under the concentration-time curve (AUC) values by 4-fold, 2.6-fold, and 2.6-fold, respectively, compared with the AUCs achieved with administration in the fasted state (8, 29). Under fasted conditions, administration of the total daily dose as divided doses increases the level of exposure to posaconazole compared with that achieved by use of a single dose (11). Thus, much is known about the pharmacokinetics of posaconazole.
However, because of the increased use of posaconazole by seriously ill patients with gastric absorption difficulties, such as those associated with GVHD, and by patients with gastrointestinal motility disorders, including chronic diarrhea and gastrointestinal reflux disease, factors that could affect the oral absorption of posaconazole should be better defined. Therefore, the purpose of the study described here was to evaluate the pharmacokinetics of posaconazole in healthy volunteers under well-controlled conditions that simulate the various clinical scenarios that may be encountered in patients who require posaconazole therapy. Specifically, the study evaluated the effects of gastric pH, the posaconazole dosing frequency and prandial state, the timing of food consumption relative to the time of posaconazole administration, and gastric motility on the absorption of posaconazole.
(The information described here was presented in part at the 18th European Congress of Clinical Microbiology and Infectious Diseases, 2008 [G. Krishna, A. Moton, L. Ma, D. Malavade, M. M. Medlock, and J. McLeod, Effect of gastric pH, dosing regimen and prandial state, food and meal timing relative to dose, and gastrointestinal motility on absorption and pharmacokinetics of the antifungal posaconazole, poster P1264].)
MATERIALS AND METHODS
Patient population.
This phase 1, mechanistic, open-label, randomized, crossover, single-center study was conducted in four parts, with a planned enrollment of 12 healthy volunteers in each part. Each subject participated in only one part.
The study was conducted in accordance with the principles of good clinical practice and the Declaration of Helsinki and was approved by an accredited institutional review board. All study participants gave written informed consent before they underwent any study-related procedures.
Inclusion and exclusion criteria.
Subjects between the ages of 18 and 55 years with a body mass index between 20 and 30 kg/m2 were eligible for enrollment. The results of the physical examination and the electrocardiogram (ECG) were required to be within normal limits, and the results of clinical laboratory tests (complete blood count, blood chemistries, and urinalysis) were also required to be within normal limits or clinically acceptable. All subjects were to have negative screens for drugs with a high potential for abuse. Women were required to be nonlactating, to test negative for pregnancy at screening, and either to be of nonchildbearing potential or to have practiced effective double-barrier contraceptive methods from at least 2 weeks prior to the start of the study and were to practice double-barrier contraception until 30 days after they took the last dose of study medication. Men were required to use a barrier method of contraception from the start of the study until 30 days after they took the last dose of study medication.
Subjects were excluded if they had a history of any significant medical disorder that required a physician's care, any clinically significant local or systemic infectious disease within 4 weeks prior to study drug administration, mental instability, or mood disorder. They were also excluded if they smoked tobacco or used other tobacco products or if they had used any drugs, including prescription, nonprescription, herbal, or mineral supplements other than acetaminophen, for at least 2 weeks prior to the study or alcohol within 48 h prior to drug administration and for the entire study period. Subjects were excluded if they had received any investigational drug within 30 days of the start of the study or were participating in another clinical trial. Subjects who tested positive for the human immunodeficiency virus, hepatitis B virus surface antigen, or hepatitis C virus antibodies were also excluded.
Treatment.
After a screening period of up to 21 days for each part of the study, the subjects were admitted to the study center on day −2 for part 1 and on day −1 for all other parts. Subjects who were scheduled for treatment in a fasted state underwent a 10-h overnight fast before they received treatment. All subjects received oral posaconazole suspension (40 mg/ml). Subjects were to refrain from tobacco use throughout the study; from alcohol, caffeine, or xanthine consumption for 48 h prior to study drug administration and during confinement at the study center; and from exercise or physical exertion for 72 h prior to each confinement period and during the confinement.
Parts 1 through 3 were conducted in a four-way crossover manner, and part 4 was conducted in a three-way crossover manner. Each treatment in parts 1, 3, and 4 was separated by a 7- to 10-day washout; in part 2, each treatment was separated by a 14- to 21-day washout.
Part 1 investigated whether there were differences in posaconazole absorption when the gastric pH was decreased via the concomitant administration of an acidic carbonated beverage or when the gastric pH was increased through pharmacologic intervention with the proton pump inhibitor (PPI) esomeprazole (Nexium; AstraZeneca, Wilmington, DE). The subjects were randomized to receive the following treatments: a single 400-mg dose of posaconazole while fasting; a single 400-mg dose of posaconazole and an acidic carbonated beverage (ginger ale, 12 oz) while fasting; a 40-mg esomeprazole capsule once in the morning for 3 days (days −2 to 1, during which scheduled meals were permitted) and on the third day (day 1) with a single 400-mg dose of posaconazole while fasting; and 40 mg esomeprazole once in the morning for 3 days (days −2 to 1, during which scheduled meals were permitted) and on the third day (day 1) with a single 400-mg dose of posaconazole and an acidic carbonated beverage (ginger ale, 12 oz) while fasting.
Part 2 examined the effects of the frequency of posaconazole administration and prandial state on posaconazole absorption. The subjects were randomized to receive the following treatments for 7 days: 400 mg posaconazole twice daily (BID) administered with a nutritional supplement (Boost [Novartis Nutrition Corporation, Fremont, MI] or an equivalent type of nutritional supplement), 400 mg posaconazole BID while fasting, 200 mg posaconazole four times daily (QID) administered with a nutritional supplement, and 200 mg posaconazole QID while fasting. The subjects remained fasted for the entire duration of each 7-day treatment period. Nonfat snacks, not to exceed a total of 800 cal per day, such as apples and oranges and/or gelatin dessert were permitted at designated times. Snacks were permitted on days 1 to 6 at 2 h following each dose for the BID treatment periods and at 2 h following the first, second, and third daily doses for the QID treatment periods; no snacks were permitted between the fourth dose of the day and first dose of the following day for the QID treatment periods. Food consumption resumed for each treatment period after the collection of the 24-h sample for pharmacokinetic analysis on day 8; no food intake was permitted after the final snack on days 6 to 8.
Part 3 assessed the effects on posaconazole absorption when the agent was administered before, during, and after a meal. The subjects were randomized to receive a single 400-mg dose of posaconazole under the following conditions: while fasting, 5 min before a high-fat meal (approximately 50 g fat), in the middle of a high-fat meal that was eaten over 20 min (with the dose taken after approximately 10 min, when half the contents of the meal had been consumed), and 20 min after a high-fat meal.
Part 4 studied the effects of gastric motility on posaconazole absorption. In addition to a single 400-mg dose of posaconazole, the subjects were randomized to receive a nutritional supplement, a nutritional supplement and 10 mg metoclopramide (Reglan; Wyeth, Madison, NJ) administered orally three times daily on the day before and the day of posaconazole dosing, and a nutritional supplement and a single dose of 4 mg loperamide given as two 2-mg tablets.
Pharmacokinetic analysis.
In each part of the study, blood samples for the determination of plasma posaconazole concentrations were collected predosing (0 h) and at 1, 2, 4, 5, 6, 8, 12, 24, 48, 72, 96, 120, 144, and 168 h postdosing. Blood collection began on the day of administration of the single posaconazole dose in parts 1, 3, and 4 and on day 7 in the morning (the subjects received only the morning dose on that day) in part 2.
For each time point, 4 ml of whole blood was collected in prechilled tubes containing EDTA. Within 30 min of collection, the blood samples were centrifuged at 1,500 × g and 4°C for approximately 15 min. Each plasma sample was collected, divided evenly, placed into two labeled, prechilled polypropylene tubes, and immediately frozen at or below −20°C. All plasma samples used for pharmacokinetic analysis were assayed for their posaconazole concentrations by a validated liquid chromatographic-tandem mass spectrometric method by the Schering-Plough Research Institute, Summit, NJ (32). The assay had a lower limit of quantitation of 5.00 ng/ml, a calibration range of 5.00 ng/ml to 5,000 ng/ml, a precision (coefficient of variation [CV]) of 6.2% to 2.5%, and an accuracy (mean percent difference) of −1.0 to 4.0. The values of the pharmacokinetic parameters were calculated by using WinNonLin software (version 4.0.1), distributed by Pharsight (Cary, NC). Plasma concentration data were used to estimate the following parameters: Cmax, the AUC to the final measurable sampling time (AUCtf), the AUC during the dosing interval (AUCτ), the time to Cmax (Tmax), t1/2, apparent total body clearance (CL/F), and the apparent volume of distribution (V/F). The pharmacokinetic parameters of primary interest were Cmax and AUC.
The terminal-phase rate constant (K) was calculated as the negative of the slope of the log-linear terminal portion of the plasma concentration-time curve by using linear regression; t1/2 was calculated as ln(2)/K; AUCtf and AUCτ were calculated by using the linear trapezoidal method and were extrapolated to infinity (AUCI) as (AUCtf/Cest-tf)/K (where Cest-tf is the estimated concentration determined from the linear regression at the final measurable sampling time); CL/F was calculated as dose/AUCI; and V/F was calculated as (CL/F)/K.
Safety analysis.
Safety and tolerability were assessed on the basis of adverse events, vital sign measurements, clinical laboratory test values, and 12-lead ECG results. Adverse events were tabulated by body system or organ class and severity and were summarized by treatment. The severities of adverse events were graded as mild, moderate, severe, or life-threatening by using the National Cancer Institute's Common Terminology Criteria for Adverse Events. Clinically significant laboratory abnormalities were to be reported as adverse events.
Statistical analysis.
The log-transformed values of the pharmacokinetic parameters were analyzed statistically by using an analysis of variance model and extracting the effects due to treatment, subject, period, and sequence. The primary comparisons were against 400 mg posaconazole under the fasted condition for parts 1 to 3 and against 400 mg posaconazole administered with a nutritional supplement for part 4. Ratio estimates and 90% confidence intervals were calculated for relative bioavailability for all primary comparisons.
RESULTS
Patient demographics.
A total of 49 healthy volunteers were enrolled in this study (Table 1). Fifty-five percent were men, 78% were white, and the mean age was 34.3 years. Twelve subjects were treated in each of parts 1 through 3, and 13 subjects were treated in part 4. Two subjects (one in part 2 and the other in part 4) withdrew consent and discontinued treatment for reasons unrelated to the study medication.
TABLE 1.
Demographic characteristicsa
| Characteristic | Part 1 (n = 12) | Part 2 (n = 12) | Part 3 (n = 12) | Part 4 (n = 13) |
|---|---|---|---|---|
| Sex (n [%]) | ||||
| Male | 9 (75) | 8 (67) | 6 (50) | 4 (31) |
| Female | 3 (25) | 4 (33) | 6 (50) | 9 (69) |
| Age (yr) | ||||
| Mean (SD) | 33.3 (13.9) | 33.5 (11.7) | 39.9 (10.5) | 30.3 (7.8) |
| Median (range) | 30.0 (18-55) | 32.0 (19-51) | 40.5 (24-52) | 29.0 (20-43) |
| Race (n [%]) | ||||
| White | 10 (83) | 8 (67) | 12 (100) | 8 (62) |
| Black | 1 (8) | 4 (33) | 0 | 5 (38) |
| Asian | 1 (8) | 0 | 0 | 0 |
| BMI (kg/m2) | ||||
| Mean (SD) | 24.50 (2.53) | 26.09 (2.50) | 25.40 (2.92) | 24.03 (2.14) |
| Median (range) | 24.75 (20.3-28.1) | 26.00 (23.0-29.3) | 26.15 (20.3-30.0) | 23.90 (20.8-28.1) |
n, number of subjects; BMI, body mass index; SD, standard deviation.
Pharmacokinetics.
Table 2 summarizes the values of the key pharmacokinetic parameters of posaconazole administered under various conditions.
TABLE 2.
Mean values of pharmacokinetic parameters
| Treatment | Cmax (ng/ml) | AUC (ng·h/ml)a | Tmaxb (h) | CL/F (liters/h) | t1/2 (h) | V/F (liters) |
|---|---|---|---|---|---|---|
| Part 1: effect of gastric pH (nc = 12) | ||||||
| Posaconazole 400 mg SDd | 151 (58)e | 5,600 (53) | 5 (3-6) | 83.6 (51) | 27.4 (22) | 3,330 (53) |
| Posaconazole 400 mg SD + acidic carbonated beverage | 286 (55) | 9,610 (49) | 4 (3-6) | 52.2 (57) | 25.2 (16) | 1,840 (50) |
| Posaconazole 400 mg SD + PPI | 76.7 (37) | 3,700 (41) | 6 (4-6) | 116 (42) | 25.7 (15) | 4,350 (51) |
| Posaconazole 400 mg SD + PPI + acidic carbonated beverage | 93.3 (34) | 4,180 (35) | 6 (4-12) | 97.4 (33) | 27.8 (36) | 3,920 (48) |
| Part 2: effect of dosing regimen (BID/QID) and prandial state (n = 12) | ||||||
| Posaconazole 400 mg BID alone (7 days)f | 982 (62) | 52,300 (89) | 6 (3-12) | 54.6 (71) | 25.7 (23) | NRg |
| Posaconazole 400 mg BID + nutritional supplement (7 days)f | 1,590 (61) | 80,600 (73)h | 6 (0-8) | 33.9 (58)h | 29.5 (28) | NR |
| Posaconazole 200 mg QID alone (7 days) | 2,300 (44) | 132,000 (54) | 4 (0-6) | 21.2 (71) | 34.7 (39) | NR |
| Posaconazole 200 mg QID + nutritional supplement (7 days)f | 2,160 (44) | 112,000 (49) | 4 (0-5) | 20.4 (53) | 27.8 (41) | NR |
| Part 3: effect of food and meal timing relative to dose (n = 12) | ||||||
| Posaconazole 400 mg SD fasted | 181 (106) | 4,280 (44) | 5 (3-8) | 110 (62) | 24.5 (26) | 3,580 (39) |
| Posaconazole 400 mg SD before meal | 274 (70) | 10,000 (68) | 7 (4-12) | 57.4 (63) | 23.9 (21) | 1,900 (55) |
| Posaconazole 400 mg SD during meal | 555 (44) | 20,900 (49) | 8 (5-12) | 24.3 (58) | 22.8 (29) | 793 (67) |
| Posaconazole 400 mg SD after meal | 544 (50) | 21,000 (48) | 6 (5-12) | 23.7 (54) | 22.6 (28) | 757 (51) |
| Part 4: effect of gastric motility (n = 13) | ||||||
| Posaconazole 400 mg SD + nutritional supplement (n = 12) | 303 (40) | 8,960 (42) | 5.5 (5-6) | 49.4 (34)i | 24.3 (15)i | 1,660 (24)i |
| Posaconazole 400 mg SD + nutritional supplement + metoclopramide (n = 13) | 232 (32) | 7,390 (45) | 5 (4-6) | 59.6 (43)j | 27.7 (29)j | 2,280 (42)j |
| Posaconazole 400 mg SD + nutritional supplement + loperamide (n = 12) | 294 (42) | 10,400 (54) | 5 (4-6) | 44.6 (43)k | 27.5 (11)k | 1,750 (41)k |
AUC to final measurable sampling time.
Values are medians (ranges).
n, number of subjects.
SD, single dose.
Unless indicated otherwise, values in parentheses are percent CV.
n = 11; no concentration data were available for one subject.
NR, not reported.
n = 10; no concentration data were available for one subject; the predose concentration was missing for one subject.
n = 9; pharmacokinetic parameters for three subjects were excluded due to extrapolated area of >25% of total AUC.
n = 12; pharmacokinetic parameters for one subject were excluded due to extrapolated area of >25% of total AUC.
n = 11; pharmacokinetic parameters for one subject were excluded due to extrapolated area of >25% of total AUC.
(i) Part 1: effect of gastric pH.
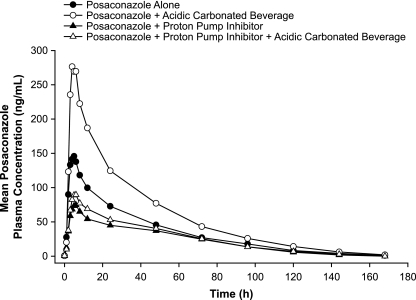
Compared with the results achieved with a single 400-mg dose of posaconazole given alone under fasting conditions, the coadministration of 400 mg of posaconazole with an acidic carbonated beverage increased the mean posaconazole Cmax and AUC by 92% and 70%, respectively (Table 3). The administration of posaconazole under increased gastric pH conditions (coadministration with esomeprazole) decreased the mean posaconazole Cmax and AUC by 46% and 32%, respectively. Similarly, the coadministration of posaconazole with an acidic carbonated beverage and esomeprazole together decreased the mean posaconazole Cmax and AUC by 33% and 21%, respectively. The plasma concentration-time profile of posaconazole is shown in Fig. 1. The posaconazole t1/2 was not appreciably affected by the different treatments (Table 2). Compared to the results achieved with posaconazole alone or with an acidic carbonated beverage, coadministration with esomeprazole (with or without an acidic carbonated beverage) reduced the intersubject variabilities (CV) for Cmax (CV, 55% to 58% versus 34% to 37%, respectively) and AUC (CV, 49% to 53% versus 35% to 41%, respectively).
TABLE 3.
Ratio estimate of log-transformed pharmacokinetic parameters
| Treatment comparison | Cmax (ng/ml) | AUC (ng·h/ml)a |
|---|---|---|
| Part 1: effect of gastric pH (n = 12) | ||
| Posaconazole 400 mg SDb + acidic carbonated beverage vs posaconazole alone | 192 (151-244)c | 170 (143-2,030) |
| Posaconazole 400 mg SD + PPI vs posaconazole alone | 54 (43-69) | 68 (57-81) |
| Posaconazole 400 mg SD + PPI + acidic carbonated beverage vs posaconazole alone | 67 (53-86) | 79 (66-94) |
| Part 2: effect of dosing regimen (BID/QID) and prandial state (n = 12) | ||
| Posaconazole 400 mg BID + nutritional supplement vs posaconazole 400 mg alone | 165 (129-211) | 166 (130-213) |
| Posaconazole 200 mg QID alone vs posaconazole 400 mg alone | 236 (184-302) | 261 (204-335) |
| Posaconazole 200 mg QID + nutritional supplement vs posaconazole 400 mg alone | 237 (186-304) | 257 (200-330) |
| Part 3: effect of food and meal timing (n = 12) | ||
| Posaconazole 400 mg SD before meal vs fasted | 196 (148-259) | 211 (160-278) |
| Posaconazole 400 mg SD during meal vs fasted | 439 (332-580) | 482 (366-635) |
| Posaconazole 400 mg SD after meal vs fasted | 433 (328-573) | 487 (370-642) |
| Part 4: Effect of gastric motility | ||
| Posaconazole 400 mg SD + nutritional supplement + metoclopramide vs posaconazole 400 mg + nutritional supplement | 79 (72-87) | 81 (72-91) |
| Posaconazole 400 mg SD + nutritional supplement + loperamide vs posaconazole 400 mg + nutritional supplement | 97 (88-107) | 111 (99-125) |
The AUC to the final measurable sampling time for parts 1, 3, and 4 and the dose-normalized AUC during the dosing interval (AUCτ/dose) for part 2.
SD, single dose.
Values in parentheses are 90% confidence intervals.
FIG. 1.
Mean posaconazole plasma concentration-time profiles following administration of a single oral dose of posaconazole at 400 mg alone, with an acidic carbonated beverage, with esomeprazole, or with both an acidic carbonated beverage and esomeprazole.
(ii) Part 2: effect of dosing regimen (BID/QID) and prandial state.
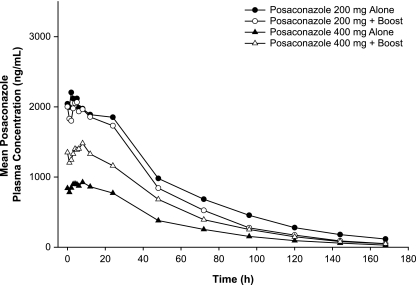
Compared with the results obtained with posaconazole given alone at 400 mg BID for 7 days under fasting conditions, the administration of posaconazole at 400 mg BID with a nutritional supplement increased the mean posaconazole Cmax and AUC by 65% and 66%, respectively (Table 3). The administration of posaconazole at 200 mg QID under fasting conditions increased the mean posaconazole Cmax and AUC by 136% and 161%, respectively. The administration of posaconazole at 200 mg QID with a nutritional supplement increased the mean posaconazole Cmax and AUC by 137% and 157%, respectively. The posaconazole plasma concentration-time profiles under these conditions are shown in Fig. 2. The median Tmax decreased from 6 h with BID dosing to 4 h with QID dosing, regardless of whether posaconazole was administered alone or with a nutritional supplement (Table 2). Compared with the results obtained with BID administration, QID administration reduced the variability in posaconazole exposure for Cmax (CV, 61% to 62% versus 44%, respectively) and AUC (CV, 73% to 89% versus 49% to 54%, respectively).
FIG. 2.
Mean posaconazole plasma concentration-time profiles following oral administration of posaconazole at 400 mg BID with or without a nutritional supplement (Boost) or at 200 mg QID with or without the nutritional supplement for 7 days.
(iii) Part 3: effect of food and meal timing relative to dose.
Compared with the results obtained with a single 400-mg dose of posaconazole administered under fasting conditions, the administration of 400 mg posaconazole 5 min before a high-fat meal increased the mean Cmax and AUC by 96% and 111%, respectively (Table 3). The administration of posaconazole during a high-fat meal increased the mean Cmax and AUC by 339% and 382%, respectively. Similarly, the administration of posaconazole 20 min after a high-fat meal increased the mean Cmax and AUC by 333% and 387%, respectively. The posaconazole plasma concentration-time profiles under different meal conditions are shown in Fig. 3. The median Tmax was the longest (8 h) when posaconazole was administered during a high-fat meal (Table 2). The CVs for the posaconazole Cmax and AUC values ranged from 44% to 106% and 44% to 68%, respectively. The relatively high intersubject variability for Cmax was due to one outlier in the fasted treatment period.
FIG. 3.
Mean posaconazole plasma concentration-time profiles following administration of a single oral dose of posaconazole at 400 mg when the subject was fasted, 5 min before a high-fat meal, during a high-fat meal, or 20 min after a high-fat meal.
(iv) Part 4: effect of gastric motility.
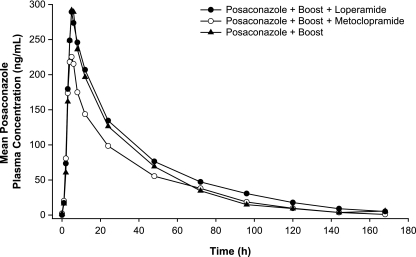
Compared with the results obtained with a single 400-mg dose of posaconazole administered with the nutritional supplement, the administration of posaconazole at 400 mg and the nutritional supplement under conditions of increased gastric motility (coadministration with metoclopramide) decreased the mean Cmax and AUC by 21% and 19%, respectively (Table 3). In contrast, the administration of posaconazole at 400 mg with the nutritional supplement under conditions of reduced gastric motility (coadministration with loperamide) decreased the mean Cmax by 3% and increased the mean AUC by 11%. The posaconazole plasma concentration-time profile for each treatment is shown in Fig. 4. The median Tmax values were similar when posaconazole was administered alone (5.5 h) and with loperamide (5 h) or metoclopramide (5 h). The CVs ranged from 32% to 42% for Cmax and from 42% to 54% for AUC (Table 2).
FIG. 4.
Mean posaconazole plasma concentration-time profiles following administration of a single oral dose of posaconazole at 400 mg with a nutritional supplement (Boost), with metoclopramide and the nutritional supplement, or with loperamide and the nutritional supplement.
Safety.
The incidence of adverse events was 25% (3/12 subjects) in part 1, 75% (9/12) in part 2, 25% (3/12) in part 3, and 38% (5/13) in part 4. All adverse events were of mild (parts 1, 3, and 4) or mild or moderate (part 2) in intensity. No subject discontinued the study due to an adverse event. In part 1, adverse events of diarrhea, upper respiratory tract infection, headache, and dry skin each occurred in no more than one subject. The most common adverse events in part 2 were dizziness (33%; 4/12), headache (25%; 3/12), and asthenia (25%; 3/12). In part 3, each adverse event occurred in no more than one subject. Common adverse events in part 4 included nausea (23%; 3/13), fatigue (15%; 2/13), and dizziness (15%; 2/13). No clinically significant changes in blood chemistry or hematologic parameters, vital signs, or ECG results were reported in any treatment group.
DISCUSSION
This phase 1, open-label, randomized, crossover, four-part study with healthy volunteers showed that posaconazole absorption is affected by gastric pH, prandial state, and the timing of dose administration relative to the time of a meal.
Part 1 of this study examined the effect of low gastric pH on posaconazole absorption. Abnormal gastric pH levels are a common clinical scenario in patients who are likely to require antifungal therapy; such individuals often present with gastrointestinal disorders that increase acid reflux and therefore are treated with PPIs. The findings of this study demonstrated that the coadministration of posaconazole with an acidic carbonated beverage that could provide a milieu of low pH increased the level of posaconazole absorption, whereas the administration of posaconazole under increased gastric pH conditions (coadministration with esomeprazole) decreased the level of absorption because of improved solubility. Since t1/2 was not appreciably affected by any of the treatments, the treatment effect on posaconazole exposure is postulated to be primarily at the absorption level (i.e., the AUC, which is likely the most useful predictor of antifungal efficacy).
The finding that posaconazole absorption is affected by gastric pH differs from the finding of a previous study in which the coadministration of posaconazole 200 mg with an antacid (maximum-strength Mylanta) increased the Cmax of posaconazole by 7% and the AUC by 15% (7), differences that were considered clinically nonsignificant. The findings of the earlier study may be attributable in part to the use of an experimental tablet formulation of posaconazole (7), whereas the subjects in the trial described here received posaconazole as an oral suspension. During the development of posaconazole, the suspension formulation was selected in preference to the tablet because greater exposure is achieved following oral administration when posaconazole is administered as a suspension (8). The findings may also be explained by differences in the degree and the duration of acid suppression between PPIs and antacids. For example, patients with gastroesophageal reflux disease who were treated with esomeprazole at 40 mg once daily for 5 days had a mean 24-h gastric pH of 4.9 and had a gastric pH greater than 4 for 16.8 h (70% of 24 h) (1). In contrast, antacids, which are inorganic salts, do not raise the gastric pH above 4 or 5 and have a duration of action of up to 3 h when they are given with or 1 h after a meal (23).
Studies have shown that gastric pH also affects the absorption of some other azole antifungals. Itraconazole, ketoconazole, and posaconazole are all poorly water soluble and are highly lipophilic; therefore, an acidic environment and the presence of food would be expected to enhance their solubility (2, 4, 13). Similar to the increased level of posaconazole absorption achieved in the present study with its coadministration with an acidic carbonated beverage, the coadministration of a 100-mg itraconazole capsule with a cola beverage was previously found to increase the Cmax and AUC of itraconazole by approximately 121% and 80%, respectively (15). The administration of ketoconazole at 200 mg to healthy fasting volunteers in a simulated state of achlorhydria induced with cimetidine and sodium bicarbonate decreased the Cmax and AUC of ketoconazole by 93% and 92%, respectively (21). In two separate studies in which healthy volunteers received 200-mg itraconazole capsules, a higher gastric pH decreased the itraconazole Cmax and AUC values by approximately 40% to 50% (20, 22). In the present study, the coadministration of posaconazole at 400 mg with an acidic carbonated beverage and esomeprazole partially restored the Cmax and AUC of posaconazole to the levels observed when it was administered alone under fasted conditions, and a similar pattern was observed when ketoconazole was coadministered with omeprazole and a cola beverage (4). In contrast, the Cmax and AUC levels were similar when itraconazole was administered alone and when it was coadministered with ranitidine and cola (20). The modest effect of low gastric acidity on the absorption of fluconazole is not clinically significant (10, 22).
Part 2 of the study examined the effects of the frequency of posaconazole administration and prandial state on posaconazole absorption. The design used established clinical conditions that simulated those of seriously ill patients who cannot eat a high-fat meal or tolerate food. The regimens of 400 mg BID and 200 mg QID (total dose, 800 mg/day) were chosen because these are the approved regimens for posaconazole for the treatment of oropharyngeal candidiasis refractory to itraconazole and/or fluconazole (United States) or refractory IFIs (European Union) (31, 33). The results showed that relative to the results obtained with posaconazole at 400 mg BID for 7 days under fasting conditions, the administration of posaconazole at 400 mg BID with a nutritional supplement increased the Cmax and the AUC by 65% and 66%, respectively; and the administration of posaconazole at 200 mg QID with and without a nutritional supplement increased the Cmax by 137% and 136%, respectively, and increased the AUC by 157% and 161%, respectively. These findings are consistent with those of earlier studies with healthy volunteers in which the administration of posaconazole at 400 mg with a liquid nutritional supplement (Boost Plus) increased the AUC and Cmax of posaconazole by 2.6-fold and 3.4-fold, respectively, an extent similar to that observed with a nonfat meal (8, 29). The differences observed between BID and QID dosing were consistent with those observed in a previous study in which Cmax increased by 64% when posaconazole was administered at 400 mg every 12 h and by 195% when posaconazole was administered at 200 mg every 6 h (11). Posaconazole is a compound with high lipophilicity, high permeation (28), and low aqueous solubility; and consequently, its absorption appears to be limited by solubility. Therefore, the splitting of an 800-mg dose into two doses given 12 h apart or four doses given 6 h apart is likely to increase the soluble fraction of posaconazole.
In part 3, relative to the results obtained with a single 400-mg dose of posaconazole administered under fasting conditions, the posaconazole Cmax and AUC increased by 96% and 111%, respectively, when posaconazole was given 5 min before a high-fat meal; 339% and 382%, respectively, when it was given during a high-fat meal; and 333% and 387%, respectively, when it was given 20 min after a high-fat meal. These findings are consistent with the results of an earlier study in which, relative to the results obtained by the administration of a single 200-mg dose of posaconazole oral suspension under fasted conditions, the administration of the posaconazole suspension 5 min after the completion of a high-fat meal increased the posaconazole Cmax and AUC by 288% and 291%, respectively (8). Thus, the coadministration of food appears to be a key factor governing enhanced posaconazole exposure (8). This is probably because a fatty meal provides an ideal oily solvent that solubilizes posaconazole into a monomeric, absorbable form. Furthermore, this study showed that the timing of the meal relative to the timing of administration of the dose is also important, with drug administration during or immediately after a meal providing a larger increase in the posaconazole AUC than that obtained by administration before a meal.
Studies with healthy volunteers have shown that food has different effects on the absorption of the other azole antifungals. Food showed inconsistent effects on Cmax and AUC levels when ketoconazole was administered at doses ranging from 200 mg to 800 mg, but overall, food appears to have little impact on ketoconazole absorption (9). Similarly, food does not have a clinically significant effect on the absorption of fluconazole (10, 40, 41). In studies that used the capsule formulation of itraconazole, the level of absorption was increased relative to that achieved in the fasted state when itraconazole was administered with a meal. The increases in the itraconazole Cmax and AUC reported previously were 71% and 63%, respectively (2); 100% and 70%, respectively (German subjects) (40); 31% and 35%, respectively (Japanese subjects) (40); 109% and 70%, respectively (41); and 244% and 163%, respectively (38). However, the level of absorption of the cyclodextrin oral solution of itraconazole is decreased when it is administered with a meal relative to the level of absorption achieved in the fasting state. In a single-dose study, compared with the results obtained in the fasted state, the administration of itraconazole solution at 100 mg immediately after a high-fat breakfast decreased the Cmax by 137% and the AUC by 39% (37). In healthy volunteers who received itraconazole solution at 200 mg once daily for 15 days (until steady state was reached), the Cmax and the AUC were decreased by 37% and 29%, respectively, when itraconazole was given with food (3). As with the itraconazole oral solution, the level of absorption of oral voriconazole is decreased when it is administered with food (24). Compared with the results obtained with the administration of voriconazole at 200 mg BID in a capsule formulation in the fasted state, steady-state administration with food decreased the voriconazole Cmax and AUC by 22% and 35%, respectively (24).
Part 4 examined whether the improved absorption of posaconazole, when it was administered with food, was the result of delayed gastric emptying or was solely the result of improved solubility, as suggested by the findings of part 3. The results showed that the coadministration of posaconazole with metoclopramide, a prokinetic agent, decreased the level of posaconazole absorption (i.e., it decreased the Cmax by 21% and the AUC by 19%). This decrease is not likely to be clinically relevant, given the wide therapeutic index of posaconazole (17, 19). This association between increased gastric emptying and decreased posaconazole exposure is consistent with the findings of an analysis of the pharmacokinetics of posaconazole in allogeneic HSCT recipients with GVHD, in which patients with diarrhea had a lower Cmax than those without diarrhea (median Cmaxs, 623 ± 685 ng/ml [n = 18] versus 1,460 ± 972 ng/ml [n = 223], respectively) (17).
In contrast, the coadministration of posaconazole with loperamide, an antikinetic agent, did not affect absorption to a relevant degree (Cmax was decreased by 3% and AUC was increased by 11%). Thus, although it has been suggested that high-fat meals increase the level of absorption by prolonging gastric residence times (8), the enhanced plasma posaconazole concentration and the enhanced exposure to posaconazole observed in this study when posaconazole was administered with a high-fat meal or a nutritional supplement are more likely the result of improved solubility, as indicated by the results of part 3.
In each part of the present study, posaconazole was well tolerated and was considered safe, and no clinically significant changes in blood chemistry or hematological parameters, vital signs, or ECG results were observed. Commonly reported adverse events, including headache, nausea, diarrhea, dizziness, asthenia, and fatigue, were also frequently reported by healthy volunteers (6, 8) and patients (5, 25, 35, 39) who received posaconazole in other studies. The higher incidence of adverse events in part 2 of this study (75% versus 25% to 38% in the other parts) may have been because of the subjects' reduced daily caloric intake, which was restricted to either a nutritional supplement alone or a nonfat diet of approximately 800 cal per day for 7 days.
The results of this study have important implications for the management of patients who are candidates for posaconazole therapy, especially those whose conditions predispose them to decreased levels of posaconazole absorption. For example, pharmacokinetic analysis of allogeneic HSCT recipients who received posaconazole prophylaxis showed a difference in median posaconazole Cmax values between patients (n = 158) with acute GVHD (1,130 ± 858 ng/ml) and patients (n = 82) with chronic GVHD (1,785 ± 1,030 ng/ml) (17). Although the median posaconazole concentrations in patients with both acute and chronic GVHD were sufficient to prevent IFIs, four of the five patients who developed IFIs while they were receiving posaconazole had acute GVHD. In these four patients, the average concentration of posaconazole ranged from 66 to 857 ng/ml, two patients experienced diarrhea at or subsequent to the baseline, and one patient had vomiting at the baseline. The patient with chronic GVHD who developed an IFI also had diarrhea at the baseline.
One component of a clinical trial of posaconazole as salvage therapy for patients with IFIs was a quartile analysis of the relationship between the steady-state Cmax or the average concentration in plasma (Cavg) and the response for the subset of patients with invasive aspergillosis (39). That analysis indicated that although posaconazole was effective at a mean Cavg of as low as 411 ng/ml with a 53% response rate in the second-lowest quartile, higher plasma posaconazole concentrations were associated with higher response rates. The response rate in the lowest quartile (mean Cmax, 142 ng/ml; mean Cavg, 134 ng/ml) was 24%, whereas the response rate in the highest quartile (mean Cmax, 1,480 ng/ml; mean Cavg, 1,250 ng/ml) was 75%. These data demonstrate the importance of optimizing posaconazole absorption in patients who are at risk for or who have IFIs.
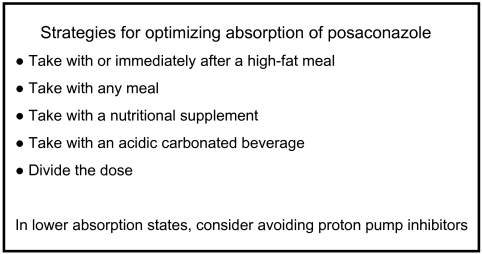
The findings of this study suggest strategies that should be used to maximize posaconazole absorption (Fig. 5). The most desirable approach is to give posaconazole during or after a high-fat meal, but administration with any meal or with a nutritional supplement is an acceptable alternative. Dividing the posaconazole dose or taking posaconazole with an acidic carbonated beverage is an appropriate strategy for increasing the level of systemic absorption in patients who cannot tolerate food. When posaconazole is administered to patients with severe GVHD or other conditions that predispose them to decreased levels of absorption, avoiding the concomitant administration of a PPI, if possible, is likely to be beneficial.
FIG. 5.
Strategies for posaconazole administration to optimize absorption
Conclusion.
Posaconazole absorption is affected by the consumption of an acidic carbonated beverage, gastric pH, prandial state, and the timing of dose administration relative to the time of a meal. The enhanced plasma posaconazole concentration and the enhanced exposure to posaconazole that are observed when posaconazole is administered with a nutritional supplement or a high-fat meal are likely the result of improved solubility rather than delayed gastric emptying. The strategies to maximize posaconazole absorption range from administration with or after a high-fat meal to the possible avoidance of PPIs if patients are likely to experience decreased absorption.
Acknowledgments
We thank Darshana Malavade, James E. Schiller, Carl Norén, and Xin Qun Wu, Schering-Plough Research Institute, for their technical help with the bioanalytical aspects of the study.
Funding for this study was supported by the Schering-Plough Research Institute.
G. Krishna, A. Moton, L. Ma, and J. McLeod are employees of Schering-Plough. M. M. Medlock is a consultant to Schering-Plough.
Footnotes
Published ahead of print on 15 December 2008.
REFERENCES
- 1.AstraZeneca. 2008. Prescribing information for Nexium (esomeprazole magnesium) delayed-release capsules/for delayed-release oral suspension. AstraZeneca, Wilmington, DE.
- 2.Barone, J. A., J. G. Koh, R. H. Bierman, J. L. Colaizzi, K. A. Swanson, M. C. Gaffar, B. L. Moskovitz, W. Mechlinski, and V. Van de Velde. 1993. Food interaction and steady-state pharmacokinetics of itraconazole capsules in healthy male volunteers. Antimicrob. Agents Chemother. 37:778-784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barone, J. A., B. L. Moskovitz, J. Guarnieri, A. E. Hassell, J. L. Colaizzi, R. H. Bierman, and L. Jessen. 1998. Food interaction and steady-state pharmacokinetics of itraconazole oral solution in healthy volunteers. Pharmacotherapy 18:295-301. [PubMed] [Google Scholar]
- 4.Chin, T. W., M. Loeb, and I. W. Fong. 1995. Effects of an acidic beverage (Coca-Cola) on absorption of ketoconazole. Antimicrob. Agents Chemother. 39:1671-1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cornely, O. A., J. Maertens, D. J. Winston, J. Perfect, A. J. Ullmann, T. J. Walsh, D. Helfgott, J. Holowiecki, D. Stockelberg, Y.-T. Goh, M. Petrini, C. Hardalo, R. Suresh, and D. Angulo-Gonzalez. 2007. Posaconazole vs. fluconazole or itraconazole prophylaxis in patients with neutropenia. N. Engl. J. Med. 356:348-359. [DOI] [PubMed] [Google Scholar]
- 6.Courtney, R., S. Pai, M. Laughlin, J. Lim, and V. Batra. 2003. Pharmacokinetics, safety, and tolerability of oral posaconazole administered in single and multiple doses in healthy adults. Antimicrob. Agents Chemother. 47:2788-2795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Courtney, R., E. Radwanski, J. Lim, and M. Laughlin. 2004. Pharmacokinetics of posaconazole coadministered with antacid in fasting or nonfasting healthy men. Antimicrob. Agents Chemother. 48:804-808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Courtney, R., D. Wexler, E. Radwanski, J. Lim, and M. Laughlin. 2003. Effect of food on the relative bioavailability of two oral formulations of posaconazole in healthy adults. Br. J. Clin. Pharmacol. 57:218-222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Daneshmend, T. K., D. W. Warnock, M. D. Ene, E. M. Johnson, M. R. Potten, M. D. Richardson, and P. J. Williamson. 1984. Influence of food on the pharmacokinetics of ketoconazole. Antimicrob. Agents Chemother. 25:1-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Debruyne, D. 1997. Clinical pharmacokinetics of fluconazole in superficial and systemic mycoses. Clin. Pharmacokinet. 33:52-77. [DOI] [PubMed] [Google Scholar]
- 11.Ezzet, F., D. Wexler, R. Courtney, G. Krishna, J. Lim, and M. Laughlin. 2005. Oral bioavailability of posaconazole in fasted healthy subjects: comparison between three regimens and basis for clinical dosage recommendations. Clin. Pharmacokinet. 44:211-220. [DOI] [PubMed] [Google Scholar]
- 12.Greenberg, R. N., K. Mullane, J.-A. H. van Burik, I. Raad, M. J. Abzug, G. Anstead, R. Herbrecht, A. Langston, K. A. Marr, G. Schiller, M. Schuster, J. R. Wingard, C. E. Gonzalez, S. G. Revankar, G. Corcoran, R. J. Kryscio, and R. Hare. 2006. Posaconazole as salvage therapy for zygomycosis. Antimicrob. Agents Chemother. 50:126-133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gubbins, P. O., G. Krishna, A. Sansone-Parsons, S. R. Penzak, L. Dong, M. Martinho, and E. J. Anaissie. 2006. Pharmacokinetics and safety of oral posaconazole in neutropenic stem cell transplant recipients. Antimicrob. Agents Chemother. 50:1993-1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Herbrecht, R. 2004. Posaconazole: a potent, extended-spectrum triazole anti-fungal for the treatment of serious fungal infections. Int. J. Clin. Pract. 58:612-624. [DOI] [PubMed] [Google Scholar]
- 15.Jaruratanasirikul, S., and A. Kleepkaew. 1997. Influence of an acidic beverage (Coca-Cola) on the absorption of itraconazole. Eur. J. Clin. Pharmacol. 52:235-237. [DOI] [PubMed] [Google Scholar]
- 16.Keating, G. M. 2005. Posaconazole. Drugs 65:1553-1567. [DOI] [PubMed] [Google Scholar]
- 17.Krishna, G., M. Martinho, P. Chandrasekar, A. J. Ullmann, and H. Patino. 2007. Pharmacokinetics of oral posaconazole in allogeneic hematopoietic stem cell transplant recipients with graft-versus-host disease. Pharmacotherapy 27:1627-1636. [DOI] [PubMed] [Google Scholar]
- 18.Krishna, G., A. Sansone-Parsons, M. Martinho, B. Kantesaria, and L. Pedicone. 2007. Posaconazole plasma concentrations in juvenile patients with invasive fungal infection. Antimicrob. Agents Chemother. 51:812-818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Krishna, G., M. A. Tarif, F. Xuan, M. Martinho, D. Angulo, and O. A. Cornely. 2008. Pharmacokinetics of oral posaconazole in neutropenic patients receiving chemotherapy for acute myelogenous leukemia or myelodysplastic syndrome. Pharmacotherapy 28:1223-1232. [DOI] [PubMed] [Google Scholar]
- 20.Lange, D., J. H. Pavao, J. Wu, and M. Klausner. 1997. Effect of a cola beverage on the bioavailability of itraconazole in the presence of H2 blockers. J. Clin. Pharmacol. 37:535-540. [DOI] [PubMed] [Google Scholar]
- 21.Lelawongs, P., J. A. Barone, J. L. Colaizzi, A. T. Hsuan, W. Mechlinski, R. Legendre, and J. Guarnieri. 1988. Effect of food and gastric acidity on absorption of orally administered ketoconazole. Clin. Pharm. 7:228-235. [PubMed] [Google Scholar]
- 22.Lim, S. G., A. M. Sawyerr, M. Hudson, J. Sercombe, and R. E. Pounder. 1993. Short report: the absorption of fluconazole and itraconazole under conditions of low intragastric acidity. Aliment. Pharmacol. Ther. 7:317-321. [DOI] [PubMed] [Google Scholar]
- 23.Maton, P. N., and M. E. Burton. 1999. Antacids revisited: a review of their clinical pharmacology and recommended therapeutic use. Drugs 57:855-870. [DOI] [PubMed] [Google Scholar]
- 24.Purkins, L., N. Wood, D. Kleinermans, K. Greenhalgh, and D. Nichols. 2003. Effect of food on the pharmacokinetics of multiple-dose oral voriconazole. Br J. Clin. Pharmacol. 56:17-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Raad, I. I., J. R. Graybill, A. B. Bustamante, O. A. Cornely, V. Gaona-Flores, C. Afif, D. R. Graham, R. N. Greenberg, S. Hadley, A. Langston, R. Negroni, J. R. Perfect, P. Pitisuttithum, A. Restrepo, G. Schiller, L. Pedicone, and A. J. Ullmann. 2006. Safety of long-term oral posaconazole use in the treatment of refractory invasive fungal infections. Clin. Infect. Dis. 42:1726-1734. [DOI] [PubMed] [Google Scholar]
- 26.Raad, I. I., R. Y. Hachem, R. Herbrecht, J. R. Graybill, R. Hare, G. Corcoran, and D. P. Kontoyiannis. 2006. Posaconazole as salvage treatment of invasive fusariosis in patients with underlying hematologic malignancy and other conditions. Clin. Infect. Dis. 42:1398-1403. [DOI] [PubMed] [Google Scholar]
- 27.Sabatelli, F., R. Patel, P. A. Mann, C. A. Mendrick, C. C. Norris, R. Hare, D. Loebenberg, T. A. Black, and P. M. McNicholas. 2006. In vitro activities of posaconazole, fluconazole, itraconazole, voriconazole, and amphotericin B against a large collection of clinically important molds and yeasts. Antimicrob. Agents Chemother. 50:2009-2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Saha, P., and J. H. Kou. 2000. Effect of solubilizing excipients on permeation of poorly water-soluble compounds across Caco-2 cell monolayers. Eur. J. Pharm. Biopharm. 50:403-411. [DOI] [PubMed] [Google Scholar]
- 29.Sansone-Parsons, A., G. Krishna, A. Calzetta, D. Wexler, B. Kantesaria, M. A. Rosenberg, and M. A. Saltzman. 2006. Effect of a nutritional supplement on posaconazole pharmacokinetics following oral administration to healthy volunteers. Antimicrob. Agents Chemother. 50:1881-1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sansone-Parsons, A., G. Krishna, J. Simon, P. Soni, B. Kantesaria, J. Herron, and R. Stoltz. 2007. Effects of age, gender, and race/ethnicity on the pharmacokinetics of posaconazole in healthy volunteers. Antimicrob. Agents Chemother. 51:495-502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schering Corporation. 2008. Prescribing information for Noxafil (posaconazole) oral suspension. Schering Corporation, Kenilworth, NJ.
- 32.Shen, J. X., G. Krishna, and R. N. Hayes. 2007. A sensitive liquid chromatography and mass spectrometry method for the determination of posaconazole in human plasma. J. Pharm. Biomed. Anal. 43:228-236. [DOI] [PubMed] [Google Scholar]
- 33.SP Europe. 2008. Noxafil 40 mg/ml oral suspension. Summary of product characteristics. SP Europe, Brussels, Belgium.
- 34.Ullmann, A. J., O. A. Cornely, A. Burchardt, R. Hachem, D. P. Kontoyiannis, K. Topelt, R. Courtney, D. Wexler, G. Krishna, M. Martinho, G. Corcoran, and I. Raad. 2006. Pharmacokinetics, safety, and efficacy of posaconazole in patients with persistent febrile neutropenia or refractory invasive fungal infection. Antimicrob. Agents Chemother. 50:658-666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ullmann, A. J., J. H. Lipton, D. H. Vesole, P. Chandrasekar, A. Langston, S. R. Tarantolo, H. Greinix, W. Morais de Azevedo, V. Reddy, N. Boparai, L. Pedicone, H. Patino, and S. Durrant. 2007. Posaconazole or fluconazole for prophylaxis in severe graft-versus-host disease. N. Engl. J. Med. 356:335-347. [DOI] [PubMed] [Google Scholar]
- 36.van Burik, J.-A. H., R. S. Hare, H. F. Solomon, M. L. Corrado, and D. P. Kontoyiannis. 2006. Posaconazole is effective as salvage therapy in zygomycosis: a retrospective summary of 91 cases. Clin. Infect. Dis. 42:e61-e65. [DOI] [PubMed] [Google Scholar]
- 37.Van de Velde, V., A. P. Van Peer, J. J. Heykants, R. J. Woestenborghs, R. P. Van, K. L. De Beule, and G. F. Cauwenbergh. 1996. Effect of food on the pharmacokinetics of a new hydroxypropyl-beta-cyclodextrin formulation of itraconazole. Pharmacotherapy 16:424-428. [PubMed] [Google Scholar]
- 38.Van Peer, A., R. Woestenborghs, J. Heykants, R. Gasparini, and G. Gauwenbergh. 1989. The effects of food and dose on the oral systemic availability of itraconazole in healthy subjects. Eur. J. Clin. Pharmacol. 36:423-426. [DOI] [PubMed] [Google Scholar]
- 39.Walsh, T. J., I. Raad, T. F. Patterson, P. Chandrasekar, G. R. Donowitz, R. Graybill, R. E. Greene, R. Hachem, S. Hadley, R. Herbrecht, A. Langston, A. Louie, P. Ribaud, B. H. Segal, D. A. Stevens, J.-A. H. van Burik, C. S. White, G. Corcoran, J. Gogate, G. Krishna, L. Pedicone, C. Hardalo, and J. R. Perfect. 2007. Treatment of invasive aspergillosis with posaconazole in patients who are refractory to or intolerant of conventional therapy: an externally controlled trial. Clin. Infect. Dis. 44:2-12. [DOI] [PubMed] [Google Scholar]
- 40.Yeates, R. A., T. Zimmermann, H. Laufen, M. Albrecht, and A. Wildfeuer. 1995. Comparative pharmacokinetics of fluconazole and of itraconazole in Japanese and in German subjects. Int. J. Clin. Pharmacol. Ther. 33:131-135. [PubMed] [Google Scholar]
- 41.Zimmermann, T., R. A. Yeates, H. Laufen, G. Pfaff, and A. Wildfeuer. 1994. Influence of concomitant food intake on the oral absorption of two triazole antifungal agents, itraconazole and fluconazole. Eur. J. Clin. Pharmacol. 46:147-150. [DOI] [PubMed] [Google Scholar]