Abstract
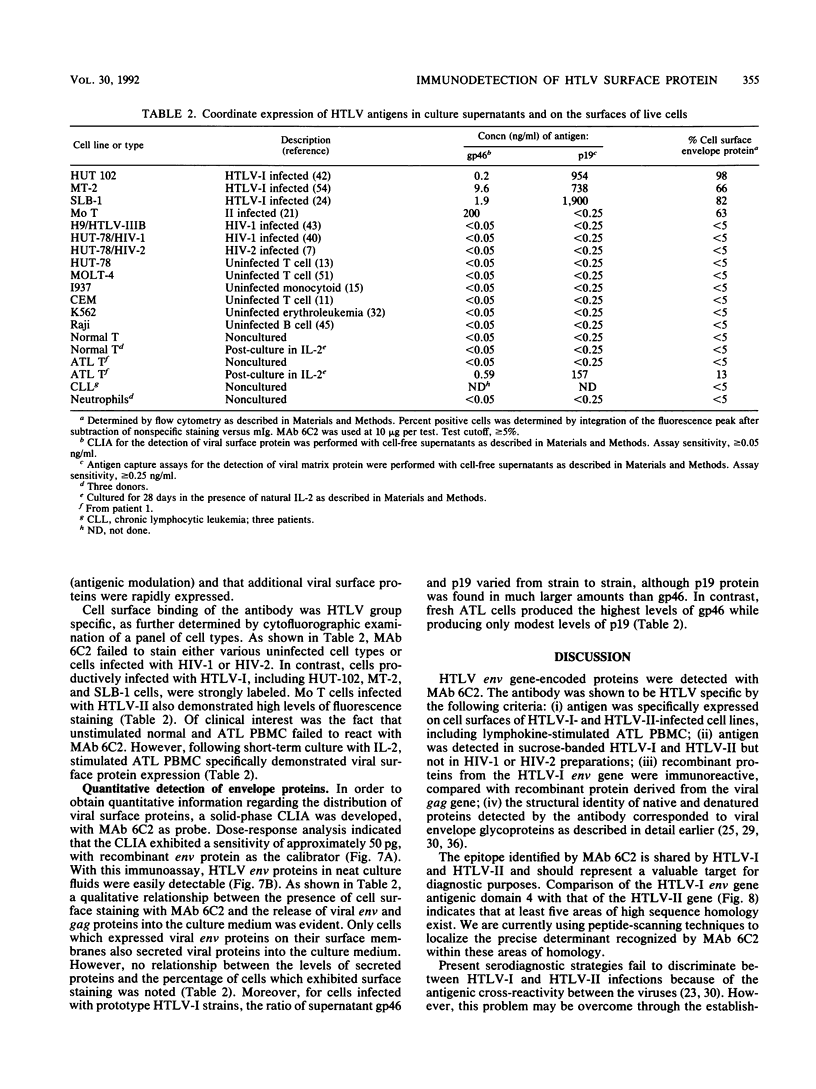
Monoclonal antibodies (MAbs) raised against human T-cell lymphotropic virus type I (HTLV-I) recognized five distinct antigenic domains of viral env gene-encoded proteins. By using recombinant env proteins and synthetic peptides as mapping antigens, it was determined that the most immunogenic region represented a central portion of the retroviral surface protein (domain 2; amino acids 165 to 191). However, only a single MAb was able to react strongly with native viral proteins. This antibody (clone 6C2) was directed to an epitope within domain 4 (amino acids 210 to 306) of the retroviral env gene and reacted with envelope proteins in both HTLV-I and HTLV-II, as determined by immunoprecipitation, solid-phase binding, and immunoblotting. No reactivity against envelope components of other human retroviruses, including human immunodeficiency virus types 1 and 2, was present. Flow cytometry data demonstrated that MAb 6C2 reacted with cell lines chronically infected with HTLV-I or HTLV-II and also with surface antigens expressed on fresh adult T-cell leukemia cells, following up-regulation with interleukin-2. By a chemiluminescence immunoassay procedure, picogram amounts of viral surface protein could be detected in the unconcentrated supernatants of HTLV-infected cell lines and in diagnostic cultures. Levels of env and gag proteins released by cells into culture supernatants were not directly related to percent expression of cell surface viral-coat proteins. Further, the molar ratio of p19 to gp46 in conditioned media varied from strain to strain, possibly reflecting differences in viral assembly or packaging mechanisms. MAb 6C2 will be of value in characterizing the biochemical and immunological behavior of retroviral env gene proteins and in studying the interaction of HTLV-I and HTLV-II with their receptors.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bhagavati S., Ehrlich G., Kula R. W., Kwok S., Sninsky J., Udani V., Poiesz B. J. Detection of human T-cell lymphoma/leukemia virus type I DNA and antigen in spinal fluid and blood of patients with chronic progressive myelopathy. N Engl J Med. 1988 May 5;318(18):1141–1147. doi: 10.1056/NEJM198805053181801. [DOI] [PubMed] [Google Scholar]
- Blattner W. A., Gibbs W. N., Saxinger C., Robert-Guroff M., Clark J., Lofters W., Hanchard B., Campbell M., Gallo R. C. Human T-cell leukaemia/lymphoma virus-associated lymphoreticular neoplasia in Jamaica. Lancet. 1983 Jul 9;2(8341):61–64. doi: 10.1016/s0140-6736(83)90056-9. [DOI] [PubMed] [Google Scholar]
- Chen Y. M., Lee T. H., Samuel K. P., Okayama A., Tachibana N., Miyoshi I., Papas T. S., Essex M. Antibody reactivity to different regions of human T-cell leukemia virus type 1 gp61 in infected people. J Virol. 1989 Nov;63(11):4952–4957. doi: 10.1128/jvi.63.11.4952-4957.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clapham P., Nagy K., Weiss R. A. Pseudotypes of human T-cell leukemia virus types 1 and 2: neutralization by patients' sera. Proc Natl Acad Sci U S A. 1984 May;81(9):2886–2889. doi: 10.1073/pnas.81.9.2886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clavel F., Guétard D., Brun-Vézinet F., Chamaret S., Rey M. A., Santos-Ferreira M. O., Laurent A. G., Dauguet C., Katlama C., Rouzioux C. Isolation of a new human retrovirus from West African patients with AIDS. Science. 1986 Jul 18;233(4761):343–346. doi: 10.1126/science.2425430. [DOI] [PubMed] [Google Scholar]
- Copeland T. D., Tsai W. P., Kim Y. D., Oroszlan S. Envelope proteins of human T cell leukemia virus type I: characterization by antisera to synthetic peptides and identification of a natural epitope. J Immunol. 1986 Nov 1;137(9):2945–2951. [PubMed] [Google Scholar]
- Ehrlich G. D., Glaser J. B., Abbott M. A., Slamon D. J., Keith D., Sliwkowski M., Brandis J., Keitelman E., Teramoto Y., Papsidero L. Detection of anti-HTLV-I Tax antibodies in HTLV-I enzyme-linked immunosorbent assay-negative individuals. Blood. 1989 Aug 15;74(3):1066–1072. [PubMed] [Google Scholar]
- Ehrlich G. D., Glaser J. B., LaVigne K., Quan D., Mildvan D., Sninsky J. J., Kwok S., Papsidero L., Poiesz B. J. Prevalence of human T-cell leukemia/lymphoma virus (HTLV) type II infection among high-risk individuals: type-specific identification of HTLVs by polymerase chain reaction. Blood. 1989 Oct;74(5):1658–1664. [PubMed] [Google Scholar]
- FOLEY G. E., LAZARUS H., FARBER S., UZMAN B. G., BOONE B. A., MCCARTHY R. E. CONTINUOUS CULTURE OF HUMAN LYMPHOBLASTS FROM PERIPHERAL BLOOD OF A CHILD WITH ACUTE LEUKEMIA. Cancer. 1965 Apr;18:522–529. doi: 10.1002/1097-0142(196504)18:4<522::aid-cncr2820180418>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- Gazdar A. F., Carney D. N., Bunn P. A., Russell E. K., Jaffe E. S., Schechter G. P., Guccion J. G. Mitogen requirements for the in vitro propagation of cutaneous T-cell lymphomas. Blood. 1980 Mar;55(3):409–417. [PubMed] [Google Scholar]
- Gessain A., Jouannelle A., Escarmant P., Calender A., Schaffar-Deshayes L., de-Thé G. HTLV antibodies in patients with non-Hodgkin lymphomas in Martinique. Lancet. 1984 May 26;1(8387):1183–1184. doi: 10.1016/s0140-6736(84)91431-4. [DOI] [PubMed] [Google Scholar]
- Gitter B. D., Finn O. J., Metzgar R. S. Cytofluorometric isolation of I937, an Ia antigen-bearing variant of the Ia-negative human monocytic cell line U937. J Immunol. 1985 Jan;134(1):280–283. [PubMed] [Google Scholar]
- Goding J. W. Antibody production by hybridomas. J Immunol Methods. 1980;39(4):285–308. doi: 10.1016/0022-1759(80)90230-6. [DOI] [PubMed] [Google Scholar]
- Greenberg S. J., Ehrlich G. D., Abbott M. A., Hurwitz B. J., Waldmann T. A., Poiesz B. J. Detection of sequences homologous to human retroviral DNA in multiple sclerosis by gene amplification. Proc Natl Acad Sci U S A. 1989 Apr;86(8):2878–2882. doi: 10.1073/pnas.86.8.2878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinuma Y., Komoda H., Chosa T., Kondo T., Kohakura M., Takenaka T., Kikuchi M., Ichimaru M., Yunoki K., Sato I. Antibodies to adult T-cell leukemia-virus-associated antigen (ATLA) in sera from patients with ATL and controls in Japan: a nation-wide sero-epidemiologic study. Int J Cancer. 1982 Jun 15;29(6):631–635. doi: 10.1002/ijc.2910290606. [DOI] [PubMed] [Google Scholar]
- Hjelle B., Scalf R., Swenson S. High frequency of human T-cell leukemia-lymphoma virus type II infection in New Mexico blood donors: determination by sequence-specific oligonucleotide hybridization. Blood. 1990 Aug 1;76(3):450–454. [PubMed] [Google Scholar]
- Jacobson S., Raine C. S., Mingioli E. S., McFarlin D. E. Isolation of an HTLV-1-like retrovirus from patients with tropical spastic paraparesis. Nature. 1988 Feb 11;331(6156):540–543. doi: 10.1038/331540a0. [DOI] [PubMed] [Google Scholar]
- Kalyanaraman V. S., Sarngadharan M. G., Robert-Guroff M., Miyoshi I., Golde D., Gallo R. C. A new subtype of human T-cell leukemia virus (HTLV-II) associated with a T-cell variant of hairy cell leukemia. Science. 1982 Nov 5;218(4572):571–573. doi: 10.1126/science.6981847. [DOI] [PubMed] [Google Scholar]
- Kanki P. J., M'Boup S., Ricard D., Barin F., Denis F., Boye C., Sangare L., Travers K., Albaum M., Marlink R. Human T-lymphotropic virus type 4 and the human immunodeficiency virus in West Africa. Science. 1987 May 15;236(4803):827–831. doi: 10.1126/science.3033826. [DOI] [PubMed] [Google Scholar]
- Kline R. L., Brothers T., Halsey N., Boulos R., Lairmore M. D., Quinn T. C. Evaluation of enzyme immunoassays for antibody to human T-lymphotropic viruses type I/II. Lancet. 1991 Jan 5;337(8732):30–33. doi: 10.1016/0140-6736(91)93343-8. [DOI] [PubMed] [Google Scholar]
- Koeffler H. P., Chen I. S., Golde D. W. Characterization of a novel HTLV-infected cell line. Blood. 1984 Aug;64(2):482–490. [PubMed] [Google Scholar]
- Koyanagi Y., Hinuma Y., Schneider J., Chosa T., Hunsmann G., Kobayashi N., Hatanaka M., Yamamoto N. Expression of HTLV-specific polypeptides in various human T-cell lines. Med Microbiol Immunol. 1984;173(3):127–140. doi: 10.1007/BF02123761. [DOI] [PubMed] [Google Scholar]
- Kwok S., Gallo D., Hanson C., McKinney N., Poiesz B., Sninsky J. J. High prevalence of HTLV-II among intravenous drug abusers: PCR confirmation and typing. AIDS Res Hum Retroviruses. 1990 Apr;6(4):561–565. doi: 10.1089/aid.1990.6.561. [DOI] [PubMed] [Google Scholar]
- Kwok S., Kellogg D., Ehrlich G., Poiesz B., Bhagavati S., Sninsky J. J. Characterization of a sequence of human T cell leukemia virus type I from a patient with chronic progressive myelopathy. J Infect Dis. 1988 Dec;158(6):1193–1197. doi: 10.1093/infdis/158.6.1193. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lee T. H., Coligan J. E., Homma T., McLane M. F., Tachibana N., Essex M. Human T-cell leukemia virus-associated membrane antigens: identity of the major antigens recognized after virus infection. Proc Natl Acad Sci U S A. 1984 Jun;81(12):3856–3860. doi: 10.1073/pnas.81.12.3856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee T. H., Coligan J. E., McLane M. F., Sodroski J. G., Popovic M., Wong-Staal F., Gallo R. C., Haseltine W., Essex M. Serological cross-reactivity between envelope gene products of type I and type II human T-cell leukemia virus. Proc Natl Acad Sci U S A. 1984 Dec;81(23):7579–7583. doi: 10.1073/pnas.81.23.7579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine P. H., Blattner W. A., Clark J., Tarone R., Maloney E. M., Murphy E. M., Gallo R. C., Robert-Guroff M., Saxinger W. C. Geographic distribution of HTLV-I and identification of a new high-risk population. Int J Cancer. 1988 Jul 15;42(1):7–12. doi: 10.1002/ijc.2910420103. [DOI] [PubMed] [Google Scholar]
- Lozzio C. B., Lozzio B. B. Cytotoxicity of a factor isolated from human spleen. J Natl Cancer Inst. 1973 Feb;50(2):535–538. doi: 10.1093/jnci/50.2.535. [DOI] [PubMed] [Google Scholar]
- Mann D. L., DeSantis P., Mark G., Pfeifer A., Newman M., Gibbs N., Popovic M., Sarngadharan M. G., Gallo R. C., Clark J. HTLV-I--associated B-cell CLL: indirect role for retrovirus in leukemogenesis. Science. 1987 May 29;236(4805):1103–1106. doi: 10.1126/science.2883731. [DOI] [PubMed] [Google Scholar]
- Minamoto G. Y., Gold J. W., Scheinberg D. A., Hardy W. D., Chein N., Zuckerman E., Reich L., Dietz K., Gee T., Hoffer J. Infection with human T-cell leukemia virus type I in patients with leukemia. N Engl J Med. 1988 Jan 28;318(4):219–222. doi: 10.1056/NEJM198801283180405. [DOI] [PubMed] [Google Scholar]
- Oroszlan S., Copeland T. D. Primary structure and processing of gag and env gene products of human T-cell leukemia viruses HTLV-ICR and HTLV-IATK. Curr Top Microbiol Immunol. 1985;115:221–233. doi: 10.1007/978-3-642-70113-9_14. [DOI] [PubMed] [Google Scholar]
- PULVERTAFT J. V. A STUDY OF MALIGNANT TUMOURS IN NIGERIA BY SHORT-TERM TISSUE CULTURE. J Clin Pathol. 1965 May;18:261–273. doi: 10.1136/jcp.18.3.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palker T. J., Tanner M. E., Scearce R. M., Streilein R. D., Clark M. E., Haynes B. F. Mapping of immunogenic regions of human T cell leukemia virus type I (HTLV-I) gp46 and gp21 envelope glycoproteins with env-encoded synthetic peptides and a monoclonal antibody to gp46. J Immunol. 1989 Feb 1;142(3):971–978. [PubMed] [Google Scholar]
- Papsidero L. D., Croghan G. A., Wang M. C., Kuriyama M., Johnson E. A., Valenzuela L. A., Chu T. M. Monoclonal antibody (F5) to human prostate antigen. Hybridoma. 1983;2(2):139–147. doi: 10.1089/hyb.1983.2.139. [DOI] [PubMed] [Google Scholar]
- Papsidero L. D., Sheu M., Ruscetti F. W. Human immunodeficiency virus type 1-neutralizing monoclonal antibodies which react with p17 core protein: characterization and epitope mapping. J Virol. 1989 Jan;63(1):267–272. doi: 10.1128/jvi.63.1.267-272.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papsidero L., Swartzwelder F., Sheu M., Montagna R., Ehrlich G., Bhagavati S., Dosik H., Sninsky J., Poiesz B. Immunodetection of human T-cell lymphotropic virus type I core protein in biological samples by using a monoclonal antibody immunoassay. J Clin Microbiol. 1990 May;28(5):949–955. doi: 10.1128/jcm.28.5.949-955.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parham P., Barnstable C. J., Bodmer W. F. Use of a monoclonal antibody (W6/32) in structural studies of HLA-A,B,C, antigens. J Immunol. 1979 Jul;123(1):342–349. [PubMed] [Google Scholar]
- Poiesz B. J., Ruscetti F. W., Gazdar A. F., Bunn P. A., Minna J. D., Gallo R. C. Detection and isolation of type C retrovirus particles from fresh and cultured lymphocytes of a patient with cutaneous T-cell lymphoma. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7415–7419. doi: 10.1073/pnas.77.12.7415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popovic M., Sarngadharan M. G., Read E., Gallo R. C. Detection, isolation, and continuous production of cytopathic retroviruses (HTLV-III) from patients with AIDS and pre-AIDS. Science. 1984 May 4;224(4648):497–500. doi: 10.1126/science.6200935. [DOI] [PubMed] [Google Scholar]
- Posnett D. N., McGrath H. A., Scott R. A., McNutt N. S., Folkl R., Hickey T. D., Macera M. J., Szabo P. Polyclonal lymphocytosis of T-cells associated with human T-cell leukemia virus I. Cancer Res. 1988 May 1;48(9):2585–2589. [PubMed] [Google Scholar]
- Ratner L., Poiesz B. J. Leukemias associated with human T-cell lymphotropic virus type I in a non-endemic region. Medicine (Baltimore) 1988 Nov;67(6):401–422. doi: 10.1097/00005792-198811000-00004. [DOI] [PubMed] [Google Scholar]
- Sahai Srivastava B. I., Minowada J. Terminal deoxynucleotidyl transferase activity in a cell line (molt-4) derived from the peripheral blood of a patient with acute lymphoblastic leukemia. Biochem Biophys Res Commun. 1973 Apr 2;51(3):529–535. doi: 10.1016/0006-291x(73)91346-6. [DOI] [PubMed] [Google Scholar]
- Samuel K. P., Lautenberger J. A., Jorcyk C. L., Josephs S., Wong-Staal F., Papas T. S. Diagnostic potential for human malignancies of bacterially produced HTLV-I envelope protein. Science. 1984 Nov 30;226(4678):1094–1097. doi: 10.1126/science.6208612. [DOI] [PubMed] [Google Scholar]
- Seiki M., Hattori S., Hirayama Y., Yoshida M. Human adult T-cell leukemia virus: complete nucleotide sequence of the provirus genome integrated in leukemia cell DNA. Proc Natl Acad Sci U S A. 1983 Jun;80(12):3618–3622. doi: 10.1073/pnas.80.12.3618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sodroski J., Patarca R., Perkins D., Briggs D., Lee T. H., Essex M., Coligan J., Wong-Staal F., Gallo R. C., Haseltine W. A. Sequence of the envelope glycoprotein gene of type II human T lymphotropic virus. Science. 1984 Jul 27;225(4660):421–424. doi: 10.1126/science.6204380. [DOI] [PubMed] [Google Scholar]
- Vaickus L., Jones V. E., Morton C. L., Whitford K., Bacon R. N. Antiproliferative mechanism of anti-class II monoclonal antibodies. Cell Immunol. 1989 Apr 1;119(2):445–458. doi: 10.1016/0008-8749(89)90257-8. [DOI] [PubMed] [Google Scholar]
- Wiley C. A., Nerenberg M., Cros D., Soto-Aguilar M. C. HTLV-I polymyositis in a patient also infected with the human immunodeficiency virus. N Engl J Med. 1989 Apr 13;320(15):992–995. doi: 10.1056/NEJM198904133201507. [DOI] [PubMed] [Google Scholar]
- Yoshida M., Miyoshi I., Hinuma Y. Isolation and characterization of retrovirus from cell lines of human adult T-cell leukemia and its implication in the disease. Proc Natl Acad Sci U S A. 1982 Mar;79(6):2031–2035. doi: 10.1073/pnas.79.6.2031. [DOI] [PMC free article] [PubMed] [Google Scholar]