Abstract
Anticardiolipin (anti-CL) antibodies, diagnostic for antiphospholipid antibody syndrome, are associated with increased risks of venous and arterial thrombosis. Because CL selectively enhances activated protein C/protein S-dependent anticoagulant activities in purified systems and because CL is not known to be a normal plasma component, we searched for CL in plasma. Plasma lipid extracts [chloroform/methanol (2:1, vol/vol)] were subjected to analyses by using TLC, analytical HPLC, and MS. A plasma lipid component was purified that was indistinguishable from reference CL (M:1448). When CL in 40 fasting plasma lipid extracts (20 males, 20 females) was quantitated by using HPLC, CL (mean ± SD) was 14.9 ± 3.7 μg/ml (range 9.1 to 24.2) and CL was not correlated with phosphatidylserine (3.8 ± 1.7 μg/ml), phosphatidylethanolamine (64 ± 20 μg/ml), or choline-containing phospholipid (1,580 ± 280 μg/ml). Based on studies of fasting blood donors, CL (≥94%) was recovered in very low density, low density, and high density lipoproteins (11 ± 5.3%, 67 ± 11.0%, and 17 ± 10%, respectively), showing that the majority of plasma CL (67%) is in low density lipoprotein. Analysis of relative phospholipid contents of lipoproteins indicated that high density lipoprotein is selectively enriched in CL and phosphatidylethanolamine. These results shows that CL is a normal plasma component and suggest that the epitopes of antiphospholipid antibodies could include CL or oxidized CL in lipoproteins or in complexes with plasma proteins (e.g., β2-glycoprotein I, prothrombin, protein C, or protein S) or with platelet or endothelial surface proteins.
Anticardiolipin (anti-CL) antibodies are associated with increased risks of venous or arterial thrombosis and ischemic coronary and cerebral disease (1–4). The titer of anti-CL antibodies usually is detected by ELISA assay by using immobilized CL and used with other tests to diagnose antiphospholipid antibody syndrome (5). The epitopes of antiphospholipid antibodies are heterogeneous and subject to controversy (5–11). Because biologically available CL is predominantly in intracellular mitochondria and is not exposed to circulating antiphospholipid (12, 13), CL has not been considered to be a physiologic or authentic antigen of antiphospholipid antibodies. Other anionic phospholipids (PL), e.g., phosphatidylserine (PS), are reported to bind to antiphospholipid antibodies (14). PS is found in lipoproteins and cell membranes (15), is accessible to circulating antibodies, and therefore has been considered a viable candidate antigen for antiphospholipid antibodies. Some antiphospholipid antibodies bind β2-glycoprotein I or complexes of protein and anionic phospholipids, such as β2-glycoprotein I-PL, prothrombin-PL, protein C-PL, protein S-PL, complement C4b-binding protein, etc. (8, 16, 17).
Anti-CL antibodies were reported to recognize oxidized phospholipids (ox-PL) (9, 10, 18). Ox-PL in plasma are derived mainly from ox-lipoproteins, especially ox-low density lipoprotein (LDL), a major risk factor for atherothrombotic disease (19–25). Ox-LDL is immunogenic, and autoantibodies recognizing epitopes of ox-LDL have been described in plasma and atherosclerotic lesions (20, 26). Moreover, higher titers of these autoantibodies against ox-LDL are present in patients with atherosclerotic diseases (27–31).
CL is an anionic phospholipid present in mitochondrial and bacterial membranes (13) and generally is not recognized as a significant physiologic plasma component (12). Phospholipids found in plasma lipoproteins include phosphatidylcholine (PC), lysoPC, phosphatidylethanolamine (PE), sphingomyelin (SM), PS, and phosphatidylinositol (32). Each subclass of phospholipid is composed of several molecular species that differ from one another in fatty acid side chains. PE in plasma is reported to be 60–90 μg/ml whereas PS is less than 15 μg/ml (15, 32–36). In plasma there is much less PE and PS than choline-containing phospholipids (c-PL), i.e., PC and SM.
In vitro studies show that PE and PS are biologically active in the blood coagulation system (37–40). PS generally is described as the most procoagulant PL in membranes (38–43). PE in multicomponent vesicles was reported to support activated protein C (APC) anticoagulant activity with little enhancement of procoagulant activity (37, 44), and we reported that CL has APC/protein S-dependent anticoagulant activities in purified clotting factor assay systems (45). These studies and the discovery that high density lipoprotein (HDL) exhibits anticoagulant cofactor activity for APC/protein S (46) stimulated us to analyze plasma for CL. To address this issue, we analyzed lipid extracts from human plasma and from purified lipoproteins by using HPLC combined with evaporative light-scattering detection (ELSD), TLC, and MS to identify and quantitate CL, PS, and PE in plasma and in lipoproteins isolated from fasting plasma. This study demonstrates that CL is a significant and normal physiologic component present in human plasma lipoproteins and that the majority of CL is found in LDL.
Materials and Methods
Materials.
Aprotinin, benzamidine, gentamicin sulfate, and chloroamphenicol were purchased from Sigma. EDTA, chloroform (molecular biology grade), methanol (HPLC grade), trifluoroacetic acid, ammonium hydroxide, and water (HPLC grade) were from Fisher. All phospholipids were from Avanti Polar Lipids.
Blood Samples.
Forty blood samples were obtained from routine venipuncture from adult healthy donors (20 male, 20 female) after an overnight fast and then mixed with 0.129 M sodium citrate (vol/vol). Plasma was prepared by centrifugation at 2,000 × g for 20 min at room temperature and then stored at −80°C. Some plasma samples also were centrifuged at 30,000 × g for 30 min to remove putative cellular debris derived from platelets, mitochondria, etc. For lipoprotein analyses, eight blood samples (four male, four female) were obtained and anticoagulated with tetrasodium EDTA (2.7 mM final). After centrifugation (2,000 × g for 30 min at 4°C), aprotinin, benzamidine, gentamicin sulfate, chloroamphenicol, and sodium azide (47–49) were added, and plasma samples were stored at −80°C for subsequent lipoprotein purification and analysis.
Preparation of Lipoprotein.
Very low density lipoprotein (VLDL) (d ≤ 1.019 g/ml), LDL (d = 1.019–1.063 g/ml), and HDL (d = 1.063–1.21 g/ml) were isolated from normal human plasma by sequential density gradient ultracentrifugation in the presence of protease inhibitors and antioxidants and then stored in 0.3 mM EDTA at 4°C as described (47, 48). The HDL fraction (d = 1.063–1.21 g/ml) contained lipoprotein(a) [Lp(a)] composed of ApoB100 and Apo(a). However, based on ELISA data [CardioCheck Lp(a); AlerCHEK, Portland, ME], the amount of Lp(a) was 2.0 ± 4.3% (n = 8) of the total protein of the HDL fraction, and Lp(a) was not sufficient to influence the lipid profile of HDL.
Lipid Extractions.
Plasma and lipoprotein lipids were extracted twice from citrated plasma or purified lipoproteins with 4 vol of chloroform/methanol (2:1, vol/vol) as described (50). After centrifugation, the lower phase (organic phase) was collected and 2 vol of chloroform was added to the other phase. Then, after mixing and centrifugation, again the lower organic phase was collected, dried by using flowing nitrogen gas, and redissolved in chloroform and combined with the first extract.
Choline-Containing Phospholipid Analysis.
Plasma c-PL concentration, essentially PC plus SM, was determined by an enzymatic colorimetric method (Phospholipids B kit; Wako).
TLC Analysis.
TLC was carried out on 5 × 5-cm HP-TLC plates (SILICA GEL 60 F-254; Merck) with chloroform/methanol/hexane/acetic acid, 50:10:30:5 (vol/vol) (acidic condition) as developing solvent. After running the sample, the TLC plate was stained by iodine vapor. For analysis of CL in plasma extracts, the area on the plate that corresponded to the migration of standard CL (0.20–0.35 Rf) was scraped off and re-extracted by using a chloroform/methanol/1% sodium chloride mixture (6:3:2, vol/vol), and the organic phase was dried under nitrogen and reconstituted by chloroform for further analysis by HPLC and MS. Another developing solvent, chloroform/methanol/ammonium hydroxide, 65:25:4 (vol/vol; basic condition), also was used for analysis on TLC plates for CL.
HPLC Analysis.
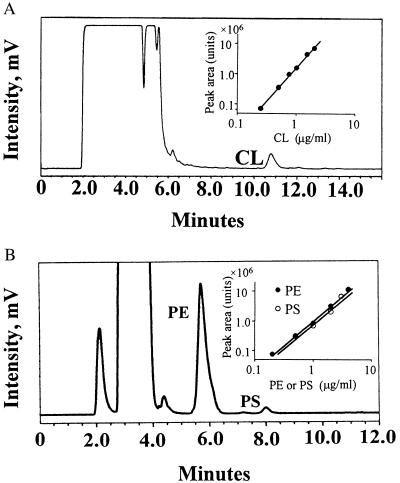
The HPLC system for PL analysis consisted of a Waters 600S controller, Waters 717plus autosampler, and an ELSD system, Sedex-55 (Sedere, Vitry sur Seine, France). The software program millennium32 (Waters) was used for data analysis. A Lichrosphere 100 Diol column (250 mm × 4.6 mm) with a guard column (Alltech Associates) was used with a linear gradient of chloroform/methanol mixtures (95:5 to 80:20%) from 0 to 16 min for CL analysis. For PE and PS analysis, a μPorosil column (300 mm × 3.9 mm) and its guard column (Waters) were used with isocratic chloroform/methanol/0.5% trifluoroacetic acid/water (79:19:1:1). The evaporation temperature of the ELSD was set to 50°C and the gain step was set to 11. The nebulization gas was nitrogen with a pressure of 2.0 bar. The flow rate in both conditions was 1 ml/min. For collection of peaks, a Micro-Splitter Valve (Western Analytical Products, Temecula, CA) was used, and 75% of the total flow was detected by ELSD and the other 25% was collected for further analysis. The identity of each peak was confirmed from the retention time of each corresponding lipid reference standard, and the enhancing technique was used as described (51). CL, PC, PE, SM, PS, phosphatidylglycerol, phosphatidylinositol, phosphatidic acid, lysoPC, and cholesterol were tested on these columns, and CL, PE, and PS peaks were clearly separated from other lipid peaks. To quantify CL, PE, and PS, calibration curves for each phospholipid were made by using standard CL, PE, and PS, which were obtained from freshly opened vials, as described (50). The calibration curve was nonlinear, and it was fitted by using the equation: phospholipid concentration = a[peak area]b (where the constant a = 0.0033, 0.000548, and 0.00026 and the exponent b = 0.448, 0.548, and 0.597 for CL, PE, and PS, respectively). See Fig. 2 Insets for a typical calibration curve.
Figure 2.
Chromatography of CL, PE, and PS of plasma lipid extract. Lipid extract from plasma was applied directly to an HPLC column as described in Materials and Methods. Peaks of CL (A), PS (B), and PE (B) are indicated. To quantify CL, PE, and PS, calibration curves for each phospholipid were constructed with purified CL, PE, and PS as described in Materials and Methods. (A and B Insets) Calibration curves on log–log plots for relating peak area to PL concentrations are shown. Each curve was fitted over the range of 0.5 to 5.0 μg of PL by using the equation as described in Materials and Methods.
MS Analysis.
The electrospray ionization (ESI) MS analysis was performed by using an API 100 Perkin–Elmer SCIEX single-quadrupole ion trap mass spectrometer. Negative and positive ESI spectra were analyzed in the mass range of 650 to 1,650 Da.
Statistical Analysis.
Statistical analyses were performed by using prism 3.0 (GraphPad, San Diego) to determine correlations and significance.
Results
Identification of CL in Normal Human Plasma.
Normal human plasma lipid extracts were analyzed by TLC as described in Materials and Methods. Under acidic conditions, CL, phosphatidylglycerol, PE, and phosphatidic acid migrated well (Rf = 0.28, 0.22, 0.19, and 0.39, respectively) whereas PC, SM, PS, and phosphatidylinositol migrated little (Rf = 0.03, 0.02, 0.05, and 0.02, respectively) and cholesterol migrated close to the solvent front (Rf = 0.84) (data not shown). After running plasma extracts on TLC, the silica gel in the area on the plate between 0.20 and 0.35 Rf corresponding to the migration of reference CL was scraped off and the lipid was extracted, dried, and used for subsequent analyses described below.
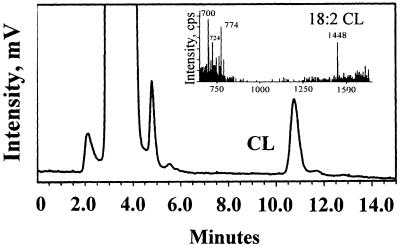
We developed a method to detect CL by using HPLC (see Materials and Methods). Under our conditions, standard CL (heart) with a retention time of 10.76 min was well separated from other lipid peaks, e.g., free fatty acids (3.97 min), and cholesterol (3.63 min). PC, SM, PE, PS, phosphatidylinositol, phosphatidic acid, and phosphatidylglycerol were not eluted under the condition employed. The material obtained from the normal plasma lipid extract isolated from the TLC plate (0.2–0.35 Rf) as described above had a peak with a retention time (10.76 min) identical to standard CL on HPLC (Fig. 1), suggesting this plasma lipid peak was CL. To confirm the identity of CL, negative ion spray MS analysis was performed. The plasma material that had been separated from the other lipids on the TLC plate showed peaks at m/z 1448 (first negative state of molecule) and 724 (second negative state of molecule) with negative electron spray (Fig. 1 Inset) that were identical to peaks observed for standard CL. Thus, plasma contains CL, and, based on molecular weight, the majority of plasma CL contains linoleic acid (18:2), which is also the major component of heart CL. Other peaks of m/z 700 and 774 in negative electron spray (Fig. 1 Inset) are likely to be other anionic phospholipids, 18:1 PA and 18:1 PG based on molecular mass, or could be CL degradation products.
Figure 1.
Identification of CL by using HPLC and MS analysis of purified human plasma lipid extract. Normal human plasma lipid was extracted by chloroform/methanol (2:1, vol/vol), and the extract was subjected to TLC as described in Materials and Methods. Subsequently, the extract from the TLC plate area corresponding to reference CL migration (0.2–0.35 Rf) was collected and analyzed by electrospray MS and analytical HPLC equipped with ELSD. HPLC analysis showed a peak with the identical retention time (10.76 min) as standard CL. (Inset) Mass spectral analysis showing a peak at m/z 1,448 for the purified plasma lipid extract, corresponding to the mass of 18:2 CL.
After chromatographic separation of CL by using either HPLC or TLC, more than 90% of both the standard heart CL and the plasma-derived CL appeared to be degraded when reanalyzed by using the same chromatographic methods. For example, post-HPLC standard CL showed various new peaks eluting at 2–6 min besides the CL peak at 10.76 min, and the peak at 10.76 min was less than 10% on the repeated HPLC chromatogram. Similarly, CL degradation patterns were observed in repeated TLC analyses, and more than 90% of the lipids eluted from the CL spot on the TLC plate migrated close to the solvent front on repeated TLC analysis. MS with positive electron spray also confirmed that the post-HPLC degradation products obtained from plasma CL were identical to those observed for post-HPLC standard CL (Avanti Polar Lipids) by their identical mass (m/z 758.5, 786.5, 806.5, 834.5, 858, and 1,175) (data not shown). These peaks likely corresponded to the new peaks eluting at 2–6 min on repeated HPLC analysis and to the new spots (close to solvent front) on repeated TLC analysis. Because these new peaks of reanalyzed CL were not observed in MS with negative electron spray, these may indicate loss of negative charge of CL by degradation during the HPLC process or second extraction of lipid. HPLC silica-diol column or TLC silica gel may act as ion exchanger, and CL would be in its unstable, salt-free form during the HPLC or TLC process, giving rise to a rapid CL degradation (52).
Quantification of Plasma CL and Phospholipids in 40 Healthy Subjects.
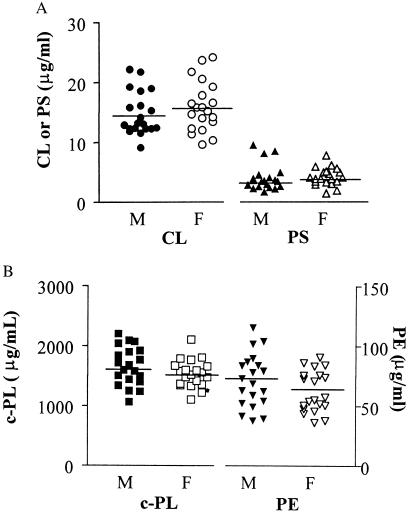
When plasma lipid extracts were subjected directly to HPLC analysis, one of the peaks had a retention time identical to standard CL (Fig. 2A). To confirm that the peak reflected CL content, we showed that addition of standard CL to plasma increased the area of this peak by as much as predicted based on the calibration curve (data not shown). When this HPLC peak was collected and analyzed by TLC using acidic and basic conditions, it gave Rf values (0.28 and 0.36, respectively) that were the same as standard CL. Thus, HPLC analysis of plasma lipid extracts permitted quantitation of CL for 40 healthy subjects. The mean value of plasma CL was 14.9 ± 3.7 μg/ml (n = 40; range, 9.1–24.2 μg/ml) (Fig. 3A) with Gaussian distribution (normality test: P < 0.01). There was no significant difference between mean values for 20 males and 20 females (P = 0.353, Student's t test). CL values for plasmas frozen at −70°C for 3–5 weeks were no different from those of fresh plasma (102 ± 5.8%, n = 6). To estimate how much CL in plasma might be derived from potential mitochondria on cell remnants, plasma was centrifuged at 30,000 × g for 30 min. Based on analysis before and after centrifugation and of the pellet, less than 1% of plasma CL (0.45 ± 0.17%, n = 4) was present in any sedimentable particles.
Figure 3.
Distribution of plasma CL, PE, PS, and choline-containing phospholipid. The concentrations of plasma CL, PS (A), PE, and c-PL (B) were determined in 40 healthy adult donors (20 males, 20 females) as described in Materials and Methods. Mean values (n = 40) for plasma CL, PS, PE, and c-PL were 14.9 ± 3.7 μg/ml (range, 9.1 to 24.2), 3.8 ± 1.7 μg/ml (range, 1.78 to 4.72), 64 ± 20 μg/ml (range, 36.2 to 115.2), and 1,580 ± 280 μg/ml (range, 1,068 to 2,200), respectively. All of the phospholipids were fitted satisfactorily with Gaussian distribution (normality test: P < 0.01), and no statistical differences were found between males and females.
The c-PL mean concentration was 1,580 ± 280 μg/ml (n = 40; range, 1,070 to 2,200 μg/ml) (Fig. 3B). The mean plasma PE level was 64 ± 20 μg/ml (n = 40; range, 36.2 to 115.2 μg/ml) (Fig. 3B), in good agreement with previous reports (33–35). Plasma PS levels are low (15) and sometimes difficult to quantitate in plasma or in lipoproteins (15). However, the method presented here readily permitted PS measurement. The plasma concentration of PS was smaller than the other phospholipids [3.8 ± 1.7 μg/ml (n = 40; range, 1.78 to 4.72 μg/ml)] (Fig. 3B). Notably, plasma contained significantly more CL than PS. The distribution pattern of PS and PE were Gaussian (normality test: P < 0.01). Neither PS nor PE showed any significant difference between mean values for males and females (P = 0.82 and 0.171, respectively, Student's t test). When various values for the 40 subjects were analyzed, plasma CL levels were not correlated with levels of c-PL, PS, or PE (as described below). Plasma PE levels correlated strongly with plasma c-PL levels (P < 0.0001, r = 0.5898) (Table 1).
Table 1.
Correlation between plasma phospholipids
| CL | PS | PE | c-PL | |
|---|---|---|---|---|
| CL | — | 0.32 | 0.78 | 0.50 |
| PS | — | 0.73 | 0.87 | |
| PE | — | <0.0001 | ||
| c-PL | — |
Correlations among phospholipids are expressed as a P value (n = 40). Statistical analysis was performed with statistical software Prism 3.0. The value of R for correlation of PE with c-PL was 0.60.
Phospholipid Composition of Human Plasma Lipoproteins.
When lipoproteins were purified from eight of 40 subjects' fasting plasmas by sequential density gradient ultracentrifugation, ≥94% CL was recovered in VLDL, LDL, and HDL (11 ± 5.3%, 67 ± 11.0%, 17 ± 10%, respectively) (Table 2). PS and PE also were recovered in VLDL, LDL, and HDL, and their distribution among the lipoprotein fractions is given in Table 2 (PE: 20 ± 11%, 47 ± 18%, and 29 ± 17%; PS: 9.0 ± 4.6%, 81 ± 9.3%, and 7.5 ± 5.1%, respectively). PE, PS, and CL are minor components of lipoproteins compared with c-PL (PC plus SM), and PE represents 3.2–4.7% of the PL in lipoproteins (Table 3). CL and PS represent less than 1% of the PL in lipoproteins (Table 3). Interestingly, the phospholipid composition of LDL had relatively higher amounts of anionic phospholipids CL and PS and less PE than the other lipoproteins. The molar ratios of CL/PS (7.0) and PE/PS (72) in HDL were three times and seven times higher, respectively, than in LDL (2.2 and 9.7, respectively), indicating that HDL is enriched selectively in CL and PE relative to VLDL or LDL (Table 4).
Table 2.
Distribution of phospholipids in plasma lipoproteins
| CL, % | PS, % | PE, % | c-PL, % | |
|---|---|---|---|---|
| VLDL | 11 ± 5.3 | 9.0 ± 4.6 | 20 ± 11 | 14 ± 7.1 |
| HDL | 17 ± 10 | 7.5 ± 5.1 | 29 ± 17 | 24 ± 13 |
| LDL | 67 ± 11 | 81 ± 9.3 | 47 ± 18 | 52 ± 14 |
| Infranatant | 5.0 ± 1.0 | 5.4 ± 6.2 | 4.2 ± 4.2 | 11 ± 2.8 |
The distributions of each phospholipid in individual lipoprotein subfractions are shown on a percentage basis for that phospholipid (mean ± SD; n = 8).
Table 3.
Phospholipid content of CL, PS, and PE in lipoprotein subfractions
| CL, % | PS, % | PE, % | |
|---|---|---|---|
| VLDL | 0.42 ± 0.04 | 0.13 ± 0.03 | 4.7 ± 0.98 |
| HDL | 0.40 ± 0.06 | 0.06 ± 0.02 | 4.2 ± 0.76 |
| LDL | 0.77 ± 0.17 | 0.36 ± 0.10 | 3.2 ± 0.64 |
Each phospholipid is expressed on a molar basis as a percentage of the total phospholipid, which was calculated as the sum PE + CL + PS + c-PL for each lipoprotein subfraction (mean ± SD; n = 8).
Table 4.
Ratios of some minor phospholipids in lipoprotein subfractions
| CL/PS | PE/PS | PE/CL | |
|---|---|---|---|
| VLDL | 3.3 ± 0.61 | 38 ± 9.7 | 11 ± 2.6 |
| HDL | 7.0 ± 2.2 | 72 ± 20 | 10 ± 1.0 |
| LDL | 2.2 ± 0.55 | 9.7 ± 3.7 | 4.5 ± 1.6 |
The ratio of minor phospholipids in lipoprotein subfractions was calculated based on data in Table 3 (mean ± SD; n = 8).
Discussion
CL, diphosphatidylglycerol, is a unique anionic phospholipid that is composed of two phosphate groups and four fatty acid side chains (13). Immobilized CL on microtiter plates is used to detect anti-CL antibodies and to help diagnose or define antiphospholipid antibody syndrome (5, 8). Epitopes of antiphospholipid antibodies have been studied for a long time because of their considerable clinical importance. Historically, the assumption regarding the identity of the primary antigen was that it was a phospholipid that was either directly antigenic or was a mandatory component of an antigenic complex. Because biologically available CL was predominantly in intracellular mitochondria (13), CL was considered not to be exposed to circulating antibodies and, therefore, not likely among the phospholipids to be an autoantigen (12). Other anionic phospholipids, such as PS, apparently also bind to some antiphospholipid antibodies. PS, which is found in lipoproteins, is accessible to circulating antibodies and therefore was considered to be a better candidate for antigen of anti-CL antibodies. The putative absence of CL in plasma stimulated a search for other “anti-CL” epitopes. In recent years, “anti-CL” antibodies have been shown to include anti-β2-glycoprotein I (7, 8, 53).
Stimulated by our interest in the anticoagulant properties of CL (45) and HDL (46), we decided to search for and to quantitate CL in plasma and lipoproteins. Using TLC, HPLC, and MS analyses, we demonstrated the presence of CL in normal human plasma. A number of technical difficulties associated with efforts to quantitate lipid components, especially trace amounts of phospholipids or glycolipids, can be overcome by using ELSD methods (50). The quantitation of CL, particularly in small amounts, was challenging because of, at least in part, its properties of easy oxidation in air and/or high lability in the absence of salt (10, 52). Nonetheless, we quantitated CL in 40 healthy adult subjects who had a mean CL level of 14.9 ± 3.7 μg/ml. The distribution of plasma CL was Gaussian, and CL levels in males were the same as in females. Notably, plasma contains more CL (15 μg/ml) than another anionic phospholipid, PS (3.8 μg/ml), and CL is a major anionic phospholipid in human plasma. Like other phospholipids, namely c-PL, PS, and PE, more than 94% of CL is located in lipoproteins (Table 2). The other 5% of CL was considered to be associated with proteins that have density higher than 1.21 g/liter, such as very high density lipoprotein or perhaps other phospholipid-binding proteins such as β2-glycoprotein I, prothrombin, protein C, protein S, complement C4-binding protein, etc.
What is the source of CL in plasma lipoproteins? Biosynthesis of lipoproteins occurs on the endoplasmic reticulum in the liver or intestine where phospholipids combine with apoproteins, e.g., apoA-I or apo B, to form nascent lipoproteins (54). Biosynthesis of CL in mammals involves the formation of CDP-diacylglycerol mediated by CDP-diacylglycerol synthase (55, 56), which is located in close association with the membrane of the endoplasmic reticulum (57). Thus, it is reasonable to speculate that the CL found in lipoproteins is combined with nascent lipoproteins as are other phospholipids in the endoplasmic reticulum of liver or intestine.
Blood coagulation pathways and the anticoagulant protein C pathway can be modulated by plasma lipids and lipoproteins. The procoagulant influences of PS and PE on the coagulation system have been well investigated in purified systems (37–44). Anionic PS is believed to be a particularly procoagulant phospholipid (38) whereas PE is apparently, under certain conditions, an anticoagulant phospholipid that acts by enhancing the anticoagulant action of APC (37, 44). We found that CL also can act anticoagulantly by selectively enhancing protein S-dependent APC activity (unpublished results; ref. 45). Because the plasma concentration of CL is similar to that of other minor phospholipids, e.g., PE or PS, CL is likely to be present in normal cell membranes as well as in lipoproteins, and CL also may exert anticoagulant activity in vivo.
Elevated LDL cholesterol or a combination of hypertriglyceridemia and low HDL are risk factors for coronary heart disease, whereas elevated HDL has an inverse correlation with incidence of coronary artery disease (58–64). HDL infusions show anti-inflammatory and antithrombotic activities in animals (65–67). HDL inhibits platelet aggregation and enhances prostacyclin production by endothelial cells (68, 69). HDL can act as an anticoagulant cofactor by enhancing protein S-dependent APC activity (46). The phospholipid composition in lipoproteins is likely to be functionally important. Phospholipids such as PS and PE exhibit significant influences on the blood coagulation reactions (37–44) and might contribute to either beneficial or deleterious properties of lipoproteins. In the analysis of lipoprotein phospholipid composition, we found significant differences in the relative phospholipid content of HDL and LDL with respect to the anionic phospholipid, PS. The relative content of PS in HDL (0.06%) is notably six times lower than that of LDL (0.36%). In contrast, the relative content of PE in each lipoprotein shows only modest variation (3.2–4.6%). CL in LDL (0.77%) is two times higher than in HDL (0.40%) (Table 3). These differences lead to significant differences in the CL/PS and PE/PS ratios for HDL compared with LDL. The molar ratios of CL/PS (7.0) and PE/PS (72) in HDL were three times and seven times higher, respectively, than ratios in LDL (2.2 and 9.7, respectively) (Table 4), indicating that HDL is selectively enriched in CL and PE compared with LDL. The relative enrichment of HDL in CL and PE might contribute to the anticoagulant properties of HDL because both CL and PE may be considered anticoagulant phospholipids. The relative distribution of the various phospholipids is quite similar in VLDL and HDL. Because apoprotein exchange occurs readily between HDL and VLDL, the apoproteins may influence the phospholipid profile of lipoproteins.
Ox-LDL is present in atherosclerotic lesions (19–25) and circulating plasma (70–72). Oxidative modification of LDL seems to be involved in the development and progression of atherosclerosis (73), the migration of macrophages, the cytotoxicity toward endothelial and foam cells, the alteration of expression of certain genes in the arterial walls (74–76), and the release of IL-1 and chemoattractant activity from macrophages (77) and T cells (78). Thus, ox-LDL can contribute to atherogenesis via many pathways. Ox-LDL is also immunogenic, and autoantibodies recognizing epitopes of highly ox-LDL have been described in plasma and atherosclerotic lesions (20, 26). Moreover, higher titers of these autoantibodies are present in patients with atherosclerotic disease (27–31). On the other hand, antiphospholipid antibodies can inhibit binding of ox-LDL to receptors and recognize ox-PL (9, 10, 18). Moreover, antibodies against ox-PL include antibodies against purified ox-CL (10). Our finding that CL is the major plasma anionic phospholipid in LDL may link these findings, and antibodies against ox-PL in ox-LDL may include antibodies against ox-CL in ox-LDL. Such antibodies may correlate with or contribute to atherogenesis. Therefore, we suggest that the epitopes of antiphospholipid antibodies could include CL or ox-CL either in lipoproteins or in complexes with various plasma proteins, e.g., β2-glycoprotein I, prothrombin, protein C, protein S, or complement C4b-binding protein, or others. Furthermore, we speculate that the epitopes of antiphospholipid antibodies could include CL or ox-CL complexed with platelet or endothelial surface proteins. Identification of the true epitopes of various so-called “anti-CL antibodies” may help clarify the pathogenic mechanisms responsible for antiphospholipid antibody-dependent ischemic diseases and venous and arterial thrombosis.
In summary, we found that CL is a normal component of human plasma lipoproteins. This discovery may lead to new insights concerning the pathogenesis of thrombosis or atherogenesis involving CL, lipoproteins, ox-lipoproteins, and antiphospholipid antibodies.
Acknowledgments
We are grateful to Dr. Ernest Beutler for helpful support of the HPLC/ELSD system, Drs. Stephen Kent and Phil Dawson for making available the mass spectrometer, and Aimie Goto for helpful technical assistance. Support was provided, in part, by National Institutes of Health Grants HL21544 (J.H.G.) and HL55517 (C.L.B), an American Heart Association Postdoctoral Research Fellowship (California Affiliate), and the Stein Endowment Fund.
Abbreviations
- CL
cardiolipin
- PL
phospholipid
- c-PL
choline-containing PL
- ELSD
evaporative light scattering detector
- PC
phosphatidylcholine
- PS
phosphatidylserine
- PE
phosphatidylethanolamine
- SM
sphingomyelin
- VLDL
very low density lipoprotein
- LDL
low density lipoprotein
- HDL
high density lipoprotein
- ox-
oxidized
- APC
activated protein C
- Lp(a)
lipoprotein(a)
References
- 1.Hughes G R. Br Med J. 1983;287:1088–1089. doi: 10.1136/bmj.287.6399.1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Harris E N, Gharavi A E, Asherson R A, Boey M L, Hughes G R. Clin Exp Rheumatol. 1984;2:47–51. [PubMed] [Google Scholar]
- 3.Edwards T, Thomas R D, McHugh N J. Lancet. 1993;342:989. doi: 10.1016/0140-6736(93)92035-r. [DOI] [PubMed] [Google Scholar]
- 4.Rand J H, Wu X X, Andree H A, Ross J B, Rusinova E, Gascon-Lema M G, Calandri C, Harpel P C. Blood. 1998;92:1652–1660. [PubMed] [Google Scholar]
- 5.Harris E N, Gharavi A E, Boey M L, Patel B M, Mackworth-Young C G, Loizou S, Hughes G R. Lancet. 1983;2:1211–1214. doi: 10.1016/s0140-6736(83)91267-9. [DOI] [PubMed] [Google Scholar]
- 6.Matsuura E, Igarashi Y, Fujimoto M, Ichikawa K, Koike T. Lancet. 1990;336:177–178. doi: 10.1016/0140-6736(90)91697-9. [DOI] [PubMed] [Google Scholar]
- 7.Galli M, Comfurius P, Maassen C, Hemker H C, de Baets M H, van Breda-Vriesman P J, Barbui T, Zwaal R F, Bevers E M. Lancet. 1990;335:1544–1547. doi: 10.1016/0140-6736(90)91374-j. [DOI] [PubMed] [Google Scholar]
- 8.Kandiah D A, Sali A, Sheng Y, Victoria E J, Marquis D M, Coutts S M, Krilis S A. Adv Immunol. 1998;70:507–563. doi: 10.1016/s0065-2776(08)60393-4. [DOI] [PubMed] [Google Scholar]
- 9.Horkko S, Miller E, Dudl E, Reaven P, Curtiss L K, Zvaifler N J, Terkeltaub R, Pierangeli S S, Branch D W, Palinski W, et al. J Clin Invest. 1996;98:815–825. doi: 10.1172/JCI118854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Horkko S, Miller E, Branch D W, Palinski W, Witztum J L. Proc Natl Acad Sci USA. 1997;94:10356–10361. doi: 10.1073/pnas.94.19.10356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rauch J. Lupus. 1998;7, Suppl. 2:S29–S31. doi: 10.1177/096120339800700207. [DOI] [PubMed] [Google Scholar]
- 12.Lockshin M D. In: The Antiphospholipid Syndrome. Asherson R A, Cervera R, Piette J C, Shoenfeld Y, editors. New York: CRC; 1999. pp. 323–329. [Google Scholar]
- 13.Marinetti G V, Erbland J, Stotz E. J Biol Chem. 1958;233:562–565. [PubMed] [Google Scholar]
- 14.Rote N S, Dostal-Johnson D, Branch D W. Am J Obstet Gynecol. 1990;163:575–584. doi: 10.1016/0002-9378(90)91201-m. [DOI] [PubMed] [Google Scholar]
- 15.Chapman M J. Methods Enzymol. 1986;128:70–143. doi: 10.1016/0076-6879(86)28063-5. [DOI] [PubMed] [Google Scholar]
- 16.Arvieux J, Pernod G, Regnault V, Darnige L, Garin J. Blood. 1999;93:4248–4255. [PubMed] [Google Scholar]
- 17.Oosting J D, Derksen R H, Bobbink I W, Hackeng T M, Bouma B N, de Groot P G. Blood. 1993;81:2618–2625. [PubMed] [Google Scholar]
- 18.Horkko S, Bird D A, Miller E, Itabe H, Leitinger N, Subbanagounder G, Berliner J A, Friedman P, Dennis E A, Curtiss L K, et al. J Clin Invest. 1999;103:117–128. doi: 10.1172/JCI4533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Palinski W, Tangirala R K, Miller E, Young S G, Witztum J L. Arterioscler Thromb Vasc Biol. 1995;15:1569–1576. doi: 10.1161/01.atv.15.10.1569. [DOI] [PubMed] [Google Scholar]
- 20.Yla-Herttuala S, Palinski W, Butler S W, Picard S, Steinberg D, Witztum J L. Arterioscler Thromb. 1994;14:32–40. doi: 10.1161/01.atv.14.1.32. [DOI] [PubMed] [Google Scholar]
- 21.Yla-Herttuala S, Palinski W, Rosenfeld M E, Parthasarathy S, Carew T E, Butler S, Witztum J L, Steinberg D. J Clin Invest. 1989;84:1086–1095. doi: 10.1172/JCI114271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Berliner J, Leitinger N, Watson A, Huber J, Fogelman A, Navab M. Thromb Haemostasis. 1997;78:195–199. [PubMed] [Google Scholar]
- 23.Navab M, Berliner J A, Watson A D, Hama S Y, Territo M C, Lusis A J, Shih D M, Van Lenten B J, Frank J S, Demer L L, et al. Arterioscler Thromb Vasc Biol. 1996;16:831–842. doi: 10.1161/01.atv.16.7.831. [DOI] [PubMed] [Google Scholar]
- 24.Navab M, Fogelman A M, Berliner J A, Territo M C, Demer L L, Frank J S, Watson A D, Edwards P A, Lusis A J. Am J Cardiol. 1995;76:18C–23C. doi: 10.1016/s0002-9149(99)80466-4. [DOI] [PubMed] [Google Scholar]
- 25.Berliner J A, Navab M, Fogelman A M, Frank J S, Demer L L, Edwards P A, Watson A D, Lusis A J. Circulation. 1995;91:2488–2496. doi: 10.1161/01.cir.91.9.2488. [DOI] [PubMed] [Google Scholar]
- 26.Palinski W, Ord V A, Plump A S, Breslow J L, Steinberg D, Witztum J L. Arterioscler Thromb. 1994;14:605–616. doi: 10.1161/01.atv.14.4.605. [DOI] [PubMed] [Google Scholar]
- 27.Salonen J T, Yla-Herttuala S, Yamamoto R, Butler S, Korpela H, Salonen R, Nyyssonen K, Palinski W, Witztum J L. Lancet. 1992;339:883–887. doi: 10.1016/0140-6736(92)90926-t. [DOI] [PubMed] [Google Scholar]
- 28.Bellomo G, Maggi E, Poli M, Agosta F G, Bollati P, Finardi G. Diabetes. 1995;44:60–66. doi: 10.2337/diab.44.1.60. [DOI] [PubMed] [Google Scholar]
- 29.Bergmark C, Wu R, de Faire U, Lefvert A K, Swedenborg J. Arterioscler Thromb Vasc Biol. 1995;15:441–445. doi: 10.1161/01.atv.15.4.441. [DOI] [PubMed] [Google Scholar]
- 30.Branch D W, Mitchell M D, Miller E, Palinski W, Witztum J L. Lancet. 1994;343:645–646. doi: 10.1016/s0140-6736(94)92639-5. [DOI] [PubMed] [Google Scholar]
- 31.Maggi E, Chiesa R, Melissano G, Castellano R, Astore D, Grossi A, Finardi G, Bellomo G. Arterioscler Thromb. 1994;14:1892–1899. doi: 10.1161/01.atv.14.12.1892. [DOI] [PubMed] [Google Scholar]
- 32.Subbaiah P V. In: Book of Lipoprotein Testing. Rifai N, Warnick G R, Dominiczak M H, editors. Washington, DC: AACC; 1997. pp. 415–429. [Google Scholar]
- 33.Vaysse J, Pilardeau P, Garnier M. Clin Chim Acta. 1985;147:183–190. doi: 10.1016/0009-8981(85)90081-6. [DOI] [PubMed] [Google Scholar]
- 34.Fournier N, Paul J L, Atger V, Cogny A, Soni T, de la Llera-Moya M, Rothblat G, Moatti N. Arterioscler Thromb Vasc Biol. 1997;17:2685–2691. doi: 10.1161/01.atv.17.11.2685. [DOI] [PubMed] [Google Scholar]
- 35.Cesar J M, Vecino A, Perez-Vaquero M, Navarro J L. Eur J Haematol. 1993;50:234–236. doi: 10.1111/j.1600-0609.1993.tb01926.x. [DOI] [PubMed] [Google Scholar]
- 36.Noël C, Marcel Y L, Davignon J. J Lab Clin Med. 1972;79:611–621. [PubMed] [Google Scholar]
- 37.Smirnov M D, Triplett D T, Comp P C, Esmon N L, Esmon C T. J Clin Invest. 1995;95:309–316. doi: 10.1172/JCI117657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pei G, Powers D D, Lentz B R. J Biol Chem. 1993;268:3226–3233. [PubMed] [Google Scholar]
- 39.Mann K G, Nesheim M E, Church W R, Haley P, Krishnaswamy S. Blood. 1990;76:1–16. [PubMed] [Google Scholar]
- 40.Nelsestuen G L, Broderius M. Biochemistry. 1977;16:4172–4177. doi: 10.1021/bi00638a006. [DOI] [PubMed] [Google Scholar]
- 41.Subbaiah P V, Bajwa S S, Smith C M, Hanahan D J. Biochim Biophys Acta. 1976;444:131–146. doi: 10.1016/0304-4165(76)90230-0. [DOI] [PubMed] [Google Scholar]
- 42.Nelsestuen G L, Kisiel W, Di Scipio R G. Biochemistry. 1978;17:2134–2138. doi: 10.1021/bi00604a017. [DOI] [PubMed] [Google Scholar]
- 43.Rosing J, Speijer H, Zwaal R F. Biochemistry. 1988;27:8–11. doi: 10.1021/bi00401a002. [DOI] [PubMed] [Google Scholar]
- 44.Smirnov M D, Esmon C T. J Biol Chem. 1994;269:816–819. [PubMed] [Google Scholar]
- 45.Fernández J A, Kojima K, Hackeng T M, Griffin J H. Thromb Haemostasis. 1995;73:1263. [PubMed] [Google Scholar]
- 46.Griffin J H, Kojima K, Banka C L, Curtiss L K, Fernandez J A. J Clin Invest. 1999;103:219–227. doi: 10.1172/JCI5006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Banka C L, Bonnet D J, Black A S, Smith R S, Curtiss L K. J Biol Chem. 1991;266:23886–23892. [PubMed] [Google Scholar]
- 48.Banka C L, Black A S, Dyer C A, Curtiss L K. J Lipid Res. 1991;32:35–43. [PubMed] [Google Scholar]
- 49.Tong H, Knapp H R, VanRollins M. J Lipid Res. 1998;39:1696–1704. [PubMed] [Google Scholar]
- 50.Bunger H, Pison U. J Chromatogr B Biomed Appl. 1995;672:25–31. doi: 10.1016/0378-4347(95)00190-t. [DOI] [PubMed] [Google Scholar]
- 51.Conforti F D, Harris C H, Rinehart J T. J Chromatogr. 1993;645:83–88. [Google Scholar]
- 52.de Haas G H, Bonsen P P, van Deenen L L. Biochim Biophys Acta. 1966;116:114–124. doi: 10.1016/0005-2760(66)90097-x. [DOI] [PubMed] [Google Scholar]
- 53.McNeil H P, Simpson R J, Chesterman C N, Krilis S A. Proc Natl Acad Sci USA. 1990;87:4120–4124. doi: 10.1073/pnas.87.11.4120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Vance J E, Vance D E. Experientia. 1990;46:560–569. doi: 10.1007/BF01939694. [DOI] [PubMed] [Google Scholar]
- 55.McMurray W C, Magee W L. Annu Rev Biochem. 1972;41:129–160. doi: 10.1146/annurev.bi.41.070172.001021. [DOI] [PubMed] [Google Scholar]
- 56.Mok A Y, McDougall G E, McMurray W C. Biochem Cell Biol. 1993;71:183–189. doi: 10.1139/o93-029. [DOI] [PubMed] [Google Scholar]
- 57.Saito S, Goto K, Tonosaki A, Kondo H. J Biol Chem. 1997;272:9503–9509. doi: 10.1074/jbc.272.14.9503. [DOI] [PubMed] [Google Scholar]
- 58.Gordon D J, Rifkind B M. N Engl J Med. 1989;321:1311–1316. doi: 10.1056/NEJM198911093211907. [DOI] [PubMed] [Google Scholar]
- 59.Barter P J, Rye K A. Atherosclerosis. 1996;121:1–12. doi: 10.1016/0021-9150(95)05675-0. [DOI] [PubMed] [Google Scholar]
- 60.Tall A R. J Clin Invest. 1990;86:379–384. doi: 10.1172/JCI114722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tribble D L, Krauss R M. Adv Intern Med. 1993;38:1–29. [PubMed] [Google Scholar]
- 62.Stein O, Stein Y. Atherosclerosis. 1999;144:285–301. doi: 10.1016/s0021-9150(99)00065-9. [DOI] [PubMed] [Google Scholar]
- 63.Brunzell J D, Hokanson J E. Am J Med. 1999;107:16S–18S. doi: 10.1016/s0002-9343(99)00139-4. [DOI] [PubMed] [Google Scholar]
- 64.Kwiterovich P O J. Am J Cardiol. 1998;82:13Q–21Q. doi: 10.1016/s0002-9149(98)00808-x. [DOI] [PubMed] [Google Scholar]
- 65.Pajkrt D, Lerch P G, van der Poll T, Levi M, Illi M, Doran J E, Arnet B, van den Ende A, ten Cate J W, van Deventer S J. Thromb Haemostasis. 1997;77:303–307. [PubMed] [Google Scholar]
- 66.Pajkrt D, Doran J E, Koster F, Lerch P G, Arnet B, van der Poll T, ten Cate J W, van Deventer S J. J Exp Med. 1996;184:1601–1608. doi: 10.1084/jem.184.5.1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Li D, Weng S, Yang B, Zander D S, Saldeen T, Nichols W W, Khan S, Mehta J L. Arterioscler Thromb Vasc Biol. 1999;19:378–383. doi: 10.1161/01.atv.19.2.378. [DOI] [PubMed] [Google Scholar]
- 68.Oravec S, Demuth K, Myara I, Hornych A. Thromb Res. 1998;92:65–71. doi: 10.1016/s0049-3848(98)00106-6. [DOI] [PubMed] [Google Scholar]
- 69.Fleisher L N, Tall A R, Witte L D, Miller R W, Cannon P J. J Biol Chem. 1982;257:6653–6655. [PubMed] [Google Scholar]
- 70.Itabe H, Yamamoto H, Imanaka T, Shimamura K, Uchiyama H, Kimura J, Sanaka T, Hata Y, Takano T. J Lipid Res. 1996;37:45–53. [PubMed] [Google Scholar]
- 71.Juul K, Nielsen L B, Munkholm K, Stender S, Nordestgaard B G. Circulation. 1996;94:1698–1704. doi: 10.1161/01.cir.94.7.1698. [DOI] [PubMed] [Google Scholar]
- 72.Palinski W, Horkko S, Miller E, Steinbrecher U P, Powell H C, Curtiss L K, Witztum J L. J Clin Invest. 1996;98:800–814. doi: 10.1172/JCI118853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Witztum J L. Lancet. 1994;344:793–795. doi: 10.1016/s0140-6736(94)92346-9. [DOI] [PubMed] [Google Scholar]
- 74.Cushing S D, Berliner J A, Valente A J, Territo M C, Navab M, Parhami F, Gerrity R, Schwartz C J, Fogelman A M. Proc Natl Acad Sci USA. 1990;87:5134–5138. doi: 10.1073/pnas.87.13.5134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Rajavashisth T B, Yamada H, Mishra N K. Arterioscler Thromb Vasc Biol. 1995;15:1591–1598. doi: 10.1161/01.atv.15.10.1591. [DOI] [PubMed] [Google Scholar]
- 76.Rajavashisth T B, Andalibi A, Territo M C, Berliner J A, Navab M, Fogelman A M, Lusis A J. Nature (London) 1990;344:254–257. doi: 10.1038/344254a0. [DOI] [PubMed] [Google Scholar]
- 77.Quinn M T, Parthasarathy S, Fong L G, Steinberg D. Proc Natl Acad Sci USA. 1987;84:2995–2998. doi: 10.1073/pnas.84.9.2995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.McMurray H F, Parthasarathy S, Steinberg D. J Clin Invest. 1993;92:1004–1008. doi: 10.1172/JCI116605. [DOI] [PMC free article] [PubMed] [Google Scholar]