Abstract
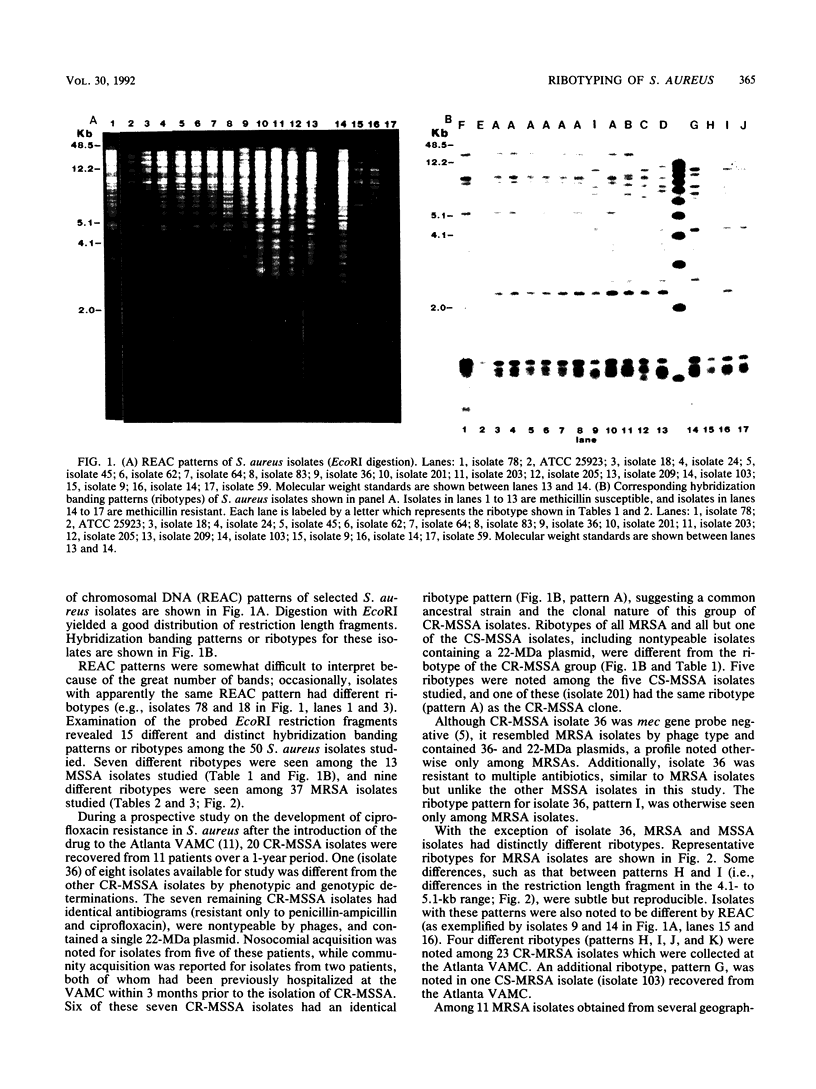
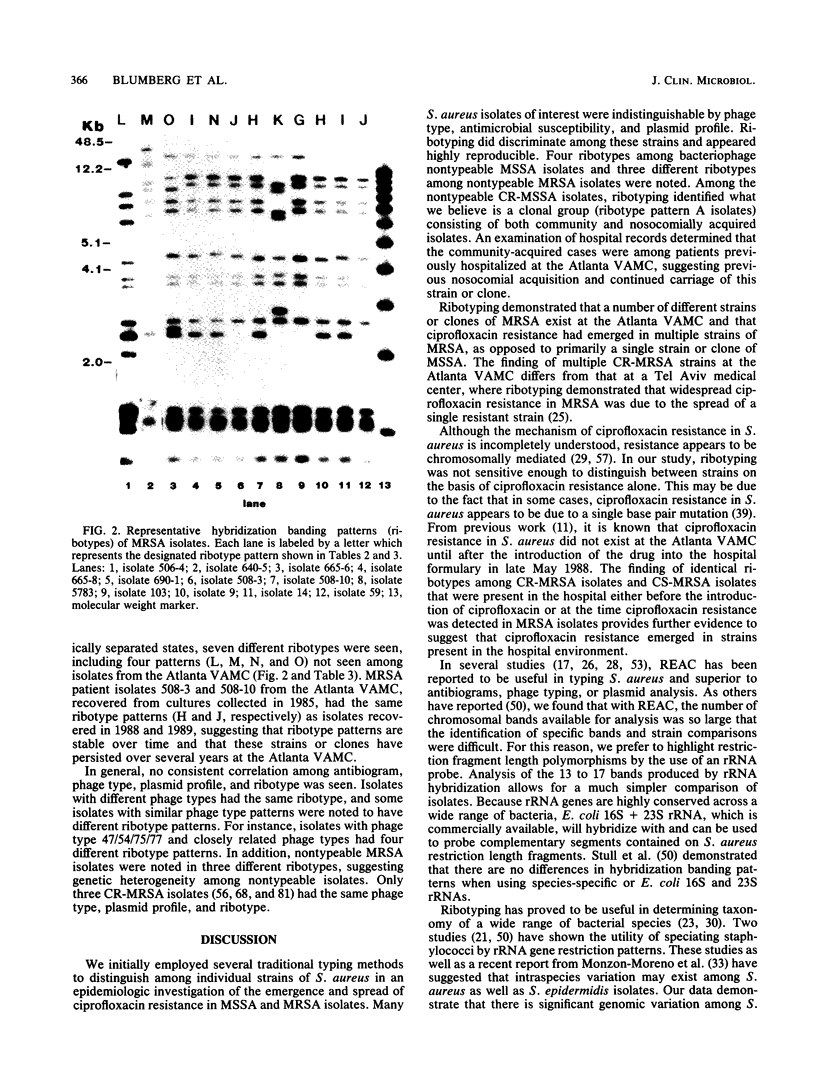
Analysis of DNA restriction fragment length polymorphisms of rRNA genes (ribotyping) was employed to assist in the epidemiologic investigation of the emergence and spread of ciprofloxacin-resistant Staphylococcus aureus at the Atlanta VA Medical Center because many isolates of interest were nontypeable by phages and harbored few plasmids useful as strain markers. Chromosomal DNAs of selected S. aureus isolates were digested initially with 20 different restriction enzymes. EcoRI appeared to give the best discrimination of hybridization banding patterns (ribotypes) and was used with all study isolates. Overall, 15 different ribotypes were seen among the 50 S. aureus isolates studied (7 ribotypes among 13 methicillin-susceptible S. aureus [MSSA] isolates and 9 ribotypes among 37 methicillin-resistant S. aureus [MRSA] isolates). Seven of eight ciprofloxacin-resistant MSSA (CR-MSSA) patient isolates had identical antibiograms, were nontypeable by phages, and had a single 22-MDa plasmid. Six of these seven CR-MSSA isolates had an identical ribotype pattern. Ribotyping distinguished this CR-MSSA strain or clone from MRSA and other MSSA isolates, including nontypeable isolates that contained a 22-MDa plasmid. Five ciprofloxacin-susceptible MSSA isolates studied had five ribotypes; one pattern was identical to the CR-MSSA clone. Twenty-three CR-MRSA isolates recovered from the Atlanta VA Medical Center had four different ribotypes. Ribotyping proved to be a useful molecular epidemiologic tool in the study of S. aureus because it differentiated isolates which were indistinguishable by more traditional methods. In addition, this technique demonstrated that at our institution, ciprofloxacin resistance emerged in multiple strains of MRSA, as opposed to primarily a single strain or clone of MSSA.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allardet-Servent A., Bouziges N., Carles-Nurit M. J., Bourg G., Gouby A., Ramuz M. Use of low-frequency-cleavage restriction endonucleases for DNA analysis in epidemiological investigations of nosocomial bacterial infections. J Clin Microbiol. 1989 Sep;27(9):2057–2061. doi: 10.1128/jcm.27.9.2057-2061.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altwegg M., Hickman-Brenner F. W., Farmer J. J., 3rd Ribosomal RNA gene restriction patterns provide increased sensitivity for typing Salmonella typhi strains. J Infect Dis. 1989 Jul;160(1):145–149. doi: 10.1093/infdis/160.1.145. [DOI] [PubMed] [Google Scholar]
- Arbeit R. D., Arthur M., Dunn R., Kim C., Selander R. K., Goldstein R. Resolution of recent evolutionary divergence among Escherichia coli from related lineages: the application of pulsed field electrophoresis to molecular epidemiology. J Infect Dis. 1990 Feb;161(2):230–235. doi: 10.1093/infdis/161.2.230. [DOI] [PubMed] [Google Scholar]
- Archer G. L., Mayhall C. G. Comparison of epidemiological markers used in the investigation of an outbreak of methicillin-resistant Staphylococcus aureus infections. J Clin Microbiol. 1983 Aug;18(2):395–399. doi: 10.1128/jcm.18.2.395-399.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arthur M., Arbeit R. D., Kim C., Beltran P., Crowe H., Steinbach S., Campanelli C., Wilson R. A., Selander R. K., Goldstein R. Restriction fragment length polymorphisms among uropathogenic Escherichia coli isolates: pap-related sequences compared with rrn operons. Infect Immun. 1990 Feb;58(2):471–479. doi: 10.1128/iai.58.2.471-479.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumberg H. M., Rimland D., Carroll D. J., Terry P., Wachsmuth I. K. Rapid development of ciprofloxacin resistance in methicillin-susceptible and -resistant Staphylococcus aureus. J Infect Dis. 1991 Jun;163(6):1279–1285. doi: 10.1093/infdis/163.6.1279. [DOI] [PubMed] [Google Scholar]
- Boyce J. M. Methicillin-resistant Staphylococcus aureus. Detection, epidemiology, and control measures. Infect Dis Clin North Am. 1989 Dec;3(4):901–913. [PubMed] [Google Scholar]
- Branger C., Goullet P., Boutonnier A., Fournier J. M. Correlation between esterase electrophoretic types and capsular polysaccharide types 5 and 8 among methicillin-susceptible and methicillin-resistant strains of Staphylococcus aureus. J Clin Microbiol. 1990 Jan;28(1):150–151. doi: 10.1128/jcm.28.1.150-151.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Branger C., Goullet P. Esterase electrophoretic polymorphism of methicillin-sensitive and methicillin-resistant strains of Staphylococcus aureus. J Med Microbiol. 1987 Nov;24(3):275–281. doi: 10.1099/00222615-24-3-275. [DOI] [PubMed] [Google Scholar]
- Branger C., Goullet P. Genetic heterogeneity in methicillin-resistant strains of Staphylococcus aureus revealed by esterase electrophoretic polymorphism. J Hosp Infect. 1989 Aug;14(2):125–134. doi: 10.1016/0195-6701(89)90115-1. [DOI] [PubMed] [Google Scholar]
- Brumfitt W., Hamilton-Miller J. Methicillin-resistant Staphylococcus aureus. N Engl J Med. 1989 May 4;320(18):1188–1196. doi: 10.1056/NEJM198905043201806. [DOI] [PubMed] [Google Scholar]
- Burnie J. P., Matthews R. C., Lee W., Murdoch D. A comparison of immunoblot and DNA restriction patterns in characterising methicillin-resistant isolates of Staphylococcus aureus. J Med Microbiol. 1989 Aug;29(4):255–261. doi: 10.1099/00222615-29-4-255. [DOI] [PubMed] [Google Scholar]
- Chambers H. F. Methicillin-resistant staphylococci. Clin Microbiol Rev. 1988 Apr;1(2):173–186. doi: 10.1128/cmr.1.2.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins J. K., Smith J. S., Kelly M. T. Comparison of phage typing, plasmid mapping, and antibiotic resistance patterns as epidemiologic markers in a nosocomial outbreak of methicillin-resistant Staphylococcus aureus infections. Diagn Microbiol Infect Dis. 1984 Jun;2(3):233–245. doi: 10.1016/0732-8893(84)90036-1. [DOI] [PubMed] [Google Scholar]
- Coudron P. E., Jones D. L., Dalton H. P., Archer G. L. Evaluation of laboratory tests for detection of methicillin-resistant Staphylococcus aureus and Staphylococcus epidermidis. J Clin Microbiol. 1986 Nov;24(5):764–769. doi: 10.1128/jcm.24.5.764-769.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Buyser M. L., Morvan A., Grimont F., el Solh N. Characterization of Staphylococcus species by ribosomal RNA gene restriction patterns. J Gen Microbiol. 1989 Apr;135(4):989–999. doi: 10.1099/00221287-135-4-989. [DOI] [PubMed] [Google Scholar]
- Goering R. V., Duensing T. D. Rapid field inversion gel electrophoresis in combination with an rRNA gene probe in the epidemiological evaluation of staphylococci. J Clin Microbiol. 1990 Mar;28(3):426–429. doi: 10.1128/jcm.28.3.426-429.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimont F., Grimont P. A. Ribosomal ribonucleic acid gene restriction patterns as potential taxonomic tools. Ann Inst Pasteur Microbiol. 1986 Sep-Oct;137B(2):165–175. doi: 10.1016/s0769-2609(86)80105-3. [DOI] [PubMed] [Google Scholar]
- Hackbarth C. J., Chambers H. F. Methicillin-resistant staphylococci: detection methods and treatment of infections. Antimicrob Agents Chemother. 1989 Jul;33(7):995–999. doi: 10.1128/aac.33.7.995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadorn K., Lenz W., Kayser F. H., Shalit I., Krasemann C. Use of a ribosomal RNA gene probe for the epidemiological study of methicillin and ciprofloxacin resistant Staphylococcus aureus. Eur J Clin Microbiol Infect Dis. 1990 Sep;9(9):649–653. doi: 10.1007/BF01964265. [DOI] [PubMed] [Google Scholar]
- Haertl R., Bandlow G. Application of small fragment restriction endonuclease analysis (SF-REA) to the epidemiological fingerprinting of Staphylococcus aureus. J Med Microbiol. 1990 Oct;33(2):91–96. doi: 10.1099/00222615-33-2-91. [DOI] [PubMed] [Google Scholar]
- Irino K., Grimont F., Casin I., Grimont P. A. rRNA gene restriction patterns of Haemophilus influenzae biogroup aegyptius strains associated with Brazilian purpuric fever. J Clin Microbiol. 1988 Aug;26(8):1535–1538. doi: 10.1128/jcm.26.8.1535-1538.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordens J. Z., Hall L. M. Characterisation of methicillin-resistant Staphylococcus aureus isolates by restriction endonuclease digestion of chromosomal DNA. J Med Microbiol. 1988 Oct;27(2):117–123. doi: 10.1099/00222615-27-2-117. [DOI] [PubMed] [Google Scholar]
- Kaatz G. W., Seo S. M., Ruble C. A. Mechanisms of fluoroquinolone resistance in Staphylococcus aureus. J Infect Dis. 1991 May;163(5):1080–1086. doi: 10.1093/infdis/163.5.1080. [DOI] [PubMed] [Google Scholar]
- Kiehlbauch J. A., Plikaytis B. D., Swaminathan B., Cameron D. N., Wachsmuth I. K. Restriction fragment length polymorphisms in the ribosomal genes for species identification and subtyping of aerotolerant Campylobacter species. J Clin Microbiol. 1991 Aug;29(8):1670–1676. doi: 10.1128/jcm.29.8.1670-1676.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LiPuma J. J., Stull T. L., Dasen S. E., Pidcock K. A., Kaye D., Korzeniowski O. M. DNA polymorphisms among Escherichia coli isolated from bacteriuric women. J Infect Dis. 1989 Mar;159(3):526–532. doi: 10.1093/infdis/159.3.526. [DOI] [PubMed] [Google Scholar]
- Monzon-Moreno C., Aubert S., Morvan A., Solh N. E. Usefulness of three probes in typing isolates of methicillin-resistant Staphylococcus aureus (MRSA). J Med Microbiol. 1991 Aug;35(2):80–88. doi: 10.1099/00222615-35-2-80. [DOI] [PubMed] [Google Scholar]
- Mulligan M. E., Arbeit R. D. Epidemiologic and clinical utility of typing systems for differentiating among strains of methicillin-resistant Staphylococcus aureus. Infect Control Hosp Epidemiol. 1991 Jan;12(1):20–28. doi: 10.1086/646234. [DOI] [PubMed] [Google Scholar]
- Mulvey M., Arbuthnott J. P., Coleman D. C. Molecular typing of methicillin and gentamicin resistant Staphylococcus aureus in Dublin. Eur J Clin Microbiol. 1986 Dec;5(6):719–725. doi: 10.1007/BF02013312. [DOI] [PubMed] [Google Scholar]
- Ohshita Y., Hiramatsu K., Yokota T. A point mutation in norA gene is responsible for quinolone resistance in Staphylococcus aureus. Biochem Biophys Res Commun. 1990 Nov 15;172(3):1028–1034. doi: 10.1016/0006-291x(90)91549-8. [DOI] [PubMed] [Google Scholar]
- Opal S. M., Mayer K. H., Stenberg M. J., Blazek J. E., Mikolich D. J., Dickensheets D. L., Lyhte L. W., Trudel R. R., Musser J. M. Frequent acquisition of multiple strains of methicillin-resistant Staphylococcus aureus by healthcare workers in an endemic hospital environment. Infect Control Hosp Epidemiol. 1990 Sep;11(9):479–485. doi: 10.1086/646215. [DOI] [PubMed] [Google Scholar]
- Owen R. J., Beck A., Dayal P. A., Dawson C. Detection of genomic variation in Providencia stuartii clinical isolates by analysis of DNA restriction fragment length polymorphisms containing rRNA cistrons. J Clin Microbiol. 1988 Oct;26(10):2161–2166. doi: 10.1128/jcm.26.10.2161-2166.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfaller M. A., Wakefield D. S., Hollis R., Fredrickson M., Evans E., Massanari R. M. The clinical microbiology laboratory as an aid in infection control. The application of molecular techniques in epidemiologic studies of methicillin-resistant Staphylococcus aureus. Diagn Microbiol Infect Dis. 1991 May-Jun;14(3):209–217. doi: 10.1016/0732-8893(91)90034-d. [DOI] [PubMed] [Google Scholar]
- Plos K., Hull S. I., Hull R. A., Levin B. R., Orskov I., Orskov F., Svanborg-Edén C. Distribution of the P-associated-pilus (pap) region among Escherichia coli from natural sources: evidence for horizontal gene transfer. Infect Immun. 1989 May;57(5):1604–1611. doi: 10.1128/iai.57.5.1604-1611.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prévost G., Pottecher B., Dahlet M., Bientz M., Mantz J. M., Piémont Y. Pulsed field gel electrophoresis as a new epidemiological tool for monitoring methicillin-resistant Staphylococcus aureus in an intensive care unit. J Hosp Infect. 1991 Apr;17(4):255–269. doi: 10.1016/0195-6701(91)90270-i. [DOI] [PubMed] [Google Scholar]
- Rhinehart E., Shlaes D. M., Keys T. F., Serkey J., Kirkley B., Kim C., Currie-McCumber C. A., Hall G. Nosocomial clonal dissemination of methicillin-resistant Staphylococcus aureus. Elucidation by plasmid analysis. Arch Intern Med. 1987 Mar;147(3):521–524. [PubMed] [Google Scholar]
- Salvi R. J., Ahroon W., Saunders S. S., Arnold S. A. Evoked potentials: computer-automated threshold-tracking procedure using an objective detection criterion. Ear Hear. 1987 Jun;8(3):151–156. [PubMed] [Google Scholar]
- Selander R. K., Caugant D. A., Ochman H., Musser J. M., Gilmour M. N., Whittam T. S. Methods of multilocus enzyme electrophoresis for bacterial population genetics and systematics. Appl Environ Microbiol. 1986 May;51(5):873–884. doi: 10.1128/aem.51.5.873-884.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Storrs M. J., Courvalin P., Foster T. J. Genetic analysis of gentamicin resistance in methicillin- and gentamicin-resistant strains of Staphylococcus aureus isolated in Dublin hospitals. Antimicrob Agents Chemother. 1988 Aug;32(8):1174–1181. doi: 10.1128/aac.32.8.1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stull T. L., LiPuma J. J., Edlind T. D. A broad-spectrum probe for molecular epidemiology of bacteria: ribosomal RNA. J Infect Dis. 1988 Feb;157(2):280–286. doi: 10.1093/infdis/157.2.280. [DOI] [PubMed] [Google Scholar]
- Thomson-Carter F. M., Carter P. E., Pennington T. H. Differentiation of staphylococcal species and strains by ribosomal RNA gene restriction patterns. J Gen Microbiol. 1989 Jul;135(7):2093–2097. doi: 10.1099/00221287-135-7-2093. [DOI] [PubMed] [Google Scholar]
- Tompkins L. S., Troup N. J., Woods T., Bibb W., McKinney R. M. Molecular epidemiology of Legionella species by restriction endonuclease and alloenzyme analysis. J Clin Microbiol. 1987 Oct;25(10):1875–1880. doi: 10.1128/jcm.25.10.1875-1880.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tveten Y., Kristiansen B. E., Ask E., Jenkins A., Hofstad T. DNA fingerprinting of isolates of Staphylococcus aureus from newborns and their contacts. J Clin Microbiol. 1991 Jun;29(6):1100–1105. doi: 10.1128/jcm.29.6.1100-1105.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wachsmuth I. K., Kiehlbauch J. A., Bopp C. A., Cameron D. N., Strockbine N. A., Wells J. G., Blake P. A. The use of plasmid profiles and nucleic acid probes in epidemiologic investigations of foodborne, diarrheal diseases. Int J Food Microbiol. 1991 Jan;12(1):77–89. doi: 10.1016/0168-1605(91)90049-u. [DOI] [PubMed] [Google Scholar]
- Wachsmuth K. Genotypic approaches to the diagnosis of bacterial infections: plasmid analyses and gene probes. Infect Control. 1985 Mar;6(3):100–109. doi: 10.1017/s0195941700062767. [DOI] [PubMed] [Google Scholar]
- Wolfson J. S., Hooper D. C. Fluoroquinolone antimicrobial agents. Clin Microbiol Rev. 1989 Oct;2(4):378–424. doi: 10.1128/cmr.2.4.378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuccarelli A. J., Roy I., Harding G. P., Couperus J. J. Diversity and stability of restriction enzyme profiles of plasmid DNA from methicillin-resistant Staphylococcus aureus. J Clin Microbiol. 1990 Jan;28(1):97–102. doi: 10.1128/jcm.28.1.97-102.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]