Abstract
There is strong evidence from randomized clinical trials that the highly selective cox-2 inhibitors (coxibs), compared with placebo, cause an excess of serious cardiovascular events that are not mitigated by low-dose acetylsalicylic acid. Both Health Canada and the Food and Drug Administration have concluded that the excess cardiovascular events may be a ‘class effect’ of all the nonsteroidal anti-inflammatory drugs (NSAIDs), including traditional NSAIDs (tNSAIDs) and coxibs, and now require appropriate black box labelling of all these agents. Celecoxib and lumiracoxib are the only coxibs remaining on the market in Canada. The prostanoid pathways, the roles of cox-1 and cox-2, as well as the inhibitory effects of acetylsalicylic acid, traditional tNSAIDs and the coxibs, are briefly reviewed. Current recommendations for the ongoing use of coxibs and the tNSAIDs are summarized.
Keywords: Clinical trials, Complications, Drugs, Epidemiology, Myocardial infarction, Thrombosis
Abstract
Un grand nombre de données provenant d’essais cliniques avec hasardisation montrent que les inhibiteurs de la cyclo-oxygénase-2 (anti-COX-2 ou « coxib »), très sélectifs, peuvent causer, par rapport au placebo, un surnombre d’événements cardiovasculaires graves, non atténués par de faibles doses d’acide acétylsalicylique. Santé Canada et la Food and Drug Administration ont conclu qu’il pouvait s’agir d’un « effet de classe » des anti-inflammatoires non stéroïdiens (AINS), y compris des AINS classiques et des anti-COX, et exigent maintenant une mise en garde entourée d’une bordure noire sur l’étiquette de tous les produits visés. Le célécoxib est le seul anti-COX à rester sur le marché au Canada. Nous ferons un survol des voies prostanoïdes et du rôle des COX-1 et -2 ainsi que des effets inhibiteurs de l’acide acétylsalicylique, des AINS classiques et des anti-COX; suivra un résumé des recommandations actuelles sur l’utilisation des anti-COX et des AINS classiques.
Nonsteroidal anti-inflammatory drugs (NSAIDs) are widely used to treat arthritis pain and inflammation, menstrual and musculoskeletal pain, as well as headache and fever. This group includes acetylsalicylic acid (in a relatively high dose), the traditional NSAIDs (tNSAIDs) (eg, naproxen, ibuprofen, indomethacin and diclofenac) and the coxibs, a group of highly selective inhibitors of the cox-2 isoform of cyclo-oxygenase (cox). The emergence of serious cardiovascular adverse events with the coxibs and concerns that tNSAIDs may have similar risks requires cardiovascular specialists to be familiar with the available evidence, as well as the balance of risk and benefit with these agents.
The cox-2 saga
Beginning in 1999, three cox-2 selective inhibitors (coxibs) came to the market in the United States (US) and Canada. Their release followed more than a decade of research designed to exploit the ‘cox-2 hypothesis’ (1,2) that the coxibs would suppress cox-2-mediated pain and inflammation, while avoiding the gastrointestinal side effects of the nonselective tNSAIDs. Rofecoxib, celecoxib and valdecoxib were licensed based on the results of several small, short-term, randomized comparisons of these agents with tNSAIDs, demonstrating equal efficacy and fewer gastrointestinal side effects with the coxibs. Although it had been anticipated that these agents would be reserved for patients at high risk for gastrointestinal side effects, or for those intolerant or unresponsive to tNSAIDs, the marketing strategies encouraged widespread use by vigorously promoting to physicians and by heavy, direct-to-consumer advertising in the US (3,4). By 2004, annual sales of these agents totalled several billion dollars.
The first suggestion of an increased risk of serious cardiovascular adverse events (cardiovascular death, myocardial infarction [MI] and stroke) came in 2000 from a study of rofe-coxib versus naproxen (5), but the observation was played down by Merck (USA), the drug’s manufacturer. However, in September 2004, a large study evaluating the effects of rofe-coxib on the incidence of colon cancer in patients with colorectal polyps was stopped early because of a twofold increase in the incidence of major adverse cardiovascular events (6). On September 30, 2004, Merck withdrew rofecoxib from the market worldwide. Shortly thereafter, a trial of celecoxib in a similar population reported a threefold increased risk of serious cardiovascular events (7). A special Food and Drug Administration (FDA) meeting was convened in February 2005 to review the evidence of cardiovascular risk associated with the coxibs and, in fact, all NSAIDs. By that time, there was evidence of excess cardiovascular risk with even short-term use of valdecoxib following coronary artery bypass surgery (8), and reports that valdecoxib also caused an increased rate of serious and potentially life-threatening skin reactions (9). The FDA recommended that celecoxib remain available, but should include a boxed warning indicating the increased risk of serious adverse cardiovascular events, that the FDA should carefully review any proposal from Merck for resumption of the marketing of rofecoxib and that Pfizer should be asked to voluntarily withdraw valdecoxib from the US market (9). The requirement for a black box warning regarding cardiovascular adverse events was extended to the prescription and over-the-counter forms of tNSAIDs. In June 2005, Health Canada convened an expert panel to review the available evidence; the panel’s recommendations were similar to those of the FDA panel (10). Health Canada has developed a Guidance for Industry document (11) that incorporates these recommendations, and does not differentiate between the tNSAIDs and the coxibs. The European Medicines Agency has made similar recommendations regarding the availability and the use of the coxibs, although it has not yet completed its deliberations on the tNSAIDs (12). Celecoxib was, for a time, the only remaining coxib available in the US and Canada, but lumiracoxib has recently been approved by Health Canada.
Mechanisms of action of acetylsalicylic acid, tNSAIDs and coxibs (2,13,14)
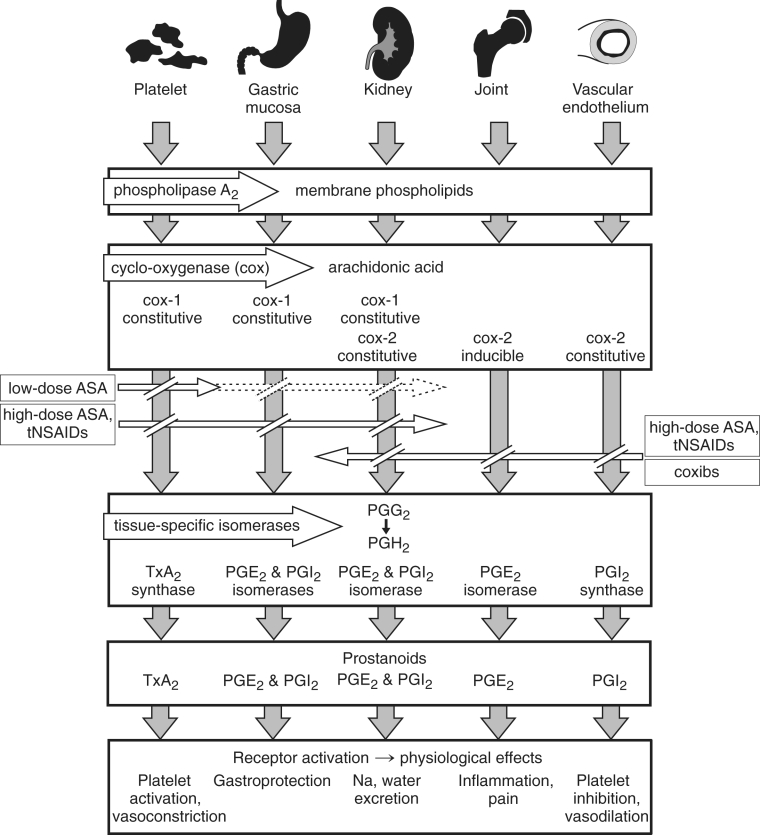
In response to cell injury or receptor activation, phospholipase A2 liberates arachidonic acid from membrane-bound phospho-lipids in a wide range of tissues (Figure 1). The enzyme cox catalyzes the transformation of arachidonic acid to prostaglandin (PG) G2 and subsequently to PGH2. Tissue-specific isomerases convert PGH2 to various prostanoids, which activate receptors and lead to physiological effects. These prostanoids include thromboxane A2 (TxA2) in platelets (platelet activation, vasoconstriction), PGE2 and PGI2 in the gastric mucosa (gas-troprotection), PGE2 and PGI2 in the kidney (salt and water excretion), PGE2 in joints (inflammation and pain), PGI2 in endothelial cells (platelet inhibition and vasodilation) and PGE2 in the central nervous system (pain and fever). The cox-1 isoform of cox is a constitutive enzyme expressed in most tissues and cell types, whereas cox-2 is inducible in inflammation and carcinogenesis.
Figure 1).
Prostanoid pathways and the effects of acetylsalicylic acid (ASA), traditional nonsteroidal anti-inflammatory drugs (tNSAIDs) and coxibs. Arachidonic acid is liberated from membrane phospholipids, is enzymatically transformed by cox-1 or cox-2 to prostaglandin (PG) G2 and then to PGH2, which is then converted by tissue-specific isomerases to a range of prostanoids that activate receptors to cause physiological effects. Low-dose ASA irreversibly inhibits cox-1, but inhibits cox-1 in the nucleated cells of the gastric mucosa and kidney in a dose-and time-dependent manner. High-dose ASA and tNSAIDs inhibit both cox-1 and cox-2. Coxibs inhibit only cox-2 (see text for details). Na Sodium; TxA2 Thromboxane A2
High-dose acetylsalicylic acid and tNSAIDs are nonselective, blocking both isoforms of cox, although the relative degrees of cox-1 and cox-2 inhibition vary substantially among these agents (Figure 1). Their therapeutic efficacy depends on their inhibition of the cox-2-mediated formation of PGE2, which causes inflammation in the joints, and pain and fever in the central nervous system. However, their inhibition of cox-1-mediated PGE2 formation in the gastric mucosa increases the risk of gastrointestinal symptoms, mucosal damage and bleeding. By contrast, the coxibs inhibit only the cox-2-mediated pathways, achieving the desired therapeutic goal of reducing inflammation and pain by blocking PGE2 formation in joints and elsewhere. The coxibs spare cox-1-mediated gastric PGE2 production, preserving its gastroprotective actions.
It is now recognized that cox-2 may be expressed constitutively by endothelial cells, and that it is required for the synthesis of vascular protective PGI2 (Figure 1). The inhibition of PGI2 by cox-2 selective agents may be an important mechanism of serious adverse cardiovascular events. PGI2 synthesis is also inhibited by high-dose acetylsalicylic acid and tNSAIDs, although possibly not to the same extent as by the cox-2 selective coxibs. Both the coxibs and the tNSAIDs inhibit renal PGE2 and PGI2, resulting in sodium and water retention, as well as elevation of blood pressure. These derangements may contribute to the cardiovascular risks of the coxibs and possibly the tNSAIDs.
At low doses (30 mg/day to 160 mg/day), acetylsalicylic acid irreversibly acetylates cox-1, eliminating its enzymatic activity (Figure 1). Cox-1 is the principal isoform of the enzyme in the platelet, and because it has no nucleus, the platelet cannot synthesize more cox-1. The consequent cessation of TxA2 synthesis persists for the life of the affected platelet, accounting for the antithrombotic effect of acetylsali-cylic acid. Although low-dose acetylsalicylic acid also irreversibly acetylates cox-1 of the gastric mucosa, these nucleated cells can synthesize more cox-1, and the inhibition is time- and dose-related. Gastrointestinal bleeding with low-dose acetyl-salicylic acid is less frequent than with higher doses (15), and when it occurs with lower doses, it is likely accounted for by the platelet antiaggregant effect, with important inhibition of gastric PGE2 occurring only at higher doses. Low-dose acetyl-salicylic acid has a minimal effect on cox-2-mediated synthesis of PGI2 in endothelial cells, which are nucleated and soon synthesize more cox-2.
At higher daily doses, the actions of acetylsalicylic acid are similar to those of other nonselective NSAIDs (Figure 1). The inhibition of platelet cox-1 does not increase, but there is more marked and sustained inhibition of cox-1 in nucleated cells, including those of the gastric mucosa, and the profile of gastrointestinal side effects becomes similar to that of tNSAIDs (the risk of bleeding may be even more marked because of irreversible platelet inhibition). The efficacy of acetylsalicylic acid in the treatment of pain, inflammation and fever at these higher doses is accounted for by cox-2 inhibition (15), as is the case for the tNSAIDs and the coxibs.
Ibuprofen binds in a dose-related, reversible manner to the cox-1 acetylation site of platelet cox-1 (16), inhibiting the access of acetylsalicylic acid. For acetylsalicylic acid to achieve its therapeutic irreversible acetylation of platelet cox-1, it must be administered in the nonenteric-coated form, approximately 2 h preceding a dose of ibuprofen. Naproxen appears to have a similar capacity to inhibit the access of acetylsalicylic acid to the cox-1 acetylation site, and similar timing of the administration sequence is necessary (17).
Evidence of cardiovascular harm from the coxibs
The FDA approvals of rofecoxib, celecoxib and valdecoxib in 1999 were based on relatively small, short-term trials showing that these agents were as effective as the tNSAIDs for the control of the symptoms of arthritis, as well as for musculoskeletal and menstrual pain, while having a much lower incidence of gastrointestinal side effects. A definitive claim of reduced gastrointestinal side effects was to be granted only after the conducting larger and longer term studies that used higher doses of the coxibs (Table 1). Accordingly, the Vioxx Gastrointestinal Outcomes Research (VIGOR) trial (5) randomly assigned 8076 patients to rofecoxib at twice the ‘maximal’ long-term dose versus naproxen, while the Celecoxib Long-term Arthritis Safety Study (CLASS) (18) randomly assigned 8059 patients to celecoxib at two to four times the usual therapeutic dose versus either diclofenac or ibuprofen. Both studies confirmed equivalent efficacy of the coxibs to the tNSAIDs for controlling the symptoms of arthritis, while causing fewer symptomatic ulcers and ulcer complications (perforation, obstruction and serious hemorrhage). Neither study had been designed to formally detect and adjudicate serious cardiovascular events. However, rofecoxib showed a 2.38-fold (P<0.001) increased risk of adverse cardiovascular events (chiefly MI, ischemic stroke and vascular death) after a mean of nine months of follow-up (19). The evidence is difficult to evaluate because the data in the original papers are incomplete, additional analyses are available in subsequently published papers (19–21) and on the FDA Web site, and there has been a dispute between the journal editors and the authors about cardiovascular events that were not included in the original report (20,21). Celecoxib had no excess cardiovascular risk in the CLASS.
TABLE 1.
Major trials of coxibs versus nonsteroidal anti-inflammatory drugs
| Trial | Population | Regimen | Follow-up | GI side effects | Cardiovascular side effects |
|---|---|---|---|---|---|
| VIGOR (5) | 8076 patients with rheumatoid arthritis | Rofecoxib (R) 50 mg/day vs naproxen (N) 1000 mg/day | Median 9 months | GI events: R=2.1%/year, N=4.5%/year, RR=0.4 (P<0.005); complicated GI events: R=0.6%/year, N=1.4%/year, RR=0.5 (P<0.001) | Serious thromboembolic events: R=1.67%/year, N=0.70%/year, RR=2.4(P<0.001) |
| CLASS (18) | 8059 patients with rheumatoid or osteoarthritis | Celecoxib (C) 800 mg/day vs either ibuprofen (I) 2400 mg/day or diclofenac (D) 150 mg/day | 6 months | Upper GI ulcers: C=2.08%/year, I/D=3.54%/year, RR=0.59 (P=0.02); complicated upper GI ulcers: C=0.76%/year, I/D=1.45%/year, RR=0.53 (P=0.09) | Cerebrovascular accident, myocardial infarction, angina: RR=0.9 (P=NS) |
| TARGET (22,23) | 18,325 patients with osteoarthritis | Lumiracoxib (L) 400 mg/day vs N 1000 mg/day or I 2400 mg/day | 1 year | Ulcer complication: L=0.32%/year, N or I=0.9%/year, HR=0.34 (P<0.0001) | Myocardial infarction, stroke, cardiovascular death: L=0.86%/year, N or I=0.75%/year, HR=1.14 (P=0.51) |
CLASS Celecoxib Long-term Arthritis Safety Study; GI Gastrointestinal; HR Hazard ratio; NS Not significant; TARGET Therapeutic Arthritis Research and Gastrointestinal Event Trial; VIGOR Vioxx Gastrointestinal Outcomes Research
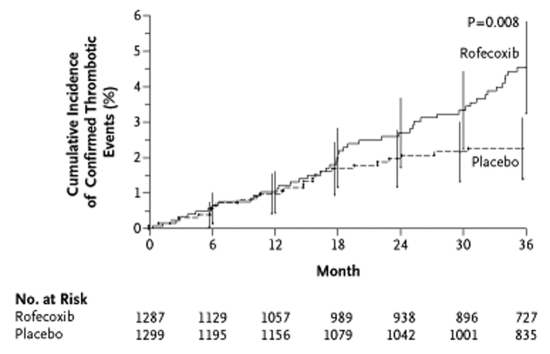
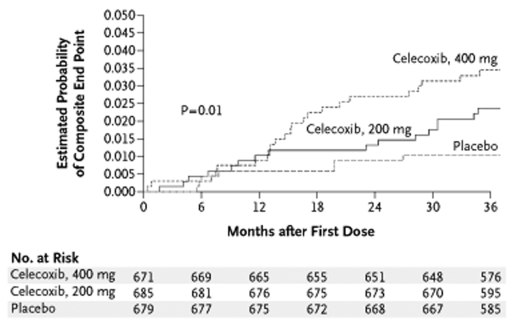
Evidence for the expression of cox-2 in neoplasms led to the design of two large, randomized, placebo-controlled trials of coxibs to prevent the recurrence of adenomatous polyps in patients with a history of colorectal adenomatous polyps (Table 2). Emerging concerns about potential serious cardiovascular adverse events prompted the inclusion of formal monitoring and adjudication procedures for serious thrombotic and cardiovascular events in both trials. Unequivocal evidence for excess serious cardiovascular events was found in both studies and led to their early termination (Table 2). The Adenomatous Polyp PRevention On Vioxx (APPROVe) trial (6) randomly assigned 2586 patients to rofecoxib in the usual therapeutic dose versus placebo. After a mean follow-up of 2.5 years, the risk ratio for thrombotic events was 1.92 (P=0.008) (Figure 2). The Adenoma Prevention with Celecoxib (APC) trial (7) randomly assigned 2035 patients to celecoxib (either 200 mg or 400 mg twice daily) or placebo. After a mean follow-up of 2.8 years, the overall hazard ratio for cardiovascular death, nonfatal MI or stroke was 2.9 (95% CI 1.2 to 7.0) (Figure 3). The APPROVe trial also found significantly increased incidences of hypertension, edema and congestive heart failure with rofecoxib, and the APC trial found an increased incidence of heart failure with celecoxib.
TABLE 2.
Major trials of coxibs versus placebo
| Trial | Population | Regimen | Follow-up | Serious cardiovascular events |
|---|---|---|---|---|
| APPROVe (6) | 2586 patients with history of colorectal adenoma | Rofecoxib (R) 25 mg/day vs placebo (Pl) | Mean R=2.4 years, Pl=2.6 years | Serious thrombotic events: R=1.50%/year, Pl=0.78%/year, HR=1.92 (P=0.008) |
| APC (7) | 2035 patients with history of colorectal adenoma | Celecoxib (C) 400 mg or 800 mg/day vs Pl | Range 2.8–3.1 years | Composite of cardiovascular death, myocardial infarction, stroke or heart failure: C=0.96%/year, Pl=0.34%/year, RR=2.8 (P=0.01) |
| Nussmeier et al (8) | 1671 patients immediately after CABG surgery | Parecoxib (P) IV for 3 days, then valdecoxib (V) for 7 days vs Pl IV, then V vs Pl IV, then Pl po | 30 days | Cardiovascular events: P or V=1.62% in 1 month (P=0.5), RR=2.9 (P=0.08) |
APC Adenoma Prevention with Celecoxib; APPROVe Adenomatous Polyp PRevention On Vioxx; CABG Coronary artery bypass graft; HR Hazard ratio; IV Intravenous; po By mouth
Figure 2).
The Adenomatous Polyp PRevention On Vioxx (APPROVe) trial (6). Kaplan-Meier estimates of the cumulative incidence of confirmed serious thrombotic events. Vertical lines indicate 95% CI. Reproduced from reference 6 with permission
Figure 3).
The Adenoma Prevention with Celecoxib (APC) trial (7). Kaplan-Meier estimates of the composite end point of death from cardiovascular causes, myocardial infarction, stroke and heart failure among patients who received celecoxib (200 mg twice daily or 400 mg twice daily) or placebo. The log-rank statistic of 8.73, which has two degrees of freedom, was used to determine the P value. Reproduced from reference 7 with permission
In a study that randomly assigned 1671 postcoronary artery bypass patients to valdecoxib plus parecoxib, valdecoxib alone or placebo, the RR for cardiovascular events over 30 days was 3.7 (P=0.03) for the combination and 2.0 (P=0.31) for valde-coxib alone (8). In a more recent trial of lumiracoxib (22,23), the RR for serious cardiovascular events was 0.76 for lumira-coxib versus ibuprofen and 1.46 for lumiracoxib versus naproxen, but neither RR was statistically significant.
In 2004, a cumulative meta-analysis of 18 randomized controlled trials of rofecoxib versus tNSAIDs or placebo reported an RR for MI of 2.3 (P=0.01) by the end of 2000 and 2.24 (P=0.007) one year later (24). The difference did not appear to depend on the control group, study duration or rofecoxib dose. When rofecoxib was compared with naproxen, there was a trend to a greater RR than when rofecoxib was compared with non-naproxen tNSAIDs. A rigorous meta-analysis of the cardiovascular risk of coxibs performed by the Oxford Clinical Trial Service Unit has shown an increased RR of such events (RR=1.42, P=0.003) when rofecoxib, celecoxib, etoricoxib, lumiracoxib and valdecoxib are compared with placebo, with no heterogeneity among the five drugs (25).
Large epidemiological studies have shown statistically significant excess cardiovascular risk for rofecoxib (26–29) and celecoxib (28,29), trends toward increased risk for both rofe-coxib (30,31) and celecoxib (26,27), as well as trends to decreased risk with celecoxib (30,31). The usual concerns about inadequate sample size, confounding, uncertain medication compliance and the use of concomitant medications in uncertain regimens must be considered in trying to draw conclusions from these studies.
Table 3 shows data comparing the absolute differences in the rates of gastrointestinal complications and cardiovascular events for rofecoxib, celecoxib, valdecoxib and lumiracoxib in studies comparing these agents with tNSAIDs and placebo. The reductions in the rates of ulcer complications are similar to the increases in the rates of cardiovascular events for rofecoxib and celecoxib, but lumiracoxib has more gastrointestinal side effects avoided than serious cardiovascular events caused. There is a dramatic increase of serious cardiovascular events in the valdecoxib study conducted among patients postcoronary artery bypass surgery.
TABLE 3.
Absolute rates of gastrointestinal benefit versus cardiovascular harm of the coxibs
| Comparison | Gastrointestinal side effects
|
Serious cardiovascular events (vascular death, nonfatal MI or nonfatal stroke) | |
|---|---|---|---|
| Ulcer symptoms or complications | Ulcer complications | ||
| Events/1000 patients/year | Events/1000 patients/year | ||
| Rofecoxib versus naproxen (5,19–21) | ↓ 24 (P<0.001) | ↓ 8 (P=0.005) | ↑ 11 (P<0.001) |
| Rofecoxib versus placebo (6) | ↑ 7.2 (P=0.008) | ||
| Celecoxib versus diclofenac or ibuprofen (18) | ↓ 14 (P=0.02) | ↓ 7 (P=0.09) | ↓ 1.0 (P=NS) |
| Celecoxib versus placebo (7) | ↑ 6.0 (P<0.05) (400 mg bid); | ||
| ↑ 4.4 (P<0.05) (200 mg bid) | |||
| Lumiracoxib versus naproxen or ibuprofen (22,23) | ↓ 8.5 (P<0.0001) | ↑ ~1.0 (P=NS) | |
| Events/1000 patients/month | Events/1000 patients/month | ||
| Valdecoxib versus placebo (8) | ↑ 2.0 (P=NS) | ↑ 11 (P=0.08) | |
↓ Decrease; ↑ Increase; bid Twice a day; MI Myocardial infarction; NS Not significant
Does the coadministration of acetylsalicylic acid with a coxib mitigate its cardiovascular risk?
Although the administration of acetylsalicylic acid would be expected to decrease the incidence of thrombotic events in patients at risk, it is not likely that the cardiovascular risk of the coxibs would be eliminated, because acetylsalicylic acid and the coxibs act on different pathways (2). Data from trials of a coxib versus placebo or a tNSAID have generally shown no mitigation of the excess cardiovascular risk of the coxibs by low-dose acetylsalicylic acid (6,7). However, the doses of acetylsalicylic acid have not been controlled or well documented, and in any case, the sample sizes were insufficient to reliably determine whether the risk of the coxibs is mitigated. There have been no controlled trials of acetylsalicylic acid versus placebo among suitable patients receiving a coxib. Although a randomized trial of acetylsalicylic acid administration may clarify the issue, it would be unjustified to withhold acetylsalicylic acid in patients at significant risk of cardiovascular outcomes. The overall risk of cardiovascular events has generally been higher among patients taking acetylsalicylic acid, likely because they have a higher baseline risk. The coadministration of acetylsalicylic acid appears to eliminate most of the benefit of reduced gastrointestinal complications with a coxib compared with a tNSAID (18,23). Among patients at lower risk who could ethically enter a trial of low-dose acetylsalicylic acid, the loss of the gastrointestinal benefit of coxibs over tNSAIDs would eliminate most of the rationale for their use.
Coadministration of gastroprotective therapy with a tNSAID
There is extensive literature documenting the efficacy of oral synthetic PGE1 analogues (misoprostol) and proton pump inhibitors (omeprazole, lansoprazole) in decreasing the incidence of gastrointestinal side effects among patients receiving tNSAIDs (32,33). Both agents are effective for patients at risk, including those with a previous ulcer or hemorrhage (33). Randomized studies comparing the use of misoprostol with a proton pump inhibitor among patients receiving a tNSAID show similar reductions of gastrointestinal side effects (33). However, miso-prostol has a less convenient dose schedule, has its own profile of side effects and patient compliance with the drug is poorer. Trials comparing a coxib with a tNSAID plus a gastroprotective agent generally show no difference in the primary or secondary prevention of gastrointestinal side effects (32,34). Cost-benefit comparisons vary from country to country (32).
Are there fewer cardiovascular adverse events with the tNSAIDs than with the coxibs?
The evaluations of the coxibs have been performed in the current era of rigorous clinical trial design, large sample sizes, and careful and prolonged follow-up of all events. The evidence for increased cardiovascular events by comparison with placebo is strong. However, the tNSAIDs were developed and released in an era of much less rigorous evaluation and surveillance, and relatively little information on cardiovascular risk is available from randomized, placebo-controlled trials. Some large epidemiological studies have concluded that the tNSAIDs have no effect on cardiovascular risk (35,36). However, several studies showed trends or significantly increased risk with certain tNSAIDs, although the effects vary among the agents (26–29). These epidemiological studies (some of them including assessments of coxibs) suffer from the deficiencies already noted above. It is possible that the cardiovascular risk of naproxen is lower than that of the other tNSAIDs (10). The Oxford Clinical Trial Service Unit meta-analysis (25) of 42 randomized trials of naproxen versus a selective cox-2 inhibitor showed an RR of 1.57 (P=0.0006) of vascular events with the coxibs. The impression that naproxen may confer less risk than the other tNSAIDs or may even be cardioprotective is supported by evidence that the standard twice daily dose regimen can provide sustained and almost complete suppression of TxA2 (37).
Direct comparisons of individual coxibs with selected NSAIDs have shown significantly more cardiovascular side effects with rofecoxib than with naproxen (5), no difference between celecoxib and either diclofenac or ibuprofen (18), nonsignificantly more cardiovascular side effects with lumira-coxib than with naproxen (22) and nonsignificantly fewer cardiovascular side effects with lumiracoxib than with ibuprofen (22). The lack of clear data demonstrating a difference in cardiovascular risk between the coxibs and the tNSAIDs led both the FDA and Health Canada to require new black box labelling of all tNSAIDs, including naproxen, in conjunction with their new requirements for labelling of the coxibs (9–11).
Is there still any therapeutic role for the coxibs?
Broad recommendations for the use of tNSAIDs and the cox-ibs are available from the deliberations of the FDA, Health Canada and the European Medicines Agency (9–11). There are many publications providing useful suggestions (38), although the American College of Rheumatology has not yet updated its guidelines since the FDA hearings and recommendations (39). The American Heart Association has provided a science advisory (40).
The main messages in recent recommendations (Table 4) have generally advised commencing management with non-drug regimens of exercise, physiotherapy, heat or cold, or others as appropriate for milder pain due to arthritis, soft tissue injury or menstruation. For more severe or persistent pain, acetaminophen is generally preferred, and in more severe pain situations, a narcotic may be appropriate. Consideration is next given to topical tNSAID therapy or possibly the intra-articular injection of a glucocorticoid for joint inflammation. When the pain is more sustained or severe, therapy with a tNSAID is advised. Given the suggestion of less cardiovascular risk with naproxen than with other tNSAIDs, it would be a reasonable first choice. The lowest effective dose for the shortest possible time is advised. Particular precautions are warranted in elderly patients. Patients should not receive any NSAIDs in the first month following coronary artery bypass surgery. A full discussion with the patient is essential to ensure their understanding of the potential for gastrointestinal and cardiovascular risks from the tNSAIDs, and to obtain their agreement that the benefit to risk ratio is appropriate. In a patient at increased risk of gastrointestinal complications (prior ulcer, advanced age, high NSAID dose, multiple NSAIDs [including acetylsalicylic acid], concomitant steroids or warfarin), the tNSAID should be accompanied by a proton pump inhibitor in preference to misoprostol because of greater convenience and fewer side effects. An inadequate response to the therapy or intolerable side effects should prompt a switch to another tNSAID, of which several effective choices are available. When low-dose acetyl-salicylic acid therapy is required for cardiovascular prophylaxis, a dose of 75 mg to 80 mg nonenteric-coated should be given daily in the morning and followed 2 h later by the tNSAID.
TABLE 4.
Summary of current recommendations for coxib use
|
There is no evidence to suggest that any of the coxibs are more efficacious for pain and inflammation than the tNSAIDs. Hence, the only rationales for their use are the decreased incidence of gastrointestinal side effects and patient preference. The absolute reduction of complicated gastrointestinal events (approximately eight of 1000 patients per year) is in the same range as the serious cardiovascular events caused (approximately four to 11 of 1000 patients per year) by rofecoxib and celecoxib (Table 3). The combination of a proton pump inhibitor with a tNSAID is as effective as a coxib in reducing gastrointestinal symptoms, although the dosage regimen is less convenient and the cost may be greater, depending on local factors. In patients requiring low-dose acetylsalicylic acid, the cox-2 advantage of reduced gastrointestinal side effects is lost. Given the strong evidence for increased cardiovascular risk with both rofecoxib and celecoxib, it seems wise to reserve the coxibs (only celecoxib and lumiracoxib remain available in Canada) for patients whose pain and inflammation are not well controlled despite the sequential use of several different tNSAIDs, and for those who are fully informed of the cardiovascular risks. Testimonials from rheumatologists and patients provide the rationale for this approach in the use of a coxib, and they strongly influenced the recommendations of the FDA and Health Canada panels, even though rigorous evidence from clinical trials was lacking (9,10,41).
CONCLUSIONS
The cox-2 hypothesis has been confirmed, but the unanticipated cardiovascular risk of coxibs has quelled enthusiasm for their use. The tNSAIDs have also come under intense scrutiny, leading to the conduct of epidemiological studies and meta-analyses of small comparison studies in the attempt to better delineate their potential cardiovascular risks. Physicians have had to sharply revise their indications for both the coxibs and the tNSAIDs in the management of pain and inflammation, and more fully engage patients in the decisions about the balance of benefits and risks. The saga of the development, evaluation, licensing and withdrawal of two of the three previously available coxibs has had a sobering effect on patients, physicians, regulatory agencies and the major pharmaceutical firms. If new coxibs with the promise of a better balance of gastrointestinal benefits and cardiovascular harm are to eventually reach the market, rigorous clinical trials, clear indications and labelling, careful postmarketing surveillance and repeated reflection on the lessons learned from the coxib saga will be essential to ensure an appropriate balance of patient benefit and risk.
REFERENCES
- 1.Bombardier C. An evidence-based evaluation of the gastrointestinal safety of coxibs. Am J Cardiol. 2002;89:3D–9D. doi: 10.1016/s0002-9149(02)02231-2. [DOI] [PubMed] [Google Scholar]
- 2.Grosser T, Fries S, FitzGerald GA. Biological basis for the cardiovascular consequences of COX-2 inhibition: Therapeutic challenges and opportunities. J Clin Invest. 2006;116:4–15. doi: 10.1172/JCI27291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Waxman HA. The lessons of Vioxx – drug safety and sales. N Engl J Med. 2005;352:2576–8. doi: 10.1056/NEJMp058136. [DOI] [PubMed] [Google Scholar]
- 4.Dai C, Stafford RS, Alexander GC. National trends in cyclooxygenase-2 inhibitor use since market release: Nonselective diffusion of a selectively cost-effective innovation. Arch Intern Med. 2005;165:171–7. doi: 10.1001/archinte.165.2.171. [DOI] [PubMed] [Google Scholar]
- 5.Bombardier C, Laine L, Reicin A, et al. Comparison of upper gastrointestinal toxicity of rofecoxib and naproxen in patients with rheumatoid arthritis. VIGOR Study Group. N Engl J Med. 2000;343:1520–8. doi: 10.1056/NEJM200011233432103. [DOI] [PubMed] [Google Scholar]
- 6.Bresalier RS, Sandler RS.Quan H, et al; Adenomatous Polyp Prevention on Vioxx (APPROVe) Trial Investigators. Cardiovascular events associated with rofecoxib in a colorectal adenoma chemoprevention trial N Engl J Med 20053521092–102.(Erratum in 2006;355:221). [DOI] [PubMed] [Google Scholar]
- 7.Solomon SD, McMurray JJ, Pfeffer MA, et al. Adenoma Prevention with Celecoxib (APC) Study Investigators Cardiovascular risk associated with celecoxib in a clinical trial for colorectal adenoma prevention. N Engl J Med. 2005;352:1071–80. doi: 10.1056/NEJMoa050405. [DOI] [PubMed] [Google Scholar]
- 8.Nussmeier NA, Whelton AA, Brown MT, et al. Complications of the COX-2 inhibitors parecoxib and valdecoxib after cardiac surgery. N Engl J Med. 2005;352:1081–91. doi: 10.1056/NEJMoa050330. [DOI] [PubMed] [Google Scholar]
- 9.Jenkins JK, Seligman PJ, FDA Memorandum Analysis and recommendations for Agency action regarding non-steroidal anti-inflammatory drugs and cardiovascular risk<www.fda.gov/ohrms/dockets/ac/06/briefing/2006-4202B1_09_FDA-Tab09.pdf> (Version current at November 30, 2006)
- 10.Health Canada Report of the Expert Advisory Panel on the Safety of Cox-2 Selective Non-steroidal Anti-inflammatory Drugs (NSAIDs)<www.hc-sc.gc.ca/dhp-mps/prodpharma/activit/sci-consult/cox2/sap_report_gcs_rapport_cox2_e.html> (Version current at November 30, 2006).
- 11.Health Canada Guidance for Industry<www.hc-sc.gc.ca/dhp-mps/alt_formats/hpfb-dgpsa/pdf/prodpharma/nsaids_ains_e.pdf> (Version current at January 16, 2007)
- 12.European Medicines Agency. Press Release: European Medicines Agency concludes action on COX-2 inhibitors<www.emea.eu.int/Cox2inhibitors.htm> (Version current at January 24, 2007)
- 13.Mitchell JA, Warner TD. COX isoforms in the cardiovascular system: Understanding the activities of non-steroidal anti-inflammatory drugs. Nat Rev Drug Discov. 2006;5:75–86. doi: 10.1038/nrd1929. [DOI] [PubMed] [Google Scholar]
- 14.FitzGerald GA, Patrono C. The coxibs, selective inhibitors of cyclooxygenase-2. N Engl J Med. 2001;345:433–42. doi: 10.1056/NEJM200108093450607. [DOI] [PubMed] [Google Scholar]
- 15.Patrono C, Coller B, FitzGerald GA, Hirsh J. Roth G. Platelet-active drugs: The relationships among dose, effectiveness, and side effects: The Seventh ACCP Conference on Antithrombotic and Thrombolytic Therapy. Chest. 2004;126(3 Suppl):234S–64S. doi: 10.1378/chest.126.3_suppl.234S. [DOI] [PubMed] [Google Scholar]
- 16.Catella-Lawson F, Reilly MP, Kapoor SC, et al. Cyclooxygenase inhibitors and the antiplatelet effects of aspirin. N Engl J Med. 2001;345:1809–17. doi: 10.1056/NEJMoa003199. [DOI] [PubMed] [Google Scholar]
- 17.Capone ML, Sciulli MG, Tacconelli S, et al. Pharmacodynamic interaction of naproxen with low-dose aspirin in healthy subjects. J Am Coll Cardiol. 2005;45:1295–301. doi: 10.1016/j.jacc.2005.01.045. [DOI] [PubMed] [Google Scholar]
- 18.Silverstein FE, Faich G, Goldstein JL, et al. Gastrointestinal toxicity with celecoxib vs. nonsteroidal anti-inflammatory drugs for osteoarthritis and rheumatoid arthritis: The CLASS study: A randomized controlled trial: Celecoxib Long-term Arthritis Safety Study. JAMA. 2000;284:1247–55. doi: 10.1001/jama.284.10.1247. [DOI] [PubMed] [Google Scholar]
- 19.Mukherjee D, Nissen SE, Topol EJ. Risk of cardiovascular events associated with selective COX-2 inhibitors. JAMA. 2001;286:954–9. doi: 10.1001/jama.286.8.954. [DOI] [PubMed] [Google Scholar]
- 20.Curfman GD, Morrissey S, Drazen JM. Expression of concern: Bombardier et al, “Comparison of upper gastrointestinal toxicity of rofecoxib and naproxen in patients with rheumatoid arthritis. VIGOR Study Group.”. N Engl J Med. N Engl J Med. 2000;2005;343353:1520–8. 2813–4. doi: 10.1056/NEJMe058314. [DOI] [PubMed] [Google Scholar]
- 21.Bombardier C, Laine L, Burgos-Vargas, et al. Response to expression of concern regarding VIGOR study. N Engl J Med. 2006;354:1196–99. doi: 10.1056/NEJMc066096. [DOI] [PubMed] [Google Scholar]
- 22.Farkouh ME, Kirshner H, Harrington RA, et al. TARGET Study Group. Comparison of lumiracoxib with naproxen and ibuprofen in the Therapeutic Arthritis Research and Gastrointestinal Event Trial (TARGET), cardiovascular outcomes: Randomised controlled trial. Lancet. 2004;364:675–84. doi: 10.1016/S0140-6736(04)16894-3. [DOI] [PubMed] [Google Scholar]
- 23.Schnitzer TJ, Burmester GR, Mysler E, et al. TARGET Study Group Comparison of lumiracoxib with naproxen and ibuprofen in the Therapeutic Arthritis Research and Gastrointestinal Event Trial (TARGET), reduction in ulcer complications: Randomised controlled trial. Lancet. 2004;364:665–74. doi: 10.1016/S0140-6736(04)16893-1. [DOI] [PubMed] [Google Scholar]
- 24.Juni P, Nartey L, Reichenbach S, Sterchi R, Dieppe PA, Egger M. Risk of cardiovascular events and rofecoxib: Cumulative meta-analysis. Lancet. 2004;364:2021–9. doi: 10.1016/S0140-6736(04)17514-4. [DOI] [PubMed] [Google Scholar]
- 25.Kearney PM, Baigent C, Godwin J, Halls H, Emberson JR, Patrono C. Do selective cyclo-oxygenase-2 inhibitors and traditional non-steroidal anti-inflammatory drugs increase the risk of atherothrombosis? Meta-analysis of randomised trials. BMJ. 2006;332:1302–8. doi: 10.1136/bmj.332.7553.1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Johnsen SP, Larsson H, Tarone RE, et al. Risk of hospitalization for myocardial infarction among users of rofecoxib, celecoxib, and other NSAIDs: A population-based case-control study. Arch Intern Med. 2005;165:978–84. doi: 10.1001/archinte.165.9.978. [DOI] [PubMed] [Google Scholar]
- 27.Hippisley-Cox J, Coupland C. Risk of myocardial infarction in patients taking cyclo-oxygenase-2 inhibitors or conventional non-steroidal anti-inflammatory drugs: Population based nested case-control analysis. BMJ. 2005;330:1366–73. doi: 10.1136/bmj.330.7504.1366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Andersohn F, Suissa S, Garbe E. Use of first- and second-generation cyclooxygenase-2-selective nonsteroidal antiinflammatory drugs and risk of acute myocardial infarction. Circulation. 2006;113:1950–7. doi: 10.1161/CIRCULATIONAHA.105.602425. [DOI] [PubMed] [Google Scholar]
- 29.Gislason GH, Jacobsen S, Rasmussen JN, et al. Risk of death or reinfarction associated with the use of selective cyclooxygenase-2 inhibitors and nonselective nonsteroidal antiinflammatory drugs after acute myocardial infarction. Circulation. 2006;113:2906–13. doi: 10.1161/CIRCULATIONAHA.106.616219. [DOI] [PubMed] [Google Scholar]
- 30.Graham DJ, Campen D, Hui R, et al. Risk of acute myocardial infarction and sudden cardiac death in patients treated with cyclo-oxygenase 2 selective and non-selective non-steroidal anti-inflammatory drugs: Nested case-control study. Lancet. 2005;365:475–81. doi: 10.1016/S0140-6736(05)17864-7. [DOI] [PubMed] [Google Scholar]
- 31.Solomon DH, Schneeweiss S, Glynn RJ, et al. Relationship between selective cyclooxygenase-2 inhibitors and acute myocardial infarction in older adults. Circulation. 2004;109:2068–73. doi: 10.1161/01.CIR.0000127578.21885.3E. [DOI] [PubMed] [Google Scholar]
- 32.Thompson AJ, Yeomans ND. COX-2 inhibition versus gastroprotection with dual COX inhibitors: An evidence-based approach. Curr Pharm Des. 2003;9:2221–8. doi: 10.2174/1381612033453956. [DOI] [PubMed] [Google Scholar]
- 33.Singh G, Triadafilopoulos G. Appropriate choice of proton pump inhibitor therapy in the prevention and management of NSAID-related gastrointestinal damage. Int J Clin Pract. 2005;59:1210–7. doi: 10.1111/j.1368-5031.2005.00660.x. [DOI] [PubMed] [Google Scholar]
- 34.Chan FK, Hung LC, Suen BY, et al. Celecoxib versus diclofenac and omeprazole in reducing the risk of recurrent ulcer bleeding in patients with arthritis. N Engl J Med. 2002;347:2104–10. doi: 10.1056/NEJMoa021907. [DOI] [PubMed] [Google Scholar]
- 35.Garcia Rodriguez LA, Varas-Lorenzo C, Maguire A, Gonzalez-Perez A. Nonsteroidal antiinflammatory drugs and the risk of myocardial infarction in the general population. Circulation. 2004;109:3000–6. doi: 10.1161/01.CIR.0000132491.96623.04. [DOI] [PubMed] [Google Scholar]
- 36.Ray WA, Stein CM, Daugherty JR, Hall K, Arbogast PG, Griffin MR. COX-2 selective non-steroidal anti-inflammatory drugs and risk of serious coronary heart disease. Lancet. 2002;360:1071–3. doi: 10.1016/S0140-6736(02)11131-7. [DOI] [PubMed] [Google Scholar]
- 37.Capone ML, Tacconelli S, Sciulli MG, et al. Clinical pharmacology of platelet, monocyte, and vascular cyclooxygenase inhibition by naproxen and low-dose aspirin in healthy subjects. Circulation. 2004;109:1468–71. doi: 10.1161/01.CIR.0000124715.27937.78. [DOI] [PubMed] [Google Scholar]
- 38.Tannenbaum H, Bombardier C, Davis P, Russell AS, Third Canadian Consensus Conference Group An evidence-based approach to prescribing nonsteroidal anti-inflammatory drugs Third Canadian Consensus Conference J Rheumat 200633140–57.(Errata in 2006;33:829, 2006;33:440) [PubMed] [Google Scholar]
- 39.American College of Rheumatology Subcommittee on Rheumatoid Arthritis Guidelines Guidelines for the management of rheumatoid arthritis: 2002 update. Arthritis Rheum. 2002;46:328–46. doi: 10.1002/art.10148. [DOI] [PubMed] [Google Scholar]
- 40.Bennett JS, Daugherty A, Herrington D, Greenland P, Roberts H, Taubert KA.The use of nonsteroidal anti-inflammatory drugs (NSAIDs): A science advisory from the American Heart Association Circulation 20051111713–6.(Erratum in 2005;111:1717). [DOI] [PubMed] [Google Scholar]
- 41.Olsen NJ. Tailoring arthritis therapy in the wake of the NSAID crisis. N Engl J Med. 2005;352:2578–80. doi: 10.1056/NEJMp058105. [DOI] [PubMed] [Google Scholar]