Abstract
BACKGROUND:
Structural and functional abnormalities of the aortic wall and disturbances of the coronary circulation with presumed microvascular complications have been reported in patients with diabetes mellitus.
OBJECTIVES:
To simultaneously establish the coronary flow velocity reserve (CFVR) and aortic distensibility indexes in type 2 diabetes mellitus patients who have normal epicardial coronary arteries by stress transesophageal echocardiography (STEE).
METHODS:
The elastic properties of the descending aorta and the CFVR were evaluated simultaneously in 18 type 2 diabetes mellitus patients who had negative coronary angiograms. These results were compared with those of 21 nondiabetic subjects with normal epicardial coronary arteries and 24 patients with left anterior descending coronary artery (LAD) stenosis. STEE was used for the evaluation of elastic moduli of the descending aorta. The CFVR was calculated as the ratio of the average peak diastolic flow velocity during hyperemia to that at rest.
RESULTS:
The CFVR of diabetic patients with normal epicardial coronary arteries and those with LAD stenosis was similarly decreased compared with the controls (2.10±0.63 and 1.78±0.47 versus 2.76±1.25, P<0.05 and P<0.001, respectively). The elastic modulus (in 103 mmHg) was similarly increased in patients with diabetes mellitus and normal epicardial coronary arteries, and in those with LAD stenosis, compared with the control subjects (0.94±0.82 and 0.91±0.59 versus 0.49±0.19, P<0.05 and P<0.05, respectively).
CONCLUSIONS:
It may be stated that reduced aortic distensibility (increased elastic modulus) and the CFVR were demonstrated simultaneously during STEE in diabetic patients compared with nondiabetic subjects with negative coronary angiograms.
Keywords: Aortic distensibility, Coronary flow velocity reserve, Diabetes mellitus, Transesophageal echocardiography
Abstract
CONTEXTE :
Des anomalies structurales et fonctionnelles de la paroi aortique et des troubles de la circulation coronarienne, liés à des complications microvasculaires présumées ont déjà été signalées chez des patients atteints de diabète sucré.
OBJECTIFS :
L’étude avait pour but d’établir simultanément les indices de capacité de dilatation aortique et de réserve coronaire (RC) chez des patients atteints de diabète de type 2 et présentant des artères coronaires épicardiques normales à l’échocardiographie transoesophagienne d’effort (ETE).
MÉTHODE :
Les propriétés élastiques de l’aorte descendante et la RC ont été évaluées en même temps chez 18 patients atteints de diabète de type 2, qui ont obtenu des résultats négatifs à la coronarographie. Les résultats ont ensuite été comparés à ceux enregistrés chez 21 sujets non diabétiques ayant des artères coronaires épicardiques normales et chez 24 patients présentant une sténose de l’artère interventriculaire antérieure gauche (AIAG). Les modules d’élasticité de l’aorte ont été évalués à l’aide de l’ETE, et la RC a été calculée comme la vitesse moyenne du flux diastolique maximal durant l’hyperémie rapportée à la valeur obtenue au repos.
RÉSULTATS :
La RC chez les patients diabétiques ayant des artères coronaires épicardiques normales et chez les patients présentant une sténose de l’AIAG a diminué à peu près pareillement par rapport à celle enregistrée chez les témoins (2,10±0,63 et 1,78±0,47 contre 2,76±1,25; p < 0,05 et p < 0,001, respectivement). Par contre, les modules d’élasticité (dans 103 mm Hg) ont augmenté à peu près pareillement chez les patients diabétiques ayant des artères coronaires épicardiques normales et chez les patients présentant une sténose de l’AIAG par rapport à ceux enregistrés chez les témoins (0,94±0,82 et 0,91±0,59 contre 0,49±0,19; p < 0,05 et p < 0,05, respectivement).
CONCLUSIONS :
Ainsi peut-on affirmer qu’une diminution de la capacité de dilatation aortique (augmentation des modules d’élasticité) et de la RC a été démontrée simultanément à l’ETE chez des patients diabétiques par rapport à des sujets non diabétiques ayant obtenu des résultats négatifs à la coronarographie.
Diabetes mellitus is associated with an increased incidence of atherosclerosis. Structural and functional abnormalities of the aortic wall and disturbances of coronary circulation with presumed microvascular complications but no significant coronary artery obstruction (macrovascular disease) have been reported in patients with diabetes mellitus.
Stress transesophageal echocardiography (STEE) is a suitable method for the simultaneous visualization of the proximal portion of the coronary arteries and the descending thoracic aorta. The coronary blood flow velocities may be measured under baseline conditions and during dipyridamole-induced coronary vasodilation during STEE (1). To characterize the vasoreactivity of the left anterior descending coronary artery (LAD), the coronary flow velocity reserve (CFVR) can be calculated as the ratio of peak to baseline diastolic coronary flow velocities. Gadallah et al (2) demonstrated a good correlation between coronary flow reserve, which was assessed by Doppler guide wire and determined by STEE (2). The elastic modulus (E[p]) and Young’s circumferential static elastic modulus (E[s]), as aortic distensibility indexes, may also be calculated simultaneously by STEE from echocardiographic aortic parameters and blood pressure data (3–5). Previous reports have demonstrated decreased CFVR and aortic distensibility (increased E[p] and E[s]), evaluated simultaneously by a STEE examination, in coronary artery disease (6), aortic stenosis (7) and aortic atherosclerosis (8).
The aim of the present study was to simultaneously evaluate the elastic properties of the descending aorta and the CFVR in the following patient populations: nondiabetic subjects with normal epicardial coronary arteries, type 2 diabetes mellitus subjects with a negative coronary angiogram and subjects with significant LAD disease but without diabetes mellitus.
METHODS
Study population
The study comprised 39 patients with chest pain without a previous myocardial infarction. All of them had undergone coronary angiography, with no evidence of significant macrovascular disease. None of the patients exhibited significant valvular heart disease. This patient population was divided into two groups, based on the presence or absence of type 2 diabetes mellitus, and the results of the 21 nondiabetic patients (group 1) were compared with those of the 18 diabetic patients (group 2). These results were then compared with those of 24 patients with LAD disease but without diabetes mellitus (group 3). The CFVR and aortic distensibility indexes were measured simultaneously by STEE. STEE and transthoracic echocardiographic (TTE) examinations were performed in all subjects. The day before the CFVR was measured, the consumption of caffeine-containing drinks was prohibited. Diabetes mellitus was defined according to the American Diabetes Association and the World Health Organization criteria (9,10). Hypertension was defined as either a systolic or a diastolic elevation of blood pressure (greater than 140/90 mmHg) or ongoing antihypertensive therapy. Hypercholesterolemia was defined as a total cholesterol level of more than 5.0 mmol/L or current treatment with lipid-lowering medication. The study complied with the Declaration of Helsinki (11). The written study protocol was approved by the locally appointed ethics committee, and all patients gave their informed consent.
TTE
A complete TTE examination was performed in all cases with commercially available echocardiographic systems (ATL Ultramark 9 HDI [ATL, USA] and Toshiba Powervision 8000 [Toshiba, Japan]). Left ventricular internal dimensions were measured by M-mode echocardiography, and ejection fraction was calculated by the method described by Teichholz et al (12).
Transesophageal echocardiography
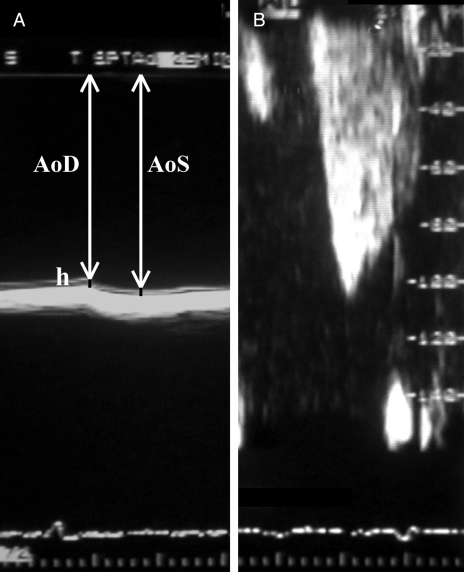
A complete transesophageal echocardiographic examination was carried out in all patients with the ATL Ultramark 9 HDI echocardiograph, using a biplane transducer or a Toshiba Powervision 8000 echocardiograph with a multiplane transducer. Blood pressure and heart rate were monitored continuously during the examinations. Systolic and diastolic aortic diameters, as well as diastolic intima-media thickness, were measured in the transversal plane behind the left atrium during the transesophageal examination (Figure 1A). The elastic properties of the descending aorta were used for the description and quantification of the physical behaviour of the vessels in response to an intraluminal force:
E(p) is an index of arterial stiffness (3): E(p) = (PS –PD)/strain, where PS is the systolic pressure and PD is the diastolic pressure. Strain was calculated as (AoS –AoD)/AoD, where AoS is the systolic aortic diameter and AoD is the diastolic aortic diameter.
E(s) was evaluated via (4) E(s) = E(p) × AoD/2h, where h is the diastolic intima-media thickness.
Figure 1).
A Pulsatile change in aortic diameter is shown (ΔD = AoS –AoD, where AoS is the systolic aortic diameter and AoD is the diastolic aortic diameter). To calculate the elastic modulus (E[p]), the measurement of pulse pressure (ΔP = PS – PD, where PS is the systolic pressure and PD is the diastolic pressure) is also needed (E[p] = (ΔP/ΔD) ×ΔAoD). Young’s circumferential static elastic modulus (E[s]) can be evaluated via E(s) = E(p) ×ΔAoD/2h, where h is the diastolic intima-media thickness. B Typical biphasic coronary waveform recorded by pulsed Doppler imaging in the left anterior descending coronary artery
Aortic atherosclerosis (AA) was graded as proposed by Fazio et al (13): grade 0, no AA; grade 1, intimal thickening; grade 2, plaque smaller than 5 mm; grade 3, plaque greater than 5 mm; grade 4, plaque with mobile parts. Parameters were considered at the ‘worst place’.
Dipyridamole STEE examinations were carried out according to the standard protocol proposed by Iliceto et al (1). Beta-blockers, nitrates and calcium antagonists were discontinued two days before the STEE examinations were performed. In all patients, the aortic root and the proximal portion of the LAD were visualized in the transversal plane. Coronary blood flow was first visualized by colour Doppler flow imaging and the phasic flow velocity waveform in the LAD was then recorded by pulsed Doppler imaging (Figure 1B). Phasic coronary flow velocity patterns were recorded under resting conditions and during hyperemia. Dipyridamole, as the vasodilating agent, was infused for 4 min at a dose of 0.56 mg/kg. Peak velocities were measured after 6 min, at maximal vasodilation. The CFVR was estimated to be the ratio of hyperemic to basal peak diastolic coronary flow velocities. All studies were recorded on super-VHS videotape and evaluated by experts in echocardiography, who were blinded to the results of coronary angiography. In each case, five consecutive cycles were measured and averaged.
Coronary angiography
Coronary angiography was performed with the Seldinger technique. Stenosis was measured by a digital caliper method with the Siemens HiCor biplane angiographic system (Siemens, Germany) and was evaluated from multiplane projections. Significant stenosis was considered to be a luminal diameter reduction of more than 50%.
Statistical analysis
All values were expressed as mean ± SD; 95% confidence limits are also included. For dichotomous values, Fisher’s exact test was used when needed. For group comparisons, ANOVA by the Bonferroni method was used. Multivariate analysis was undertaken using age, sex, diabetes, hypertension and hypercholesterolemia, and different drugs as independent factors for prediction of group 2. P<0.05 indicated a significant difference. SPSS 9.0 software (SPSS Inc, USA) was used for statistical calculations. Numerical correlations were established by Spearman correlation.
RESULTS
Patient demographic and clinical data are shown in Table 1. All diabetic patients had mild to severe nephropathy and retinopathy. In group 2, mild retinopathy was found in 12 patients, while six subjects showed moderate or severe retinopathy. The mean creatinine level was 160±32 μmol/L, while the mean urea nitrogen level was 18±2 mmol/L (mild nephropathy). Waist and hip circumferences were not measured at the time of the study.
TABLE 1.
Demographic and clinical data
| Group 1 (no diabetes) | Group 2 (type 2 diabetes) | Group 3 (LAD disease without diabetes) | |
|---|---|---|---|
| Patients, n | 21 | 18 | 24 |
| Men, n (%) | 9 (43) | 7 (39)**** | 19 (79)* |
| Age, mean ± SD (years) | 47.4±7.8 | 59.1±11.0*** | 57.5±10.5** |
| Hypertension, n (%) | 10 (48) | 16 (89)****** | 12 (50) |
| Hypercholesterolemia, n (%) | 11 (52) | 4 (22) | 12 (50) |
| Body mass index, mean ± SD (kg/m2) | 24.3±2.3 | 29.2±4.1 | 25.2±1.9 |
P<0.05 versus group 1;
P<0.01 versus group 1;
P<0.001 versus group 1;
P<0.01 versus group 3. LAD Left anterior descending coronary artery
Medication administration data are detailed in Table 2. The use of nitrates and acetylsalicylic acid was significantly more frequent in the patients with LAD disease without diabetes mellitus (group 3). Regarding the inclusion criteria, oral antidiabetics (55%) and insulin (56%) were used only in group 2. Standard TTE data were similar among the patient groups (Table 3).
TABLE 2.
Medication administration among study groups
| Group 1 (no diabetes) | Group 2 (type 2 diabetes) | Group 3 (LAD disease without diabetes) | |
|---|---|---|---|
| Patients, n | 21 | 18 | 24 |
| Beta-blockers, n (%) | 13 (62) | 13 (72) | 17 (71) |
| ACE inhibitors, n (%) | 11 (52) | 11 (61) | 18 (75) |
| Nitrates, n (%) | 7 (33) | 8 (44) | 17 (71)* |
| Calcium antagonists, n (%) | 6 (29) | 5 (28) | 8 (33) |
| Lipid-lowering therapy, n (%) | 8 (38) | 6 (33) | 12 (50) |
| Acetylsalicylic acid, n (%) | 7 (33) | 5 (28) | 24 (100)***** |
| Coumarin, n (%) | 1 (5) | 2 (11) | 4 (17) |
| Diuretics, n (%) | 10 (48) | 9 (50) | 12 (50) |
P<0.05 versus group 1;
P<0.001 versus group 1;
P<0.001 versus group 2. ACE Angiotensin-converting enzyme; LAD Left anterior descending coronary artery
TABLE 3.
Transthoracic echocardiographic data
| Group 1 (no diabetes) | Group 2 (type 2 diabetes) | Group 3 (LAD disease without diabetes) | |
|---|---|---|---|
| End-diastolic diameter (mm) | 49.3±7.4 | 54.4±6.3 | 53.6±5.9 |
| Interventricular septum (mm) | 9.9±1.4 | 10.9±2.8 | 10.1±1.4 |
| Posterior wall (mm) | 9.6±1.4 | 10.8±2.4 | 9.9±1.2 |
| Ejection fraction (%) | 68.6±6.7 | 60.6±10.8 | 62.2±9.3 |
Values are presented as mean ± SD. LAD Left anterior descending coronary artery
During dipyridamole STEE, no patients displayed significant limiting side effects or ST-T changes on electrocardiogram. The mean resting systolic velocities were similar in the three patient groups. The average systolic velocity measured at peak stress was decreased in group 3, but the difference did not reach the level of significance. The ratio of systolic flow velocity measured at peak stress to the resting systolic flow velocity was nonsignificantly reduced in group 3 compared with groups 1 and 2. The mean resting diastolic velocity was increased, while the mean diastolic velocity measured at peak stress was decreased in group 3 compared with group 1. The CFVR was significantly lower in groups 2 and 3 than in group 1. E(p) was similarly increased in groups 2 and 3. E(s) was higher in group 3 than group 1, while no significant difference was found between groups 1 and 2 (Table 4). Negative correlations were found between the CFVR and elastic moduli (E[p] and E[s]) (r=–0.269, P=0.047 and r=–0.286, P=0.034, respectively). In the multivariate analysis model, after entering the potential factors as independent factors (age, sex, hypertension, hypercholesterolemia and different drugs), diabetes remained the only significant predictor in group 2 (P<0.01).
TABLE 4.
Coronary flow velocities, coronary flow velocity reserves (CFVRs) and aortic distensibility indexes
| Group I (no diabetes) | Group 2 (type 2 diabetes) | Group 3 (LAD disease without diabetes) | |
|---|---|---|---|
| Srest (cm/s) | 26.8±9.2 | 25.8±9.5 | 27.2±12.2 |
| Smax (cm/s) | 62.5±26.6 | 57.2±32.8 | 50.2±33.3 |
| Smax/Srest ratio | 2.52±1.21 | 2.23±1.09 | 1.84±0.66 |
| Drest (cm/s) | 53.4±18.0 | 58.6±20.1 | 58.6±26.4 |
| Dmax (cm/s) | 134.2±39.5 | 118.0±41.5 | 94.4±31.0** |
| CFVR | 2.76±1.25 | 2.10±0.63* | 1.78±0.47*** |
| AoS – AoD (mm) | 1.74±1.56 | 1.16±0.47 | 1.23±0.49 |
| E(p) (103 mmHg) | 0.49±0.19 | 0.94±0.82* | 0.91±0.59* |
| E(s) (103 mmHg) | 5.08±2.26 | 8.07±6.09 | 9.41±7.19* |
| Mean grade of AA | 0.62±0.74 | 1.06±0.77 | 1.18±0.73* |
Values are presented as mean ± SD.
P<0.05 versus group 1;
P<0.01 versus group 1;
P<0.001 versus group 1. AA Aortic atherosclerosis; AoD Diastolic aortic diameter; AoS Systolic aortic diameter; Dmax Diastolic flow velocity measured at peak stress; Drest Resting diastolic flow velocity; E(p) Elastic modulus; E(s) Young’s circumferential static elastic modulus; Smax Systolic flow velocity measured at peak stress; Srest Resting systolic flow velocity
DISCUSSION
To the best of our knowledge, this is the first simultaneous evaluation of E(p), E(s) and CFVR in type 2 diabetes mellitus patients with no evidence of significant coronary artery stenosis. The results demonstrated reduced CFVR and aortic distensibility (increased elastic moduli) in type 2 diabetic patients compared with nondiabetic patients; these data were similar to those in the LAD stenosis patients without diabetes mellitus.
In diabetic heart disease, the myocardial, interstitial, coronary and neural structures are frequently involved. A markedly reduced CFVR has been observed in type 2 diabetic patients with angiographically normal coronary arteries, demonstrating the functional existence of presumed coronary small-vessel disease in the diabetic heart (14–16). Yokoyama et al (17) found that control of the blood glucose concentration, rather than insulin resistance, is most probably related to the reduced CFVR in type 2 diabetes. The results of Pitkanen et al (18) demonstrated an impaired CFVR in young male adults with type 1 diabetes, with no or only minimal microvascular complications and without any evidence of coronary artery disease. Previous studies have demonstrated that the hyperemic flow is 20% lower in diabetics than in healthy control subjects (18,19). Di Carli et al (19) found that diabetics with evidence of cardiac sympathetic nerve dysfunction exhibit an impaired sympathetically mediated dilation of the coronary resistance vessels.
However, aside from these factors, abbreviation of diastole and reduced coronary diastolic perfusion pressure can have also an effect on coronary flow (20,21). It has been demonstrated that during stress-induced myocardial ischemia, coronary obstruction and diastolic perfusion time are factors that limit subendocardial perfusion (20). After maximal coronary vasodilation or reduced coronary perfusion pressure, a close relation between the decreases in diastolic perfusion time and subendocardial perfusion was reported (22,23).
Tentolouris et al (24) demonstrated that type 2 diabetes is associated with a significant reduction in aortic distensibility. Moreover, the known duration of diabetes and the presence of cardiac autonomic neuropathy are the main predictors of aortic distensibility in subjects with type 2 diabetes. Endothelial dysfunction and diffuse arterial wall stiffening and thickening can be found in type 1 diabetes, and both may contribute to the excess cardiovascular mortality in such patients (25). Hu et al (26) observed a difference in aortic stiffness among women with type 1 diabetes during pregnancy and those with uncomplicated pregnancy. This may be interpreted as an altered cardiovascular adaptation to pregnancy in women with diabetes. Increased aortic wall stiffness was found to be related to the duration of diabetes (27).
Previous studies have verified that STEE may be used for the simultaneous evaluation of aortic distensibility indexes and CFVR (6–8). First results demonstrated a reduced CFVR and increased E(p) and E(s) (decreased aortic distensibility) in patients with LAD disease compared with patients with normal epicardial coronary arteries (6). No further changes in these parameters were found in patients with multivessel disease. In patients with nonsignificant coronary artery stenosis, the CFVR and stiffness moduli were between those of patients with negative angiography and those of patients with LAD disease and multivessel disease. A reduced CFVR has also been demonstrated in aortic stenosis patients with normal epicardial coronary arteries, characterizing the diminished reserve capacity of the LAD, as well as altered aortic distensibility (increased E[p] and E[s]) relative to those in patients without valvular heart disease or significant coronary artery disease (7). The CFVR was reduced in patients with even minimal grade AA in the event of aortic intimal thickening relative to subjects without relevant AA (8). No further decrease was observed in patients with higher grades of AA (in the presence of different grades of aortic plaque). The indexes of aortic distensibility increased continuously parallel to the aortic grade, and significant differences were found between patients with grade 2 or 3 AA and those with grade 0 or 1 AA.
The prevalence of hypertension was frequent in our diabetic patients; this is a common finding in diabetes mellitus. Notwithstanding, in the present study, diabetes remained the only significant predictor of the diabetic group (patients with diabetes mellitus and a negative coronary angiogram). Structural and functional properties of the arterial wall have been reported to be altered in hypertension, even at early stages of the disease (28–31). Blood pressure lowering in the hypertensive patients was associated with a significant reduction in aortic stiffness distinct from its acute depressor effect (32). Previous findings also indicate that hypertension is associated with reduced CFVR (33–35). Hamouda et al (33) found impaired CFVR that was evaluated by STEE, which was more obvious in hypertensive patients with LVH than in those without hypertrophy. The CFVR in hypertensive patients was inversely related to the severity of LVH, which is in agreement with our previous results (33,35). Moreover, impaired CFVR was found very early in hypertension, before hypertrophy was apparent (34). These results warrant further investigation on simultanous evaluation of these functional parameters in nondiabetic patients.
Clinical implications
The purpose of the present study was to assess the effects of diabetes mellitus on the coronary and aortic distensibility simultaneously evaluated by STEE. The aortic distensibility and LAD CFVR were reduced in type 2 diabetic patients without epicardial coronary artery stenosis. Diabetic autonomic neuropathy and endothelial dysfunction can play a role in this phenomenon. These results can suggest the overall effect of diabetes mellitus on the function and structure of the descending aorta and coronary arteries. However, the authors do not think that STEE is the best choice for the vascular examination of diabetic patients. Further examinations are warranted for the examination of stiffened aorta on coronary function in patients with different kinds of cardiovascular risk factors, even in diabetes mellitus.
Study limitations
Blood flow velocities, but not the blood flow itself, were measured by STEE. The measurement of coronary blood flow requires an evaluation of a luminal cross-sectional area. Furthermore, because there is an angle between the ultrasound beam and the vessel direction, blood flow velocities measured by this approach can be lower than the real values. However, both the numerator and the denominator in the formula for the CFVR are measured at the same angle, and the ratio is not appreciably influenced by the angle or the vessel direction. Control subjects (group 1) were significantly younger than patients in groups 2 and 3, which can affect the results.
In calculation of E(p) and E(s), it was assumed that the pulse pressure (PP) in the aorta was the same as the PP in the brachial artery. PP is not constant throughout the large artery tree; brachial artery PP may not be a good surrogate for central aortic PP (36). The difference between the aortic and brachial artery pressures is difficult to predict because it is influenced by many factors, such as body height, age, heart rate, stroke volume, ejection time, etc. An accurate, noninvasive method to measure aortic pressures serves as important clinical evidence.
CONCLUSIONS
Reduced aortic distensibility (increased elastic modulus) and CFVR, evaluated simultaneously by STEE, can be demonstrated in type 2 diabetes mellitus patients with a negative coronary angiogram compared with nondiabetic subjects.
Acknowledgments
The authors gratefully acknowledge the skilled assistance of the nursing staff of the Stress Echocardiographic Laboratory and Cardiac Catheterization Unit at the Albert Szent-Györgyi Medical and Pharmaceutical Center (Szeged, Hungary).
Footnotes
GRANT SUPPORT: ETT 408 06/2003 (Budapest)
REFERENCES
- 1.Iliceto S, Marangelli V, Memmola C, Rizzon P. Transesophageal Doppler echocardiography evaluation of coronary blood flow velocity in baseline conditions and during dipyridamole-induced coronary vasodilation. Circulation. 1991;83:61–9. doi: 10.1161/01.cir.83.1.61. [DOI] [PubMed] [Google Scholar]
- 2.Gadallah S, Thaker KB, Kawanishi D, et al. Comparison of intracoronary Doppler guide wire and transesophageal echocardiography in measurement of flow velocity and coronary flow reserve in the left anterior descending coronary artery. Am Heart J. 1998;135:38–42. doi: 10.1016/s0002-8703(98)70340-0. [DOI] [PubMed] [Google Scholar]
- 3.Peterson LN, Jensen RE, Parnell R. Mechanical properties of arteries in vivo. Circ Res. 1960;8:622–39. [Google Scholar]
- 4.Nichols WW, O’Rourke MF, editors. McDonald’s Blood Flow in Arteries. Philadelphia: Lea & Febiger; 1989. Properties of the arterial wall; pp. 77–124. [Google Scholar]
- 5.Pearson AC, Guo R, Orsinelli DA, Binkley PF, Pasierski TJ. Transesophageal echocardiographic assessment of the effects of age, gender, and hypertension on thoracic aortic wall size, thickness, and stiffness. Am Heart J. 1994;128:344–51. doi: 10.1016/0002-8703(94)90488-x. [DOI] [PubMed] [Google Scholar]
- 6.Nemes A, Forster T, Gruber N, Csanady M. Coronary flow velocity reserve and indices describing aortic distensibility in patients after coronary angiography. Int J Cardiol. 2004;96:29–33. doi: 10.1016/j.ijcard.2003.06.009. [DOI] [PubMed] [Google Scholar]
- 7.Nemes A, Forster T, Csanady M. Decreased aortic distensibility and coronary flow velocity reserve in patients with significant aortic valve stenosis with normal epicardial coronary arteries. J Heart Valve Dis. 2004;13:567–73. [PubMed] [Google Scholar]
- 8.Nemes A, Forster T, Csanady M, Gruber N. Indices of aortic distensibility and coronary flow velocity reserve in patients with different grades of aortic atherosclerosis. Int J Cardiovasc Imag. 2004;20:271–7. doi: 10.1023/b:caim.0000041935.28173.55. [DOI] [PubMed] [Google Scholar]
- 9.American Diabetes Association All about diabetes<www.diabetes.org/about-diabetes.jsp> (Version current at December 5, 2006).
- 10.World Health Organization Diabetes programme: What is diabetes?<www.who.int/diabetes/BOOKLET_HTML/en/index4.html> (Version current at December 5, 2006).
- 11.The World Medical Association Policy: Declaration of Helsinki –ethical principles for medical research involving human subjects<www.wma.net/e/policy/b3.htm> (Version current at December 6, 2006).
- 12.Teichholz LE, Cohen MV, Sonnenblick EH, Gorlin R. Study of the left ventricular geometry and function by B-scan ultrasonography in patients with and without asynergy. N Engl J Med. 1974;291:1220–6. doi: 10.1056/NEJM197412052912304. [DOI] [PubMed] [Google Scholar]
- 13.Fazio GP, Redberg RF, Winslow T, Schiller NB. Transesophageal echocardiographically detected atherosclerotic aortic plaque is a marker for coronary artery disease. J Am Coll Cardiol. 1993;21:144–50. doi: 10.1016/0735-1097(93)90729-k. [DOI] [PubMed] [Google Scholar]
- 14.Fang ZY, Prins JB, Marwick TH. Diabetic cardiomyopathy: Evidence, mechanisms, and therapeutic implications. Endocr Rev. 2004;25:543–67. doi: 10.1210/er.2003-0012. [DOI] [PubMed] [Google Scholar]
- 15.Kranidis A, Zamanis N, Mitrakou A, et al. Coronary microcirculation evaluation with transesophageal echocardiography Doppler in type II diabetics. Int J Cardiol. 1997;59:119–24. doi: 10.1016/s0167-5273(97)02935-5. [DOI] [PubMed] [Google Scholar]
- 16.Strauer BE, Motz W, Vogt M, Schwartzkopff B. Evidence for reduced coronary flow reserve in patients with insulin-dependent diabetes. A possible cause for diabetic heart disease in man. Exp Clin Endocrinol Diabetes. 1997;105:15–20. doi: 10.1055/s-0029-1211722. [DOI] [PubMed] [Google Scholar]
- 17.Yokoyama I, Ohtake T, Momomura S, et al. Hyperglycemia rather than insulin resistance is related to reduced coronary flow reserve in NIDDM. Diabetes. 1998;47:119–24. doi: 10.2337/diab.47.1.119. [DOI] [PubMed] [Google Scholar]
- 18.Pitkanen OP, Nuutila P, Raitakari OT, et al. Coronary flow reserve is reduced in young men with IDDM. Diabetes. 1998;47:248–54. doi: 10.2337/diab.47.2.248. [DOI] [PubMed] [Google Scholar]
- 19.Di Carli MF, Bianco-Batlles D, Landa ME, et al. Effects of autonomic neuropathy on coronary blood flow in patients with diabetes mellitus. Circulation. 1999;100:813–9. doi: 10.1161/01.cir.100.8.813. [DOI] [PubMed] [Google Scholar]
- 20.Ferro G, Duilio C, Spinelli L, Liucci GA, Mazza F, Indolfi C. Relation between diastolic perfusion time and coronary artery stenosis during stress-induced myocardial ischemia. Circulation. 1995;92:342–7. doi: 10.1161/01.cir.92.3.342. [DOI] [PubMed] [Google Scholar]
- 21.Buckberg GD, Towers B, Paglia DE, Mulder DG, Maloney JV. Subendocardial ischemia after cardiopulmonary bypass. J Thorac Cardiovasc Surg. 1972;64:669–84. [PubMed] [Google Scholar]
- 22.Flynn AE, Coggins DL, Goto M, et al. Does systolic subepicardial perfusion come from retrograde subendocardial flow? Am J Physiol. 1992;262:H1759–69. doi: 10.1152/ajpheart.1992.262.6.H1759. [DOI] [PubMed] [Google Scholar]
- 23.Bache RJ, Cobb FR. Effect of maximal coronary vasodilation on transmural myocardial perfusion during tachycardia in the awake dog. Circ Res. 1977;41:648–53. doi: 10.1161/01.res.41.5.648. [DOI] [PubMed] [Google Scholar]
- 24.Tentolouris N, Liatis S, Moyssakis I, et al. Aortic distensibility is reduced in subjects with type 2 diabetes and cardiac autonomic neuropathy. Eur J Clin Invest. 2003;33:1075–83. doi: 10.1111/j.1365-2362.2003.01279.x. [DOI] [PubMed] [Google Scholar]
- 25.Wilkinson IB, MacCallum H, Rooijmans DF, et al. Increased augmentation index and systolic stress in type 1 diabetes mellitus. QJM. 2000;93:441–8. doi: 10.1093/qjmed/93.7.441. [DOI] [PubMed] [Google Scholar]
- 26.Hu J, Bjorklund A, Nyman M, Gennser G. Mechanical properties of large arteries in mother and fetus during normal and diabetic pregnancy. J Matern Fetal Investig. 1998;8:185–93. [PubMed] [Google Scholar]
- 27.Ahlgren AR, Sundkvist G, Wollmer P, Sonesson B, Lanne T. Increased aortic stiffness in women with type 1 diabetes mellitus is associated with diabetes duration and autonomic nerve function. Diabet Med. 1999;16:291–7. doi: 10.1046/j.1464-5491.1999.00079.x. [DOI] [PubMed] [Google Scholar]
- 28.Safar ME. Pulse pressure in essential hypertension: Clinical and therapeutical implications. J Hypertens. 1989;7:769–76. doi: 10.1097/00004872-198910000-00001. [DOI] [PubMed] [Google Scholar]
- 29.Laurent S, Kingwell B, Bank A, Weber M, Struijker-Boudier H. Clinical applications of arterial stiffness: Therapeutics and pharmacology. Am J Hypertens. 2002;15:453–58. doi: 10.1016/s0895-7061(01)02329-9. [DOI] [PubMed] [Google Scholar]
- 30.Asmar R, Benetos A, London G, et al. Aortic distensibility in normotensive, untreated and treated hypertensive patients. Blood Press. 1995;4:48–54. doi: 10.3109/08037059509077567. [DOI] [PubMed] [Google Scholar]
- 31.Heintz B, Gillessen T, Walkenhorst F, et al. Evaluation of segmental elastic properties of the aorta in normotensive and medically treated hypertensive patients by intravascular ultrasound J Hypertens 1993111253–8.(Erratum in 1993;11:following H71). [PubMed] [Google Scholar]
- 32.Ichihara A, Hayashi M, Koura Y, Tada Y, Hirota N, Saruta T. Long-term effects of intensive blood-pressure lowering on arterial wall stiffness in hypertensive patients. Am J Hypertens. 2003;16:959–65. doi: 10.1016/s0895-7061(03)01004-5. [DOI] [PubMed] [Google Scholar]
- 33.Hamouda MS, Kassem HK, Salama M, et al. Evaluation of coronary flow reserve in hypertensive patients by dipyridamole transesophageal doppler echcoardiography. Am J Cardiol. 2000;86:305–8. doi: 10.1016/s0002-9149(00)00919-x. [DOI] [PubMed] [Google Scholar]
- 34.Laine H, Raitakari OT, Niinikoski H, et al. Early impairment of coronary flow reserve in young men with borderline hypertension. J Am Coll Cardiol. 1998;32:147–53. doi: 10.1016/s0735-1097(98)00222-8. [DOI] [PubMed] [Google Scholar]
- 35.Nemes A, Neu K, Forster T, Kovacs Z, Csanady M. Coronary flow velocity reserve is diminished in hypertensive left ventricular hypertrophy. Kardiol Pol. 2005;62:1–5. [PubMed] [Google Scholar]
- 36.Pauca AL, Wallenhaupt SL, Kon ND, Tucker WY. Does radial artery pressure accurately reflect aortic pressure? Chest. 1992;102:1193–8. doi: 10.1378/chest.102.4.1193. [DOI] [PubMed] [Google Scholar]