Abstract
BACKGROUND:
Atrial fibrillation (AF) is the most common adult arrhythmia, and significantly increases the risk of ischemic stroke. Oral anticoagulation may be underused and may be less effective in community settings than clinical trial settings.
OBJECTIVES:
To determine the rates of thromboembolism and bleeding in an ambulatory cohort of patients with AF.
METHODS:
Observational study of Nova Scotian residents with AF identified by electrocardiogram in ambulatory settings between November 1999 and January 2001. Main outcome measures were rates of thromboembolism and bleeding over two years.
RESULTS:
Four hundred twenty-five patients were included in the study. The mean (±SD) age was 70.6±11.1 years, and 40% were women. Warfarin therapy was used by 68% of patients. Sixty-two per cent of patients had hypertension, 21% had a previous stroke or transient ischemic attack, 44% had congestive heart failure and 20% were diabetic. The overall rate of thromboembolic events was 2.7% in warfarin users and 8.5% in nonwarfarin users over two years, with an RR reduction of 68% (OR 0.31, 95% CI 0.09 to 0.91; P=0.047). The annual rate of ischemic stroke was 1.2% and 3.1% in warfarin and nonwarfarin users, respectively, with an RR reduction of 62% (OR 0.29, 95% CI 0.08 to 1.04; P=0.057). The overall rate of major bleeding was 2.6% in warfarin users and 1.4% in nonwarfarin users (P=0.667). The annual mortality rate was 7.79% in warfarin users and 9.93% in nonwarfarin users (P=0.192).
CONCLUSIONS:
Warfarin use was found to significantly reduce the rate of thromboembolic events without a concomitant increase in hemorrhagic events. The present study confirms the effectiveness of warfarin therapy in a population-based cohort.
Keywords: Atrial fibrillation, Hemorrhage, Mortality, Stroke
Abstract
CONTEXTE :
La fibrillation auriculaire (FA) est l’arythmie la plus fréquente chez les adultes et elle augmente sensiblement le risque d’accident vasculaire cérébral (AVC) ischémique. Le traitement anticoagulant par voie orale peut être insuffisant et peut se révéler moins efficace en pratique communautaire que dans les essais cliniques.
BUT :
L’étude avait pour but de déterminer les taux de thromboembolies et d’hémorragies dans une cohorte de patients ambulatoires, atteints de FA.
MÉTHODE :
Une étude d’observation de la population de la Nouvelle-Écosse atteinte de FA, repérée par électrocardiographie en milieu ambulatoire, a été menée entre novembre 1999 et janvier 2001. Les mesures des principaux résultats cliniques étaient les taux de thromboembolies et d’hémorragies sur une période de deux ans.
RÉSULTATS :
Quatre cent vingt-cinq patients ont été sélectionnés en vue de l’étude. L’âge moyen (±écart type) était de 70,6±11,1 ans, et 40 % des sujets étaient des femmes. Un traitement à la warfarine a été prescrit à 68 % des patients. Soixante-deux pour cent des patients étaient atteints d’hypertension, 21 % avaient déjà subi un AVC ou un accident ischémique transitoire, 44 % souffraient d’insuffisance cardiaque congestive et 20 % étaient diabétiques. Le taux global d’accidents thromboemboliques s’est élevé à 2,7 % dans le groupe traité à la warfarine et à 8,5 % dans le groupe non traité à la warfarine, sur la période de deux ans, ce qui donne une réduction de 68 % du RR (RR approché : 0,31; IC à 95 % : 0,09 à 0,91; p = 0,047). Le taux annuel d’AVC ischémiques s’est établi à 1,2 % et à 3,1 % dans le groupe traité à la warfarine et dans le groupe non traité à la warfarine respectivement, ce qui donne une réduction de 62 % du RR (RR approché : 0,29; IC à 95 % : 0,08 à 1,04; p = 0,057). Le taux global d’hémorragies importantes a atteint 2,6 % dans le groupe traité à la warfarine et 1,4 % dans l’autre groupe (p = 0,667). Enfin, le taux annuel de mortalité était de 7,79 % dans le groupe traité à la warfarine et de 9,93 % dans l’autre groupe (p = 0,192).
CONCLUSION :
La warfarine diminue de façon significative le taux d’accidents thromboemboliques sans augmenter pour autant le nombre d’accidents hémorragiques. La présente étude confirme l’efficacité du traitement à la warfarine dans une cohorte fondée sur une population.
A trial fibrillation (AF), the most common adult arrhythmia, is a growing epidemiological problem affecting more than 200,000 Canadians and up to 10% of octogenerians (1–3). Its presence is associated with a stroke rate that is up to five times that of patients without AF (4). Several randomized clinical trials have studied the beneficial effects of warfarin on the reduction in stroke and thromboembolism (5–8). Selection of patients and management of anticoagulation may limit the applicability of the outcomes observed in these trials to those outside a clinical trial setting. In particular, only 20% of participants in clinical trials were older than 75 years of age, and they had relatively fewer comorbidities than are typically seen in patients in the real-world setting. In addition, oral anticoagulation in these studies was monitored closely by a clinical trial team rather than busy community family physicians. No large assessments of the community effectiveness of warfarin in Canada have been conducted to date.
Anticoagulant use in patients with AF in Nova Scotia has been previously studied and was found to be greater than 70%, with a higher proportion considered to be appropriate (9). We studied an ambulatory cohort of AF patients in Nova Scotia to determine whether warfarin reduced the rates of thromboembolic events to the extent seen in clinical trials, without a corresponding increase in hemorrhagic complications.
METHODS
Participants
Eligible patients were identified through electrocardiogram (ECG) departments in hospitals and community health care facilities throughout Nova Scotia. Patients with ECG evidence of AF on an outpatient, emergency room or preadmission ECG were potentially eligible for the present study. For those patients who met the inclusion criteria, the following were excluding characteristics: patients who were not residents of Nova Scotia, family physician refusal to consent to a patient’s participation, patient refusal or inability to give informed consent, inability to speak English and being a nursing home resident. The reasons for patient ineligibility were recorded in the exclusion log book. This protocol was approved by the Research Ethics Board of the Queen Elizabeth II Health Sciences Centre in Halifax, Nova Scotia.
Family physicians of identified patients were contacted for consent regarding their patients’ enrolment in the study. Following family physician approval, identified patients were sent an information package about the study, including a consent form, date and time of proposed interview, as well as the nature of information they would be asked to provide. Patients were encouraged to have a family member present to assist with the interview, if necessary, and to have all current medications available for review. Consenting patients completed a telephone questionnaire regarding demographic characteristics, stroke risk factors, hemorrhagic risk factors, prescription and nonprescription drug use, and factors that may influence their compliance with medication. Family physician offices were contacted to confirm information, if needed.
Antithrombotic therapy
The decision to use antithrombotic therapy was made by the family physician or specialist involved in the patient’s care. Monitoring and dose adjustment were performed either by the family physician’s office or the anticoagulation clinic at the Queen Elizabeth II Health Sciences Centre.
Follow-up
Patients were contacted at six-month intervals to determine whether there had been any changes in their medication, risk factors for stroke or contraindications to anticoagulation. Patients were also questioned as to whether they developed symptoms of thromboembolic or bleeding complications, or whether they required hospitalization or medical attention in the intervening period. In the event of hospitalization, records were retrieved to determine whether thromboembolic or bleeding complications occurred.
End points
The outcome measures included the rates of cardioembolic and major bleeding complications in this cohort of patients.
Stroke was defined as the development of focal neurological symptoms lasting longer than 24 h. Stroke was considered ‘definite’ by confirmation of a new abnormality on a computed tomography scan or magnetic resonance imaging consistent with infarction or hemorrhage. Stroke was considered ‘probable’ if a neurologist or internist made the diagnosis without radiographic confirmation.
Cardioembolism was defined by the development of acute ischemic events involving the mesentery, limbs or kidneys, which was verified by angiography, surgery or autopsy. An adjudication committee, blinded as to whether patients were receiving antithrombotic therapy, reviewed the clinical and radiographic details of all suspected outcome events and made the final determination of outcome events.
Major bleeding was defined using previously described criteria. In brief, bleeding was considered major if it was overt and associated with the need for surgical intervention, hospitalization, major patient morbidity or death.
Statistical analysis
Baseline data were reported using simple descriptive statistics. Student’s t test was used to compare continuous variables, and the χ2 test was used to assess differences between categorical variables. Multivariate logistic regression models were used to determine which factors were independently associated with the risk of cardioembolic and major bleeding complications. All tests were two-sided, and P<0.05 was considered to represent a statistically significant difference.
RESULTS
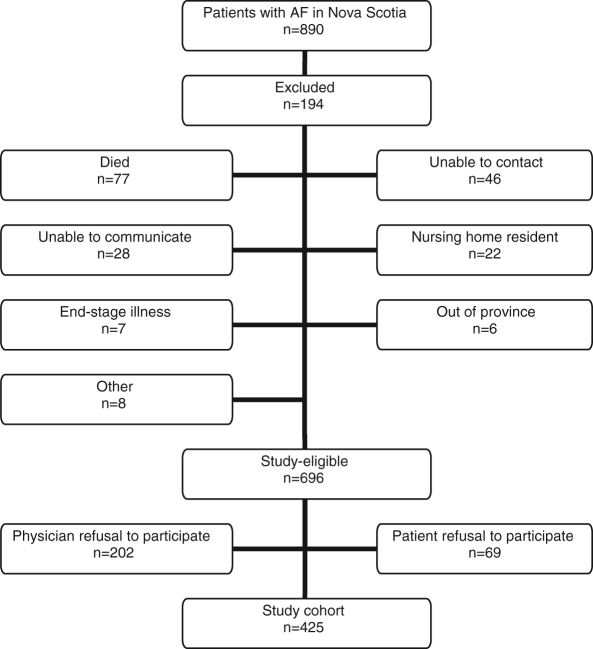
Eight-hundred ninety patients with AF were identified across Nova Scotia between November 1999 and January 2001. Of these patients, 194 were excluded: 77 patients had died between the time of the ECG recording and when their physician was contacted by the study team, 46 patients were unable to be contacted and the remaining 71 patients met at least one of the exclusion criteria. These included communication problems (28 patients), nursing home residents (22 patients), end-stage illness (seven patients), moving out of the province (six patients) and other reasons (eight patients). Of the remaining 696 study-eligible patients, primary care physicians requested that we not contact 202 patients (29%), and 69 patients (10%) refused to participate in the study.
A total of 425 patients (61% of those eligible) who enrolled in the study (Figure 1) (9) were enrolled in this cross-sectional survey (Table 1). Mean (± SD) patient age was 70.6±11.1 years, and 156 patients (37%) were older than 75 years of age. There were 170 women (40%), two-thirds of patients were hypertensive, 21% had a history of stroke or transient ischemic attack, 44% had a history of congestive heart failure and 20% were diabetic. At baseline, 295 patients (69%) were on warfarin and 31 patients (7%) were concomitantly taking acetylsalicylic acid. Eighty-five patients (20%) were on acetylsalicylic acid alone, and 45 patients (11%) were not receiving any antithrombotic therapy.
Figure 1).
Patient selection process. AF Atrial fibrillation
TABLE 1.
Baseline patient characteristics
| Characteristic | All patients (n=425) | Warfarin (n=295) | No warfarin (n=130) |
|---|---|---|---|
| Mean age, years | 70.6 | 71.3 | 69.2 |
| ≥75 years of age, % | 42 | 64.4 | 56.2 |
| Female sex, % | 40 | 40.0 | 40.0 |
| Hypertension, % | 62 | 62.4 | 61.4 |
| Congestive heart failure, % | 44 | 46.3 | 26.2 |
| Current smoker, % | 12 | 7.08 | 6.13 |
| Previous stroke/TIA, % | 21 | 23.4 | 17.7 |
| Diabetes mellitus, % | 20 | 17.9 | 23.9 |
| Angina, % | 22 | 26.0 | 25.3 |
| Myocardial infarction, % | 27 | 34.8 | 26.0 |
| Valvular disease, % | 10 | 12.5 | 4.6 |
TIA Transient ischemic attack
Examination of the appropriateness of therapy using the American College of Chest Physicians (10) guidelines reveals that 88% of patients were on appropriate therapy. Of 132 patients who were not on warfarin, 61% were not on warfarin appropriately; there is no information as to why the remaining 39% were not on appropriate therapy. Of these, 37 of 52 were not on warfarin when they should have been, and 15 of 52 were on warfarin when the guidelines suggested that this was not indicated.
End points
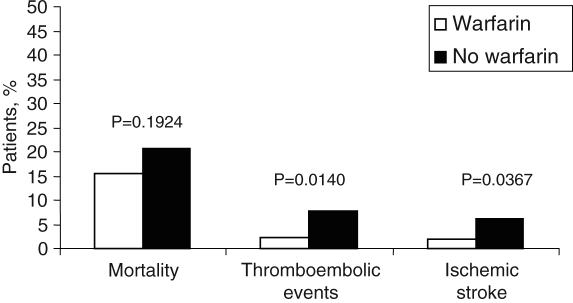
A total of 19 patients (4.5%) suffered a thromboembolic event. Fourteen of these were confirmed by in-hospital investigations, and five were highly suspicious based on available information. Six patients (32.0%) were on warfarin at the time of the event, while 13 (68.0%) were not. Of those patients on warfarin, five had an ischemic stroke and one had a transient ischemic attack. International normalized ratios (INRs) were available for all patients on warfarin at the time of the event, and only three were in the therapeutic range of 2.0 to 3.0. Thirteen patients who were not receiving warfarin therapy had a thromboembolic event; five of these patients were on acetylsalicylic acid alone. Ten of these events were ischemic strokes, two were transient ischemic attacks and one patient had a deep venous thrombosis or pulmonary embolism. In the population-based study, RR reductions of 68% (OR 0.31, 95% CI 0.09 to 0.91; P=0.047) for thromboembolic events and 63% (OR 0.29, 95% CI 0.08 to 1.04; P=0.057) for ischemic strokes were observed among patients on warfarin therapy compared with those who were not.
Over a two-year follow-up period, 73 patients (17.1%) died, 46 (10.8%) of whom were on warfarin and 27 (6.4%) of whom were not. In the warfarin group, annual mortality was not significantly different from the group not on warfarin, at 7.8% and 9.9%, respectively (P=0.192) (Figure 2).
Figure 2).
Mortality and thromboembolic events in patients on antithrombotic therapy
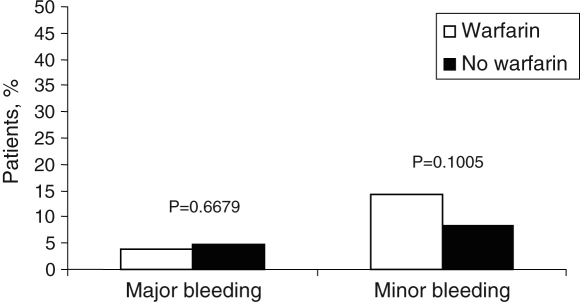
There were a total of 20 major bleeding events in 17 patients (4.0%); 14 events occurred in 11 patients (2.6%) on warfarin and six events occurred in six patients (1.4%) not on warfarin. INRs were available for 11 of the 20 cases at the time of the event: the INR was less than 2.0 in four cases, 2.0 to 3.0 in one case and more than 3.0 in six cases. In 14 of the bleeding cases (70.0%), patients were receiving warfarin, thereby resulting in an annual major bleeding rate of 1.9% in those on warfarin and 2.2% in those not receiving warfarin (P=0.667) (Figure 3). There were 13 gastrointestinal bleeds, one intracranial hemorrhage, three cases of gross hematuria, two cases of epistaxis and one bleed secondary to a motor vehicle accident. Minor bleeds occurred in 53 patients (12.5%), for a total of 70 events. There were four vaginal bleeds, 21 cases of epistaxis, 12 gastrointestinal bleeds, 24 episodes of hematuria and nine other causes of minor bleeding. Forty-two of these patients (79.0%) were on warfarin therapy (P=0.097) (Table 2).
Figure 3).
Major and minor bleeding among patients on antithrombotic therapy
TABLE 2.
Major and minor bleeding
| Type of bleed | Major bleeds (n=20) | Minor bleeds (n=70) |
|---|---|---|
| Gastrointestinal | 13 | 12 |
| Hematuria | 3 | 24 |
| Epistaxis | 2 | 21 |
| Trauma | 1 | 0 |
| Gynecological | 0 | 4 |
| Intracranial | 1 | 0 |
| Other | 0 | 9 |
Predictors of events
The use of warfarin significantly reduced the risk of thromboembolic events, (OR 0.31, 95% CI 0.09 to 0.91; P=0.047) (Table 3). History of heart failure, hypertension, stroke, diabetes mellitus, age as defined by the Congestive heart failure, Hypertension, Age ≥75, Diabetes mellitus, and prior Stroke or transient ischemic attack (CHADS2) variable, and myocardial infarction were not found to affect the risk of thromboembolic events in this high-risk population. Significant predictors of major bleeding on multivariate analysis included history of valvular disease (OR 4.06, P=0.01) and congestive heart failure (OR 2.32, P=0.04).
TABLE 3.
Multivariate predictors of ischemic stroke
| Variable | OR | 95% CI | P |
|---|---|---|---|
| Warfarin use | 0.24 | (0.07–0.87) | 0.0296 |
| History of stroke | 2.47 | (0.92–6.63) | 0.0722 |
| Acetylsalicylic acid use | 0.81 | (0.21–3.08) | 0.7563 |
| Diabetes mellitus | 0.26 | (0.03–2.06) | 0.2015 |
| Cardiac disease* | 1.55 | (0.33–7.22) | 0.5769 |
Includes a history of any one of the following: ischemic heart disease, hypertension, valvular disease or heart failure
Predictors of mortality included older age (OR 2.98, 95% CI 1.58 to 5.63; P=0.0008) and male sex (OR 2.10, 95% CI 1.18 to 3.73; P=0.01). Stroke and diabetes showed a trend toward significance, at P=0.07 and P=0.06, respectively. Warfarin and a history of cardiac disease (heart failure, myocardial infarction) were not associated with mortality.
DISCUSSION
The present study found that the risk of thromboembolic events in a population-based cohort of patients with AF was reduced in those taking warfarin, while the risk of hemorrhagic complications was not found to be increased. Finally, in this cohort, mortality was not affected by warfarin therapy.
Pooled data from clinical trials have reported a 62% to 68% risk reduction in all strokes with the use of warfarin for cardioembolic prophylaxis in patients with AF (11–13). In our population, a 68% risk reduction in stroke was observed. Mortality, however, was relatively high in our cohort, with an annual rate of 7.9% in warfarin users and 9.9% in nonwarfarin users. This was a significantly higher mortality than that observed in pooled clinical trial data, in which annual mortality is reported to be 3.6% and 5.4%, respectively. Major bleeding rates were slightly higher than those reported in clinical trials, 1.9% versus 0.6% to 1.3% in pooled data (13).
Some differences in the results can be explained by observed differences between our population and those enrolled in the clinical trials that assessed warfarin efficacy. Specifically, our population contained a greater proportion of patients older than 75 years of age (42% versus 20%), more women (40% versus 25%), and more patients with a prior history of cerebrovascular disease (21% versus 6%), congestive heart failure (44% versus 19%), myocardial infarction (27% versus 12%) and hypertension (62% versus 12%) than participants in AF clinical trials (13). The higher comorbidity rates in our population may explain the relatively higher mortality rate. The increased bleeding may have been due to supratherapeutic INR values, which may occur more often in the community setting than in a clinical trial.
A few studies have examined whether the established efficacy of warfarin translates into effectiveness in clinical practice (14–17). Caro et al (14) evaluated the outcomes of 221 outpatients with AF identified at two Montreal (Quebec) hospitals. Approximately 30% of these patients received the oral anticoagulant warfarin. This study reported that warfarin use was associated with a significant reduction in stroke risk versus no therapy (1.8% versus 5.9%, RR 0.31; 95% CI 0.1% to 1.0%). Kalra et al (16) followed 167 patients with AF who were managed at a district hospital in Britain. Two per cent of patients had cardioembolic events, and 1.4% experienced major bleeding episodes. A single large study by Go et al (15) examined this issue in ambulatory patients in a managed care setting in the United States. In 11,526 patients, warfarin therapy was associated with a 51% reduction (95% CI 39% to 60%) in the risk of thromboembolism compared with no therapy.
Previous population-based cohort studies have observed similar findings of relatively higher mortality rates but have also documented similar risk reductions in stroke with warfarin compared with clinical trials. As in our study, Go et al (15) found more comorbidities, a greater proportion of patients older than 75 years of age and more women among their ambulatory population than in the clinical trials. Our study did not find warfarin to be associated with a lower mortality rates. This may reflect a relatively higher level of comorbidity rates in the patients on warfarin, such that any potential benefit in mortality reduction from the drug was offset by an increased mortality due competing conditions. In addition, our study was also likely underpowered to detect a mortality benefit.
The major limitation of the present study was that we were unable to ascertain the quality of anticoagulation management between clinical events. There may have been some selection bias in the enrolment of patients because family physician consent was required for patients to participate in the study. Furthermore, information obtained through self-reporting from individual patients might have been prone to recall bias.
The study also has several strengths. It is the largest Canadian prospective cohort study to examine rates of thromboembolic and hemorrhagic events in a population-based, ambulatory cohort of patients who are most likely to reflect those being managed in actual practice. Rates of thromboembolic and hemorrhagic events, as well as the effectiveness of warfarin as measured in the present study, are therefore more likely to represent those experienced in the real-world setting. Finally, we obtained complete follow-up data of all enrolled patients, and outcome events were comprehensively recorded with blinded adjudication of events.
CONCLUSION
The present study provides data on the effectiveness of antithrombotic therapy in a population-based cohort in Nova Scotia and confirms that the risk reduction of stroke observed in clinical trial settings is also observed in the nontrial setting. In addition, our results highlight the observation that the patients likely to be seen in community practice have a higher risk clinical profile than those enrolled in randomized controlled trials and, as such, are most likely to benefit from appropriate antithrombotic therapy.
Footnotes
FUNDING: This study was supported by a research grant from the Heart and Stroke Foundation of Nova Scotia.
REFERENCES
- 1.Kerr CR, Roy D. 2004 Canadian Cardiovascular Society Consensus Conference: Atrial fibrillation. Can J Cardiol. 2005;11(Suppl B):9B–10B. [PubMed] [Google Scholar]
- 2.Krahn AD, Manfreda J, Tate RB, Mathewson FA, Cuddy TE. The natural history of atrial fibrillation: Incidence, risk factors, and prognosis in the Manitoba Follow-Up Study. Am J Med. 1995;98:476–84. doi: 10.1016/S0002-9343(99)80348-9. [DOI] [PubMed] [Google Scholar]
- 3.Kannel WB, Abbott RD, Savage DD, McNamara PM. Epidemiologic features of chronic atrial fibrillation: The Framingham Study. N Engl J Med. 1982;306:1018–22. doi: 10.1056/NEJM198204293061703. [DOI] [PubMed] [Google Scholar]
- 4.Wolf PA, Abbott RD, Kannel WB. Atrial fibrillation as an independent risk factor for stroke: The Framingham Study. Stroke. 1991;22:983–8. doi: 10.1161/01.str.22.8.983. [DOI] [PubMed] [Google Scholar]
- 5.Adjusted-dose warfarin versus low-intensity, fixed-dose warfarin plus aspirin for high-risk patients with atrial fibrillation: Stroke Prevention in Atrial Fibrillation III randomised clinical trial. Lancet. 1996;348:633–8. [PubMed] [Google Scholar]
- 6.The effect of low-dose warfarin on the risk of stroke in patients with nonrheumatic atrial fibrillation The Boston Area Anticoagulation Trial for Atrial Fibrillation Investigators. N Engl J Med. 1990;323:1505–11. doi: 10.1056/NEJM199011293232201. [DOI] [PubMed] [Google Scholar]
- 7.Secondary prevention in non-rheumatic atrial fibrillation after transient ischaemic attack or minor stroke EAFT (European Atrial Fibrillation Trial) Study Group. Lancet. 1993;342:1255–62. [PubMed] [Google Scholar]
- 8.Ezekowitz MD, Bridgers SL, James KE, et al. Warfarin in the prevention of stroke associated with nonrheumatic atrial fibrillation. Veterans Affairs Stroke Prevention in Nonrheumatic Atrial Fibrillation Investigators N Engl J Med 19923271406–12. (Erratum in 1993;328:148). [DOI] [PubMed] [Google Scholar]
- 9.Anderson DR, Gardner MJ, Putnam W, et al. Population-based evaluation of the management of antithrombotic therapy for atrial fibrillation. Can J Cardiol. 2005;21:257–66. [PubMed] [Google Scholar]
- 10.Albers GW, Dalen JE, Laupacis A, Manning WJ, Petersen P, Singer DE. Antithrombotic therapy in atrial fibrillation. Chest. 2001;119:194S–204S. doi: 10.1378/chest.119.1_suppl.194s. [DOI] [PubMed] [Google Scholar]
- 11.van Walraven C, Hart RG, Singer DE, et al. Oral anticoagulants vs aspirin in nonvalvular atrial fibrillation: An individual patient meta-analysis. JAMA. 2002;288:2441–8. doi: 10.1001/jama.288.19.2441. [DOI] [PubMed] [Google Scholar]
- 11.Hart RG, Benavente O, McBride R, Pearce LA. Antithrombotic therapy to prevent stroke in patients with atrial fibrillation: A meta-analysis. Ann Intern Med. 1999;131:492–501. doi: 10.7326/0003-4819-131-7-199910050-00003. [DOI] [PubMed] [Google Scholar]
- 12.Risk factors for stroke and efficacy of antithrombotic therapy in atrial fibrillation. Analysis of pooled data from five randomized controlled trials Arch Intern Med 19941541449–57.(Erratum in 1994;154:2254). [PubMed] [Google Scholar]
- 13.Caro JJ, Flegel KM, Orejuela ME, Kelley HE, Speckman JL, Migliaccio-Walle K.Anticoagulant prophylaxis against stroke in atrial fibrillation: Effectiveness in actual practice CMAJ 1999161493–7.(Erratum in 2000;162:973). [PMC free article] [PubMed] [Google Scholar]
- 14.Go AS, Hylek EM, Chang Y, et al. Anticoagulation therapy for stroke prevention in atrial fibrillation: How well do randomized trials translate into clinical practice? JAMA. 2003;290:2685–92. doi: 10.1001/jama.290.20.2685. [DOI] [PubMed] [Google Scholar]
- 15.Kalra L, Yu G, Perez I, Lakhani A, Donaldson N. Prospective cohort study to determine if trial efficacy of anticoagulation for stroke prevention in atrial fibrillation translates into clinical effectiveness. BMJ. 2000;320:1236–9. doi: 10.1136/bmj.320.7244.1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Frykman V, Beerman B, Ryden L, Rosenqvist M, Medical Products Agency; Swedish Society of Cardiology Management of atrial fibrillation: Discrepancy between guideline recommendations and actual practice exposes patients to risk for complications. Eur Heart J. 2001;22:1954–9. doi: 10.1053/euhj.2000.2300. [DOI] [PubMed] [Google Scholar]