Abstract
Chronic excess alcohol use is a well-established cause of dilated cardiomyopathy. The clinical features are variable because patients may be asymptomatic despite there being evidence of severe left ventricular dysfunction. Although the mechanism of alcohol-induced cardiomyopathy is not clearly understood, abstinence from alcohol has been associated with improvement in left ventricular function. Conversely, patients with ongoing alcohol abuse and dilated cardiomyopathy have a poor prognosis, with progressive biventricular failure and, ultimately, death.
A case of rapid reversal of alcohol-induced cardiomyopathy with abstinence is reviewed. The present case highlights the acute toxic nature of alcohol and the potential for rapid functional recovery.
Keywords: Alcohol, Cardiomyopathy, Heart failure
Abstract
La consommation excessive d’alcool chronique est une cause bien établie de myocardiopathie dilatée. Les caractéristiques cliniques sont variables, parce que les patients peuvent être asymptomatiques malgré des constatations de grave dysfonction ventriculaire gauche. Bien qu’on ne comprenne pas clairement le mécanisme de myocardiopathie induite par l’alcool, l’abstinence de l’alcool s’associe à une amélioration de la fonction ventriculaire gauche. Par contre, les patients qui continuent à consommer trop d’alcool et à souffrir d’une myocardiopathie dilatée présentent un mauvais pronostic, soit une insuffisance biventriculaire évolutive et, finalement, la mort.
On examine un cas de disparition rapide d’une myocardiopathie induite par l’alcool grâce à l’abstinence. Le présent cas souligne la nature toxique aiguë de l’alcool et le potentiel de rétablissement fonctionnel rapide.
A 48-year-old woman presented to the emergency department with confusion and shortness of breath. She admitted to an eight-year history of the ingestion of more than 600 mL of vodka per day. Within the month before presentation, she had increased her alcohol intake by drinking a large glass of 70% ethanol per day.
Before this medical admission, the patient had two previous admissions for acute pancreatitis due to ethanol abuse. On both occasions, she had normal cardiac enzyme levels and no evidence of cardiac dysfunction, and a chest x-ray revealed no cardiomegaly or pulmonary edema. Her most recent admission with pancreatitis had occurred four months before the present admission.
The patient came to the emergency room with a decreased level of consciousness, hallucinations and convulsions after 24 h to 48 h of abstinence from alcohol. Her clinical assessment was consistent with the symptoms of delirium tremens.
Her baseline laboratory evaluation showed pancytopenia, abnormal liver function tests (Table 1) and elevated cardiac enzyme levels (Table 2). The toxicology screen was negative (Table 1). The initial chest x-ray revealed a normal cardiothoracic ratio and no evidence of heart failure. Her electrocardiogram showed sinus tachycardia, a nonspecific T-wave abnormality and right axis deviation. The right axis deviation was unchanged from a previous electrocardiogram. She received aggressive volume resuscitation, and 24 h after admission, she developed severe dyspnea. A subsequent chest x-ray after fluid resuscitation revealed pulmonary edema.
TABLE 1.
Basic laboratory and toxicology tests
| On admission | In hospital, first week | In hospital, second week | On discharge | |
|---|---|---|---|---|
| Hemoglobin, g/L (115 g/L – 155 g/L) | 112 | 106 | 106 | 130 |
| Mean corpuscular volume, fL (80 fL – 100 fL) | 115 | 115 | 119.8 | 105 |
| White blood cells, ×109/L (3×109/L – 10.5×109/L) | 2.9 | 4.4 | 4.8 | 4.8 |
| Platelets, ×109/L (125×109/L – 400×109/L) | 73 | 87 | 363 | 396 |
| Total bilirubin, μmol/L | 98 | 139 | 79 | – |
| AST, U/L (14 U/L – 41 U/L) | 189 | 104 | 98 | 61 |
| GGT, U/L (7 U/L – 50 U/L) | 1699 | 1090 | 512 | 352 |
| Folate, nmol/L (215 nmol/L – 1292 nmol/L) | 677 | – | – | – |
| Vitamin B12, pmol/L (133 pmol/L – 674 pmol/L) | 211 | – | – | – |
| Ferritin, pmol/L (10 pmol/L – 190 pmol/L) | 186 | – | – | – |
| Osmolality, mmol/kg (280 mmol/kg – 295 mmol/kg) | 283 | – | – | – |
| Glucose, mmol/L (3.8 mmol/L – 6 mmol/L) | 11.4 | – | – | – |
| Anion gap, mmol/L (6 mmol/L – 15 mmol/L) | 17 | – | – | – |
| Acetaminophen, μmol/L | <66 | – | – | – |
| Salicylic acid, mmol/L (0.5 mmol/L – 2.2 mmol/L) | <0.3 | – | – | – |
| Ethanol, mmol/L | <1 | – | – | – |
Normal values are presented in parentheses. AST Aspartate aminotransferase; GGT Gamma glutamyltransferase
TABLE 2.
Cardiac enzyme levels
| Cardiac enzyme | On admission | 8 h after admission | 18 h after admission |
|---|---|---|---|
| Creatine kinase, U/L (20 U/L – 160 U/L) | 293 | 463 | 403 |
| Troponin T, μg/L (0.00 μg/L – 0.1 μg/L) | 0.24 | 0.19 | 0.11 |
Normal values are presented in parentheses
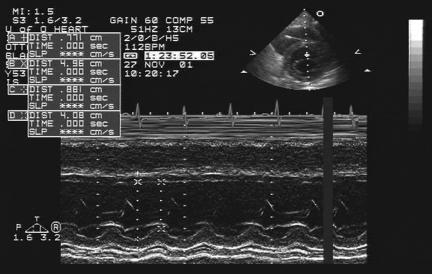
An echocardiogram performed within 24 h of admission and reviewed by two independent echocardiographers demonstrated severe global left ventricular systolic dysfunction, with an ejection fraction of 20% by modified Simpson’s biplane method. The left ventricle was not dilated, and the right ventricle had normal function. The end-systolic dimension was 4.1 cm and the end-diastolic dimension was 5.0 cm (Figure 1).
Figure 1).
Echocardiogram within 24 h of admission
The patient’s delirium tremens was treated with benzodiazepines, and her congestive heart failure was treated with diuretics and an angiotensin-converting enzyme (ACE) inhibitor. After diuresis, her chest x-ray returned to normal. The pancytopenia and elevated liver enzyme levels resolved within a few days of hospital admission, with abstinence from alcohol. A dipyridamole stress test performed seven days after admission revealed no myocardial ischemia. The patient’s ejection fraction was calculated at 58%, and she was discharged on a diuretic, an ACE inhibitor and a beta-blocker.
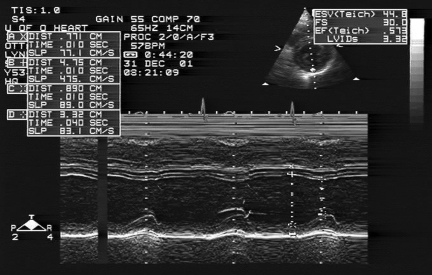
After one month of abstaining from alcohol, the patient was asymptomatic. A repeat echocardiogram revealed normal left ventricular function, with an ejection fraction of 62% by modified Simpson’s biplane method. The end-systolic dimension was 3.3 cm and the end-diastolic dimension was 4.8 cm (Figure 2). Her cardiac medications were subsequently discontinued.
Figure 2).
Echocardiogram one month after admission
DISCUSSION
Alcohol effects on left ventricular function can be reversible. A number of small studies (1–3) have demonstrated that if it is started before fibrosis, abstinence from alcohol can result in significant improvement in left ventricular function. A short duration of symptoms of heart failure and abstinence from alcohol are the two factors associated with favourable outcomes (4). The prognosis of patients with alcohol-induced cardiomyopathy is poor with the continued use of alcohol; the reported mortality rates are as high as 42% at three years (5).
Improvement in left ventricular function has been observed as early as six months after abstinence from alcohol, and complete recovery can be achieved in 18 months (5,6). In an echocardiographic study of 13 patients with alcohol-induced cardiomyopathy, five demonstrated the normalization of left ventricular function after total abstinence for six months (6).
In our patient, acute myocardial injury and cardiomyopathy improved significantly with abstinence from alcohol. Compared with previously reported cases (5,6) of recovery of left ventricular function with abstinence from alcohol, the present report shows the prompt recovery (within one month) of a patient with acute alcohol intoxication and cardiomyopathy who attained completely normal left ventricular function.
The diagnosis of alcohol-induced cardiomyopathy in our patient relied on the absence of known causes of dilated cardiomyopathy, the identification of excessive alcohol consumption and the improvement of cardiac function after the abstention from alcohol ingestion. Specifically, there was no evidence of a preceding viral infection or presence of another toxin.
The distinction between alcohol-induced cardiomyopathy and dilated cardiomyopathy due to other causes (eg, idiopathic or viral) may be difficult. Patients with dilated cardiomyopathy are apt to have history of some ethanol consumption, and the histological changes of both diseases are nonspecific. Teragaki et al (6) studied the clinical and histological findings of patients with cardiomyopathy and significant alcohol consumption compared with patients with cardiomyopathy and no alcohol intake. Histologically, myocyte fibrosis and nuclear changes were found to be less significant, while the potential for clinical reversibility of left ventricular dysfunction was greater in patients whose alcohol abuse was associated with cardiomyopathy.
The exact mechanism of alcoholic cardiomyopathy is unknown. Electron microscopic studies (7,8) of biopsies from patients with alcohol-induced cardiomyopathy have shown evidence of damage to the myofibres, including separation of filaments and loss of striation. In animal studies, loss of contractile proteins and defects in myocardial protein synthesis may partly explain the altered contractility. These studies have demonstrated that acute alcohol ingestion directly reduces contractile protein synthesis in vivo by approximately 25%. Various studies have shown that alcohol exerts a negative inotropic effect on the myocardium. It is possible that changes in calcium hemostasis may contribute toward this phenomenon, because this divalent cation plays an integral part in transmembrane ion movement and muscle contraction (9). Some have also suggested that lipid peroxidation may play a role in the pathogenesis of alcoholic cardiomyopathy (10). In conditions of acute alcohol toxicity, ethanol has been shown to increase circulating catecholamine, which may play a role in myocardial damage.
Thiamine deficiency (beriberi) is a nutritional factor that may be associated with heart muscle diseases, particularly in alcoholics. However, it is now widely accepted that thiamine deficiency is a contributory factor in only a small proportion of alcohol abusers. Other agents, such as cobalt (previously used as a foaming agent in beer) can cause cardiotoxicity in heavy drinkers. In general, nutritional deficiencies have been dismissed in the pathogenesis of alcohol-induced cardiomyopathy (9).
Excess acute alcohol intoxication causes troponin release. Earlier studies by Puszkin and Rubin (10) were the first to suggest that alcohol had effects on the regulatory proteins, troponins or tropomyosins. They found that high concentrations of alcohol (150 mmol to 180 mmol) administered acutely inhibited calcium binding to troponin-tropomyosin protein complexes in vitro. Also, acute alcohol administration in a rat model significantly raised plasma cardiac troponin T level after 2.5 h (11). In our patient, the elevated troponin T is suggestive of acute myocardial damage. However, the falling level of troponin T in hospital suggested that the myocardial damage had occurred before admission. In the absence of other causes, this was likely due to alcohol toxicity.
The association between alcohol-induced cardiomyopathy and myocarditis is controversial. In one six-patient study (12) focusing on alcoholic cardiomyopathy, the surprising histological findings on endomyocardial biopsy of two patients was found to be myocarditis with lymphocytic infiltration in association with myocyte degeneration or focal necrosis. However, no other biopsy study of patients with presumed alcohol-induced cardiomyopathy has found this. It is likely that those two patients were incorrectly labelled with alcohol-induced cardiomyopathy.
In the absence of myocardial biopsy, the diagnosis of myocarditis is always questionable. In our patient, myocardial biopsy was contemplated, but given the rapid recovery of ventricular function, its diagnostic limitations and the absence of its clinical implications, the risk of this procedure outweighed its benefits, and thus, it was not performed. However, given the characteristic rise and fall of cardiac enzyme levels, this supports the diagnosis of acute alcohol-induced myocardial damage.
One interesting aspect of the present case is that although the patient had been a heavy user of alcohol for many years, there had been no previous evidence of cardiomyopathy. It was only after the recent significant increase in alcohol intake that the myocardial dysfunction became apparent. It is unclear whether it was the cumulative dose or the increased daily dose of alcohol that precipitated the left ventricular dysfunction.
In the present report, the short history of patient symptoms, the failed but not dilated or thinned left ventricle, the elevated cardiac enzyme levels and the rapid reversal of left ventricular systolic dysfunction suggest acute alcohol toxicity.
In patients with alcohol-induced cardiomyopathy, the mainstay and goal of therapy is abstinence from alcohol. As with all patients with congestive heart failure, ACE inhibitors and beta-blockers should be prescribed as initial therapy.
REFERENCES
- 1.Regan TJ. Alcoholic cardiomyopathy. Prog Cardiovasc Dis. 1984;27:141–52. doi: 10.1016/0033-0620(84)90001-x. [DOI] [PubMed] [Google Scholar]
- 2.Renault A, Mansourati J, Genet L, Blanc JJ. [Dilated cardiomyopathies in severe cardiac failure in chronic alcoholics: Clinical course after complete withdrawal. ] Rev Med Interne. 1993;14:942. doi: 10.1016/s0248-8663(05)80064-5. [DOI] [PubMed] [Google Scholar]
- 3.Guillo P, Mansourati J, Maheu B, et al. Long-term prognosis in patients with alcoholic cardiomyopathy and severe heart failure after total abstinence. Am J Cardiol. 1997;79:1276–8. doi: 10.1016/s0002-9149(97)00101-x. [DOI] [PubMed] [Google Scholar]
- 4.Demakis JG, Proskey A, Rahimtoola SH, et al. The natural course of alcoholic cardiomyopathy. Ann Intern Med. 1974;80:293–7. doi: 10.7326/0003-4819-80-3-293. [DOI] [PubMed] [Google Scholar]
- 5.Nethala V, Brown EJ, Jr, Timson CR, Patcha R. Reversal of alcoholic cardiomyopathy in a patient with severe coronary artery disease. Chest. 1993;104:626. doi: 10.1378/chest.104.2.626. [DOI] [PubMed] [Google Scholar]
- 6.Teragaki M, Takeuchi K, Takeda T. Clinical and histologic features of alcohol drinkers with congestive heart failure. Am Heart J. 1993;125:808–17. doi: 10.1016/0002-8703(93)90175-9. [DOI] [PubMed] [Google Scholar]
- 7.Fernandez-Sola J, Estruch R, Grau JM, Parc JC, Rubin E, Urbano-Marquez A. The relation of alcoholic myopathy to cardiomyopathy. Ann Intern Med. 1994;120:529–36. doi: 10.7326/0003-4819-120-7-199404010-00001. [DOI] [PubMed] [Google Scholar]
- 8.Alexander CS. Electron microscopic observations in alcoholic heart disease. Br Heart J. 1967;29:200–6. doi: 10.1136/hrt.29.2.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Patel VB, Why HJ, Richardson PJ, Preedy VR. The effects of alcohol on the heart. Adverse Drug React Toxicol Rev. 1997;16:15–43. [PubMed] [Google Scholar]
- 10.Puszkin S, Rubin E. Adenosine diphosphate effect on contractility of human muscle actomyosin: inhibition by ethanol and acetaldehyde. Science. 1975;188:1319–20. doi: 10.1126/science.124949. [DOI] [PubMed] [Google Scholar]
- 11.Piano MR, Schwertz DW. Alcoholic heart disease: A review. Heart Lung. 1994;23:3–17. [PubMed] [Google Scholar]
- 12.Patel VB, Ajmal R, Sherwood RA, Sullivan A, Richardson PJ, Preedy VR. Cardioprotective effect of propranolol from alcohol-induced heart muscle damage as assessed by plasma cardiac troponin-t. Alcohol Clin Exp Res. 2001;25:882–9. [PubMed] [Google Scholar]
- 13.Wilke A, Kaiser A, Ferency I, Maisch B. [Alcohol and myocarditis. ] Herz. 1996;21:248–57. [PubMed] [Google Scholar]