SUMMARY
The global increase of the human parasite, the common bed bug Cimex lectularius, calls for specific pest control target sites. The bed bug is also a model species for sexual conflict theory which suggests seminal fluids may be highly diverse. The species has a highly unusual sperm biology and seminal proteins may have unique functions. 1-D PAGE gels showed 40 to 50% band sharing between C. lectularius and another cimicid species, Afrocimex constrictus. However, adult, sexually rested C. lectularius males were found to store 5 to 7μg of seminal protein and with only 60μg of protein we obtained informative 2-D PAGE gels. These showed 79% shared protein spots between two laboratory populations, and more than half of the shared protein spots were detected in the mated female. Further analysis using liquid chromatography electrospray ionisation tandem mass spectrometry revealed that 26.5% of the proteins had matches among arthropods in data bases and 14.5% matched Drosophila proteins. These included ubiquitous proteins but also those more closely associated with reproduction such as moj 29, ubiquitin, the stress-related elongation factor EF-1alpha, a protein disulfide isomerase and an antioxidant, Peroxiredoxin 6.
Keywords: accessory glands, Heteroptera, proteomics, reproductive tract, sperm
Introduction
Seminal fluid proteins of male insects have a multitude of functions and many alter the female reproductive physiology to the male’s benefit (Wolfner 2002; Gillott 2003; Chapman and Davies 2004; Poiani 2006). However, in Drosophila, seminal fluids also accelerated female mortality directly (Chapman et al. 1993, Rice 1996). Because of such direct or indirect interference with the female reproductive output, i.e. her evolutionary fitness, seminal proteins have become the target of researchers interested in evolutionary biology and bioinformatics. Genes coding for seminal proteins evolve with an unusually rapid rate (Civetta and Singh 1995; Swanson et al. 2001; Andres et al. 2006), like many other reproductive traits. Rapid evolution of reproductive traits has been shown to arise when male adaptations suppress female fitness (Rice 1992; 1996).
For many insect taxa the knowledge about seminal fluids is relatively scarce and “pre-proteomic”, i.e. not amenable to bioinformatic analyses (e.g. Chen 1984; Lange and Loughton 1984, 1985; Cheeseman and Gillott 1989; Hartmann and Loher 1999). Genome sequencing has verified evolutionary predictions about the rapid evolution and high diversity at the genetic and RNA level in Drosophila species (Fiumera et al. 2005; Mueller et al. 2005, Haerty et al. 2007) and Anopheles (Dottorini et al 2007). The coupling of mass spectrometry and bioinformatics tools (e.g. Domon and Aebersold 2006) will allow such tests at the protein level and will open avenues into research of other important organisms.
Here we address the suitability of mass spectrometry and bioinformatics to characterise the seminal proteome of the common bed bug, Cimex lectularius. This was stimulated by three visions. First, the current increase in bed bug infestations throughout much of the western world (see Reinhardt and Siva-Jothy 2007 for a review of such studies) calls for pest control measures that specifically target the reproduction of bed bugs. Seminal proteins may play an important role in such specific targets. It is therefore, desirable, that target proteins are those not found in other taxa, i.e. producing no returns in Drosophila databases.
Second, bed bugs are a model system for sexual conflict (Chapman 2006) because several studies have detailed the co-evolution between male and female physiological, morphological and behavioural traits (Stutt and Siva-Jothy 2000; Morrow and Arnqvist 2003; Reinhardt et al. 2003; 2005; Siva-Jothy 2006). As sexual conflict generally leads to very rapid evolution of reproductive traits (Rice 1992; 1996; Holland and Rice 1998) seminal proteins were expected to evolve rapidly in bed bugs. Thirdly, the sperm biology of the bed bug is highly unusual (reviewed Davis 1966; Reinhardt and Siva-Jothy 2007). During copulation the male deposits sperm and seminal fluid into a female organ, the spermalege which is unique to the Cimicoidea. From the spermalege, sperm travel through the hemolymph of the female, through the walls of the oviduct until they reach either the ovaries or a secondary storage organ. During this passage sperm cells aggregate and re-aggregate and are being attacked by female cells (haemocytes) (see references in Davis 1966; Reinhardt and Siva-Jothy 2007). While the interest in the seminal proteome of the honey bee is largely governed by identifying proteins that assist in the extraordinarily long sperm storage (Collins et al. 2006 and references therein), seminal fluid properties of the bed bug are more likely to have evolved to assisting sperm in withstanding the rather harsh female environments.
MATERIAL AND METHODS
Study species and rearing
Three populations/ species of bed bugs were used, two populations of the common bed bug, Cimex lectularius, henceforth Cimex A and Cimex B and Afrocimex constrictus, a species parasitic on fruit bat in Africa (Reinhardt et al. 2007).
Cimex A originates from a donation by the Medical Entomology Centre, Cambridge, UK in 1998. The bugs where maintained in an incubator at 26±1°C, at 70% relative humidity with a light cycle of L:D 12h:12h. Bugs were fed weekly using established protocols (Reinhardt et al. 2003) until two weeks after adult eclosion. Females were separated from males in order to ensure virginity. Males were not fed for three weeks just prior to the sampling of the seminal fluid.
Cimex B originates from a donation by Insect Control & Research, Inc. Maryland Inc. (USA) in June 2003. Individuals were cultured under identical conditions as Cimex A. Individuals of a related species, Afrocimex constrictus, were collected in a cave in Kenya and transported live to the laboratory in the UK (see Reinhardt et al. 2007 for details). Eight males that were kept in sexual isolation for two months were killed and their seminal fluid containers dissected out and used for further analysis.
Bed bug anatomy
The anatomy of male Cimex lectularius is detailed in Usinger (1966). Briefly, in the male one seminal vesicle descends from each of the two testes. Sperm is constantly produced by the testes and sperm accumulates and is stored in the seminal vesicles (fig.1). Another paired structure, the accessory glands (or mesadenal glands sensu Usinger 1966) produce the seminal fluid which is stored until copulation separately from sperm in a pair of containers called seminal reservoir. During a 60-second mating, males transfer approximately 20% of their seminal fluids to the female (Reinhardt, unpubl. data). Female anatomy is also reviewed in Usinger (1966) and involves a secondary genitalic organ into which the male deposits his sperm. The sperm then travel freely through the female body (see Introduction).
Figure 1.
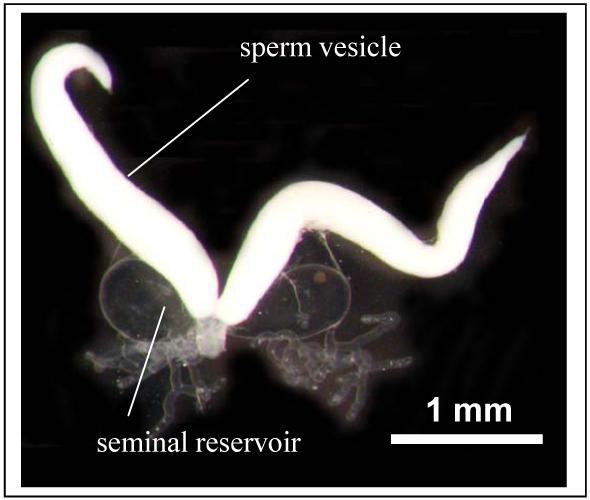
The reproductive tract of a virgin male bed bug, Cimex lectularius (30 days old. Note the sperm and seminal fluid containers are clearly separated.
Dissection
All Cimex males were sexually rested for two weeks, Afrocimex for two months (sse above). Sperm and seminal fluid containers are well separated and can be dissected out separately (Fig.1). The containers were placed into an Eppendorf tube on ice until used further.
Measurement of protein content
Protein content was determined using the Bradford method. Fifteen accessory containers (removed from 17 males) in Cimex A, 8 containers removed from 8 males in Afrocimex constrictus and 9 containers from 9 males in Cimex B. All males had not fed for four weeks. Containers were placed in 100 μl PBS with protease inhibitors (2mM PMSF, and 1mM EDTA) in Eppendorf tubes. The tubes were briefly vortexed, spun down and the supernatant used for Bradford reaction. Distilled water was added to the pellet and all samples were placed in a hotblock (100°C) in order to denature the proteins.
No attempt was made to measure the protein content of sperm but individual males of this age contain approximately 0.08 mg of sperm dry weight (K. Reinhardt, unpubl.).
1-D gel electrophoresis
One third of each of the samples was run on a ready-made gel (NuPage, Invitrogen) at 200 V for 40 minutes. The gel was Coomassie stained and fixed with acetic acid. The gel was scanned and the image saved as a .jpg file. Bands were scored independently by two observers (KR, SAG) after manually adjusting the contrasts and the brightness using Photoshop CS2 (Adobe).
2-D electrophoresis
20 accessory containers of Cimex A, 20 containers from Cimex B were used.
In order to get information about how many proteins found in the seminal containers are also found in the female, we dissected the spermalege from 19 virgin females and from 20 females 30 to 45 minutes after mating (a time at which sperm and seminal fluid are still in the spermalege). The spermalege tissues of all virgin females were pooled as were the tissues of all mated females. During mating obviously sperm is passed to female alongside seminal fluid. We therefore used sperm from 3 males in strain A and used in the electrophoresis. Note that the analysis of sperm proteins was not intended (as by Dorus et al. 2006) but merely used as a control to see whether any proteins detected in the mated female by our method could have been attached to or transferred by sperm.
The organs were briefly rinsed in PBS and placed in 100 μl PBS containing protease inhibitors (2mM PMSF, and 1mM EDTA) in Eppendorf tubes. The tubes were vortexed for few seconds and centrifuged for 30 seconds at 14,000 rpm to separate cellular components. The supernatant was frozen at -80° C until further use.
Proteins were resolved in the first dimension by isoelectric focusing (IEF) using 18cm, pH 3-10 IPG strips (Amersham Biosciences) for a total of 33500Vhrs, in an IPGphor Isolectric Focusing system (Amersham Biosciences). The IEF program started with 500V for 500Vhrs, followed by a step-and-hold increase to 1000V for 1000Vhrs, and finally to a step-and-hold increase to 8000V for 32,000Vhrs. After focusing, IPG strips were equilibrated to reduce protein disulfide bonds in 10 ml of equilibrating solution per strip [6M Urea, 30% (v/v) glycerol, 2% (w/v) SDS, 50mM Tris-HCl, pH 8.8 and 0.25% (w/v) bromophenol blue and 1% (w/v) DTT] with gentle rocking for 15 mins. The free cysteine residues of proteins were then alkylated to prevent reformation of disulfide bonds by rocking each strip for 15 mins in 10 ml of solution containing 6M Urea, 30% (v/v) glycerol, 2% (w/v) SDS, 50mM Tris-HCl, pH 8.8 and 0.25% (w/v) bromophenol blue and 2.5% (w/v) iodoacetamide. These strips were then affixed onto homogeneous 12.5% polyacrylamide SDS-PAGE slab gels (2550 × 2100 × 1mm). The second dimension was performed in the EttanDalt vertical system (Amersham Biosciences) at 25°C. The resolved 2D spots were visualized with SYPRO Ruby fluorescent dye (Bio-Rad) according to the manufacturer’s instruction.
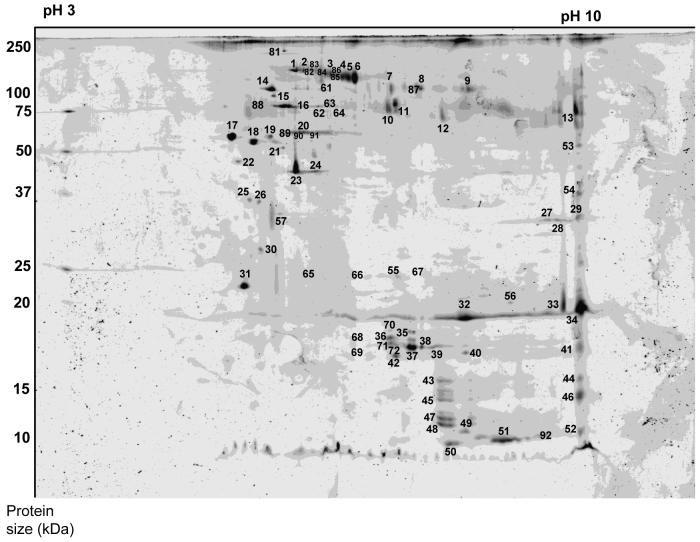
The absence and presence of protein spots were scored by eye (see fig. 3) independently by two observers (KR, CHW) after manually adjusting the contrasts and the brightness using Photoshop CS2 (Adobe).
Mass spectrometry and protein identification
Differentially expressed proteins were excised from the gel, and spots were incubated with acetonitrile (ACN) for 15 mins at room temperature. ACN was subsequently removed and the spots were dried in a vacuum centrifuge. Dried spots were stored at 4°C until required. Proteins were digested with 20 ng/microlitre of sequencing grade modified trypsin (Promega, Southampton, UK) in 50mM ammonium bicarbonate at 37°C for 12 hours. The supernatant from trypsin digest was transferred to a siliconised microcentrifuge tube. Peptides were sequentially extracted three times by incubation with peptide extraction solution, consisting of 25mM ammonium bicarbonate (10 mins at room temperature), 5% formic acid (15 mins at 37°C) and ACN (15 mins at 37°C). Each extraction was followed by centrifugation and removal of supernatants. The original supernatant and the supernatants from the three sequential extractions were combined and dried in a vacuum centrifuge for 4-6 hrs. The dried peptides were dissolved in 7 l of 0.1% (v/v) formic acid in 3% (v/v) ACN in water. Samples were centrifuged for 5 mins at 12,000 x g and the supernatants were subjected to liquid chromatography electrospray ionisation tandem mass spectrometry (LC-ESI-MS/MS).
Liquid chromatographic (LC) separations of the tryptic digests were performed using a reverse phase CapLC™ system (Waters, Manchester, UK). Peptides were desalted by a PepMap C18 microguard column (300 μm internal diameter x 1mm) (LC-Dionex, Leeds, UK) and were then transferred to the analytical column (PepMap C18; 75 μm internal diameter x 15cm column, LC-Dionex). The peptides were eluted in a 60 min gradient. The compositions of the hydrophilic and hydrophobic solvents were 5% ACN, 0.1% formic acid and 95% ACN, 0.1% formic acid. The column eluent was sprayed directly into the nanoESI source of a Q-TOF micro (Waters). An initial MS scan was performed and selection of ions for collision induced dissociation (CID) was automated by Mass Lynx software (Waters). CID selection criteria were set for 2+ and 3+ ions within the range of 400-2000 m/z above 10 ion counts.
Spectra were searched against the National Centre for Biotechnology Information non-redundant protein data bases NCBInr and MSDB in a sequence query search using MASCOT 2.0 software (www.matrixscience.com). The taxonomy was limited to filter for all Metazoa but only Arthropoda were considered, with special reference to Drosophila and to Acyrthosiphon pisum, the closest sequenced relative of the bedbug. Trypsin was used as the cleavage enzyme, with two missed cleavage sites allowed. The peptide tolerance was set to 1.0Da and the MS/MS tolerance was set to 0.3Da. Carbamidomethyl modification of cysteine and oxidised methionine were set as variable modifications. Peptide/protein matches were only considered significant for mowse scores p<0.05 (Pappin et al. 1993). Mowse scores and p values were generated by MASCOT software. Briefly, based on the total protein mass, Mowse scores use empirically determined factors to statistically weight each individual peptide match from a protein sequence. Statistical significance can be derived by calculating the probability that an observed match is a random event. MASCOT provides mowse scores as -10*LOG10(P), where P is the absolute probability, i.e. a probability of 10-4 corresponds to a mowse score of 40. In MSDB mowse scores above 45 are a significant hit.
RESULTS
Bradford determination revealed the following average amounts of seminal fluid proteins per male: Cimex A: 7.4μg, Afrocimex 5 μg and Cimex B 5.4 μg).
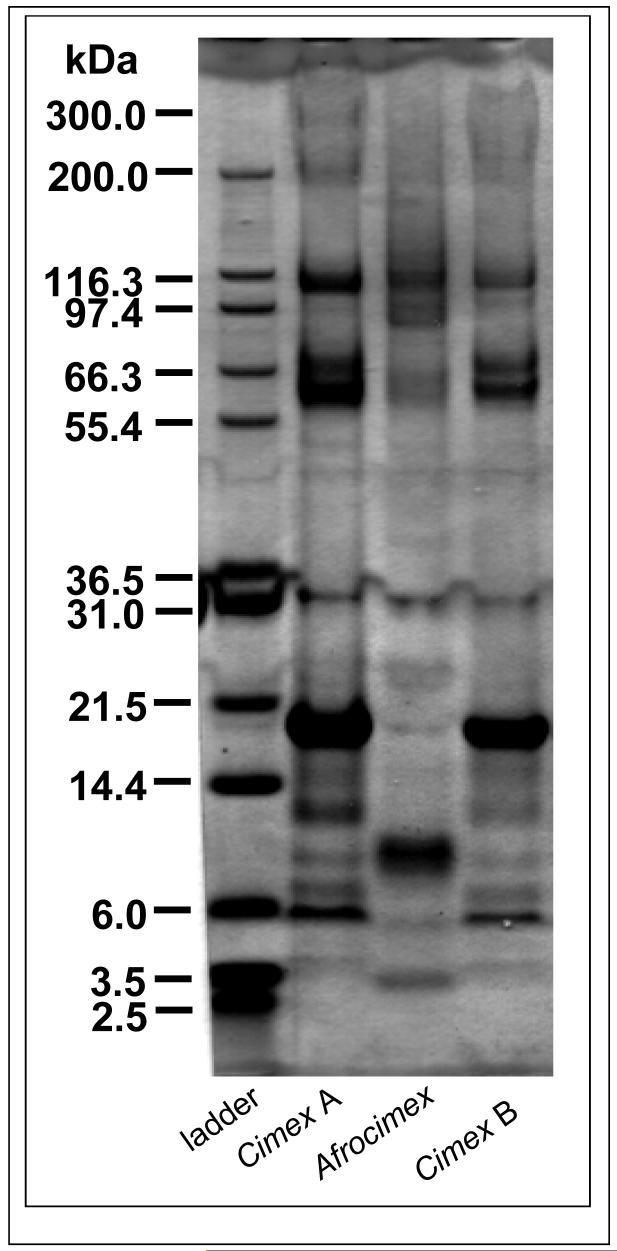
Twelve bands were found on the 1-D gel for Cimex A. Of these, all but one (approximately 300 kDa) were also found in Cimex B (Fig.2). One protein band at 97 kDa was found in Cimex B but not in Cimex A. Afrocimex shared 5 bands with Cimex A and an additional one with Cimex B but had 6 that it did not share with Cimex.
Figure 2.
1-D PAGE showing differences between two species of cimicid bugs, Cimex lectularius and Afrocimex constrictus.
Using 20 seminal fluid containers, and thus only about 54 to 74 μg of protein, we were able to obtain informative gels: 2-D PAGE revealed at least 69 protein spots in the seminal fluid of Cimex A, 58 of which were also found in Cimex B (Fig. 3). Seven proteins found in Cimex B were not detectable in Cimex A. Thus even the small amounts of protein used resulted in 79% shared protein spots by both samples (Note the similar difference in the 1-D PAGE). The low protein amounts of 30 μg available for Afrocimex did not produce protein spots.
Out of the 76 spots found in the seminal fluid, 41 were found in the mated female, two of which were only found in Cimex A seminal fluid. Of these 41 proteins, 32 were not found in virgin females. Of the nine proteins that were found in virgin females only one was identified, the gag protein, which with a mowse score of 36 was not significant.
Two control runs with sperm did not return any protein spots with the method used here. Therefore, most proteins are from the seminal fluid and up to 32 seminal fluid proteins may be transferred from male to female during mating.
Out of the 69 proteins that were analysed by mass spectrometry, 18 (26.5%) returned entries in the protein data bases when tested against all arthropods, 17 (24.6%) against all insects, 10 (14.5%) against Drosophila and 3 (4.3%) against other Hemiptera species (Table 1). Many of them, however, were ubiquituous and cytoskeletal proteins and fewer had - in Drosophila - a distinct link to reproduction (see Discussion). One protein (spot 31), predicted Fkbp13 CG9847-PA, isoform A (accession number gi|17352457) had a mowse score of 38 and so was just below the 5% significance level.
Table 1.
Predicted proteins found in the seminal fluid of bed bugs, Cimex lectularius. Numbers in column 1 refer to the protein number on the 2D gel shown in figure 3. Also provided are the theoretical molecular mass of the protein, the arthropod species whose protein provided the closest match in the database, as well as the respective mowse score and protein coverage. Some proteins were found in females, either in mated or virgin ones. The predicted protein function is given in the last column
| # in Fig.3 | Accession # in NCBI | Predicted protein | Theoretical mass (Da) | Arthropod species | Mowse score | % protein coverage | Found in female? | Protein function (Uniprot database annotation) |
|---|---|---|---|---|---|---|---|---|
| 8 | gi|34597154 | Elongation factor | 23474 | Cleidogona major | 53 | 4 | No | Involved in protein synthesis, cell metabolism, translation |
| 16 | gi|157658 | Heat shock cognate 72 | 72190 | D. melanogaster | 153 | 7 | Mated | Chaperone |
| 17 | gi|6063416 | Calreticulin | 46780 | D. melanogaster 1 | 46 | 2 | No | Molecular Ca-binding chaperone promoting folding and influencing gene expression and cell adhesion. |
| 18 | gi|112984454 | Protein disulfide isomerase | 55554 | Bombyx mori 2 | 66 | 2 | No | Usually involved in cell redox homeostasis |
| 22 | gi|158451643 | Putative enolase protein | 40841 | Lacosoma chiridota | 62 | 3 | No | Glycolytic enzyme |
| 23 | gi|19365716 | Actin | 41759 | Acyrthosiphon pisum | 202 | 15 | Mated | Involved in various types of cell motility. Ubiquitously expressed in all eukaryotic cells |
| 24 | gi|13430414 | Actin E2 | 41653 | D. virilis | 267 | 12 | Mated | See above |
| 25 | gi|85165 | Tropomyosin, exon9B | 32870 | D. melanogaster 3 | 252 | 15 | Mated | Central role in the calcium dependent regulation of muscle contraction. |
| 26 | gi|24647095 | Tropomyosin 2 CG4843-PB, isoform B | 32786 | D. melanogaster 4 | 195 | 18 | No | See above |
| 27 | gi|85164 | Tropomyosin, cytoskeletal | 29348 | D. melanogaster | 61 | 7 | No | See above |
| 30 | gi|121543925 | putative 14-3-3 protein | 28057 | Maconellicoccus hirsutus | 178 | 14 | No | Interacts with target proteins by phosphorylation |
| 53 | gi|7915 | EF-1-alpha | 50250 | D. melanogaster | 74 | 4 | Mated | Promotes binding of aminoacyl-tRNA to ribosomes during protein biosynthesis. |
| 54 | Q1EPM0_BOMMO | Glyceraldehyde-3-phosphate dehydrogenase | 35406 | Bombyx mori | 105 | 4 | No | Glycolytic enzyme |
| 56 | gi|71840902 | Moj29 | 18078 | D. arizonae 5 | 122 | 17 | No | PPIases accelerate the folding of proteins. |
| 61 | gi|10959 | Paramyosin | 102162 | D. melanogaster | 198 | 3 | No | Major structural component of many thick filaments in muscles. |
| 62 | gi|24657014 | Ubiquitin-63E CG11624-PA, isoform A | 85746 | D. melanogaster | 65 | 2 | No | Marking proteins for proteolysis, regulation of cell cycle, DNA repair, embryogenesis, transcription and apoptosis. |
| 65 | Q17IM5_AEDAE | Peroxiredoxin 6 | 24880 | Anopheles aegypti | 56 | 4 | No | Detoxification of peroxide |
| 72 | Q7PVJ6_ANOGA | ENSANGP00000012313 (Fragment) | 41263 | Anopheles gambiae str. PEST | 54 | 6 | No | ? |
also found in Culex pipiens quinquefasciatus (gi|170042204), mowse score 64, protein coverage 3%
found with similar parameters in Anopheles gambiae str. PEST, Tribolium castaneum
found with similar parameters in many basal insects (e.g. Periplaneta americana, gi|4378573), mowse score 290, protein coverage 16%)
also found in Acyrthosiphon pisum (gi|193704626), predicted mass 32294 kDa, mowse score 182, protein coverage 15%
also found in Anopheles gambiae str. PEST (accession Q7QIV1_ANOGA), predicted mass 21802 kDa, mowse score 80, protein coverage 8%
DISCUSSION
This study supports the notion that proteomics of seminal plasma is a fruitful technology to discover proteins that have not been detected by genomic analysis (see also Collins et al. 2006, Walker et al. 2006, Braswell et al. 2006, Findlay et al. 2008). Such studies add to an increasing knowledge about the diversity of seminal plasma, about further development of methods and about the function of specific components of the semen. These three areas are discussed below.
Seminal protein diversity
The low overall amount of protein in our samples did not allow quantification in terms of higher or lower expression of a particular protein. Likewise, absent spots cannot be used as evidence for the absence of proteins. However, we can rely on proteins that have been detected. Such positive identification (independently of any undetected proteins) is in agreement with the notion that seminal fluids differ strongly between species, i.e. evolve rapidly for three reasons. First, there was a high departure between Cimex and Drosophila (only 14% shared proteins in data bases) and between Cimex and all other arthropods (26% shared protein entries). Second, using a different method, the differences were substantially smaller, but still considerable, between Cimex and Afrocimex which are members of the same family (Cimicidae) - about 60% of the 1-D gel bands were shared. Thirdly, the 1-D and 2-D gel analysis suggests that even between two separated laboratory populations of the same species only between 90% and 80% bands and protein spots, respectively, are shared.
Using genomic analysis these levels of similarity compare positively with Andrés et al. (2006) who did not find shared transcripts of accessory proteins between Drosophila and field crickets, Gryllus spp., while Braswell et al. (2006) found 7-40% similarity between Drosophila and either Gryllus or Allonemobius. The level of similarity is less comparable to that between D. melanogaster and the honey bee semen where a staggering 96% of the proteins could be matched to Drosophila proteins (Collins et al. 2006, but see Findlay et al. 2008 for additional proteins). Again using genomic analyses, the within-genus comparison between Drosophila melanogaster and D. pseudoobscura 58% shared putative seminal proteins genes were found (Mueller et al. 2005), being closer to the 1-D gel similarity found between Cimex and Afrocimex.
Methodology
We have also demonstrated that with just 20 seminal fluid containers, i.e. around 60 μg of protein can be obtained. Although we note that a third population of the same species might further improve the repeatability and confidence in the data we suggest that our method may be used to compare bed bug samples from the “field”, i.e. by using the seminal fluid containers of only about 20 males per infestation.
Furthermore, the abundance of many proteins in our 2-D gels (Fig. 3) strongly suggests that the proteins can be subjected to fragmentation. This may increase the return rate in data bases and, given good bed bug breeding facilities, may also enable the purification of larger amounts of some proteins. Larger amounts of proteins will enable the assessment of more detailed physiological properties of bed bug seminal fluid, given that in other insects seminal fluids affect ovulation (Qazi et al. 2003), increase the oviposition rate (Wolfner 2002; Gillot 2003; Chapman and Davies 2004; Poiani 2006), reduce female attractiveness (Tram and Wolfner 1998) and egg hatchability immediately after mating (Prout and Clark 2000), influence sperm storage (Neubaum and Wolfner 1999), affect paternity by differential spermicide (Fry and Wilkinson 2004), influence sperm organisation in the female (Viscuso et al. 2001) and sperm motility (Ruknudin and Raghavan 1988).
Our study has identified several predicted proteins in both the seminal fluid and in the mated (but not the unmated) female. These are candidate proteins for being actually transferred during mating. Given the complex sperm biology of the bed bug (Davis 1966; Rao and Davis 1969; Reinhardt and Siva-Jothy 2007) and that the female copulatory organ of bed bugs is capable of phagocytosing sperm (see references in Reinhardt and Siva-Jothy 2007) one may speculate about a sperm protective functions of several of the proteins.
Specific functions of some identified predicted proteins
The presence of actin, calreticulin, tropomyosin or paramyosin indicates a cellular component of the seminal fluid analysed here. These proteins unlikely originate from sperm cells because seminal plasma and sperm are stored separately in the bed bug (Fig. 1) and because sperm material analysed in the same way as seminal fluids did not produce any protein spots. It is possible that these mostly cytoskeletal proteins escaped from the cells of the seminal fluid container during centrifugation. On the other hand, intracellular material can be found in gland discharges as shown in the careful study by Walker et al. (2006) who found calreticulin in male accessory glands of Drosophila.
Even if we assume (as a worst case scenario) that all proteins that were identified represent cellular contaminants, only unidentified protein spot may then represent seminal fluid proteins, i.e. a maximum of 80.3% out of 76 (61 proteins). This number would still resemble the number of proteins found in honey bee semen (Collins et al. 2006), were higher than that reported by Walker et al. (2006) but much lower than that reported by Findlay et al. (2008).
Others of the predicted proteins have been directly associated with insect reproduction. The gene moj 29 which was found mostly expressed in the testis and/or the accessory glands of Drosophila mojavensis and D. arizonae (Wagstaff and Begun 2005) but its function is unknown. A proteomic study of Drosophila seminal fluid (Walker et al. 2006) reported several proteins that were also found in the present study, including the Fkbp13 protein (a non-significant hit in our study), the protein disulfide isomerase, and a similar sized heat shock protein.
Ubiquitin is very abundant in seminal plasma of humans (Lippert et al. 1993) and is here reported from the bed bug. Its extracellular role is currently debated. While the amount of ubiquitin per ejaculate can be positively correlated with the proportion of abnormal sperm (Sutovsky et al. 2003, 2004a) other studies do not support such notion (Muratori et al. 2005) and show that ubiquitin enables or facilitates fertilisation (Sutovsky et al. 1999, 2004b; Sawada et al. 2002). In mammalian males ubiquitinated sperm are phagocytosed (Sutovsky et al., 2001) and may, therefore, serve as a control mechanism ensuring that fertilisation-competent cells are being ejaculated.
A similar controversy revolves around the elongation factor EF-1alpha which we detected in the seminal fluid. This factor is involved in protein synthesis, regulation of apoptosis and interacts with actin and the ubiquitin-dependent proteolysis. Some, but not all, genotypes bearing additional EF-1 alpha gene copies showed enhanced lifespan (Stearns and Kaiser 1993; Shikama et al. 1994). While such lines do not necessarily express more EF-1 alpha (Shikama et al. 1994), other studies show that increased expression of EF-1 alpha under stress lead to lifespan extension (Wang et al. 2004; Talapatra et al. 2002).
Finally, the occurrence of peroxiredoxin, an antioxidant (e.g. Kawazu et al. 2008), in the seminal plasma suggests poses the question whether this protects sperm or benefits the female is an exciting future research topic and requires the separation of the effect of other beneficial ejaculate substances (see above).
While female lifespan prolongation due to ejaculate substances has been found in a cricket species (Wagner et al. 2001) there is much less evidence for that in bed bugs. One study found the contribution of copulatory wounding to female mortality was larger than the total negative effect of copulation on females (Morrow and Arnqvist 2003) raising the possibility of a beneficial effect of the male’s ejaculate (Reinhardt and Siva-Jothy 2007 counter to Morrow’s and Arnqvist’s (2003) suggestion). This intriguing possibility is currently being tested directly. Given sufficient protein material the method presented here may, in the future, enable us to reveal whether such proposed effect is due to EF-1 alpha or other proteins.
Conclusion
We have shown that even the small amount of seminal fluid used here can provide informative gels. The proteins positively identified in the bed bug (Cimex lectularius) can be used as a reference gel (mass spectrometry files in .pkl format are available from KR on request) and be further analysed by fragmentation and bioinformatic tools. Comparisons with other species pose a challenge for interfering with reproductive pathways in the bed bug because the predicted rapid evolution of seminal proteins will result in divergence between different populations. It is desirable to focus further analyses and direct physiological assays towards proteins that are found conserved within the family of the Cimicidae or even within the species Cimex lectularius.
Acknowledgments
Sveta Sedelnikova, University of Sheffield, carried out the Bradford analysis and run the 1-D gel. The study was funded through a VIP award by the Wellcome Trust to KR. We thank Richard Naylor, Matt Pitts-Tucker and Mike Siva-Jothy for help in collecting Afrocimex constrictus. We also thank Robin Todd, Insect Control & Research, Inc., Baltimore, Maryland and Woodbridge Foster, Ohio State University, for donation of bed bugs. Richard Naylor helped with some of the dissections. Christian Wegener provided helpful comments on an earlier version.
REFERENCES
- Andrés JA, Maroja LS, Bogdanowicz SM, Swanson WJ, Harrison RG. Molecular Evolution of Seminal Proteins in Field Crickets. Molecular Biology and Evolution. 2006;23:1574–1584. doi: 10.1093/molbev/msl020. [DOI] [PubMed] [Google Scholar]
- Braswell WE, Andrés JA, Maroja LS, Harrison RG, Howard DJ, Swanson WJ. Identification and comparative analysis of accessory gland proteins in Orthoptera. Genome. 2006;49:1069–1080. doi: 10.1139/g06-061. [DOI] [PubMed] [Google Scholar]
- Chapman T. Sexual conflict. Nature. 2006;439:537. [Google Scholar]
- Chapman T, Davies SJ. Functions and analysis of the seminal fluid proteins of male Drosophila melanogaster fruit flies. Peptides. 2004;25:1477–1490. doi: 10.1016/j.peptides.2003.10.023. [DOI] [PubMed] [Google Scholar]
- Chapman T, Hutchings J, Partridge L. No reduction in the cost of mating for Drosophila melanogaster females mating with spermless males. Proceedings of the Royal Society of London B, Biological series. 1993;253:211–217. doi: 10.1098/rspb.1993.0105. [DOI] [PubMed] [Google Scholar]
- Cheeseman MT, Gillott C. Long hyaline gland discharge and multiple spermatophore formation by the male grasshopper, Melanoplus sanguinipes. Physiological Entomology. 1989;14:257–264. [Google Scholar]
- Chen PS. The functional morphology and biochemistry of insect male accessory glands and their secretions. Annual Review of Entomology. 1984;29:233–255. [Google Scholar]
- Civetta A, Singh RS. High divergence of reproductive tract proteins and their association with postzygotic reproductive isolation in Drosophila melanogaster and Drosophila virilis group species. Journal of Molecular Evolution. 1995;41:1085–1095. doi: 10.1007/BF00173190. [DOI] [PubMed] [Google Scholar]
- Collins AM, Caperna TJ, Williams V, Garrett WM, Evans JD. Proteomic analyses of male contributions to honey bee sperm storage and mating. Insect Molecular Biology. 2006;15:541–549. doi: 10.1111/j.1365-2583.2006.00674.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis NT. Reproductive physiology. 1966. pp. 167–182. R. L. Usinger Monograph of the Cimicidae. [Google Scholar]
- Domon B, Aebersold R. Review - Mass spectrometry and protein analysis. Science. 2006;312:212–217. doi: 10.1126/science.1124619. [DOI] [PubMed] [Google Scholar]
- Dorus S, Busby SA, Gerike U, Shabanowitz J, Hunt DF, Karr TL. Genomic and functional evolution of the Drosophila melanogaster sperm proteome. Nature Genetics. 2006;38:1440–1445. doi: 10.1038/ng1915. [DOI] [PubMed] [Google Scholar]
- Dottorini T, Nicolaides L, Ranson H, Rogers DW, Crisanti A, Catteruccia F. A genome-wide analysis in Anopheles gambiae mosquitoes reveals 46 male accessory gland genes, possible modulators of female behavior. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:16215–16220. doi: 10.1073/pnas.0703904104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiumera AC, Dumont BL, Clark AG. Sperm competitive ability in Drosophila melanogaster associated with variation in male reproductive proteins. Genetics. 2005;169:243–257. doi: 10.1534/genetics.104.032870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fry CL, Wilkinson GS. Sperm survival in female stalk-eyed flies depends on seminal fluid and meiotic drive. Evolution. 2004;58:1622–1626. doi: 10.1111/j.0014-3820.2004.tb01743.x. [DOI] [PubMed] [Google Scholar]
- Gillott C. Male accessory gland secretions: modulators of female reproductive physiology and behavior. Annual Review of Entomology. 2003;48:163–184. doi: 10.1146/annurev.ento.48.091801.112657. [DOI] [PubMed] [Google Scholar]
- Hartmann R, Loher W. Post-mating effects in the grasshopper, Gomphocerus rufus L. mediated by the spermatheca. Journal of comparative Physiology A. 1999;184:325–332. [Google Scholar]
- Haerty W, Jagadeeshan S, Kulathinal RJ, Wong A, Ram KR, Sirot LK, Levesque L, Artieri CG, Wolfner MF, Civetta A, Singh RS. Evolution in the fast lane: Rapidly evolving sex-related genes in drosophila. Genetics. 2007;177:1321–1335. doi: 10.1534/genetics.107.078865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland B, Rice WR. Perspective: Chase-away sexual selection: antagonistic seduction versus resistance. Evolution. 1998;52:1–7. doi: 10.1111/j.1558-5646.1998.tb05132.x. [DOI] [PubMed] [Google Scholar]
- Kawazu S, Komaki-Yasuda K, Oku H, Kano S. Peroxiredoxins in malaria parasites: Parasitologic aspects. Parasitology International. 2008;57:1–7. doi: 10.1016/j.parint.2007.08.001. [DOI] [PubMed] [Google Scholar]
- Lange AB, Loughton BG. An analysis of the secretions of the male accessory reproductive gland of the African migratory locust. International Journal of Invertebrate Reproduction and Development. 1984;7:73–81. [Google Scholar]
- Lange AB, Loughton BG. An oviposition-stimulating factor in the male accessory reproductive gland of the locust, Locusta migratoria. General and Comparative Endocrinology. 1985;57:208–215. doi: 10.1016/0016-6480(85)90265-5. [DOI] [PubMed] [Google Scholar]
- Lippert T, Seeger H, Schieferstein G, Voelter W. Immunoreactive ubiquitin in human seminal plasma. Journal of Andrology. 1993;14:130–131. [PubMed] [Google Scholar]
- Morrow EH, Arnqvist G. Costly traumatic insemination and a female counteradaptation in bed bugs. Proceedings of the Royal Society of London B, Biological series. 2003;270:2377–2381. doi: 10.1098/rspb.2003.2514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller JL, Ravi-Ram K, McGraw LA, Qazi MCB, Siggia ED, Clark AG, Aquadro CF, Wolfner MF. Cross-species comparison of Drosophila male accessory gland protein genes. Genetics. 2005;171:131–143. doi: 10.1534/genetics.105.043844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muratori M, Marchiani S, Forti G, Baldi E. Sperm ubiquitination positively correlates to normal morphology in human semen. Human Reproduction. 2005;20:1035–1043. doi: 10.1093/humrep/deh678. [DOI] [PubMed] [Google Scholar]
- Neubaum DM, Wolfner MF. Wise, winsome, or weird? Mechanisms of sperm storage in female animals. Current Topics in Developmental Biology. 1999;41:67–97. doi: 10.1016/s0070-2153(08)60270-7. [DOI] [PubMed] [Google Scholar]
- Pappin DJ, Hojrup P, Bleasby AJ. Rapid identification of proteins by peptide-mass fingerprinting. Curr Biol. 1993;3:327–332. doi: 10.1016/0960-9822(93)90195-t. [DOI] [PubMed] [Google Scholar]
- Poiani A. Complexity of seminal fluid: a review. Behavioral Ecology and Sociobiology. 2006;60:289–310. [Google Scholar]
- Prout T, Clark AG. Seminal fluid causes temporarily reduced egg hatch in previously mated females. Proceedings of the Royal Society of London B, Biological series. 2000;267:201–203. doi: 10.1098/rspb.2000.0988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qazi MCB, Heifetz Y, Wolfner MF. The developments between gametogenesis and fertilization: ovulation and female sperm storage in Drosophila melanogaster. Developmental Biology. 2003;256:195–211. doi: 10.1016/s0012-1606(02)00125-2. [DOI] [PubMed] [Google Scholar]
- Rao HV, Davis NT. Sperm activation and migration in bed bugs. Journal of Insect Physiology. 1969;15:1815–1832. [Google Scholar]
- Reinhardt K, Siva-Jothy MT. Biology of the bed bugs (Cimicidae) Annual Review of Entomology. 2007;52:351–374. doi: 10.1146/annurev.ento.52.040306.133913. [DOI] [PubMed] [Google Scholar]
- Reinhardt K, Naylor RA, Siva-Jothy MT. Reducing a cost of traumatic insemination: female bedbugs evolve a unique organ. Proceedings of the Royal Society of London B, Biological series. 2003;270:2371–2375. doi: 10.1098/rspb.2003.2515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinhardt K, Naylor RA, Siva-Jothy MT. Potential sexual transmission of environmental microbes in a traumatically inseminating insect. Ecological Entomology. 2005;30:607–611. [Google Scholar]
- Reinhardt K, Naylor RA, Siva-Jothy MT. Estimating the mean abundance and feeding rate of a temporal ectoparasite in the wild: Afrocimex constrictus (Heteroptera: Cimicidae) International Journal for Parasitology. 2007;37:937–942. doi: 10.1016/j.ijpara.2007.01.013. [DOI] [PubMed] [Google Scholar]
- Rice WR. Sexually antagonistic genes: experimental evidence. Science. 1992;256:1436–1439. doi: 10.1126/science.1604317. [DOI] [PubMed] [Google Scholar]
- Rice WR. Sexually antagonistic male adaptation triggered by experimental arrest of female evolution. Nature. 1996;381:232–234. doi: 10.1038/381232a0. [DOI] [PubMed] [Google Scholar]
- Ruknudin A, Raghavan VV. Initiation, maintenance and energy-metabolism of sperm motility in the bed bug, Cimex hemipterus. Journal of Insect Physiology. 1988;34:137–142. [Google Scholar]
- Sawada H, Sakai N, Abe Y, Tanaka E, Takahashi Y, Fujino J, Kodama E, Takizawa S, Yokosawa H. Extracellular ubiquitination and proteasome-mediated degradation of the ascidian sperm receptor. Proceedings of the National Academy of Sciences of the United States of America. 2002;99:1223–1228. doi: 10.1073/pnas.032389499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shikama N, Ackermann R, Brack C. Protein synthesis elongation factor EF-1a expression and longevity in Drosophila melanogaster. Proceedings of the National Academy of Sciences of the United States of America. 1994;91:4199–4203. doi: 10.1073/pnas.91.10.4199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siva-Jothy MT. Trauma, disease and collateral damage: conflict in cimcids. Philosophical Transactions of the Royal Society of London B, Biological series. 2006;361:269–275. doi: 10.1098/rstb.2005.1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stearns SC, Kaiser M. The effects of enhanced expression factor EF-1α on lifespan in Drosophila melanogaster. IV. A summary of three experiments. Genetica. 1993;91:167–182. doi: 10.1007/BF01435996. [DOI] [PubMed] [Google Scholar]
- Stutt AD, Siva-Jothy MT. Traumatic insemination and sexual conflict in the bed bug Cimex lectularius. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:5683–5687. doi: 10.1073/pnas.101440698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutovsky P. Ubiquitin-dependent proteolysis in mammalian spermatogenesis, fertilization, and sperm quality control: Killing three birds with one stone. Microscopy Research and Technique. 2003;61:88–102. doi: 10.1002/jemt.10319. [DOI] [PubMed] [Google Scholar]
- Sutovsky P, Hauser R, Sutovsky M. Increased levels of sperm ubiquitin correlate with semen quality in men from an andrology laboratory clinic population. Human Reproduction. 2004a;19:628–638. doi: 10.1093/humrep/deh131. [DOI] [PubMed] [Google Scholar]
- Sutovsky P, Manandhar G, McCauley TC, Caamano JN, Sutovsky M, Thompson WE, Day BN. Proteasomal interference prevents zona pellucida penetration and fertilization in mammals. Biology of Reproduction. 2004b;71:1625–1637. doi: 10.1095/biolreprod.104.032532. [DOI] [PubMed] [Google Scholar]
- Sutovsky P, Moreno R, Ramalho-Santos J, Dominko T, Winston WE, Schatten G. A putative, ubiquitin-dependent mechanism for the recognition and elimination of defective spermatozoa in the mammalian epididymis. Journal of Cell Science. 2001;114:1665–1675. doi: 10.1242/jcs.114.9.1665. [DOI] [PubMed] [Google Scholar]
- Sutovsky P, Turner RM, Hameed S, Sutovsky M. Differential ubiquitination of stallion sperm proteins: Possible implications for infertility and reproductive seasonality. Biology of Reproduction. 2003;68:688–698. doi: 10.1095/biolreprod.102.005306. [DOI] [PubMed] [Google Scholar]
- Swanson WJ, Clark AG, Waldrip-Dail HM, Wolfner MF, Aquadro CF. Evolutionary EST analysis identifies rapidly evolving male reproductive proteins in Drosophila. Proceedings of the National Academy of Sciences of the United States of America. 2001;95:4051–4054. doi: 10.1073/pnas.131568198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talapatra S, Wagner JDO, Thompson CB. Elongation factor-1 alpha is a selective regulator of growth factor withdrawal and ER stress-induced apoptosis. Cell Death and Differentiation. 2002;9:856–861. doi: 10.1038/sj.cdd.4401078. [DOI] [PubMed] [Google Scholar]
- Tram U, Wolfner MF. Seminal fluid regulation of female sexual attractiveness in Drosophila melanogaster. Proceedings of the National Academy of Sciences of the United States of America. 1998;95:4051–4054. doi: 10.1073/pnas.95.7.4051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viscuso R, Narcisi L, Sottile L, Brundo MV. Role of male accessory glands in spermatodesm reorganization in Orthoptera Tettigonioidea. Tissue & Cell. 2001;33:33–39. doi: 10.1054/tice.2000.0147. [DOI] [PubMed] [Google Scholar]
- Usinger RL. Monograph of the Cimicidae. Entomological Society of America; Philadelphia: 1966. [Google Scholar]
- Wagner WE, Kelley RJ, Tucker KR, Haper CJ. Females receive a life-span benefit from male ejaculates in a field cricket. Evolution. 2001;55:994–1001. doi: 10.1554/0014-3820(2001)055[0994:fralsb]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Wagstaff BJ, Begun DJ. Molecular population genetics of accessory gland protein genes and testis-expressed genes in Drosophila mojavensis and D. arizonae. Genetics. 2005;171:1083–1101. doi: 10.1534/genetics.105.043372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker MJ, Rylett CM, Keen JN, Audsley N, Sajid M, Shirras AD, Isaac RE. Proteomic identification of Drosophila melanogaster male accessory gland proteins, including a pro-cathepsin and a soluble gamma-glutamyl transpeptidase. Proteome Science. 2006;2:4.9. doi: 10.1186/1477-5956-4-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang HD, Kazemi-Esfarjani P, Benzer S. Multiple-stress analysis for isolation of Drosophila longevity genes. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:12610–12615. doi: 10.1073/pnas.0404648101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfner MF. The gifts that keep on giving: physiological functions and evolutionary dynamics of male seminal proteins in Drosophila. Heredity. 2002;88:85–93. doi: 10.1038/sj.hdy.6800017. [DOI] [PubMed] [Google Scholar]