Abstract
Phosphatidylinositol 3-kinase (PI 3-kinase) is a signaling molecule that controls numerous cellular properties and activities. The oncogene v-p3k is a homolog of the gene coding for the catalytic subunit of PI 3-kinase, p110α. P3k induces transformation of cells in culture, formation of hemangiosarcomas in young chickens, and myogenic differentiation in myoblasts. Here, we describe a role of PI 3-kinase in angiogenesis. Overexpression of the v-P3k protein or of cellular PI 3-kinase equipped with a myristylation signal, Myr-P3k, can induce angiogenesis in the chorioallantoic membrane (CAM) of the chicken embryo. This process is characterized by extensive sprouting of new blood vessels and enlargement of preexisting vessels. Overexpression of the myristylated form of the PI 3-kinase target Akt, Myr-Akt, also induces angiogenesis. Overexpression of the tumor suppressor PTEN or of dominant-negative constructs of PI 3-kinase inhibits angiogenesis in the yolk sac of chicken embryos, suggesting that PI 3-kinase and Akt signaling is required for normal embryonal angiogenesis. The levels of mRNA for vascular endothelial growth factor (VEGF) are elevated in cells expressing activated PI 3-kinase or Myr-Akt. VEGF mRNA levels are also increased by insulin treatment through the PI 3-kinase-dependent pathway. VEGF mRNA levels are decreased in cells treated with the PI 3-kinase inhibitor LY294002 and restored by overexpression of v-P3k or Myr-Akt. Overexpression of VEGF by the RCAS vector induces angiogenesis in chicken embryos. These results suggest that PI 3-kinase plays an important role in angiogenesis and regulates VEGF expression.
Keywords: Akt, tumor suppressor, transdominant negative mutant
Phosphatidylinositol 3-kinase (PI 3-kinase) is activated by insulin, by various growth factors, and by cytokines (1). PI 3-kinase catalyzes the phosphorylation of inositol phospholipids at the 3 position to generate phosphatidylinositol 3,4,5-trisphosphate and phosphatidylinositol 3,4-bisphosphate. PI 3-kinase has been implicated in a number of cellular functions, including cell adhesion, vesicular trafficking, protein synthesis, and cell survival (1). An oncogenic form of PI 3-kinase has been isolated from an avian retrovirus, ASV16, and termed v-P3k (2). The oncogene v-p3k codes for a constitutively active form of the catalytic subunit p110α of PI 3-kinase (2). Expression of the v-P3k protein induces oncogenic transformation of chicken embryo fibroblasts in culture, hemangiosarcomas in young chickens, and formation of myotubes and myogenic differentiation in chicken embryo myoblast cultures (2, 3).
The serine-threonine kinase Akt is a downstream target of PI 3-kinase. Akt is regulated by binding of its pleckstrin homology domain to the lipid products of PI 3-kinase and by phosphorylation at Thr-308 and Ser-473 residues by two phosphoinositide-dependent protein kinases, PDK1 and PDK2 (4, 5). Akt controls cell survival, glycogen metabolism, cellular transformation, and myogenic differentiation (6–10).
PI 3-kinase is activated by a variety of growth factors binding to their receptors. Several of these growth factors [e.g., fibroblast growth factors, epidermal growth factor, vascular endothelial growth factor (VEGF), hepatocyte growth factor, and IL-8] are known to induce angiogenesis. Angiogenesis is required for the progression of normal physiological events, such as embryonic development, as well as for pathogenic processes, e.g., tumorigenesis. In this report, we show that constitutively active PI 3-kinase and Akt induce angiogenesis. Inhibition of PI 3-kinase signaling interferes with angiogenesis. PI 3-kinase signaling also mediates VEGF expression in endothelial cells.
Materials and Methods
Plasmid Construction and Retrovirus Preparation.
The plasmid constructs used are v-P3k, p85ΔiSH2, Myr-Akt, and quail VEGF expressed by the avian retrovirus vector RCAS as previously described (2, 3, 10, 11). PTEN (12, 13) and Myr-P3k, c-P3k fused with the amino-terminal sequences of c-Src, were subcloned into an adaptor vector pBSFI and then inserted into a modified avian retrovirus vector RCAS.Sfi (10). Chicken embryo fibroblasts (CEF) were plated at 2 × 105 cells/well in six-well plates the day before the transfection, and transfected with 2 μg of plasmid DNA by using the Lipofectamine reagent (Life Technologies, Gaithersburg, MD). After transfection, the cells were passaged for 2 wk to ensure that the actively replicating retroviral vector spreads through the culture. Infectious virus was harvested from the cell culture medium and concentrated by centrifugation for 2 h at 23,000 rpm in a Beckman SW28 rotor at 4°C. The virus pellet was resuspended in Ham's F10 medium and stored at −70°C.
Angiogenesis Assay in Chicken Embryos.
Fertilized chicken eggs (SPAFAS, Preston, CT) were incubated at 37°C with 70% humidity for 9 days. An artificial air sac was created over a region containing small blood vessels in the chicken chorioallantoic membrane (CAM) as described (14). A small window was cut in the shell over the artificial air sac. The CAM was infected with 15 μl of RCAS, RCAS-v-P3k, or RCAS-Myr-Akt viruses containing about 1–7 × 108 infectious units per ml. Angiogenesis was monitored by photography 5, 7, and 9 days after infection. The relative angiogenesis index was determined by measuring the percentage of a unit area taken up by blood vessels (15) on days 5 and 7 after infection by using the KS300 imaging system (Zeiss).
Immunohistologic Analysis.
CAM fragments were removed from embryos 5 or 7 days after infection, and embedded in Tissue-Tek O.C.T. compound (Sakura Finetek, Torrance, CA). The CAMs were snap frozen, and 6-μm cryostat sections were cut and fixed in 3.7% formaldehyde in PBS. The CAM sections were blocked for 1 h with 2% goat serum in PBS and incubated for 1 h with a 1:50 dilution of rabbit anti-factor VIII antibodies (BioGenex Laboratories, San Ramon, CA). After three 10-min washes with PBS-0.2% Tween buffer, the sections were incubated with a 1:100 dilution of FITC-conjugated goat anti-rabbit IgG (Sigma) for 1 h. After washes as above, the sections were mounted in and examined with a fluorescence microscope (Zeiss).
Northern Blot Analysis.
CEF and chicken endothelial cells prepared from the CAM (CAME) were infected with virus expressing RCAS alone, v-P3k, Myr-P3k, or Myr-Akt. CEF were cultured in cloning medium consisting of 83% Ham's F-10, 10% calf serum, 4.5% chicken serum, 1% of ×100 vitamin solution, 1% of 1.8 mM folic acid, and 0.5% DMSO. CAME cells were cultured in cloning medium supplemented with 1% of endothelial cell growth supplement (Upstate Biotechnology, Lake Placid, NY). Total RNA was isolated by using the RNA STAT-60 reagent (Tel-Test, Friendswood, TX). Poly(A)+ RNA was isolated from total RNA by using Oligotex suspension (Qiagen, Valencia, CA). Aliquots of 0.5 μg poly(A)+ RNA were fractionated by formaldehyde gel electrophoresis and transferred to a nylon membrane (Schleicher & Schuell). The blots were hybridized with probes of quail VEGF cDNA (11) or actin cDNA, and analyzed by autoradiography.
Results and Discussion
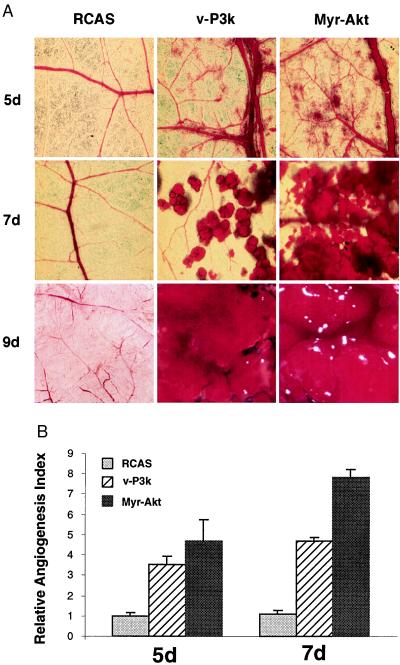
We used the oncoprotein v-P3k to investigate possible effects of PI 3-kinase on angiogenesis in the chicken CAM. Expression of the proteins was mediated by the avian retroviral vector RCAS, which produces infectious progeny virus that can spread in the CAM. CAMs of 9-day chicken embryos (SPAFAS) were infected with approximately 1.5–10.5 × 106 infectious units of RCAS virus expressing v-P3k (2, 16, 17). The empty RCAS retroviral vector served as control. High levels of v-P3k protein were detected in the CAMs 3 days after infection with RCAS-v-P3k. On day 5, an increase in the number and size of blood vessels was evident in the v-P3k-expressing CAMs (Fig. 1A). Sprouting of new vessels and enlargement of preexisting vessels were observed. The expansion of the vasculature continued rapidly, and, by day 7, small hemangiosarcomas could be detected. On day 9, these had fused into one or a few large tumors on each v-P3k-expressing CAM. In contrast, the CAMs of control embryos infected with vector RCAS virus showed a mature vascular system without excessive sprouting and branching of blood vessels. The effect of v-P3k on CAMs was quantified by determining the relative angiogenesis index (15, 18), which is based on the number of blood vessels per unit area on days 5 and 7 after infection, normalized to that of CAMs infected with the RCAS vector. Expression of v-P3k increased the angiogenesis index 3- to 5-fold relative to vector alone (Fig. 1B). Another constitutively active form of PI 3-kinase, consisting of avian cellular p110α with an amino-terminal myristylation signal, Myr-P3k, also stimulated angiogenesis and induced hemangiosarcomas when expressed by RCAS in the CAM.
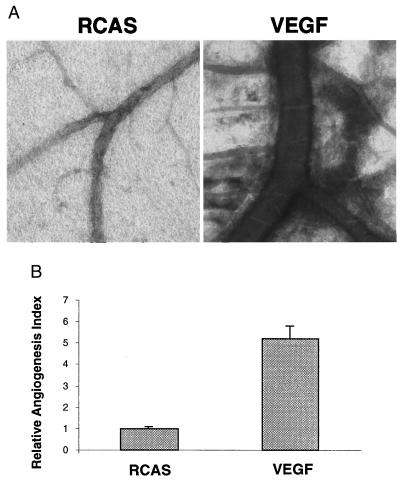
Figure 1.
Constitutively active PI 3-kinase and Akt induce angiogenesis in the CAM of chicken embryos. (A) Angiogenesis was assayed in 9-day-old chicken embryos with 15 μl of RCAS, RCAS-v-P3k, or RCAS-Myr-Akt viruses containing about 1–7 × 108 infectious units per ml. The inocula were placed on the CAM in a region devoid of blood vessels (15, 18). Angiogenesis was monitored by photography 5, 7, and 9 days after infection. The experiments were repeated at least four times with four to seven embryos for each virus, and representative portion on infected CAMs were photographed. The 5-day (5d) and 7-day (7d) photos were taken with ×6.5 magnification. The 9-day (9d) images were taken with ×3 magnification. (B) The relative angiogenesis index was determined by measuring the percentage of a unit area taken up by blood vessels (15) on day 5 (5d) and 7 (7d) using the KS300 imaging system (Zeiss). Data are expressed as mean from replicate experiments and normalized to results obtained with CAMs infected by RCAS alone (=1).
A downstream target of PI 3-kinase is the serine-threonine protein kinase Akt, which serves as an essential component for the transmission of anti-apoptotic (6–8), myogenic, and tumorigenic signals from PI 3-kinase (9, 10). To examine the possible role of Akt in angiogenesis, CAMs from 9-day-old chicken embryos were infected with 1.5–10.5 × 106 infectious units of RCAS virus expressing Myr-Akt, a constitutively active form of Akt generated by fusing a myristylation signal to the amino terminus of the mouse cellular Akt. The development of neovascularization and enlargement of existing vessels in these CAMs were equal to or exceeded that seen with v-P3k or Myr-P3k (Fig. 1).
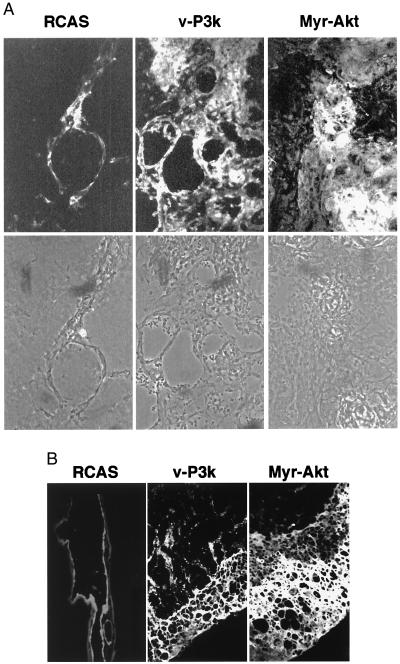
Endothelial cells of blood vessels express factor VIII of the blood clotting cascade and Flk-1, the receptor of VEGF. These two proteins served as endothelial cell-specific markers (19, 20). Their expression in CAMs was examined in cryostat sections stained with fluorescent antibodies directed against factor VIII or Flk-1. In control experiments, it was established that these antibodies stain chicken endothelial cells prepared from normal CAMs, but fail to stain CEF. CAMs expressing v-P3k or Myr-Akt were 6- to 10-times thicker than those expressing RCAS alone 9 days after infection, and showed many abnormally large capillaries and excessive proliferation of endothelial cells leading to the formation of large luminal structures. Staining with factor VIII antibodies, which marked the endothelial linings of the blood vessels, was greatly increased (Fig. 2). Identical results were obtained with antibodies against Flk-1.
Figure 2.
Constitutively active forms of PI 3-kinase and Akt induce multiple abnormally large capillaries and proliferation of endothelial cells. The CAMs infected with virus as above were snap frozen, and 6-μm cryostat sections of CAMs were fixed in 3.7% formaldehyde and stained by immunofluorescence with factor VIII antibodies (BioGenex). (A) Representative fields showing CAMs 7 days after infection by RCAS, RCAS-v-P3k, and RCAS-Myr-Akt virus (×40 objective). (Upper) Immunofluorescence with factor VIII antibodies. (Lower) Phase-contrast. (B) Representative fields were stained with factor VIII antibodies, showing increased numbers of endothelial cells that form large luminal structures 9 days after infection.
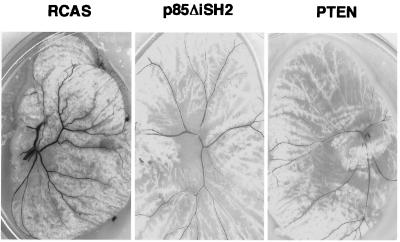
PI 3-kinase is activated by growth factors through the interaction with the regulatory subunit p85. Mutants of p85 that fail to bind to the catalytic subunit p110α function as dominant negatives of PI 3-kinase signaling (3, 21). Another antagonist of PI 3-kinase is PTEN, a phosphatase that removes the phosphate at the 3 position of phosphatidylinositol-3,4,5-trisphosphate (22–24). Introduction of either dominant-negative p85 or PTEN into 4-day-old chicken embryos with the RCAS vector inhibited the formation of normal vascular networks and caused decreased numbers and sizes of blood vessels in yolk sac development (Fig. 3), presumably by interfering with endogenous PI 3-kinase and Akt signaling. These results suggest that PI 3-kinase signaling is required for normal embryonal angiogenesis.
Figure 3.
Overexpression of PI 3-kinase dominant negative constructs p85ΔiSH2 and tumor suppressor PTEN inhibit angiogenesis in the chicken yolk sac. CAMs of 4-day-old chicken embryos were infected with RCAS virus or RCAS carrying p85ΔiSH2 or PTEN. Angiogenesis in yolk sac was monitored 9 days after infection, and representative fields were photographed.
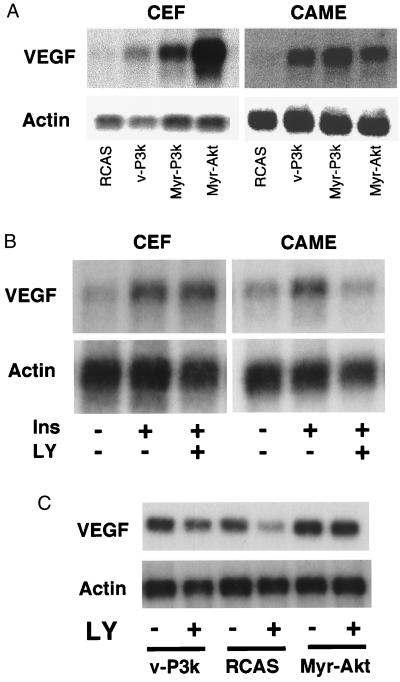
PI 3-kinase and Akt probably stimulate angiogenesis through the activation of one or several downstream mediators. A candidate for this mediator function is VEGF, which specifically stimulates endothelial cell mitogenesis. VEGF is induced by v-Src and by activated Ras (25–28), both are known to activate PI 3-kinase. We therefore expressed v-P3k, Myr-P3k, or Myr-Akt with the RCAS vector in CEF and in cultures of CAME cells. Northern blot analysis demonstrated that activated PI 3-kinase and Akt both increased the levels of VEGF mRNA (Fig. 4A). To test whether insulin can induce VEGF expression in a PI 3-kinase-dependent manner, CEF and CAME cells were switched to serum-free Ham's F10 medium for 24 h and then stimulated with 150 nM insulin for 16 h in the presence or absence of PI 3-kinase inhibitor LY294002. VEGF mRNA was induced by insulin in both CAME and CEF cells. The inhibitor LY294002 prevented VEGF expression specifically in CAME cells but not in CEF (Fig. 3B). The reduction in VEGF expression by LY294002 was partially relieved by v-P3k and was completely reversed by Myr-Akt (Fig. 4C). The effect of VEGF overexpression was investigated by infecting CAMs with the RCAS-VEGF construct (11). Seven days after inoculation, extensive sprouting of blood vessels and expansion of preexisting vessels was observed, but overexpression of VEGF failed to induce fusion of blood vessels or hemangiosarcomas (Fig. 5).
Figure 4.
VEGF expression is mediated by PI 3-kinase signaling. (A) Induction of VEGF by PI 3-kinase and Akt. CEF and CAME cells were infected with virus RCAS alone, or RCAS expressing v-P3k, Myr-P3k, or Myr-Akt. CEF were cultured for 2 wk in cloning medium consisting of 83% Ham's F-10, 10% calf serum, 4.5% chicken serum, 1% of ×100 vitamin solution, 1% of 1.8 mM folic acid, and 0.5% DMSO. CAME cells were cultured in cloning medium supplemented with 1% of endothelial cell growth supplement (Upstate Biotechnology). mRNA isolated from these cultures was analyzed in Northern blots. The blots were hybridized with quail VEGF cDNA (11) or actin cDNA. (B) VEGF expression is induced by insulin in CAME cells and CEF, and is inhibited in CAME cells but not in CEF by the PI 3-kinase inhibitor, LY294002. Primary cultures of CAME and CEF cells were switched to serum-free Ham's F-10 medium for 24 h, and remained untreated or were treated for 16 h with 150 nM insulin in the presence of 40 μM LY294002 or DMSO solvent. Northern blots were performed as above. (C) v-P3k and Myr-Akt reverse the LY294002-induced inhibition of VEGF expression. CAME cells were cultured in cloning medium supplemented as described and infected with RCAS, RCAS-v-P3k, or RCAS-Myr-Akt. Two weeks after infection, the cells were switched to serum-free Ham's F10 for 24 h and treated with 150 nM insulin in the presence of 40 μM LY294002 (LY, +) or DMSO (LY, −).
Figure 5.
Induction of angiogenesis by RCAS-VEGF. (A) Angiogenesis was assayed in 9-day-old chicken embryos with 15 μl of concentrated RCAS or RCAS-VEGF virus as in Fig. 1. Angiogenesis was monitored by photomicroscopy 7 days after infection. The experiments were repeated four times with four to eight inoculated embryos per experiment. Representative fields were photographed (×2.5 objective, phase contrast). (B) The number and extent of branching of blood vessels were scored as relative angiogenesis index as in Fig. 1. Data are expressed as mean from the four replicate experiments and normalized to results obtained with CAMs infected by RCAS virus.
Angiogenesis is critical in the development of tumors (29–33), in ocular neovascularization (29, 34, 35), and in inflammation (36, 37). The results described in this paper identify signals from PI 3-kinase and from its target Akt as regulators of angiogenesis. Activated PI 3-kinase and Akt are strong inducers of neovascularization and endothelial cell proliferation. Embryonal angiogenesis is interfered with by dominant-negative PI 3-kinase and by the PI 3-kinase antagonist PTEN. RNA levels of VEGF are elevated in cells expressing activated PI 3-kinase and Akt either by enhanced transcription or increased RNA stability. Transcription of a reporter construct containing promoter-enhancer sequences of the human VEGF gene is stimulated by PI 3-kinase and Akt (data not shown). The induction of VEGF by insulin is inhibited by the PI 3-kinase inhibitor LY294002 in endothelial cells, and this inhibition can be relieved or completely abolished by v-P3k or Myr-Akt. The induction of VEGF by platelet-derived growth factor also requires PI 3-kinase (38). VEGF has been shown to induce the activation of PI 3-kinase and Akt in human umbilical vein endothelial cells (39, 40). Although VEGF treatment of CAMs induced angiogenesis, it did not increase Akt activity in CAME cell culture (data not shown), suggesting that in this cell system there is no feedback regulatory loop that includes VEGF and PI 3-kinase. PI 3-kinase also plays an important role in embryonal angiogenesis mediated by VE-cadherin (41) and by the receptor tyrosine kinase Tie2 (42). Although VEGF is up-regulated by PI 3-kinase and Akt and induces angiogenesis in the CAM, it may not be the only angiogenesis-related target of PI 3-kinase signaling. Overexpression of VEGF with the RCAS vector does not induce hemangiosarcomas; therefore, additional factor(s) must mediate the oncogenic effect of PI 3-kinase and of Akt on endothelial cells. An important open question concerns the downstream components of the angiogenic PI 3-kinase/Akt signals, especially the identity of the transcriptional regulators that affect VEGF expression in response to PI 3-kinase and Akt signaling. Hypoxia-inducible factor 1 (43) and activator protein-1 (44) have been implicated in the transcriptional activation of VEGF. Experiments are in progress to define the PI 3-kinase-responsive element in the VEGF promoter and identify the relevant transactivators.
Acknowledgments
We thank W. K. Cavenee for PTEN, and W. Risau and I. Flamme for quail VEGF expression plasmids. This work was supported by U.S. Public Health Service Grants CA 42564 and 78230 (to P.K.V.) and the Sam and Rose Stein Endowment Fund. B.-H.J. is the recipient of National Research Service Award postdoctoral fellowship CA 77892 from the National Cancer Institute.
Abbreviations
- PI 3-kinase
phosphatidylinositol 3-kinase
- CEF
chicken embryo fibroblasts
- CAM
chicken chorioallantoic membrane
- CAME
chicken chorioallantoic membrane-derived endothelial cells
- VEGF
vascular endothelial growth factor
- PTEN
phosphatase and tensin homolog deleted on chromosome 10
Footnotes
Article published online before print: Proc. Natl. Acad. Sci. USA, 10.1073/pnas.040560897.
Article and publication date are at www.pnas.org/cgi/doi/10.1073/pnas.040560897
References
- 1.Toker A, Cantley L C. Nature (London) 1997;387:673–676. doi: 10.1038/42648. [DOI] [PubMed] [Google Scholar]
- 2.Chang H W, Aoki M, Fruman D, Auger K R, Bellacosa A, Tsichlis P N, Cantley L C, Roberts T M, Vogt P K. Science. 1997;276:1848–1850. doi: 10.1126/science.276.5320.1848. [DOI] [PubMed] [Google Scholar]
- 3.Jiang B H, Zheng J Z, Vogt P K. Proc Natl Acad Sci USA. 1998;95:14179–14183. doi: 10.1073/pnas.95.24.14179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Franke T F, Kaplan D R, Cantley L C, Toker A. Science. 1997;275:665–668. doi: 10.1126/science.275.5300.665. [DOI] [PubMed] [Google Scholar]
- 5.Alessi D R, James S R, Downes C P, Holmes A B, Gaffney P R, Reese C B, Cohen P. Curr Biol. 1997;7:261–269. doi: 10.1016/s0960-9822(06)00122-9. [DOI] [PubMed] [Google Scholar]
- 6.Franke T F, Kaplan D R, Cantley L C. Cell. 1997;88:435–437. doi: 10.1016/s0092-8674(00)81883-8. [DOI] [PubMed] [Google Scholar]
- 7.Hemmings B A. Science. 1997;275:628–630. doi: 10.1126/science.275.5300.628. [DOI] [PubMed] [Google Scholar]
- 8.Kennedy S G, Wagner A J, Conzen S D, Jordan J, Bellacosa A, Tsichlis P N, Hay N. Genes Dev. 1997;11:701–713. doi: 10.1101/gad.11.6.701. [DOI] [PubMed] [Google Scholar]
- 9.Jiang B H, Aoki M, Zheng J Z, Li J, Vogt P K. Proc Natl Acad Sci USA. 1999;96:2077–2081. doi: 10.1073/pnas.96.5.2077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Aoki M, Batista O, Bellacosa A, Tsichlis P, Vogt P K. Proc Natl Acad Sci USA. 1998;95:14950–14955. doi: 10.1073/pnas.95.25.14950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Flamme I, von Reutern M, Drexler H C, Syed-Ali S, Risau W. Dev Biol. 1995;171:399–414. doi: 10.1006/dbio.1995.1291. [DOI] [PubMed] [Google Scholar]
- 12.Li J, Yen C, Liaw D, Podsypanina K, Bose S, Wang S I, Puc J, Miliaresis C, Rodgers L, McCombie R, et al. Science. 1997;275:1943–1947. doi: 10.1126/science.275.5308.1943. [DOI] [PubMed] [Google Scholar]
- 13.Furnari F B, Lin H, Huang H S, Cavenee W K. Proc Natl Acad Sci USA. 1997;94:12479–12484. doi: 10.1073/pnas.94.23.12479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Eliceiri B P, Klemke R, Stromblad S, Cheresh D A. J Cell Biol. 1998;140:1255–1263. doi: 10.1083/jcb.140.5.1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brooks P C, Clark R A, Cheresh D A. Science. 1994;264:569–571. doi: 10.1126/science.7512751. [DOI] [PubMed] [Google Scholar]
- 16.Morgan B A, Fekete D M. Methods Cell Biol. 1996;51:185–218. doi: 10.1016/s0091-679x(08)60629-9. [DOI] [PubMed] [Google Scholar]
- 17.Bronner-Fraser M. Methods Cell Biol. 1996;51:61–79. doi: 10.1016/s0091-679x(08)60622-6. [DOI] [PubMed] [Google Scholar]
- 18.Friedlander M, Brooks P C, Shaffer R W, Kincaid C M, Varner J A, Cheresh D A. Science. 1995;270:1500–1502. doi: 10.1126/science.270.5241.1500. [DOI] [PubMed] [Google Scholar]
- 19.Kumar P, Erroi A, Sattar A, Kumar S. Cancer Res. 1985;45:4339–4348. [PubMed] [Google Scholar]
- 20.Yamaguchi T P, Dumont D J, Conlon R A, Breitman M L, Rossant J. Development (Cambridge, UK) 1993;118:489–498. doi: 10.1242/dev.118.2.489. [DOI] [PubMed] [Google Scholar]
- 21.Rodriguez-Viciana P, Warne P H, Khwaja A, Marte B M, Pappin D, Das P, Waterfield M D, Ridley A, Downward J. Cell. 1997;89:457–467. doi: 10.1016/s0092-8674(00)80226-3. [DOI] [PubMed] [Google Scholar]
- 22.Maehama T, Dixon J E. J Biol Chem. 1998;273:13375–13378. doi: 10.1074/jbc.273.22.13375. [DOI] [PubMed] [Google Scholar]
- 23.Haas-Kogan D, Shalev N, Wong M, Mills G, Yount G, Stokoe D. Curr Biol. 1998;8:1195–1198. doi: 10.1016/s0960-9822(07)00493-9. [DOI] [PubMed] [Google Scholar]
- 24.Myers M P, Pass I, Batty I H, Van der Kaay J, Stolarov J P, Hemmings B A, Wigler M H, Downes C P, Tonks N K. Proc Natl Acad Sci USA. 1998;95:13513–13518. doi: 10.1073/pnas.95.23.13513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jiang B H, Agani F, Passaniti A, Semenza G L. Cancer Res. 1997;57:5328–5335. [PubMed] [Google Scholar]
- 26.Arbiser J L, Moses M A, Fernandez C A, Ghiso N, Cao Y, Klauber N, Frank D, Brownlee M, Flynn E, Parangi S, et al. Proc Natl Acad Sci USA. 1997;94:861–866. doi: 10.1073/pnas.94.3.861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mazure N M, Chen E Y, Laderoute K R, Giaccia A J. Blood. 1997;90:3322–3331. [PubMed] [Google Scholar]
- 28.Mukhopadhyay D, Tsiokas L, Sukhatme V P. Cancer Res. 1995;55:6161–6165. [PubMed] [Google Scholar]
- 29.Folkman J. Nat Med. 1995;1:27–31. doi: 10.1038/nm0195-27. [DOI] [PubMed] [Google Scholar]
- 30.Hanahan D. Science. 1997;277:48–50. doi: 10.1126/science.277.5322.48. [DOI] [PubMed] [Google Scholar]
- 31.Folkman J, D'Amore P A. Cell. 1996;87:1153–1155. doi: 10.1016/s0092-8674(00)81810-3. [DOI] [PubMed] [Google Scholar]
- 32.Fidler I J, Ellis L M. Cell. 1994;79:185–188. doi: 10.1016/0092-8674(94)90187-2. [DOI] [PubMed] [Google Scholar]
- 33.Risau W. Nature (London) 1997;386:671–674. doi: 10.1038/386671a0. [DOI] [PubMed] [Google Scholar]
- 34.Cursiefen C, Schonherr U. Klin Monatsbl Augenheilkd. 1997;210:341–351. doi: 10.1055/s-2008-1035072. [DOI] [PubMed] [Google Scholar]
- 35.Casey R, Li W W. Am J Ophthalmol. 1997;124:521–529. doi: 10.1016/s0002-9394(14)70868-2. [DOI] [PubMed] [Google Scholar]
- 36.Majno G. Am J Pathol. 1998;153:1035–1039. doi: 10.1016/S0002-9440(10)65648-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Djuric S, Winkler J, Glaser K. Inflamm Res. 1999;48:101–103. doi: 10.1007/s000110050430. [DOI] [PubMed] [Google Scholar]
- 38.Wang D, Huang H J, Kazlauskas A, Cavenee W K. Cancer Res. 1999;59:1464–1472. [PubMed] [Google Scholar]
- 39.Gerber H P, McMurtrey A, Kowalski J, Yan M, Keyt B A, Dixit V, Ferrara N. J Biol Chem. 1998;273:30336–30343. doi: 10.1074/jbc.273.46.30336. [DOI] [PubMed] [Google Scholar]
- 40.Thakker G D, Hajjar D P, Muller W A, Rosengart T K. J Biol Chem. 1999;274:10002–10007. doi: 10.1074/jbc.274.15.10002. [DOI] [PubMed] [Google Scholar]
- 41.Carmeliet P, Lampugnani M G, Moons L, Breviario F, Compernolle V, Bono F, Balconi G, Spagnuolo R, Oostuyse B, Dewerchin M, et al. Cell. 1999;98:147–157. doi: 10.1016/s0092-8674(00)81010-7. [DOI] [PubMed] [Google Scholar]
- 42.Kontos C D, Stauffer T P, Yang W P, York J D, Huang L, Blanar M A, Meyer T, Peters K G. Mol Cell Biol. 1998;18:4131–4140. doi: 10.1128/mcb.18.7.4131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Forsythe J A, Jiang B H, Iyer N V, Agani F, Leung S W, Koos R D, Semenza G L. Mol Cell Biol. 1996;16:4604–4613. doi: 10.1128/mcb.16.9.4604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Damert A, Ikeda E, Risau W. Biochem J. 1997;327:419–423. doi: 10.1042/bj3270419. [DOI] [PMC free article] [PubMed] [Google Scholar]