Abstract
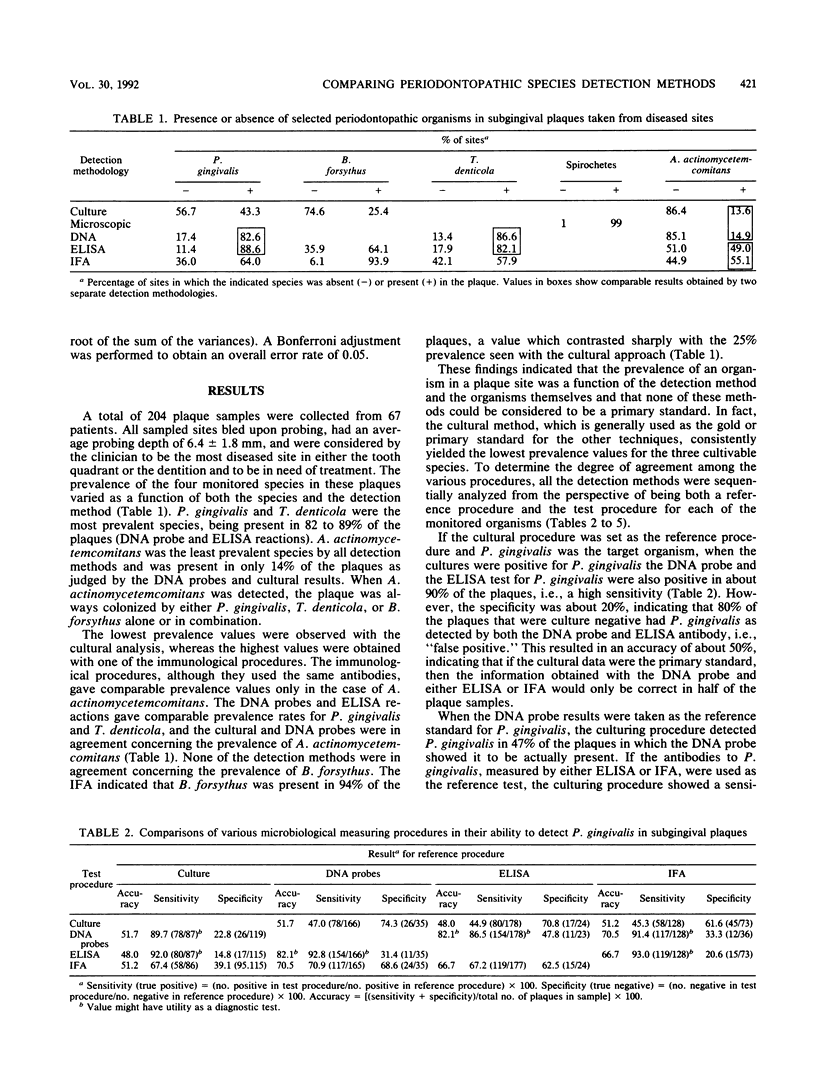
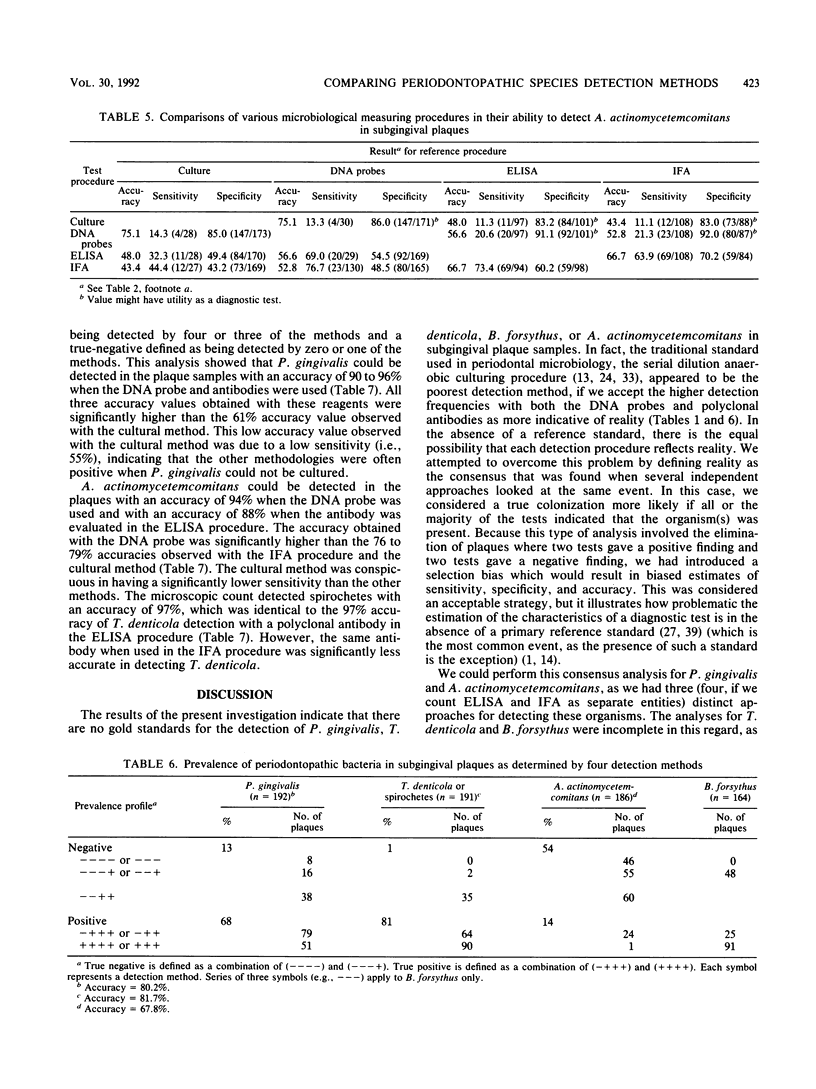
The development of diagnostic tests for a periodontal infection raises the issue as to what the appropriate reference standard, or "gold standard," should be for the evaluation of a new test. The present research was initiated to compare the ability of several detection methods, i.e., a serial dilution anaerobic culture and/or microscopic procedure, a DNA probe procedure, and immunological reagents using both an enzyme-linked immunosorbent assay and an indirect immunofluorescence assay to detect Treponema denticola, Porphyromonas gingivalis, Bacteroides forsythus, and Actinobacillus actinomycetemcomitans in subgingival plaque samples taken from 204 periodontally diseased tooth sites. The prevalence of the four monitored species varied as a function of both the species and the detection method. Spirochetes were present in 99% of the plaques, whereas A. actinomycetemcomitans was detected at the lowest frequency. The culture method yielded the lowest prevalence values for the three cultivable species. This raised the question as to which results, those obtained by culture or those obtained by the DNA probes and the immunological reagents, were the most reliable. This issue was addressed by looking at the prevalence profile of the monitored organisms, as determined by all the detection methods. If the species was detected by three or four of the detection methods, then it was considered present, whereas if it was absent by three or four of the detection methods, then it was considered absent. This approach showed the DNA probes and immunological reagents to be significantly superior (P less than 0.05) to the culture approach for the detection of P. gingivalis, A. actinomycetemcomitans, and B. forsythus and to be comparable to the microscopic approach in the detection of T. denticola.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Begg C. B. Biases in the assessment of diagnostic tests. Stat Med. 1987 Jun;6(4):411–423. doi: 10.1002/sim.4780060402. [DOI] [PubMed] [Google Scholar]
- Bonta Y., Zambon J. J., Genco R. J., Neiders M. E. Rapid identification of periodontal pathogens in subgingival plaque: comparison of indirect immunofluorescence microscopy with bacterial culture for detection of Actinobacillus actinomycetemcomitans. J Dent Res. 1985 May;64(5):793–798. doi: 10.1177/00220345850640050201. [DOI] [PubMed] [Google Scholar]
- Bretz W. A., Lopatin D. E., Loesche W. J. Benzoyl-arginine naphthylamide (BANA) hydrolysis by Treponema denticola and/or Bacteroides gingivalis in periodontal plaques. Oral Microbiol Immunol. 1990 Oct;5(5):275–279. doi: 10.1111/j.1399-302x.1990.tb00425.x. [DOI] [PubMed] [Google Scholar]
- Cochrane A. L., Holland W. W. Validation of screening procedures. Br Med Bull. 1971 Jan;27(1):3–8. doi: 10.1093/oxfordjournals.bmb.a070810. [DOI] [PubMed] [Google Scholar]
- Dahlén G., Renvert S., Wikström M., Egelberg J. Reproducibility of microbiological samples from periodontal pockets. J Clin Periodontol. 1990 Feb;17(2):73–77. doi: 10.1111/j.1600-051x.1990.tb01065.x. [DOI] [PubMed] [Google Scholar]
- Dan M., Richardson J., Miliotis M. D., Koornhof H. J. Comparison of preservation media and freezing conditions for storage of specimens of faeces. J Med Microbiol. 1989 Feb;28(2):151–154. doi: 10.1099/00222615-28-2-151. [DOI] [PubMed] [Google Scholar]
- Dix K., Watanabe S. M., McArdle S., Lee D. I., Randolph C., Moncla B., Schwartz D. E. Species-specific oligodeoxynucleotide probes for the identification of periodontal bacteria. J Clin Microbiol. 1990 Feb;28(2):319–323. doi: 10.1128/jcm.28.2.319-323.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dzink J. L., Socransky S. S., Haffajee A. D. The predominant cultivable microbiota of active and inactive lesions of destructive periodontal diseases. J Clin Periodontol. 1988 May;15(5):316–323. doi: 10.1111/j.1600-051x.1988.tb01590.x. [DOI] [PubMed] [Google Scholar]
- French C. K., Savitt E. D., Simon S. L., Eklund S. M., Chen M. C., Klotz L. C., Vaccaro K. K. DNA probe detection of periodontal pathogens. Oral Microbiol Immunol. 1986 Nov;1(1):58–62. doi: 10.1111/j.1399-302x.1986.tb00320.x. [DOI] [PubMed] [Google Scholar]
- Gilmour M. N., Turner G., Berman R. G., Krenzer A. K. Compact liquid nitrogen storage system yielding high recoveries of gram-negative anaerobes. Appl Environ Microbiol. 1978 Jan;35(1):84–88. doi: 10.1128/aem.35.1.84-88.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gmür R. Applicability of monoclonal antibodies to quantitatively monitor subgingival plaque for specific bacteria. Oral Microbiol Immunol. 1988 Dec;3(4):187–191. doi: 10.1111/j.1399-302x.1988.tb00008.x. [DOI] [PubMed] [Google Scholar]
- Gordon D. F., Stutman M., Loesche W. J. Improved isolation of anaerobic bacteria from the gingival crevice area of man. Appl Microbiol. 1971 Jun;21(6):1046–1050. doi: 10.1128/am.21.6.1046-1050.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hui S. L., Walter S. D. Estimating the error rates of diagnostic tests. Biometrics. 1980 Mar;36(1):167–171. [PubMed] [Google Scholar]
- Hujoel P. P., Loesche W. J., DeRouen T. A. Assessment of relationships between site-specific variables. J Periodontol. 1990 Jun;61(6):368–372. doi: 10.1902/jop.1990.61.6.368. [DOI] [PubMed] [Google Scholar]
- Loesche W. J., Bretz W. A., Kerschensteiner D., Stoll J., Socransky S. S., Hujoel P., Lopatin D. E. Development of a diagnostic test for anaerobic periodontal infections based on plaque hydrolysis of benzoyl-DL-arginine-naphthylamide. J Clin Microbiol. 1990 Jul;28(7):1551–1559. doi: 10.1128/jcm.28.7.1551-1559.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loesche W. J., Hockett R. N., Syed S. A. The predominant cultivable flora of tooth surface plaque removed from institutionalized subjects. Arch Oral Biol. 1972 Sep;17(9):1311–1325. doi: 10.1016/0003-9969(72)90164-1. [DOI] [PubMed] [Google Scholar]
- Loesche W. J., Lopatin D. E., Giordano J., Alcoforado G., Hujoel P. Comparison of the benzoyl-DL-arginine-naphthylamide (BANA) test, DNA probes, and immunological reagents for ability to detect anaerobic periodontal infections due to Porphyromonas gingivalis, Treponema denticola, and Bacteroides forsythus. J Clin Microbiol. 1992 Feb;30(2):427–433. doi: 10.1128/jcm.30.2.427-433.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loesche W. J., Syed S. A., Schmidt E., Morrison E. C. Bacterial profiles of subgingival plaques in periodontitis. J Periodontol. 1985 Aug;56(8):447–456. doi: 10.1902/jop.1985.56.8.447. [DOI] [PubMed] [Google Scholar]
- Loesche W. J. The role of spirochetes in periodontal disease. Adv Dent Res. 1988 Nov;2(2):275–283. doi: 10.1177/08959374880020021201. [DOI] [PubMed] [Google Scholar]
- Lopatin D. E., Smith F. N., Syed S. A., Morrison E. C. The effect of periodontal therapy on lymphocyte blastogenesis to plaque associated microorganisms. J Periodontal Res. 1983 Jan;18(1):93–102. doi: 10.1111/j.1600-0765.1983.tb00340.x. [DOI] [PubMed] [Google Scholar]
- Mangan D. F., Laughon B. E., Bower B., Lopatin D. E. In vitro lymphocyte blastogenic responses and titers of humoral antibodies from periodontitis patients to oral spirochete isolates. Infect Immun. 1982 Aug;37(2):445–451. doi: 10.1128/iai.37.2.445-451.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mombelli A., Minder C. E., Gusberti F. A., Lang N. P. Reproducibility of microscopic and cultural data in repeated subgingival plaque samples. J Clin Periodontol. 1989 Aug;16(7):434–442. doi: 10.1111/j.1600-051x.1989.tb01672.x. [DOI] [PubMed] [Google Scholar]
- Moore W. E. Microbiology of periodontal disease. J Periodontal Res. 1987 Sep;22(5):335–341. doi: 10.1111/j.1600-0765.1987.tb01595.x. [DOI] [PubMed] [Google Scholar]
- Obernesser M. S., Socransky S. S., Stashenko P. Limit of resolution of flow cytometry for the detection of selected bacterial species. J Dent Res. 1990 Sep;69(9):1592–1598. doi: 10.1177/00220345900690091101. [DOI] [PubMed] [Google Scholar]
- Payne W. J., Jr, Marshall D. L., Shockley R. K., Martin W. J. Clinical laboratory applications of monoclonal antibodies. Clin Microbiol Rev. 1988 Jul;1(3):313–329. doi: 10.1128/cmr.1.3.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SOCRANSKY S. S., GIBBONS R. J., DALE A. C., BORTNICK L., ROSENTHAL E., MACDONALD J. B. The microbiota of the gingival crevice area of man. I. Total microscopic and viable counts and counts of specific organisms. Arch Oral Biol. 1963 May-Jun;8:275–280. doi: 10.1016/0003-9969(63)90019-0. [DOI] [PubMed] [Google Scholar]
- Salvador S. L., Syed S. A., Loesche W. J. Comparison of three dispersion procedures for quantitative recovery of cultivable species of subgingival spirochetes. J Clin Microbiol. 1987 Nov;25(11):2230–2232. doi: 10.1128/jcm.25.11.2230-2232.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savitt E. D., Strzempko M. N., Vaccaro K. K., Peros W. J., French C. K. Comparison of cultural methods and DNA probe analyses for the detection of Actinobacillus actinomycetemcomitans, Bacteroides gingivalis, and Bacteroides intermedius in subgingival plaque samples. J Periodontol. 1988 Jul;59(7):431–438. doi: 10.1902/jop.1988.59.7.431. [DOI] [PubMed] [Google Scholar]
- Simonson L. G., Goodman C. H., Bial J. J., Morton H. E. Quantitative relationship of Treponema denticola to severity of periodontal disease. Infect Immun. 1988 Apr;56(4):726–728. doi: 10.1128/iai.56.4.726-728.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slots J., Hafström C., Rosling B., Dahlén G. Detection of Actinobacillus actinomycetemcomitans and Bacteroides gingivalis in subgingival smears by the indirect fluorescent-antibody technique. J Periodontal Res. 1985 Nov;20(6):613–620. doi: 10.1111/j.1600-0765.1985.tb00845.x. [DOI] [PubMed] [Google Scholar]
- Slots J. Rapid identification of important periodontal microorganisms by cultivation. Oral Microbiol Immunol. 1986 Nov;1(1):48–57. doi: 10.1111/j.1399-302x.1986.tb00318.x. [DOI] [PubMed] [Google Scholar]
- Socransky S. S., Haffajee A. D., Smith G. L., Dzink J. L. Difficulties encountered in the search for the etiologic agents of destructive periodontal diseases. J Clin Periodontol. 1987 Nov;14(10):588–593. doi: 10.1111/j.1600-051x.1987.tb01520.x. [DOI] [PubMed] [Google Scholar]
- Strzempko M. N., Simon S. L., French C. K., Lippke J. A., Raia F. F., Savitt E. D., Vaccaro K. K. A cross-reactivity study of whole genomic DNA probes for Haemophilus actinomycetemcomitans, Bacteroides intermedius, and Bacteroides gingivalis. J Dent Res. 1987 Oct;66(10):1543–1546. doi: 10.1177/00220345870660100601. [DOI] [PubMed] [Google Scholar]
- Syed S. A., Svanberg M., Svanberg G. The predominant cultivable dental plaque flora of beagle dogs with gingivitis. J Periodontal Res. 1980 Mar;15(2):123–136. doi: 10.1111/j.1600-0765.1980.tb00266.x. [DOI] [PubMed] [Google Scholar]
- Tanner A. C., Goodson J. M. Sampling of microorganisms associated with periodontal disease. Oral Microbiol Immunol. 1986 Nov;1(1):15–22. doi: 10.1111/j.1399-302x.1986.tb00310.x. [DOI] [PubMed] [Google Scholar]
- Tanner A. C., Strzempko M. N., Belsky C. A., McKinley G. A. API ZYM and API An-Ident reactions of fastidious oral gram-negative species. J Clin Microbiol. 1985 Sep;22(3):333–335. doi: 10.1128/jcm.22.3.333-335.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tenover F. C. Diagnostic deoxyribonucleic acid probes for infectious diseases. Clin Microbiol Rev. 1988 Jan;1(1):82–101. doi: 10.1128/cmr.1.1.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson R. F., Woods A., Ashley F. P. The effect of storage in liquid nitrogen on the recovery of human dental plaque bacteria. Arch Oral Biol. 1984;29(11):941–944. doi: 10.1016/0003-9969(84)90095-5. [DOI] [PubMed] [Google Scholar]
- Zambon J. J., Reynolds H. S., Chen P., Genco R. J. Rapid identification of periodontal pathogens in subgingival dental plaque. Comparison of indirect immunofluorescence microscopy with bacterial culture for detection of Bacteroides gingivalis. J Periodontol. 1985 Nov;56(11 Suppl):32–40. doi: 10.1902/jop.1985.56.11s.32. [DOI] [PubMed] [Google Scholar]
- van Poperin N., Lopatin D. E. Slot immunoblot assay for detection and quantitation of periodontal disease-associated microorganisms in dental plaque. J Clin Microbiol. 1991 Nov;29(11):2554–2558. doi: 10.1128/jcm.29.11.2554-2558.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]



