Abstract
Background
Systematic reviews have found that luteinizing hormone–releasing hormone (LHRH) agonists are effective in treating premenopausal women with early breast cancer.
Methods
We conducted long-term follow-up (median 12 years) of 2706 women in the Zoladex In Premenopausal Patients (ZIPP), which evaluated the LHRH agonist goserelin (3.6 mg injection every 4 weeks) and tamoxifen (20 or 40 mg daily), given for 2 years. Women were randomly assigned to receive each therapy alone, both, or neither, after primary therapy (surgery with or without radiotherapy/chemotherapy). Hazard ratios and absolute risk differences were used to assess the effect of goserelin treatment on event-free survival (breast cancer recurrence, new tumor or death), overall survival, risk of recurrence of breast cancer, and risk of dying from breast cancer, in the presence or absence of tamoxifen.
Results
Fifteen years after the initiation of treatment, for every 100 women not given tamoxifen, there were 13.9 (95% confidence interval [CI] = 17.5 to 19.4) fewer events among those who were treated with goserelin compared with those who were not treated with goserelin. However, among women who did take tamoxifen, there were 2.8 fewer events (95% CI = 7.7 fewer to 2.0 more) per 100 women treated with goserelin compared with those not treated with goserelin. The risk of dying from breast cancer was also reduced at 15 years: For every 100 women given goserelin, the number of breast cancer deaths was lower by 2.6 (95% CI = 6.6 fewer to 2.1 more) and 8.5 (95% CI = 2.2 to 13.7) in those who did and did not take tamoxifen, respectively, although in the former group the difference was not statistically significant.
Conclusions
Two years of goserelin treatment was as effective as 2 years of tamoxifen treatment 15 years after starting therapy. In women who did not take tamoxifen, there was a large benefit of goserelin treatment on survival and recurrence, and in women who did take tamoxifen, there was a marginal potential benefit on these outcomes when goserelin was added.
Context and Caveats
Prior knowledge
Ovarian suppression by treatment with goserelin had been shown to reduce the risk of mortality in premenopausal breast cancer patients, but data on the long-term benefit of this treatment were lacking.
Study design
The analysis was based on a randomized trial with 2 × 2 factorial assignment of breast cancer patients younger than 50 years to no hormonal therapy or 2 years of treatment with tamoxifen alone, goserelin alone, or goserelin plus tamoxifen. Kaplan–Meier curves were constructed and hazard ratios and absolute risk reductions were calculated to compare treatment groups in terms of survival and recurrence outcomes.
Contribution
This study quantified the absolute risk reduction of mortality and recurrence conferred by goserelin treatment among women who did and did not take tamoxifen based on long-term follow-up (mean follow-up time = 11 years).
Implications
For every 100 women younger than 50 years and not treated with tamoxifen, 2 years of goserelin treatment would result in 8.5 fewer breast cancer deaths compared to those not given goserelin. The benefit of goserelin treatment in women treated with tamoxifen, if any, would be smaller (possibly 2.6 fewer deaths).
Limitations
Women in the trial were treated with tamoxifen for 2 years, which is currently not standard practice. The trial did not address the question of the optimal frequency and duration of goserelin treatment.
From the Editors
Estrogen plays a fundamental role in the pathogenesis and development of breast cancer, and the risk of this disease is associated with several factors related to the level and length of exposure to this hormone (1,2). In women diagnosed with breast cancer, after initial treatment with surgery, the concentration of circulating estrogen can be reduced by ovarian ablation using surgery, irradiation, or luteinizing hormone–releasing hormone (LHRH) agonists. These treatments, designed to prevent recurrence after surgery, have a clear beneficial effect in premenopausal women with breast cancer. In 2005, the Early Breast Cancer Trialists’ Collaborative Group (EBCTCG) conducted a meta-analysis of several randomized trials investigating the effect of ovarian ablation or suppression. They reported a 17% reduction in recurrence and a 13% reduction in breast cancer mortality among women younger than 50 years with estrogen receptor (ER)–positive or unknown disease (3). The meta-analysis was based on an average follow-up of 8 years for trials of surgery or irradiation and 5 years for trials of LHRH agonists.
In 2007, a more detailed systematic review and analysis focused on adjuvant randomized trials that used LHRH agonists (4) and was based largely on 9022 women with tumors positive for ER, progesterone receptor, or both. In these trials, women were randomly assigned to receive an LHRH agonist or not, with other comparisons based on chemotherapy or tamoxifen. The analysis revealed statistically significant reductions in recurrence and death after recurrence of 13% and 15%, respectively, with the addition of an LHRH agonist to women who received tamoxifen, chemotherapy, or both.
The Zoladex in Premenopausal Patients (ZIPP) study evaluated goserelin and tamoxifen separately and in combination in premenopausal women with early breast cancer (5). We have obtained long-term follow-up data on all the patients, probably longer than any other trial of an LHRH agonist, allowing us to examine differences in survival and risk of recurrence many years after the start of therapy.
Methods
The ZIPP trial was conducted jointly by 4 breast cancer study groups from Cancer Research UK (CRUK, formerly Cancer Research Campaign), Gruppo Interdisciplinare Valutazione Interventi in Oncologia (GIVIO) Italy, South East Sweden, and Stockholm. Details of its design and initial results have been presented elsewhere (5). The aim was to determine whether the addition of goserelin (Zoladex) to local treatment, with or without tamoxifen, provided a benefit in survival or recurrence among pre- or perimenopausal women with operable, early breast cancer. The investigators in each location used the same protocol and worked together when setting up and conducting the trial. The sample size was based on all groups together. The local ethics committee for each center taking part in the trial approved the protocol, and all patients provided informed consent.
Trial Design and Subsequent Modifications
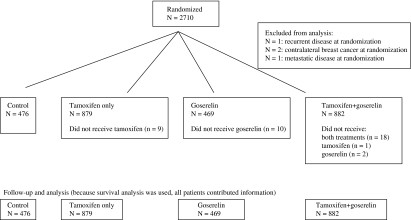
A total of 2710 premenopausal women were recruited from August 27, 1987, to March 22, 1999, and randomly assigned to receive goserelin alone (3.6-mg subcutaneous depot injection into the abdominal wall every 4 weeks), tamoxifen alone (20 or 40 mg daily), both treatments, or no endocrine therapy (Figure 1). Most women were younger than 50 years, with invasive, operable breast cancer confined to one breast without evidence of metastatic disease. Treatments were administered for 2 years. Women received primary therapy: surgery with or without radiotherapy and adjuvant systemic chemotherapy, where appropriate. Chemotherapy was perioperative cyclophosphamide, or six cycles of cyclophosphamide, methotrexate, and 5-fluorouracil (CMF) or 5-fluorouracil, epirubicin, and cyclophosphamide (FEC). The proportions of patients who received surgery, radiotherapy, or chemotherapy were evenly distributed between the goserelin groups (Supplementary Table 1, available online), so these treatments would not be expected to affect the results.
Figure 1.
Distribution of patients in the Zoladex in Premenopausal Patients (ZIPP) trial (CONSORT flow diagram).
All patients in the Stockholm and GIVIO trials were included in the 2 × 2 factorial random assignment. Patients in the CRUK and South East Sweden trials were initially randomly assigned into four arms, but with the publication of the data on tamoxifen in younger patients (6), investigators were permitted to give tamoxifen electively, following random assignment to goserelin or no goserelin. This did not affect the results because the numbers of women treated and not treated with tamoxifen were balanced between the trial arms: Of those electively given tamoxifen, 432 were randomly assigned to goserelin and 428 to no goserelin, and of those electively not given tamoxifen, 25 were randomly assigned to goserelin and 25 to no goserelin.
Four patients were subsequently found to have been ineligible at the time of random assignment (two had contralateral breast cancer, one had metastatic disease, and one had recurrent disease) and were excluded from this analysis. The numbers of women analyzed in the control, tamoxifen-only, goserelin-only, and both therapies arms were 476, 879, 469, and 882, respectively (2706 in total).
Outcomes
The following four outcome measures were examined: (1) Event-free survival (EFS), the main trial endpoint and defined as a recurrence, new tumor, or death. (2) Overall survival (OS, death from any cause). (3) Risk of recurrence [recurrence could be local or distant and among 10 women who died from breast cancer with no previously recorded recurrence, it was assumed that they had a distant recurrence, assumed to occur on the date of death; consistent with the assumptions of the EBCTCG overview (3)]. (4) Risk of dying from breast cancer. There were 520 women known to have died from breast cancer. There were also 110 whose cause of death was unknown, but these deaths also were assumed to be due to breast cancer because among those with a known cause of death, 90% were due to breast cancer. In addition, there were 13 women who died from other known causes but in whom breast cancer was present at death; these women were censored at the date of first recurrence because according to the EBCTCG overview (3), cause of death may not be reliably recorded after a recurrence.
Statistical Analysis
The sample size target was set at 2700 women to detect an absolute difference in the 5-year EFS rate of 5% (70% vs 75% in the control and treated group, respectively), with 83% power and alpha set at .05. Simple randomization was used in each of the four trial groups to allocate patients to the trial arms with a block size of four.
Kaplan–Meier survival curves and hazard ratios (HRs) were used to compare treatment groups based on intention-to-treat analyses. We examined the interaction between goserelin and tamoxifen and whether the effect of goserelin differed between subgroups of patients for four prognostic factors: age (<40 or ≥40 years), nodal status (positive or negative), ER status (positive, negative, or unknown), and previous adjuvant systemic chemotherapy (yes or no). Although age was not prespecified in the original trial protocol, the intention was to include it in the long-term follow-up analysis, particularly because it was examined in detail in the recent LHRH overview (4). All analyses were stratified by trial group. The assumption of proportional hazards was verified using a plot of the Schoenfeld residuals (7). Statistical analyses were performed using SAS 9.1 (8). Because tamoxifen is the current standard of treatment, most analyses were presented according to whether women received tamoxifen or not, allowing an examination of the effect of goserelin in addition to tamoxifen. Relative or proportional risk reductions, based on hazard ratios, are indicated by percentages (%), whereas absolute risk differences at specified time points are indicated by percentage points (ie, the number of events prevented among every 100 treated women). All statistical tests were two-sided, and P values less than .05 were considered statistically significant.
Results
The median length of follow-up was 12 years, with 26 545 person-years in total. The numbers of EFS events (recurrence, second primary tumor, or death), deaths, breast cancer recurrences, and breast cancer deaths were 1148, 690, 941, and 630, respectively (Supplementary Table 2, available online).
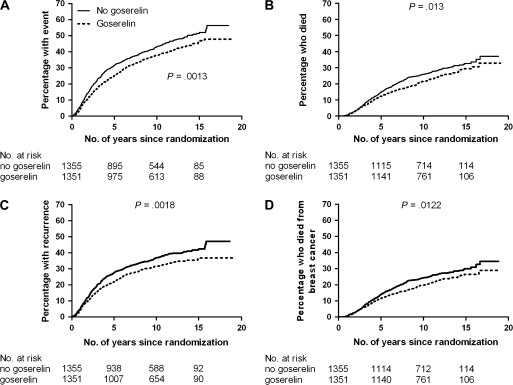
HRs associated with goserelin treatment according to trial group and overall for the four outcome measures are shown in (Supplementary Table 3, available online. Goserelin was associated with a risk reduction in all four endpoints: the risk of having an EFS event (HR = 0.82, 95% confidence interval [CI] 0.73 to 0.92, P = .001), overall mortality (HR = 0.83, 95% CI 0.71 to 0.96, P = .013), the risk of recurrence (HR = 0.81, 95% CI 0.71 to 0.92, P = .001), and breast cancer mortality (HR = 0.82, 95% CI 0.70 to 0.96, P = 0.03). Although the point estimates of HR for EFS, OS, recurrence, and breast cancer mortality in the GIVIO trial appeared to differ from those of the other trials, the confidence intervals contained the overall estimate for each outcome, suggesting that the differences may have been due to chance. There was a clear benefit on survival and recurrence 10 or 15 years after randomization (Figure 2).
Figure 2.
Survival and disease outcomes according to goserelin treatment. A) The percentages (95% confidence interval [CI]) of women treated with goserelin who experienced death, had recurrence, or had a new tumor at 5, 10, and 15 years were 25 (23 to 27), 38 (35 to 41), and 47 (43 to 51), respectively; corresponding percentages in control women were 32 (29 to 35), 43 (40 to 46), and 52 (48 to 55). B) The percentages (95% CI) of women treated with goserelin who died at 5, 10, and 15 years were 12 (10 to 14), 21 (19 to 23), and 29 (26 to 32), respectively; the corresponding percentages in control women were 15 (13 to 17), 26 (23 to 28), and 33 (30 to 36). C) The percentages (95% CI) of women treated with goserelin who had a recurrence at 5, 10, and 15 years were 22 (20 to 24), 31 (28 to 34), and 36 (33 to 39), respectively; the corresponding percentages in control women were 27 (24 to 29), 37 (34 to 40), and 42 (39 to 45). D) The percentages (95% CI) of women treated with goserelin who died from breast cancer at 5, 10, and 15 years were 11 (9 to 13), 20 (18 to 22), and 26 (23 to 29), respectively; the corresponding percentages in control women were 14 (12 to 16), 24 (22 to 26), and 30 (27 to 33). Two-sided P values from log-rank tests are shown.
The effect of goserelin depended on whether women received tamoxifen (Table 1). The test for interaction was statistically significant for EFS and recurrence, and although the P values were greater than .05 for OS and breast cancer mortality, the HRs were in a direction similar to the other endpoints, that is, women who did not receive tamoxifen experienced a greater benefit from goserelin than those who received tamoxifen. Thus, in women who did not receive tamoxifen, goserelin was associated with a 33% reduction in the risk of having an EFS event, a 29% reduction in risk of overall mortality, a 34% reduction in risk of recurrence, and a 29% reduction in risk of breast cancer mortality. In women who received tamoxifen, there was a much smaller benefit due to goserelin: 8% reduction in risk of EFS events, 10% reduction in risk of overall mortality, 9% reduction in risk of recurrence, and 11% reduction in risk of breast cancer mortality. Table 1 also shows that goserelin was as effective as tamoxifen; the hazard ratios for each treatment on its own were similar, when compared with the control group.
Table 1.
Examination of the interaction between goserelin and tamoxifen
| Hazard ratios* (95% confidence interval) |
||||
| Treatment | Any event‡ | Death from any cause | Breast cancer recurrence‡ | Death from breast cancer‡ |
| Tamoxifen† | ||||
| No | 0.67 (0.56 to 0.81) | 0.71 (0.56 to 0.91) | 0.66 (0.53 to 0.81) | 0.71 (0.55 to 0.92) |
| Yes | 0.92 (0.79 to 1.06) | 0.90 (0.74 to 1.09) | 0.91 (0.77 to 1.07) | 0.89 (0.73 to 1.09) |
| No endocrine therapy | 1 (referent) | 1 | 1 | 1 |
| Tamoxifen alone | 0.71 (0.60 to 0.84) | 0.74 (0.60 to 0.91) | 0.69 (0.57 to 0.83) | 0.72 (0.58 to 0.90) |
| Goserelin alone | 0.67 (0.56 to 0.81) | 0.71 (0.56 to 0.91) | 0.66 (0.53 to 0.81) | 0.71 (0.55 to 0.92) |
| Both tamoxifen and goserelin | 0.65 (0.55 to 0.78) | 0.66 (0.54 to 0.83) | 0.63 (0.52 to 0.76) | 0.64 (0.51 to 0.81) |
| Tamoxifen alone | 1 (referent) | 1 | 1 | 1 |
| Both tamoxifen and goserelin | 0.92 (0.80 to 1.07) | 0.90 (0.75 to 1.09) | 0.91 (0.78 to 1.07) | 0.89 (0.73 to 1.09) |
| Goserelin alone | 1 | 1 | 1 | 1 |
| Both tamoxifen and goserelin | 0.97 (0.81 to 1.17) | 0.93 (0.74 to 1.18) | 0.95 (0.78 to 1.17) | 0.90 (0.70 to 1.16) |
The hazard ratios can be converted to percentage reduction or increase in risk by subtracting 1 and multiplying by 100.
The hazard ratios are for goserelin vs no goserelin. The P values for the test of interaction between goserelin and tamoxifen were .01 (any event), .13 (death from any cause), .016 (breast cancer recurrence), and .17 (death from breast cancer).
Recurrence, new tumor, or death.
Kaplanmeier curves for the four treatment arms are shown in Figure 3. Taking either tamoxifen or goserelin had similar and large effects, but using both was associated with a possible small additional benefit that was not statistically significant. We calculated absolute risk differences associated with goserelin treatment at 5, 10, and 15 years after random assignment among women who did or did not take tamoxifen (Table 2). Among women who did not take tamoxifen, there was a clear and substantial benefit associated with goserelin treatment for all four endpoints. For example, the estimated EFS absolute risk difference at 15 years indicates that for every 100 such women treated with goserelin, there could be 13.9 fewer with an event (recurrence, new tumor or death), compared with those not given goserelin (risk difference −13.9 percentage points, 95% CI −19.4 to −7.5). This corresponds to a number needed to treat of 7. Among women who took tamoxifen, the benefit was smaller and not statistically significant: For every 100 such women treated with goserelin, there could be 2.8 fewer with an event (95% CI −7.7 to 2.0). With regards to the effect on breast cancer mortality, 12 patients needed to be treated with goserelin (and not tamoxifen) to avoid one woman dying (risk difference −8.5 percentage points, 95% CI −13.7 to −2.2), compared with no goserelin, whereas 38 needed to be treated with both goserelin and tamoxifen to avoid one death (risk difference −2.6 percentage points, 95% CI −6.6 to 2.1). Risk differences and NNTs are likely to be different in populations with much lower or higher baseline risks than in the ZIPP trial.
Figure 3.
Survival and disease outcomes according to each of the four treatment groups. Outcomes considered were death, recurrence, or new tumor (A); death from any cause (B); breast cancer recurrence (C); and death due to breast cancer (D). Two-sided P values from log-rank tests.
Table 2.
Estimated absolute risk difference (percentage points) between goserelin and no goserelin for four outcomes
| Women who did not take tamoxifen (n = 945) |
Women who took tamoxifen (n = 1761) |
|||
| No. of years after randomization | Absolute risk difference* (95% confidence interval) | Number needed to treat† | Absolute risk difference (95% confidence interval) | Number needed to treat |
| Any event (recurrence, new tumor, or death) | ||||
| 5 | −10.7 (−14.7 to −6.0) | 9 | −1.9 (−5.1 to 1.4) | 53 |
| 10 | −12.8 (−17.7 to −7.0) | 8 | −2.5 (−6.8 to 1.8) | 40 |
| 15 | −13.9 (−19.4 to −7.5) | 7 | −2.8 (−7.7 to 2.0) | 36 |
| Death from any cause | ||||
| 5 | −4.6 (−7.1 to −1.4) | 23 | −1.3 (−3.4 to 1.1) | 77 |
| 10 | −7.6 (−11.9 to −2.3) | 13 | −2.1 (−5.6 to 1.8) | 48 |
| 15 | −9.2 (−14.4 to −2.7) | 11 | −2.5 (−6.8 to 2.2) | 40 |
| Breast cancer recurrence | ||||
| 5 | −10.1 (−14.3 to −5.4) | 10 | −1.9 (−5.0 to 1.4) | 53 |
| 10 | −11.9 (−17.1 to −6.4) | 8 | −2.5 (−6.6 to 1.9) | 40 |
| 15 | −12.8 (−18.4 to −6.8) | 8 | −2.8 (−7.5 to 2.1) | 36 |
| Death from breast cancer | ||||
| 5 | −4.4 (−7.0 to −1.2) | 23 | −1.3 (−3.3 to 1.1) | 77 |
| 10 | −7.3 (−11.7 to −1.9) | 14 | −2.2 (−5.4 to 1.7) | 45 |
| 15 | −8.5 (−13.7 to −2.2) | 12 | −2.6 (−6.6 to 2.1) | 38 |
If P is the event rate in the no goserelin arm (eg, EFS rate or risk of recurrence) at a specified time point, then the absolute difference at this time is P − exp[hazard ratio × logeP ]. The hazard ratios were taken from Table 1 (tamoxifen = “No” or “Yes”).
Number needed to treat = 100% (absolute risk difference). The expected number of women who need to be treated with goserelin to avoid one event at the specified time point.
We also examined the absolute risk difference according to age (Table 3). Older women (≥40 years) who did not take tamoxifen benefited the most: The absolute risk difference was 14.8 percentage points after 15 years. In younger women (<40 years), goserelin decreased the event rate by 4–5 percentage points at 15 years, but the confidence intervals were wide, which may partly reflect the smaller sample size in these subgroups. The least benefit associated with goserelin treatment appeared to be among older women who took tamoxifen (the absolute risk was 1.7 percentage points lower).
Table 3.
Estimated absolute risk difference (percentage points) for any event (recurrence, new tumour or death) between women treated with goserelin and those not treated with goserelin, according to age and whether women took tamoxifen or not.
| Absolute risk difference (95% confidence interval) * | ||||
| No. of years since randomisation | Women who did not take tamoxifen |
Women who took tamoxifen |
||
| Age <40 years (n = 191) | Age ≥ 40 years (n = 754) | Age < 40 years (n = 415) | Age ≥ 40 years (n = 1346) | |
| 5 | −3.6 (−15.0 to +10.1) | −11.2 (−15.8 to −6.2) | −4.3 (−11.0 to +3.8) | −1.1 (−4.3 to +2.9) |
| 10 | −4.0 (−17.3 to +10.9) | −13.4 (−19.2 to −7.3) | −5.1 (−13.2 to +4.3) | −1.5 (−6.1 to +3.9) |
| 15 | −4.2 (−18.6 to +11.0) | −14.8 (−21.5 to −7.9) | −5.5 (−14.7 to +4.5) | −1.7 (−7.0 to +4.4) |
| Hazard ratio (95% CI) | 0.89 (0.58–1.35) | 0.65 (0.52–0.80) | 0.86 (0.66–1.13) | 0.95 (0.80–1.14). |
If P = the event rate in the ‘no goserelin’ arm at a specified time point, then the absolute risk difference at this time is P minus exp[hazard ratio x logeP].
Among younger women who had prior chemotherapy, the hazard ratio for goserelin treatment (compared with those not given goserelin) in relation to breast cancer mortality was 0.66 (95% CI 0.44 to 0.98), and this reduction in risk did not seem to depend on whether they took tamoxifen or not (HRs 0.66 and 0.63, respectively). The corresponding hazard ratio in younger women without prior chemotherapy was 0.86 (95% CI 0.55 to 1.34). These observations could be partly explained by the fact that younger women derive greater benefits from adjuvant chemotherapy. Among older women who had prior chemotherapy, the HR was 0.87 (95% CI 0.68 to 1.13), although the effect might be greater when they also took tamoxifen (HRs of 0.80 and 0.93, with and without tamoxifen, respectively). Among older women who did not have prior chemotherapy, the effect of goserelin depended on whether they took tamoxifen (HR for the effect of goserelin for older women who did or did not receive tamoxifen was 1.06 [95% CI = 0.75 to 1.52] and 0.60 [95% CI = 0.39 to 0.93], respectively).
We investigated interactions between goserelin and four prognostic factors (Supplementary Table 4, available online). The effect of goserelin was greatest among women who were node negative, those with ER-positive tumors, and those who had received no prior chemotherapy (although age and tamoxifen use influenced this). Similar to other studies, a benefit of goserelin in women with ER-positive tumors was observed, whether they took tamoxifen or not. It is possible that the interaction between goserelin and nodal status might have been influenced by prior chemotherapy because women with node-positive tumors were more likely to receive chemotherapy. However, when patients were stratified by nodal status and prior chemotherapy, there was still a tendency for the effect of goserelin to be greater among node-negative women, although those who were node positive who had prior chemotherapy did not appear to benefit from goserelin (Supplementary Table 5, available online).
Discussion
To our knowledge, the ZIPP trial is the largest that has investigated an LHRH agonist (2706 women) and the only factorial study that has evaluated goserelin and tamoxifen simultaneously with a control group of women who received no endocrine therapy. Recurrence and breast cancer mortality rates in both the tamoxifen and control arms were similar to those from the EBCTCG overview (3).
The overview of LHRH agonists (4), based on an average follow-up of 6.8 years, reported data on recurrence, death from any cause, and death after recurrence. The main conclusions were that LHRH agonists had an effect on reducing the risk of these events similar to that for chemotherapy (such as FEC or CMF), and could be used as an effective treatment in women with ER-positive tumors, either alone or in combination with chemotherapy or tamoxifen. Furthermore, there seemed to be a particular benefit among younger women (<40 years) who had received chemotherapy.
Although our earlier data were included in the overview (4), our results from the long-term follow-up (average follow-up is 12 years) add further information on the effect of LHRH agonists and allow estimates of the absolute risk difference at 10 and 15 years after the start of treatment. In addition to information on recurrence and death from any cause, we analyzed the effect of goserelin on the chance of dying from breast cancer and the chance of having any event (recurrence, new tumor, or death). Our analysis also quantified the long-term effect of goserelin separately among women who did or did not have tamoxifen. Because tamoxifen is now offered routinely to many women, it is important to know what the additional benefit of goserelin is 10 and 15 years after the initiation of treatment.
Among women who did not receive tamoxifen, goserelin was associated with large reductions in the event rate, the chance of dying from any cause or from breast cancer, and the risk of having a recurrence; the effect remained substantial 15 years later. Although the effect of goserelin was smaller (and not statistically significant) among women who took tamoxifen, a difference in risk of 2–3 percentage points in absolute risk at 15 years might be important, given the high incidence of early breast cancer. This would correspond to treating 33–50 women with tamoxifen and goserelin to avoid one woman having a recurrence, new tumor, or death. Indeed, the number needed to treat at 15 years was 18 in women younger than 40 years who also took tamoxifen (Table 3).
The effect of goserelin was greatest in women who were node negative and in those with ER-positive tumors. Our results were also consistent with the observation that the effect of goserelin may be greater in younger women who had prior chemotherapy (4).
A limitation of our trial is that it was based on 2 years of tamoxifen treatment, like many other studies at the time, though it is now standard practice to treat women for 5 years. Data from the INT 0101 trial, in which all women received chemotherapy, showed that the combination of 5 years of goserelin and tamoxifen was associated with a 9-year disease-free rate that was 9 and 7 percentage points higher among younger (<40 years) and older (≥40) women, respectively, compared with those treated with goserelin alone (9). Furthermore, there is evidence that a large proportion of women discontinue tamoxifen before 5 years. In a cohort of 2816 women in Ireland, 20% had stopped by 3.5 years with no further hormonal therapy (10). Therefore, the effect of 2 years of goserelin or tamoxifen therapy might still be relevant in these women.
Data on the tolerability of goserelin were presented in our first report (5). The most common side effect was hot flashes: This was experienced by none of the control patients, 26% of patients treated with goserelin, 17% of those treated with tamoxifen, and 44% of those who received goserelin plus tamoxifen. In a trial of 874 women with lymph node–negative breast cancer randomly assigned to receive six courses of CMF chemotherapy, goserelin for 2 years, or CMF followed by 18 months of goserelin, a greater proportion of women who received goserelin alone had hot flashes compared with those treated with CMF alone (11). However, in the goserelin group, the incidence 3 years later was similar to that in baseline, but not in those who received CMF. This effect is expected, given that amenorrhea can be induced by either chemotherapy or goserelin, but is reversible after stopping goserelin but permanent after chemotherapy. Goserelin was also associated with an improvement in quality-of-life measures such as mood, coping effort, tiredness, nausea and/or vomiting, and overall subjective assessment of health, compared with CMF alone (11).
Treatment-induced bone loss is a side effect of ovarian ablation or aromatase inhibitor therapy, which increases the risk of fractures or spinal cord compression (12). In a subgroup of patients randomly assigned to receive goserelin (n = 53) for 2 years or CMF chemotherapy for 6 months (n = 43), loss of bone mineral density (BMD) was greater in the goserelin group (13). Although there was partial recovery 1 year after stopping goserelin, the bone loss in the CMF group persisted. Although the combination of goserelin and tamoxifen may have a small additional benefit compared with either alone, there may be a further case for recommending both therapies because of the possible mitigating effect of tamoxifen on BMD. Markers of bone health were not measured in all women in the ZIPP trial, but the effects of 2 years of treatment with goserelin and tamoxifen on BMD were examined in a subgroup (n = 89) of patients in the Stockholm group (14). The reduction in BMD was 5% in the patients treated with goserelin (with partial recovery 1 year after stopping therapy) and 1.4% in the patients treated with tamoxifen and goserelin.
There is accumulating evidence that zoledronic acid combined with endocrine therapy can also help prevent BMD loss. In a trial of 401 patients treated with goserelin, women were randomly assigned to receive 3 years of either tamoxifen or anastrazole, each with or without zoledronic acid (15). There were reductions in lumbar spine and trochanter BMD when tamoxifen or anastrazole was used alone, but no material reduction when zoledronic acid was added. A combined interim analysis of two recent randomized trials, Z-FAST and ZO-FAST (total from both trials, n = 1667), in which all women received adjuvant letrozole, compared giving zoledronic acid either at the time of random assignment or only if their bone density fell below a prespecified cutoff or they suffered a nontraumatic fracture (16). After 1 year, the score for lumbar spine BMD was 5.2% higher in the patients given zoledronic acid at the time of random assignment, and the total hip score was 3.5% higher. There was also evidence that the risk of recurrence was lower in these patients. The effect of zoledronic acid on survival and recurrence has been confirmed in a large trial of 1801 premenopausal women randomly assigned to receive endocrine therapy (tamoxifen or anastrozole), with or without zoledronic acid, with all women receiving goserelin (17). The risk of death or recurrence was reduced by 35% (95% CI = 8% to 54%) among women who were given zoledronic acid. Given its benefits in terms of both bone health and clinical outcome, zoledronic acid could be considered as an additional treatment to goserelin.
The optimal frequency and duration of treatment with LHRH agonists are unknown. In the ZIPP trial, goserelin was given monthly for 2 years. Evidence from a randomized trial of 599 women with node-positive breast cancer indicated that a less frequent schedule (3-monthly depot of the LHRH agonist leuprorelin acetate, 11.25 mg) may be as beneficial as CMF chemotherapy in terms of the effect—5-year recurrence-free survival (HR 0.97) and after 2 years OS was better in patients given leuprorelin acetate (HR 0.64, 95% CI 0.46 to 0.90) (18). Nevertheless, no trial has specifically addressed the question of the optimal frequency and duration of goserelin treatment.
In summary, long-term follow-up of our large trial showed that goserelin had a demonstrable effect on survival and recurrence 15 years after starting treatment and is as effective as tamoxifen when each are given for 2 years. The benefit was greatest among women who did not receive tamoxifen (among every 100 women treated with goserelin, there could be 8.5 fewer breast cancer deaths), and there was a possible gain in those who did receive tamoxifen (among every 100 women treated with goserelin, there could be 2.6 fewer breast cancer deaths). It may be that women who are unlikely to complete 5 years of tamoxifen tablets may prefer 2 years of goserelin injections. It may also be reasonable to recommend both therapies to minimize the reduction in BMD associated with endocrine treatment.
Funding
Free drug was supplied by ICI (now AstraZeneca) (GIVIO) for the UK and Gruppo Interdisciplinare Valutazione Interventi in Oncologia trials. The UK trial was supported by a grant from Cancer Research UK (formerly Cancer Research Campaign). In Italy, the coordination of the trial was supported by an educational grant from AstraZeneca (the manufacturer of goserelin). The Stockholm trial had received funding from the King Gustaf V Jubilee Fund and an unrestricted research grant from AstraZeneca.
In 2007, AstraZeneca provided a grant to collect and collate the long-term follow-up data from the four study groups, and the statistical analysis was performed in the Cancer Research UK & UCL Cancer Trials Centre. (Grant number 45034) M. Baum has been a principal investigator for other trials sponsored by AstraZeneca and spoken at events funded by the company. AstraZeneca was not involved in either the analysis or the writing of the paper, or the decision to publish.
Supplementary Material
Footnotes
The trial was originally designed by M. Baum, R. Sainsbury, T. Fornander, B. Nordenskjold, and A. Nicolucci as part of a collaborative group. M. Baum, R. Sainsbury, T. Fornander, B. Nordenskjold, A. Nicolucci, and A. Hackshaw decided to examine the long-term effects. K. Monson, S. Forsyth, K. Reczko, U. Johansson, H. Fohlin, and M. Valentini were responsible for collecting the follow-up data for the trial in their country (UK, Sweden, and Italy, respectively). The statistical analyses were performed by A. Hackshaw. All authors were involved in writing the paper and gave approval of the final version.
We would also like to thank the following clinicians in each participating center.
Cancer Research UK, UK: C. Teasdale (Derrifold Hospital, Plymouth); W. Odling-Smee (City Hospital, Belfast); A. Wilkinson (Royal Victoria Hospital, Belfast); W. P. Abram (Belvoir Park, Belfast); F. MacNeill, N. Orr (Colchester District General Hospital (DGH); A. Yelland, B. Hogbin (Royal Sussex County Hospital, Brighton); K. Vellacott (Royal Gwent Hospital, Newport); R. Hall (York District Hospital); D. Berstock (Clatterbridge General); D. B. Mackie (Salisbury General Infirmary); M. Hawe (Mid-Ulster Hospital, Magherafelt, County Londonderry); S. Ebbs (Mayday University Hospital, Surrey); R. M. Rainsbury (Hampshire County Hospital, Winchester); O. H. Mitchell (Ards Hospital, Newtonards, County Down); J. Tobias (Middx/Whittington Hospital); J. Ledermann (Whittington Hospital); M. Perry (Queen Alexandra Hospital, Portsmouth); A. Howell (Withington & Christie Hospital, Manchester); D. J. Pinto (Tyrone County Hospital, Portsmouth); J. Elder (North Staffs Royal Infirmary Stoke); R. Marcus (Stratford-on-Avon Hospital); R. Brookstein (Darlington Memorial Hospital); R Matheiem, M. Coibion, D. Hertens (Institut Jules Bordet, Belgium); F. Rodesch (Erasme, Belgium); J. Roberts, J. Dawson (Kings College Hospital); A. Peel (North Tees General Hospital, Cleveland); S. Ide (Catholic University of Louvain, Leuven, Belgium); A. Nejim (Airedale DGH); I. Hutchinson (Airedale General Hospital, Keighley); M. Baum (Royal Marsden, later University College London); N. Sacks (Royal Marsden, St Grorge's Hospital); A. McKinna (Royal Marsden); R. Sainsbury (Huddersfield Royal Infirmary); I. R. Morris (Barnsley District Hospital); S. Myint (Southport & Formby DGH); P. Barker (Haslar, Gosport); S. Korzeniowski (Centre of Oncology, Krakow, Poland); M. Holmes (Princess Royal Hospital, Hull); B. Utracka-Hutka (Gliwice, Poland); T. Pienkowski (Warsaw, Poland); M. Krzakowski (Warsaw, Poland); S. Leinster (Royal Liverpool Hospital); T. Bates (William Harvey, Ashford); Y. Lau (Hong Kong); G. Mair (Royal London Hospital); A. Bulman (Norfolk & Norwich Hospital).
Gruppo Interdisciplinare Valutazione Interventi in Oncologia (GIVIO), Italy: M. Belfiglio, A. Nicolucci, F. Pellegrini, M. Sacco, M. Valentini (GIVIO Coordinating Center, Consorzio Mario Negri Sud, S. Maria Imbaro); M. Cucchi, F. Grasso, F. Di Vito (Aosta); G. Di Biagio (Assisi PG); F. Testore, M. Ceste (Asti); G. D. Beretta (Bergamo); P. Marpicati, G. Marini, E. Simoncini (Brescia); S. Pasqualucci, C. Floris, M. C. Cherchi, E. Valle (Cagliari); A. Tedde, A. Desogus, M. Pintus (Cagliari); A. Ferrari, V. Corsetti, I. Capatà (Calcinato BS); G. Serravezza, A. Elia (Casarano LE); A. Ferragni (Cremona); F. Peradotto (Cuorgnè TO); P. Garattini, A. Bertoni (Gardone Val Trompia BS); D. Belluardo, G. Franciolini (Gavardo BS); L. Isa (Gorgonzola MI); G. M. Baratelli (Gravedona CO); A. D’Arrigo (Ivrea TO); E. Locatelli (Milano); L. Armaroli, C. Iotti, A. Romeo (Reggio Emilia); G. M. Corradini, P. Candido, G. Pavia (Rho MI); M. Antimi, M. Minelli, V. Bellini (Roma); N. Olmeo, A. Contu, M. Piras, T. Scotto (Sassari); L. Galletto, M. Sussio (Savigliano CN); G. Richiardi, S. Danese, G. Giardina (Torino); M. Molteni, A. Richetti (Varese); E. Ansaldi (Venaria Reale TO).
South East Sweden: L.-G. Arnesson, T. Hatschek, A. Malmström, B. Nordenskjöld (Linköping); H. Bång (Motala); E. Einarsson (Eksjö); A.-C. Källström (Norrköping); B. Norberg (Jönköping); G. Tejler (Västervik); P. Skoog (Värnamo); M. Sundquist (Kalmar).
Stockholm: L.-E. Rutqvist, T. Fornander, J. Einhorn, J. Bergh, S. Rotstein, L. Perbeck, B. Cedermark, U. Ringborg, N. Wilking, L. Skoog, M. Iiristo, G. Svane, I. Fredriksson, P. Nikolaidis, J. Frisell, K. Sandelin, P. Gunnarsson, E. Goransson, T. Bondesson (Karolinska University Hospital); U. Glas, A. Somell, F. Celebioglu (Södersjukhuset Hospital); R. Fernstad, L. Löfgren (St Görans Hospital); M.-L. Hjalmar, K. Dalberg (Danderyd Hospital); E. af Trampe (Sophiahemmet); E. Lenner (Visby Hospital); C. Wadström (Stockholms Bröstklinik); M. al Haider (Södertälje Hospital); T. Theve (Sabbatsberg Hospital); J. Askegren (Huddinge Hospital).
References
- 1.Kelsey JL, Bernstein L. Epidemiology and prevention of breast cancer. Annu Rev Public Health. 1996;17(1):47–67. doi: 10.1146/annurev.pu.17.050196.000403. [DOI] [PubMed] [Google Scholar]
- 2.Hankinson SE, Willett WC, Colditz GA, et al. Circulating concentrations of insulin-like growth factor-I and risk of breast cancer. Lancet. 1998;351(9113):1393–1396. doi: 10.1016/S0140-6736(97)10384-1. [DOI] [PubMed] [Google Scholar]
- 3.Early Breast Cancer Trialists’ Collaborative Group (EBCTCG). Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trials. Lancet. 2005;365(9472):1687–1717. doi: 10.1016/S0140-6736(05)66544-0. [DOI] [PubMed] [Google Scholar]
- 4.LHRH-agonists in Early Breast Cancer Overview Group. Use of luteinising hormone-releasing hormone (LHRH) agonists as adjuvant treatment in premenopausal patients with hormone-receptor-positive breast cancer: a meta-analysis of individual patient data from randomised adjuvant trials. Lancet. 2007;369(9574):1711–1723. doi: 10.1016/S0140-6736(07)60778-8. [DOI] [PubMed] [Google Scholar]
- 5.Baum M, Hackshaw A, Houghton J, et al. Adjuvant goserelin in premenopausal patients with early breast cancer: results from the ‘Zoladex’ in premenopausal patients (ZIPP) trial. Eur J Cancer. 2006;42(7):895–904. doi: 10.1016/j.ejca.2005.12.013. [DOI] [PubMed] [Google Scholar]
- 6.Early Breast Cancer Trialists’ Collaborative Group (EBCTCG) Tamoxifen for early breast cancer: an overview of the randomised trials. Lancet. 1998;351(9114):1451–1467. [PubMed] [Google Scholar]
- 7.Collett D. Modelling Survival Data in Medical Research. UK: Chapman and Hall; 1994. [Google Scholar]
- 8.Statistical Analysis Software (SAS), Version 9.1. Cary, NC: SAS Institute Inc; [Google Scholar]
- 9.Davidson NE, O’Neill AM, Vukov AM, et al. Chemoendocrine therapy for premenopausal women with axillary lymph node-positive, steroid hormone receptor-positive breast cancer: results from INT 0101 (E5188) J Clin Oncol. 2005;23(25):5973–5982. doi: 10.1200/JCO.2005.05.551. [DOI] [PubMed] [Google Scholar]
- 10.Barron TI, Connolly RM, Bennett K, Feely J, Kennedy MJ. Early discontinuation of tamoxifen. Cancer. 2007;109(5):832–839. doi: 10.1002/cncr.22485. [DOI] [PubMed] [Google Scholar]
- 11.Bernhard J, Zahrieh D, Castiglione-Gertsch M, et al. Adjuvant chemotherapy followed by goserelin compared with either modality alone: the impact on amenorrhea, hot flashes, and quality of life in premenopausal patients—The International Breast Cancer Study Group Trial VIII. J Clin Oncol. 2007;25(3):263–270. doi: 10.1200/JCO.2005.04.5393. [DOI] [PubMed] [Google Scholar]
- 12.Aapro MS. Long-term complications of bone loss in breast cancer. Breast. 2004;13(suppl 1) doi: 10.1016/j.breast.2004.09.005. s29–s37. [DOI] [PubMed] [Google Scholar]
- 13.Fogelman I, Blake GM, Palmer M, et al. Bone mineral density in premenopausal women treated for node-negative early breast cancer with 2 years of goserelin or 6 months of cyclophosphamide, methotrexate and 5-fluorouracil (CMF) Osteoporos Int. 2003;14(12):1001–1006. doi: 10.1007/s00198-003-1508-y. [DOI] [PubMed] [Google Scholar]
- 14.Sverrisdottir A, Fornander T, Jacobsson H, von Schoultz E, Rutqvist LE. Bone mineral density among premenopausal women with early breast cancer in a randomised trial of adjuvant endocrine therapy. J Clin Oncol. 2004;22(18):3694–3699. doi: 10.1200/JCO.2004.08.148. [DOI] [PubMed] [Google Scholar]
- 15.Gnant MFX, Mlineritsch B, Luschin-Ebengreuth G, et al. Zoledronic acid prevents cancer treatment-induced bone loss in premenopausal women receiving adjuvant endocrine therapy for hormone-responsive breast cancer: a report from the Austrian Breast and Colorectal Cancer Study Group. J Clin Oncol. 2007;25(7):820–828. doi: 10.1200/JCO.2005.02.7102. [DOI] [PubMed] [Google Scholar]
- 16.Brufsky A, Bundred N, Coleman R, et al. for the Z-FAST and ZO-FAST Study Groups. Integrated analysis of zoledronic acid for prevention of aromatase inhibitor-associated bone loss in postmenopausal women with early breast cancer receiving adjuvant letrozole. Oncologist. 2008;13(5):503–514. doi: 10.1634/theoncologist.2007-0206. [DOI] [PubMed] [Google Scholar]
- 17.Gnant M, Mlineritsch B, Schippinger W, et al. on behalf of the ABCSG. Adjuvant ovarian suppression combined with tamoxifen or anastrozole, alone or in combination with zoledronic acid, in premenopausal women with hormone-responsive, stage I and II breast cancer: first efficacy results from ABCSG-12. J Clin Oncol. 2008;(suppl):26. Abstract LBA4. [Google Scholar]
- 18.Schmid P, Untch M, Kosse V, et al. Leuprorelin acetate every-3-months depot versus cyclophosphamide, methotrexate, and fluorouracil as adjuvant treatment in premenopausal patients with node-positive breast cancer: the TABLE study. J Clin Oncol. 2007;25(18):2509–2515. doi: 10.1200/JCO.2006.08.8534. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.