Abstract
Purpose
To investigate whether the variable forms of putative iron deposition seen with susceptibility weighted imaging (SWI) will lead to a set of multiple sclerosis (MS) lesion characteristics different than that seen in conventional MR imaging.
Materials and Methods
Twenty-seven clinically definite MS patients underwent brain scans using magnetic resonance imaging including: pre- and post-contrast T1-weighted, T2-weighted, FLAIR, and SWI at 1.5T, 3T and 4T. MS lesions were identified separately in each imaging sequence. Lesions identified in SWI were re-evaluated for their iron content using the SWI filtered phase images.
Results
There were a variety of new lesion characteristics identified by SWI and these were classified into six types. A total of 75 lesions were seen only with conventional imaging, 143 only with SWI and 204 by both. From the iron quantification measurements, a moderate linear correlation between signal intensity and iron content (phase) was established.
Conclusion
The amount of iron deposition in the brain may serve as a surrogate biomarker for different MS lesion characteristics. SWI showed many lesions missed by conventional methods and six different lesion characteristics. SWI was particularly effective at recognizing the presence of iron in MS lesions and in the basal ganglia and pulvinar thalamus.
Keywords: multiple sclerosis, iron deposition, susceptibility weighted imaging, phase imaging
Introduction
Multiple sclerosis (MS) is an inflammatory demyelinating and neurodegenerative disease of the central nervous system (1,2). Most patients start with a relapsing-remitting course which has a clearly defined episode of neurological disability and recovery. The pathological hallmark of multiple sclerosis is the demyelinated plaque, a well-demarcated hypo-cellular area characterized by the loss of myelin, axonal loss due to Wallerian degeneration (3,4) and the formation of astrocytic scars. The etiologic mechanism underlying MS is generally believed to be autoimmune inflammation (5). Nevertheless, what initiates the disease and the sequence of events underlying the development of MS is not yet well-established (6).
Conventional MRI has been used routinely to diagnose and monitor the disease spatially and temporally. The use of conventional MRI to measure disease activity and assess effects of therapy is now standard in clinical practice and drug trials (7). T2-weighted imaging (T2WI) is highly sensitive in the detection of hyperintensities in white matter. However, hyperintensities on T2WI can correspond to a wide spectrum of pathology, ranging from edema and mild demyelination to lesions in which the neurons and supporting glial cells are replaced by glial scars or liquid necrosis (8–14). Gadolinium enhancement on T1-weighted imaging (T1WI) suggests acute inflammation, which is a marker of disease activity (15). Newer MRI techniques, including magnetization transfer ratio (MTR) imaging, magnetic resonance spectroscopy (MRS), diffusion tensor imaging (DTI) and quantitative analysis of changes in brain volume (brain atrophy) are applied in MS to detect diffuse damage of axons and neurons. These MRI techniques have limited and different specificities toward various elements of MS pathology (16–19).
It is becoming a consensus among many studies that iron is enriched within oligodendrocytes and myelin in both normal and diseased tissue (20–23). One explanation for such findings proposes that iron is associated with the biosynthetic enzymes of myelinogenesis (24). In the case of demyelinating diseases, the mechanism of damage to the brain by iron might be related to oxidative stress induced by the generation of toxic free radicals (20). Brain iron accumulation has been shown histologically in neurodegenerative diseases, including MS (25,26), and has been specifically seen in the vessel walls of veins (27). Recently, more studies have been investigating hypointensities on T2WI, suggesting iron deposition in the dentate nucleus, the cortex and adjacent subcortical white matter, the brain stem, the basal ganglia and the thalamus. The results of these studies have suggested that hypointensities in T2WI were highly related to brain atrophy, disease course and physical disability (28–30). However, such studies were based on conventional or fast-spin-echo T2WI, which is not sufficient for detecting a subtle iron component that may be associated with lesion development and progression. A fairly new technique, magnetic field correlation imaging (31), has been used to quantitatively assess iron accumulation in the deep gray matter. This is a low-resolution technique that attempts to measure local magnetic field inhomogeneities to assess iron accumulation. Susceptibility weighted imaging (SWI) has been shown to be very sensitive to iron in the form of hemosiderin, ferritin and deoxyhemoglobin (32,33), offering the ability to measure iron on the order of just 1µg/g of tissue in vivo (34). SWI is a 3D, high-resolution, fully flow-compensated gradient echo sequence that uses magnitude and phase data both separately and together to enhance information about local tissue susceptibility.
In the past, phase images were seldom used because artifacts from the background field destroyed the integrity of small changes seen in pristine tissue. As we know now, phase images contain a wealth of information that may not be observed from the magnitude image. Recently, SWI filtered phase images were used to map out putative iron content in the brain (35). Phase images are a direct measure of the sources of local susceptibility changes (34,36,37). In this study, we explore the new contrast and information provided by SWI as it applies to imaging multiple sclerosis lesions. Given the perivascular (venous) relationship with MS, specifically the fact that there is vessel wall breakdown in the veins (27), we hypothesize that signal measured on SWI phase would provide a unique signature for iron accumulation. The goal of this study is to investigate whether the variable forms of putative iron deposition seen in SWI will lead to a set of lesion characteristics different than that seen in conventional MR imaging.
Materials and Methods
Twenty-seven clinically definite MS patients (21 females and 6 males aged from 21 to 71 years old with a mean age of 45 years old, all patients signed an IRB-approved consent form) (38) underwent clinical brain MRI scans including: 3D T1-weighted MPRAGE, axial T2W, FLAIR and contrast-enhanced T1W imaging at 1.5T, 3T and 4T. (The 3T data sets did not have FLAIR images.) Detailed parameters for these sequences are provided in Table 1– Table 3. In addition to these conventional MRI sequences, patients underwent 3D SWI to acquire simultaneously phase and magnitude images. A special high-pass (homodyne) filter was used to remove most of the low spatial frequency background field artifacts (4,19,39). Usually, a 64 × 64 low spatial frequency kernel is used to complex-divide into the original data to create an effective high pass filtered phase image. The resulting image is referred to as the “SWI filtered phase image.”
Table 1.
Imaging parameters for conventional sequences at 1.5T.
| 1.5T | ||||||||
|---|---|---|---|---|---|---|---|---|
| # of Slices | Slice Thickness (mm) |
FOV (mm) |
TR (ms) |
TE (ms) |
BW (Hz/pixel) |
FA | Resol. (mm) |
|
| T1-pre | 45 | 3 | 256 | 630 | 15 | 110 | 90° | 1×1×3 |
| T2 | 45 | 3 | 256 | 2800 | 16 | 100 | 180° | 1×1×3 |
| FLAIR | 45 | 3 | 240 | 8800 | 125 | 130 | 180° | 1×1×3 |
| T1-post | 45 | 3 | 256 | 630 | 15 | 110 | 90° | 1×1×3 |
Table 3.
Imaging parameters for conventional sequences at 4T.
| 4T | ||||||||
|---|---|---|---|---|---|---|---|---|
| # of Slices | Slice Thickness (mm) |
FOV (mm) |
TR (ms) |
TE (ms) |
BW (Hz/pixel) |
FA | Resol. (mm) |
|
| T1-pre | 32 | 2 | 256 | 28 | 15 | 80 | 12° | 1×1×2 |
| T2 | 29 | 4 | 256 | 4000 | 72 | 120 | 150° | 0.8×0.8×4 |
| FLAIR | 24 | 4 | 256 | 6870 | 116 | 200 | 150° | 0.5×0.5×4 |
| T1-post | 32 | 2 | 256 | 28 | 15 | 80 | 12° | 1×1×2 |
1.5T
Fourteen patients (aged 21 to 68 years old with a mean age of 44 years old) were scanned on a 1.5T Sonata (Siemens, Erlangen, Germany). The image parameters for SWI were: a slab of 64 slices with 2 mm thickness, no gap, a FOV of 256 mm, an acquisition matrix of 512 × 256, a TR/TE of 85/35 ms and a flip angle of 25°. This was a turbo-SWI segmented echo-planar-like sequence with 5 echoes and phase encoding between echoes to save time. The total scan time for all sequences was about 45 minutes.
3.0T
Seven patients (aged 41 to 67 years old with a mean age of 50 years old) were scanned on a 3.0T Trio (Siemens, Erlangen, Germany). The image parameters for SWI were: a slab of 64 slices with 2 mm thickness, no gap, a FOV of 220 mm, an acquisition matrix of 512 × 512, a TR/TE of 50/25 ms and a flip angle of 20°. This was a conventional (single echo) gradient echo acquisition. The total scan time for all sequences was about 45 minutes.
4.0T
Six patients (aged 21 to 71 years old with a mean age of 42 years old) were scanned on a 4T magnet (Brucker/Siemens). The image parameters for SWI were: a slab of 64 slices with 2 mm thickness, no gap, a FOV of 256 mm, an acquisition matrix of 512 × 256, a TR/TE of 24/15 ms and a flip angle of 12°. This was a conventional single echo acquisition. The total scan time for all sequences was about 40 minutes.
Lesion identification
MS lesions on conventional MRI sequences were identified by an experienced neuroradiologist. Lesions on SWI phase images were identified by consensus between three experienced MR researchers after consultation with the neuroradiologist. SWI was compared with conventional T2W, T1W and FLAIR (except at 3T). Lesions seen on SWI were hand-drawn, overlaid onto conventional T2W and FLAIR images, and their shapes and patterns were compared.
Iron quantification
Phase is determined by the following function (for a left-handed system):
| [1] |
where γ is the gyromagnetic ratio [MHz/T], ΔB [T] is the change in magnetic field between tissues and TE is the echo time [ms]. Iron is a paramagnetic element and aligns along the main magnetic field producing a larger field, i.e., ΔB is positive. At a given echo time, the more iron content in the tissue, the more the phase differs from zero. Therefore, the contrast seen in a brain image, for example, will depend on how much iron is present. This can be expressed as follows:
| [2] |
| [3] |
where c is the concentration of iron and Δχ is the change in susceptibility (in ppm). Thus, any changes in the amount of iron will lead to changes in the phase of the tissue relative to its surroundings. From [1] and [2], it is evident that phase will remain invariant if the product of Bo and TE remains constant.
Siemens uses the following phase convention:
| [4] |
In addition to showing that 3 Siemens phase units (Φ) correspond to 1 µg Fe/g tissue, a recent study has established a baseline of phase differences between tissues in a number of brain regions for normal people (35). To evaluate the iron content in MS patients, regions of interest (ROIs) were chosen in three separate areas: lesions, the area immediately surrounding lesions and normal-appearing white and gray matter (using T2W and FLAIR images to distinguish between white and gray matter). The area immediately surrounding lesions was defined by carefully tracing lesion boundaries seen in each slice. Around this was drawn another larger boundary—creating effectively an annular boundary region. Combining this evaluation with a comparison of lesions’ appearance on conventional images allowed the areas inside and outside of lesion boundaries to be well-characterized. The average phase value of each ROI was measured using homemade software called SPIN (Signal Processing In NMR). The iron content on SWI phase images was then correlated with the signal intensity of the same ROI measured on T2W images using a simple linear regression.
Of the 27 patient data sets processed, one had widespread lesions covering the entire white matter structures and was not analyzed for the purpose of this investigation. Another patient did not show any lesions in any clinical MRI sequence, as well as SWI, and was thus excluded from our study.
Results
Phase data were compared between 1.5T, 3T and 4T. The imaging parameters for SWI were designed so that the product of B0 and TE remained constant. An example phase image from the same volunteer at 1.5T and 3T (Figure 1) shows that the phase is invariant as expected. For example, the phase in the motor cortex was measured to be 2155 ± 31 units for 1.5T and 2120 ± 17 units or 3T. After reviewing the data, it was evident that there were lesions clearly seen with SWI but not with FLAIR or T2WI and also those seen with FLAIR or T2WI but not with SWI. There was a variety of lesion characteristics seen in the SWI phase images (see Table 4) and we were able to categorize them according to the following six properties: a) uniform darkening of lesions in phase; b) lesions seen in the magnitude SWI data but not in the phase; c) lesions associated with veins; d) lesions surrounded by a rim of hypointense signal; e) lesions with a central darkening of signal; and f) gray matter lesions (including one in the basal ganglia). Some representative examples of these lesions and their comparisons with conventional imaging are shown in Figure 2 through Figure 6.
Figure 1.
Phase images at 1.5T (a) and 3T (b) of the same patient with B0TE kept constant. The central sulcus (arrow) is clearly seen in both individuals. The gray/white matter contrast in these images comes from the increased MR visible iron content in the gray matter giving it an appearance similar to a T1-weighted scan.
Table 4.
Counts and categories of lesions seen on SWI
| Category | Description | 1.5T | 3T | 4T |
|---|---|---|---|---|
| A | Uniform darkening of lesions in phase | 101 (63m) | 46 (38m) | 72 (33m) |
| B | Magnitude lesions not seen with phase | 7 | 32 | 31 |
| C | Lesions associated with veins | 6 | 3 | 4 |
| D | Lesions surrounded by a rim of hypointense signal | 7 | 1 | 3 |
| E | Lesions with central darkening of the signal | 4 | 1 | 1 |
| F | Gray matter lesions (including the basal ganglia) | 16 | 6 (1m) | 5 |
| Total | 141 | 90 | 116 |
m = magnitude
Figure 2.
Two SWI processed images of adjacent slices acquired at 3T. Note the connectivity between the iron-containing lesion (the dark nodule, long arrow) and a peripheral vein that curls up toward the lateral right side of the brain (a, short arrow) and a vein that connects to the putamen (b, short arrow).
Figure 6.
Possible gray matter lesions seen in an SWI phase image (a) and a T2W image (b) for data acquired at 1.5T.
A total of 422 lesions were identified by all methods; 75 were not seen with SWI, and 143 were not seen with conventional methods but were detected by SWI. A total of 204 lesions were seen with both methods. A detailed summary of all lesions measured for each field strength appears in Table 5, Table 6 and Table 7. In each table, the number in parentheses followed by an “m” represents how many of the phase lesions were also seen in the magnitude data.
Table 5.
Lesion counts for 14 patients at 1.5T
| T2 | T2-FLAIR | FLAIR | SWI only | Total | |
|---|---|---|---|---|---|
| Seen on SWI | 30 | 30 | 3 | 78 | 141 |
| Not seen on SWI | 18 | 27 | 3 | 48 | |
| Total | 48 | 57 | 6 | 78 | 189 |
Table 6.
Lesion counts for 7 patients at 3T
| T2 | SWI only | Total | |
|---|---|---|---|
| Seen on SWI | 38p+32m | 20 | 90 |
| Not seen on SWI | 21 | 21 | |
| Total | 91 | 20 | 111 |
m = magnitude, p = phase
Table 7.
Lesion counts for 6 patients at 4T
| T2 | FLAIR | SWI only | Total | |
|---|---|---|---|---|
| Seen on SWI | 33p+18m | 20 | 45 | 116 |
| Not seen on SWI | 6 | 6 | ||
| Total | 51 | 26 | 51 | 122 |
m = magnitude, p = phase
Iron quantification using SWI phase images
A total of 199 uniform phase lesions were evaluated from eight patients who had enough lesions to draw a correlation. These patients were imaged at various field strengths. The phase value in the lesions was 2186 ± 42 while the surrounding normal-appearing white matter had an average phase value of 2044 ± 20. The difference between these two values is 142 units, representing an average iron content of 47 µg Fe/g tissue (34). (In most of the cases, the region adjacent to the lesion had iron concentrations slightly lower than those in the white matter.) A histogram of the iron content in 177 of the most well-defined lesions from the 26 analyzed patients (measured at 1.5T, 3T and 4T) is shown in Figure 7.
Figure 7.
Histogram showing the distribution of iron deposition in well-defined lesions of the 26 analyzed patients. (These concentrations were calculated assuming that 180 Siemens phase units correspond to 60 µg Fe/g tissue.)
The signal intensity changes in lesions seen with both SWI and T2 relative to the surrounding normal-appearing white matter were seen to be moderately linearly correlated. Specifically, the SWI filtered phase was compared to the signal intensity in T2W images. The correlations between T2 signal intensity and phase/iron are summarized in Table 8 and two example plots are shown in Figure 8 and Figure 9.
Table 8.
Correlations between T2 Signal Intensity and Phase/Iron Content
| Patient | rT2 | pT2 |
|---|---|---|
| 1 | −0.87 | 0.010 |
| 2 | −0.34 | 0.230 |
| 3 | −0.39 | 0.270 |
| 4 | −0.59 | 0.020 |
| 5 | −0.11 | 0.680 |
| 6 | −0.34 | 0.320 |
| 7 | −0.78 | 0.005 |
| 8 | −0.83 | 0.022 |
Figure 8.
A plot of signal intensity from T2 versus phase and a plot of phase converted into iron content.
Figure 9.
A plot of signal intensity from T2 versus phase and a plot of phase converted into iron content.
Discussion
Susceptibility weighted imaging offers a unique way to view tissue affected by iron deposition whether in the form of deoxyhemoglobin, ferritin or hemosiderin. Not only have we demonstrated that there are nearly 50% more lesions seen in total combining conventional imaging with SWI, but the iron content that makes lesions visible in SWI can also be quantified. The distribution of iron in the lesions in Figure 7 shows that the peak iron can reach 60µg Fe/g tissue. This is as large as the iron content expected in the motor cortex. With the imaging parameters used here, recent results suggest that at 1.5T in a region-of-interest of 100 pixels, it is possible to determine changes in iron of just 1µg Fe/g tissue (35). This may serve as a means to monitor iron changes over time in the lesions.
Of the six different types of lesions observed, most seem to have a fairly uniform distribution of iron. In 13 cases, we can see the direct connectivity of lesions with veins. In six others, only the center of the lesion is dark. There were, however, lesions that exhibited a ring-like structure of high iron content (11 cases). This may be similar to the ring-like effects seen both pathologically in leukoencephalopathy and also sometimes visible on FLAIR and T2WI. The ability to see these rims of iron (Figure 3) may also have an impact on disease progress (15). Finally, there is some evidence of gray matter abnormality.
Figure 3.
The rims of lesions (arrows) are seen more clearly in SWI phase (a) than in FLAIR (b). The rims are not defined in magnitude (c) or T2 (d). This data was acquired at 4T.
Differentiating simple changes in phase from veins was done by looking for connectivity. Usually, it was fairly easy to discriminate between signal changes caused by veins and those corresponding to lesions because we could view the minimum intensity projection (mIP) of three or more slices centered on the slice of interest. These mIP images show the connectivity of the vessels and make it clear if the vessel runs through the lesion of interest. Most of the lesions, however, showed fairly large non-vascular structures that were not circular in nature. Since these lesions often sat in white matter, and since the phase of white matter is close to zero, it is fairly obvious, with practice, what represents abnormal phase signal and hence its probability of being a lesion. We read the phase images separately and then compared the results to the FLAIR or T2 data. Since many of the lesions do overlap with T2 lesions, this gave us good confidence that our interpretations of these new findings were likely correct. As a comparison with T1, T2 and FLAIR, we used a “copy ROI” feature of our software to ensure the appropriate interpretation and registration of the lesions. Since all sequences were run with the same FOV, this was a particularly easy way to ensure that we were looking at the same lesions. When these ROIs were copied from one image to the next, we observed that the shapes of the ring lesions seen on the SWI data were essentially identical to the shape on the corresponding T2W images.
Why is this new biomarker for iron potentially important in the study of MS with SWI? The current MR imaging biomarkers of MS pathology focus on: the breakdown of the blood brain barrier, multi-focal inflammation, demyelination, oligodendrocyte loss, axonal and neuronal degeneration, gliosis, and remyelination and repair (40). In a systematic analysis of all studies published in the last 20 years, it was found that none of the proposed biomarkers could serve as a surrogate marker for clinical outcome (41). In a disease with a complex pathogenesis, such as MS, an individual biomarker is likely to reflect only one aspect of many pathogenic processes. The ability to predict the outcome of MS is complicated due to the underlying diversity and variability of the lesions. Although clinical judgment and experience provide the foundation for medical decisions, advances in neuroimaging may enhance the management of these patients if more specific biomarkers can be found.
Iron may be yet another critical means by which to assess the status of MS patients. Iron is a paramagnetic substance that reduces T2 relaxation time resulting in hypointensity on T2-weighted images. The different types of non-heme iron in the brain include low-molecular-weight complexes, ionic iron, metalloproteins such as transferrin, melanotransferrin and lactoferrin, as well as storage proteins such as ferritin and hemosiderin (34). Transferrin carries iron from the blood into tissues, while ferritin stores excess iron atoms that are not immediately engaged in metabolic activities. There can be up to 4500 iron atoms stored in the 8-nm-diameter internal cavity of one ferritin protein (34). Hemosiderin is considered to be a water-soluble iron storage molecule that is a breakdown product of ferritin and appears to be associated with iron overload disorders and hemorrhage (34). Brain iron accumulation has been shown histologically in MS and recently, an iron increase from 24% to 39.5% was reported in the deep gray matter in MS patients compared to control subjects (25,26).
The source of iron deposition may be myelin/oligodendrocyte debris (17), concentrated iron in the macrophages (that phagocytize the destructed myelin/oligodendrocyte), or the product of hemorrhages from damaged brain vessels. The mechanism of direct damage to the brain by iron might be related to oxidative stress and the generation of toxic free radicals (12). The amount of iron deposition could reflect the extent of tissue damage, thus iron could be used as a biomarker to predict clinical outcome. This is a reasonable hypothesis given recent findings (27), which show very clear iron deposition encircling dilated veins in MS. The source of this iron is still unclear, but it could result from microhemorrhaging and hemosiderin buildup (27). Additionally, our results appear to indicate that chronic lesions may vanish on T2WI in some instances. If this is the case, then it may explain why the number of lesions on T2WI has not been very specific to the severity of the disease.
Apart from signal-to-noise, one of the key points about phase contrast is that it is independent of field strength if the product of field strength and echo time is kept constant. Therefore, for the first time, it is possible to make comparisons of studies across systems and across field strengths and reasonably expect to get the same images. This should make SWI globally applicable in clinical trials on all manufacturers’ systems.
In conclusion, we have shown that SWI has the potential to recognize the presence of iron in MS lesions, visualize lesions missed by conventional methods and visualize different lesion characteristics. The iron may be from blood or other iron sources sequestered by macrophages in the form of hemosiderin. Future studies should focus on monitoring iron levels along with cognitive and motor evaluations of the patient as a possible means to have a more specific imaging test of the patient’s clinical status.
Figure 4.
Lesions with high phase/iron content, as shown in SWI filtered phase images (a) are either not visible or less clearly seen in SWI magnitude (b), T2-weighted (c) or FLAIR images (d) at 4T.
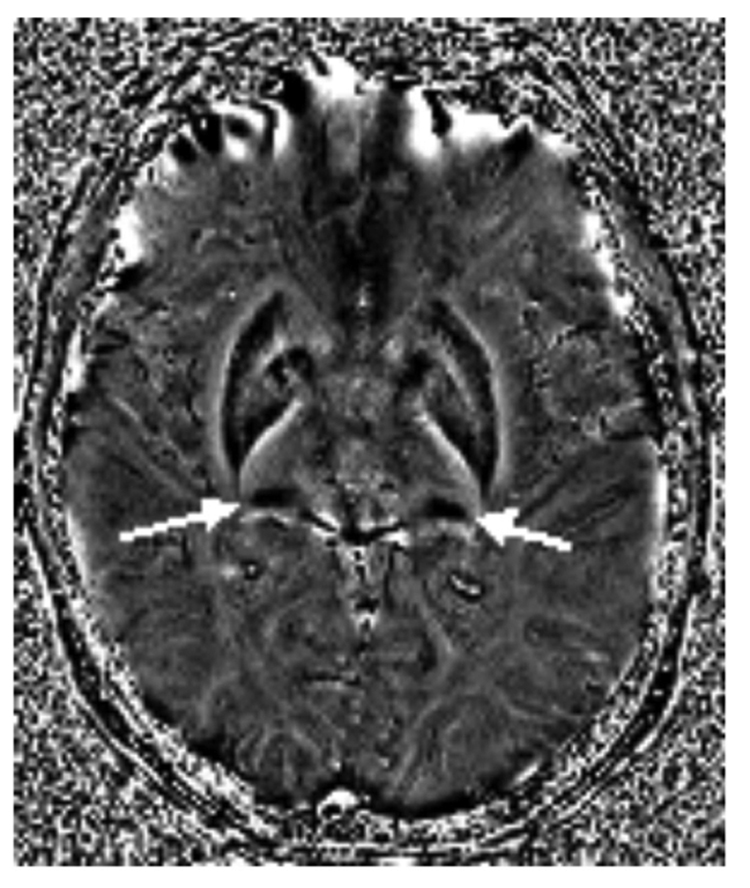
Figure 5.
Filtered phase SWI image acquired at 3T showing high iron deposition (white arrows) in the left and right pulvinar thalamus.
Table 2.
Imaging parameters for conventional sequences at 3T.
| 3T* | ||||||||
|---|---|---|---|---|---|---|---|---|
| # of Slices | Slice Thickness (mm) |
FOV (mm) |
TR (ms) |
TE (ms) |
BW (Hz/pixel) |
FA | Resol. (mm) |
|
| T1-pre | 50 | 3 | 220 | 499 | 7 | 7 | 90° | 0.5×0.5×3 |
| T2 | 96 | 3 | 256 | 9860 | 13 | 13 | 121° | 1×1×3 |
| T1-post | 50 | 3 | 220 | 499 | 7 | 7 | 90° | 0.5×0.5×3 |
FLAIR scans were not performed.
Acknowledgements
The authors would like to thank Alexander S. Boikov and Charbel A. Habib for their assistance in editing and revising this work. We also thank Siemens Medical Solutions for their continued support of our research.
Grant Support:
This work was supported in part by the National Institutes of Health grant # R01NS029029, the State of Michigan grant # 085P5200251 and the Multiple Sclerosis Society grant # CA1042-A-8.
References
- 1.Noseworthy JH, Wolinsky JS, Lublin FD, et al. Linomide in relapsing and secondary progressive MS, part I: trial design and clinical results. North American Linomide Investigators. Neurology. 2000;54:1726–1733. doi: 10.1212/wnl.54.9.1726. [DOI] [PubMed] [Google Scholar]
- 2.Weinshenker BG, Rice GP, Noseworthy JH, et al. The natural history of multiple sclerosis: a geographically based study. 3. Multivariate analysis of predictive factors and models of outcome. Brain. 1991;114:1045–1056. doi: 10.1093/brain/114.2.1045. [DOI] [PubMed] [Google Scholar]
- 3.Keegan BM, Noseworthy JH. Multiple sclerosis. Annu Rev Med. 2002;53:285–302. doi: 10.1146/annurev.med.53.082901.103909. [DOI] [PubMed] [Google Scholar]
- 4.Evangelou N, Esiri MM, Smith S, Palace J, Matthews PM. Quantitative pathological evidence for axonal loss in normal appearing white matter in multiple sclerosis. Ann Neurol. 2000;47:391–395. [PubMed] [Google Scholar]
- 5.Hohlfeld R. Biotechnological agents for the immunotherapy of multiple sclerosis: principles, problems and perspectives. Brain. 1997;120:865–916. doi: 10.1093/brain/120.5.865. [DOI] [PubMed] [Google Scholar]
- 6.Haacke EM, Wang Y, Yu Y, et al. Artery and vein separation using susceptibility-dependent phase in contrast-enhanced MRA. J Magn Reson Imaging. 2000;12:661–670. doi: 10.1002/1522-2586(200011)12:5<661::aid-jmri2>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 7.Tjoa CW, Benedict RH, Weinstock-Guttman B, Fabiano AJ, Bakshi R. MRI T2 hypointensity of the dentate nucleus is related to ambulatory impairment in multiple sclerosis. J Neurol Sci. 2005;234:17–24. doi: 10.1016/j.jns.2005.02.009. [DOI] [PubMed] [Google Scholar]
- 8.MacKay A, Laule C, Vavasour I, Bjarnason T, Kolind S, Mädler B. Insights into brain microstructure from the T2 distribution. Magn Reson Imaging. 2006;24:515–525. doi: 10.1016/j.mri.2005.12.037. [DOI] [PubMed] [Google Scholar]
- 9.Laule C, Leung E, Lis DK, et al. Myelin water imaging in multiple sclerosis: quantitative correlations with histopathology. Multiple Sclerosis. 2006;12:747–753. doi: 10.1177/1352458506070928. [DOI] [PubMed] [Google Scholar]
- 10.Jensen JH, Lu H, Inglese M. Microvessel density estimation in the human brain by means of dynamic contrast-enhanced echo-planar imaging. Magn Reson Med. 2006;56:1145–1150. doi: 10.1002/mrm.21052. [DOI] [PubMed] [Google Scholar]
- 11.Inglese M, van Waesberghe JH, Barkhof F, et al. The effect of interferon beta-1b on quantities derived from MT MRI in secondary progressive MS. Neurology. 2003;60:853–860. doi: 10.1212/01.wnl.0000049929.27032.29. [DOI] [PubMed] [Google Scholar]
- 12.de Groot CJ, Bergers E, Kamphorst W, et al. Post-mortem MRI-guided sampling of multiple sclerosis brain lesions: increased yield of active demyelinating and (p)reactive lesions. Brain. 2001;124:1635–1645. doi: 10.1093/brain/124.8.1635. [DOI] [PubMed] [Google Scholar]
- 13.van Waesberghe JH, Kamphorst W, de Groot C, et al. Axonal loss in multiple sclerosis lesions: magnetic resonance imaging insights into substrates of disability. Ann Neurol. 1999;46:747–754. doi: 10.1002/1531-8249(199911)46:5<747::aid-ana10>3.3.co;2-w. [DOI] [PubMed] [Google Scholar]
- 14.van Walderveen MA, Barkhof F, Hommes OR, et al. Correlating MRI and clinical disease activity in multiple sclerosis: relevance of hypointense lesions on short-TR/short-TE (T1-weighted) spin-echo images. Neurology. 1995;45:1684–1690. doi: 10.1212/wnl.45.9.1684. [DOI] [PubMed] [Google Scholar]
- 15.Morgen K, Jeffries NO, Stone R, et al. Ring enchancement in multiple sclerosis: marker of disease severity. Multiple Sclerosis. 2001;7:167–171. doi: 10.1177/135245850100700306. [DOI] [PubMed] [Google Scholar]
- 16.Filippi M, Rocca MA, Rovaris M. Clinical trials and clinical practice in multiple sclerosis: conventional and emerging magnetic resonance imaging technologies. Curr Neurol Neurosci Rep. 2002;2:267–276. doi: 10.1007/s11910-002-0086-2. [DOI] [PubMed] [Google Scholar]
- 17.Fu L, Matthews PM, de Stefano N, et al. Imaging axonal damage of normal-appearing white matter in multiple sclerosis. Brain. 1998;121:103–113. doi: 10.1093/brain/121.1.103. [DOI] [PubMed] [Google Scholar]
- 18.Zivadinov R. Can imaging techniques measure neuroprotection and remyelination in multiple sclerosis? Neurology. 2007;68:72–82. doi: 10.1212/01.wnl.0000275236.51129.d2. [DOI] [PubMed] [Google Scholar]
- 19.Pike GB, de Stefano N, Narayanan S, et al. Combined magnetization transfer and proton spectroscopic imaging in the assessment of pathologic brain lesions in multiple sclerosis. AJNR Am J Neuroradiol. 1999;20:829–837. [PMC free article] [PubMed] [Google Scholar]
- 20.Schenck JF, Zimmerman EA. High-field magnetic resonance imaging of brain iron: birth of a biomarker? NMR Biomed. 2004;17:433–445. doi: 10.1002/nbm.922. [DOI] [PubMed] [Google Scholar]
- 21.LeVine SM, Macklin WB. Iron-enriched oligodendrocytes: a reexamination of their spatial distribution. J Neurosci Res. 1990;26:508–512. doi: 10.1002/jnr.490260415. [DOI] [PubMed] [Google Scholar]
- 22.Dwork AJ, Schon EA, Herbert J. Nonidentical distribution of transferrin and ferric iron in human brain. Neuroscience. 1988;27:333–345. doi: 10.1016/0306-4522(88)90242-4. [DOI] [PubMed] [Google Scholar]
- 23.Francois C, Nguyen-Legros J, Percheron G. Topographical and cytological localization of iron in rat and monkey brains. Brain Res. 1981;215:317–322. doi: 10.1016/0006-8993(81)90510-2. [DOI] [PubMed] [Google Scholar]
- 24.Connor JR, Snyder BS, Arosio P. A quantitative analysis of isoferritins in select regions of aged, parkinsonian, and Alzheimer's diseased brains. J Neurochem. 1995;65:717–724. doi: 10.1046/j.1471-4159.1995.65020717.x. [DOI] [PubMed] [Google Scholar]
- 25.Levine SM, Chakrabarty A. The role of iron in the pathogenesis of experimental allergic encephalomyelitis and multiple sclerosis. Ann N Y Acad Sci. 2004;1012:252–266. doi: 10.1196/annals.1306.021. [DOI] [PubMed] [Google Scholar]
- 26.Craelius W, Migdal MW, Luessenhop CP, et al. Iron deposits surrounding multiple sclerosis plaques. Arch Pathol Lab Med. 1982;106:397–399. [PubMed] [Google Scholar]
- 27.Zamboni P. The Big Idea: iron-dependent inflammation in venous disease and proposed parallels in multiple sclerosis. J R Soc Med. 2006;99:589–593. doi: 10.1258/jrsm.99.11.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tozer DJ, Davies GR, Altmann DR, Miller DH, Tofts PS. Correlation of apparent myelin measures obtained in multiple sclerosis patients and controls from magnetization transfer and multicompartmental T2 analysis. Magn Reson Med. 2005;53:1415–1422. doi: 10.1002/mrm.20479. [DOI] [PubMed] [Google Scholar]
- 29.Bakshi R, Ariyaratana S, Benedict RH, Jacobs L. Fluid attenuated inversion recovery magnetic resonance imaging detects cortical and juxtacortical multiple sclerosis lesions. Arch Neurol. 2001;58:742–748. doi: 10.1001/archneur.58.5.742. [DOI] [PubMed] [Google Scholar]
- 30.Drayer BP, Burger P, Hurwitz B, et al. Reduced signal intensity on MR images of the thalamus and putamen in multiple sclerosis: increased iron content? AJR Am J Roentgenol. 1987;149:357–363. doi: 10.2214/ajr.149.2.357. [DOI] [PubMed] [Google Scholar]
- 31.Ge Y, Jensen JH, Lu H, et al. Quantitative assessment of iron accumulation in the deep gray matter of multiple sclerosis by magnetic field correlation imaging. AJNR Am J Neuroradiol. 2007;28:1639–1644. doi: 10.3174/ajnr.A0646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Haacke EM, Xu Y, Cheng YC, Reichenbach JR. Susceptibility weighted imaging. Magn Reson Med. 2004;52:612–618. doi: 10.1002/mrm.20198. [DOI] [PubMed] [Google Scholar]
- 33.Reichenbach JR, Venkatesan R, Yablonskiy DA, Thompson MR, Lai S, Haacke EM. Theory and application of static field inhomogeneity effects in gradient-echo imaging. J Magn Reson Imaging. 1997;7:266–279. doi: 10.1002/jmri.1880070203. [DOI] [PubMed] [Google Scholar]
- 34.Haacke EM, Cheng NY, House MJ, et al. Imaging iron stores in the brain using magnetic resonance imaging. Magn Reson Imaging. 2005;23:1–25. doi: 10.1016/j.mri.2004.10.001. [DOI] [PubMed] [Google Scholar]
- 35.Haacke EM, Ayaz M, Khan A, et al. Establishing a baseline phase behavior in magnetic resonance imaging to determine normal vs. abnormal iron content in the brain. J Magn Reson Imaging. 2007;26:256–264. doi: 10.1002/jmri.22987. [DOI] [PubMed] [Google Scholar]
- 36.Duyn J, van Gelderen P, Li TQ, de Zwart J, Koretsky AP, Fukunaga M. High-field MRI of brain cortical substructure based on signal phase. Proc Natl Acad Sci U S A. 2007;104:11796–11801. doi: 10.1073/pnas.0610821104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Haacke EM, Lai S, Yablonskiy DA, Lin W. In vivo validation of the BOLD mechanism: a review of signal changes in gradient echo functional MRI in the presence of flow. Intl J Imaging Sys Technol. 1995;6:153–163. [Google Scholar]
- 38.McDonald WI, Compston A, Edan G, et al. Recommended diagnostic criteria for multiple sclerosis: guidelines from the international panel on the diagnosis of multiple sclerosis. Ann Neurol. 2001;50:121–127. doi: 10.1002/ana.1032. [DOI] [PubMed] [Google Scholar]
- 39.Bielekova B, Martin R. Development of biomarkers in multiple sclerosis. Brain. 2004;127:1463–1478. doi: 10.1093/brain/awh176. [DOI] [PubMed] [Google Scholar]
- 40.Prineas JW, Kwon EE, Cho ES, et al. Immunopathology of secondary-progressive multiple sclerosis. Ann Neurol. 2001;50:646–657. doi: 10.1002/ana.1255. [DOI] [PubMed] [Google Scholar]
- 41.Lublin FD, Reingold SC. Defining the clinical course of multiple sclerosis: results of an international survey. National Multiple Sclerosis Society (USA) Advisory Committee on Clinical Trials of New Agents in Multiple Sclerosis. Neurology. 1996;46:907–911. doi: 10.1212/wnl.46.4.907. [DOI] [PubMed] [Google Scholar]