Abstract
We examined the gene expression and regulation of Type III interferon (IFN), IFN-λ, in human neuronal cells. Human neuronal cells expressed endogenous IFN-λ1 but not IFN-λ2/3. Upon the activation of Toll-like receptor (TLR)-3 expressed in the neuronal cells by polyriboinosinic polyribocytidylic acid (PolyI:C), both IFN-λ1 and IFN-λ2/3 expression was significantly induced. The activation of TLR-3 also exhibited antiviral activity against pseudotyped HIV-1 infection of the neuronal cells. Human neuronal cells also expressed functional IFN-λ receptor complex, interleukin-28 receptor α subunit (IL-28Rα) and IL-10Rβ, as evidenced by the observations that exogenous IFN-λ treatment inhibited pseudotyped HIV-1 infection of the neuronal cells and induced the expression of APOBEC3G/3F, the newly identified anti-HIV-1 cellular factors. These data provide direct and compelling evidence that there is intracellular expression and regulation of IFN-λ in human neuronal cells, which may have an important role in the innate neuronal protection against viral infections in the CNS.
Keywords: Interferon (IFN), Toll-like receptor (TLR), NT2-N, Neuron, Virus, HIV
Interferon lambda (IFN-λ), the newly discovered type III family of IFNs (Kotenko et al., 2003, Sheppard et al., 2003), comprises three structurally related cytokines, IFN-λ1, IFN-λ2 and IFN-λ3. A gene array experiment covering the whole human genome revealed that all the IFN-λinduced genes could also be activated by type I IFNs (Zhou et al., 2007). In addition, IFN-λ has recently been demonstrated to have a similar action as type II IFN, as certain chemokines elevated by IFN-λ are considered exclusively to be induced by IFN-γ in human peripheral blood mononuclear cells (Pekarek et al., 2007). IFN-λ gene expression has been identified mainly in antigen-presenting cells, such as monocyte-derived dendritic cells, plasmacytoid dendritic cells (Coccia et al., 2004, Siren et al., 2005), and in a number of cancer cell lines after Toll-like receptor (TLR) triggering or a panel of different virus infection (Uze and Monneron, 2007). TLRs are a family of pattern recognition receptors that mediate innate immune responses to stimuli from pathogens or endogenous signals (Aravalli et al., 2007). TLR has the ability to activate type I IFNs (IFN-alpha/beta), providing a crucial mechanism of antiviral defense (Colonna, 2007). Agonists of TLRs 3, 4, 7, 8, 9, which have the ability to induce type I IFN expression, also induce IFN-λ production in macrophages or plasmacytoid dendritic cells (Siren et al., 2005, Ank et al., 2006). These findings suggest that IFN-λ is likely to share strikingly similar patterns of signaling pathway with type I IFNs.
The biological functions of IFN-λs are dependent on their receptors composed of two chains, IL-28Rα that is specific for IFN-λ and IL-10Rβ that is shared among IL-10, IL-22 and IL-26 (Donnelly et al., 2004). IL-10Rβ is ubiquitously expressed, while IL-28Rα expression is cell-type dependent. IL-28Rα gene expression is not detected in several cell types, mainly fibroblastic and endothelial cells (Brand et al., 2005, Lasfar et al., 2006). By signaling through the heterodimeric IL-28Rα/IL-10Rβ complex, IFN-λ exerts its antiviral, antitumor and immunoregulatory activities (Lasfar et al., 2006, Uze and Monneron, 2007, Srinivas et al., 2008). It has been shown that IFN-λ-mediated antiviral activity is linked to its ability to activate type I IFN-stimulated gene factor 3 (ISGF3) and several antiviral genes (Kotenko et al., 2003, Doyle et al., 2006). In addition, IFN-λ has the ability to activate both janus kinases-signal transducers and activators of transcription (Jak-STAT) pathway (Kotenko et al., 2003, Sheppard et al., 2003, Dumoutier et al., 2004) and the mitogen-activated protein (MAP) kinases pathway (Zhou et al., 2007). Although the expression and regulatory mechanism of IFN-λ is now being unclosed, most of the studies have focused on immune cells. More recently, a bias towards action of IFN-λ on epithelial cells has been suggested in the mouse (Sommereyns et al., 2008). However, little is known about whether central nerve system (CNS), more specifically, human neuronal cell, expresses IFN-λ and their receptors. It also remains to be determined whether IFN-λ expression in human neuron relies on the TLR ligands as those inducing type I IFN expression in immune cells. In the present study, we examined whether human neuronal cells possess IFN-λ/receptor machinery. We also determined whether the activation of TLR-3 could induce IFN-λ expression and activate innate antiviral activity in human neuronal cells.
EXPERIMENTAL PROCEDURES
Reagents
Recombinant human IFN-λ1 and IFN-λ2 were purchased from PeproTech Inc. (Rocky Hill, NJ). Poly I:C was purchased from Invitrogen (San Diego, CA, USA). Anti-IL-10Rβ antibody was purchased from R&D Systems Inc. (Minneapolis, MN, USA).
Primary cells
Human brain tissues used were obtained from the National Neurological Research Specimen Bank (Los Angeles, CA). Primary neuronal cell cultures were prepared from 16 to 18-week-old human fetal brain tissues, as described previously (Hu et al., 2002). In brief, human fetal brain cortical tissue obtained under a protocol approved by the Human Subjects Research Committee of the University of Minnesota were dissociated, trypsinized and resuspended in DMEM containing 10% heat-inactivated FBS plus penicillin (100 U/mL) and streptomycin (100 mg/mL) and plated onto collagen-coated plates (1×106 cells/well in 12-well plates). On day 5, cell cultures were treated with uridine (33.6 mg/mL) and fluorodeoxyuridine (13.6 mg/mL), followed by replacement with DMEM with 10% FBS on day 6 and every 4 days thereafter. On day 12, the neuronal cell cultures consisting of about 80–90% neurons (stained by anti-NeuN or anti-MAP-2 antibodies), 10–15% astrocytes (stained by anti-GFAP antibody), and 2–5% microglial cells (stained by anti-CD68 antibody) were used for the experiments.
Cell lines
Human neuronal cells (NT2-N) were derived from differentiated Ntera-2clD/1 (NT2) cells (Andrews, 1984) and were cultured as described previously (Guo et al., 2003). In brief, NT2 cells were plated at a density of 2.3 × 106 per T75 flask and fed twice weekly with DMEM containing high glucose (Gibco, Grand Island, NY, USA) and 10% FBS (Hyclone, Iogan, UT, USA) with 100 U/ml penicillin plus 100 μg/ml streptomycin (Gibco) and 10−5M retinoic acid (RA) (Sigma-Aldrich, ST. Louis, MO, USA) for 5 weeks. The cells were then divided (1:4) and grown for an additional 48 h in identical media without RA. Neuronal cells growing above a monolayer of non-neuronal cells were dislodged with trypsin and plated at a density of 0.5 × 106 cells per well in a 24-well plate for this study. NT2-N neurons have morphologic features similar to primary human neurons, and have processes that differentiate into axons and dendrites (Andrews, 1984). NT2-N neurons also express cytoskeletal proteins, secretory markers, and surface markers, which are characteristics of neurons. They also express functional neuropeptides (Guillemain et al., 2000) and N-methyl-D-aspartate (NMDA) as well as non-NMDA glutamate receptors (Younkin et al., 1993). Undifferentiated human NT2 cells grafted into mouse brain differentiated into neuronal and glial cells (Ferrari et al., 2000).
The human neuroblastoma cell lines (CHP212 and SH-SY5Y) were purchased from American Type Tissue Culture (ATCC; Manassas, VA). CHP212 cells were cultured in Eagle’s MEM-Ham F12 (1:1) media containing 10% FBS, 0.1 mM non-essential amino acid and 1.0 mM sodium pyruvate. SH-SY5Y cells were propagated in DMEM with high glucose containing 10% FBS.
Pseudotyped HIV-1 inhibiton Assay
HIV-1 virions pseudotyped with the envelope from amphotropic murine leukemia virus (MLV) (HIV-1 entry receptor independent) were used to study the impact of PolyI:C and IFN- λ on viral replication. The plasmid encoding MLV Env gene was provided by John Moore (Aaron Diamond AIDS Research Center, New York, NY). The Env-deleted luciferase reporter gene containing plasmid (PNL-Luc-E-R+) was cotransfected into 293T cells along with the plasmids encoding the MLV Env genes as described (Connor et al., 1995, Lai et al., 2001). Pseudotyped HIV-1 was prepared as described (Wang et al., 2006). NT2-N cells plated in 24-well plates (3 × 105 cells/well) were pretreated with PolyI:C (0.1, 1, 10 μg/ml) or IFN-λ1 (100 ng/ml) or IFN-λ2 (100 ng/ml) for 24 h. The cells were then infected with the pseudotyped HIV-1 (p24 20ng/ml) for 24 h. After wash with plain DMEM, IFN-λ1 (100 ng/ml) and IFN-λ2 (100 ng/ml) was added in the culture. The cells were lysed in 100 μl of 1x reporter lysis buffer (Promega Corp.) 72 h postinfection. Lysate (50 μl) was mixed with 100 μl of luciferase substrate (Promega Corp., Madison, WI) and luciferase activity was then determined in a TD-20/20 Luminometer (Turner Designs, Sunnyvale, CA).
RNA extraction and RT-PCR
Total RNA was extracted from cell culture with Tri-Reagent (Molecular Research Center, Cincinnati, OH), following the manufacturer’s instructions. The real time RT-PCR for this study was described previously (Guo et al., 2004). The primer sequences for IFN-λ1, λ2/3, TLR-3, IL-28Rα, IL-10Rβ, APOBEC3G and APOBEC3F were published previously (Liu et al., 2002, Coccia et al., 2004, Chen et al., 2006, Ge et al., 2006, Tanaka et al., 2006). The oligonucleotide primers were synthesized by Integrated DNA Technologies Inc. (Coralville, IA). PCR was performed with the Brilliant SYBR Green Master Mix (Bio-Rad Laboratories, Hercules, CA). All values were expressed as the increase relative to the expression of GAPDH mRNA. The mean value of the replicates for each sample was calculated and expressed as the cycle threshold (CT; cycle number at which each PCR reaches a predetermined fluorescence threshold, set within the linear range of all reactions). The levels of gene expression were then calculated as the difference between the CT of the sample for the target gene and the mean CT of that sample for the endogenous control. In some cases, the RT-PCR-amplified products were analyzed by 2% ultra pure agarose gel electrophoresis.
Data analysis
Where appropriate, data were expressed as mean ± SD. For comparison of the mean of the two groups, statistical significance was measured by Student’s t test. Calculations were performed with Stata Statistical Software (StataCorp, College Station, TX). Statistical significance was defined as P<0.05.
RESULTS
IFN-λ and IFN-λ receptor expression in human neuronal cells
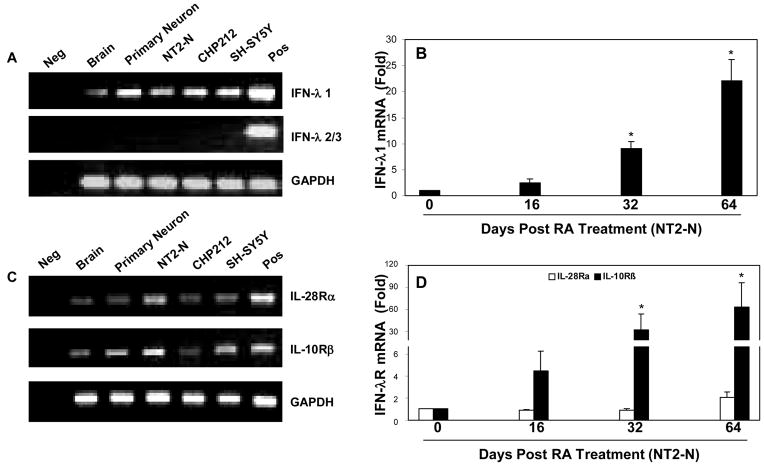
In order to determine whether human neuronal cells express IFN-λ and IFN-λ receptor, we first examined endogenous IFN-λ mRNA expression in human brain tissue and a set of human neuronal cells, including primary human neurons, NT2-N neurons and neuroblastoma cell lines (CHP212, and SH-SY5Y). Because human IFN-λ2 and IFN-λ3 genes are almost identical, sharing 96% amino acid identity (Sheppard et al., 2003), we use one pair of the specific primers for both IFN-λ2 and IFN-λ3. Human brain, primary neurons, NT2-N and neuronal cell lines (CHP212 and SH-SY5Y) expressed IFN-λ1 mRNA. However, IFN-λ2/3 mRNA was not detectable in these cells (Fig. 1A). Since fully differentiated NT2-N cells are considered as a model system for neuronal differentiation (Hohjoh and Fukushima, 2007), we also examined IFN-λ expression during the course of NT2-N differentiation. There is a gradual and steady increase in the levels of IFN-λ1 (Fig. 1B), while IFN-λ2/3 mRNA was not detected during the course of the differentiation (data not shown). Since the biological functions of IFN-λ are dependent on their receptors: IL-28Rα and IL-10Rβ, we also examined the expression of IFN-λ receptors in human brain tissue and neuronal cells. Human brain, primary neuronal cells and the neuronal cell lines expressed mRNA for both IL-28Rα and IL-10Rβ (Fig. 1C). Moreover, we observed a gradual and steady increase in the levels of IL-28Rα and IL-10Rβ during the course of neuronal differentiation (Fig. 1D). The specificity of the PCR-amplified IFN-λ and IFN-λ receptor genes was confirmed by the sequencing analysis (data not shown).
Fig. 1. Expression of IFN-λ and IFN-λ receptors (IL-28Rα and IL-10Rβ) in human brain tissues and neuronal cells.
A+C: IFN-λ and IFN-λ receptor expression in brain tissues and neuronal cells. RNA isolated from human brain tissues, primary neurons, NT2-N, CHP212 and SH-SY5Y cells was subjected to the real-time RT-PCR with the specific primers for IFN-λ1, λ2/3, IL-28Rα, IL-10Rβ and the internal control GAPDH. Reverse transcription product without template was used as a negative control (Neg). PolyI:C treated human macrophages and hepatocytes were used as a positive control (Pos) for A and C, respectively. The RT-PCR-amplified products were analyzed by 2% agarose electrophoresis. B+D: IFN-λ1 and IFN-λ receptor (IL-28Rα, IL-10Rβ) expression during the course of differentiation of NT2 to NT2-N cells. Undifferentiated NT2 cells (day 0) were cultured in vitro for 64 days, and RNA was collected at indicated time points for the real time RT-PCR analysis. The expression of IFN-λ1, IL-28Rα, IL-10Rβ mRNA was indicated as the increase in induction (n-fold) relative to day 0 NT2 cells, which is normalized to GAPDH levels. Value is mean ± SEM of three different experiments (*, P<0.05).
TLR-3 expression in human neuronal cells
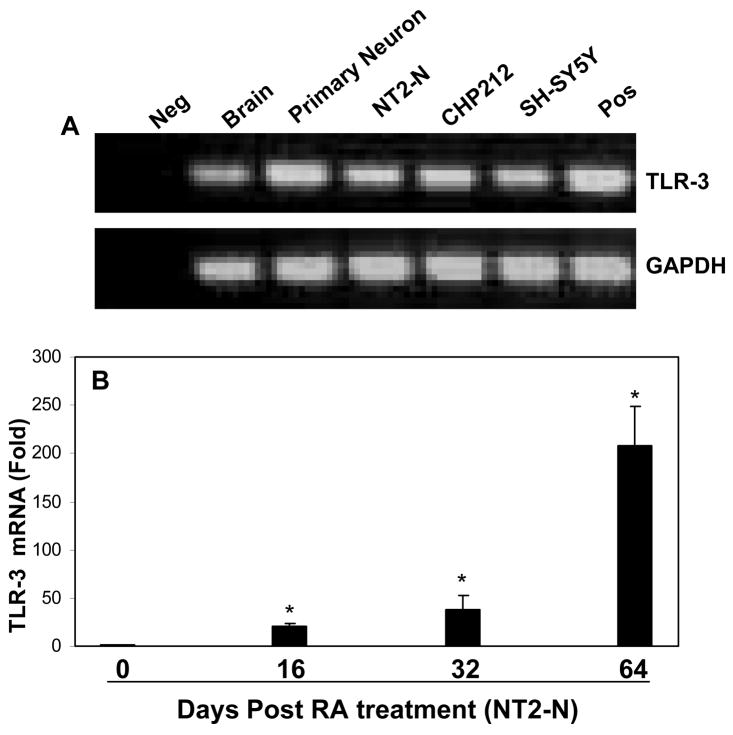
TLRs are now considered as the primary sensors for the host cells to generate innate immune response (Akira et al., 2006). We investigated TLR-3 expression in human brain tissues and neuronal cells. As shown in Fig. 2A, human brain tissues, primary neurons, NT2-N and neuronal cell lines (CHP212 and SH-SY5Y) expressed TLR-3 mRNA. The observation that human neuronal cells express TLR-3 was further confirmed by the finding that there was a steady increase in TLR-3 expression during NT2-N neuronal differentiation (Fig. 2B).
Fig. 2. Expression of TLR-3 in human brain tissues and neuronal cells.
A: TLR-3 expression. RNA isolated from human brain tissues, primary neurons, NT2-N, CHP212 and SH-SY5Y cells was subjected to the real-time RT-PCR with the specific primers for TLR-3, and the internal control GAPDH. Reverse transcription product without template was used as a negative control (Neg). PolyI:C treated human macrophages were used as a positive control (Pos). The RT-PCR-amplified products were analyzed by 2% agarose electrophoresis. B: TLR-3 expression during the course of differentiation of NT2 to NT2-N cells. The expression of TLR-3 was indicated as the increase in induction (n-fold) relative to day 0 NT2 cells, which is normalized to GAPDH levels. Value is mean ± SEM of three different experiments (*, P<0.05).
TLR- 3 activation induced IFN-λ expression
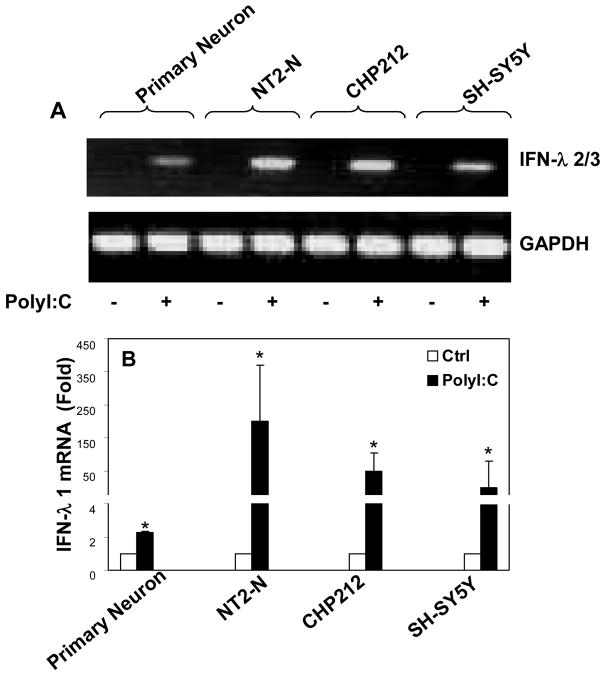
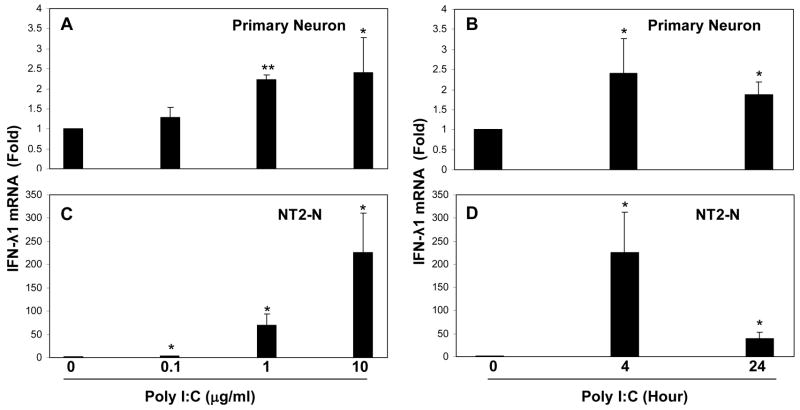
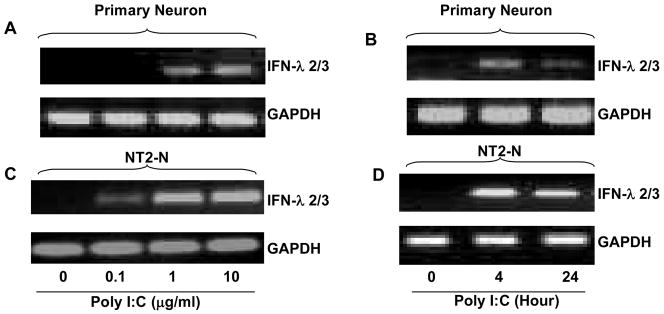
Since TLR-3 plays an essential role in initiating intracellular type I IFN-mediated innate immunity against virus infections, we investigated whether the activation of TLR-3 could induce IFN-λ expression in human neuronal cells. We treated with PolyI:C, the ligand to TLR-3. The neuronal cells (primary human neurons, NT2-N, CHP212 and SH-SY5Y) that had undetectable levels of IFN-λ2/3 became positive for IFN-λ2/3 after PolyI:C treatment (Fig. 3A). The cells treated with PolyI:C expressed significantly higher levels of IFN-λ1 than untreated cells (Fig. 3B). This PolyI:C-mediated induction of IFN-λ1 was dose- and time- dependent in both primary neurons (Fig. 4A+4B) and NT2-N cells (Fig. 4C+4D). Similarly, the effect of PolyI:C on IFN-λ2/3 expression was dose-and time-dependent in primary human neurons (Fig. 5A+5B) and NT2-N cells (Fig. 5C+5D). Because the control (untreated) cell cultures had undetectable levels of IFN-λ2/3, the data presented in Fig. 5 could not be quantitatively analyzed. This PolyI:C-mediated regulation of IFN-λ was specific, as other TLR ligands (LPS, R837, ssRNA and CpG) had little impact on both IFN-λ1 and IFN-λ2/3 expression (data not shown).
Fig. 3. Effect of TLR-3 activation on IFN-λ expression.
Primary neurons, NT2-N, CHP212, SH-SY5Y cells were treated with or without PolyI:C (10 μg/ml) for 4 h. Total cellular RNA was extracted for the real time RT-PCR. Data is expressed as the PCR-amplified products analyzed by 2% agarose electrophoresis (A) or the increase of IFN- λ1 mRNA in induction (n-fold) relative to the control (B). Value is mean ± SEM of three different experiments (*, P<0.05).
Fig. 4. Effect of PolyI:C on IFN-λ1 expression.
Primary neurons (A+B) and NT2-N cells (C+D) were treated with PolyI:C at indicated doses for 4 h, or treated with polyI:C at 10 μg/ml for the indicated timepoints posttreatment. Total cellular RNA was extracted for the real time RT-PCR. Data is expressed as the increase of IFN- λ1 mRNA in induction (n-fold) relative to the control. Value is mean ± SEM of three different experiments (*, P<0.05).
Fig. 5. Effect of PolyI:C on IFN-λ2/3 expression.
Primary neurons (A+B) and NT2-N cells (C+D) were treated with PolyI:C at indicated doses for 4 h, or treated with polyI:C at 10 μg/ml for the indicated timepoints posttreatment. Total cellular RNA was extracted for the real time RT-PCR. The PCR-amplified products of IFN-λ2/3 was analyzed by 2% agarose electrophoresis. Similar results were obtained in three separate experiments.
TLR-3 activation inhibited pseudotyped HIV-1 replication
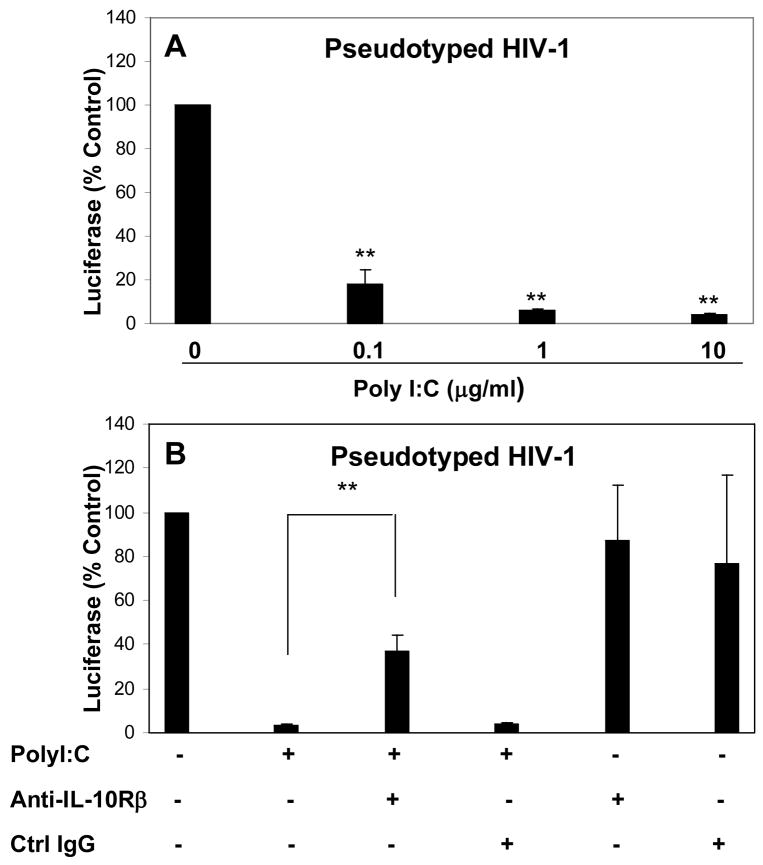
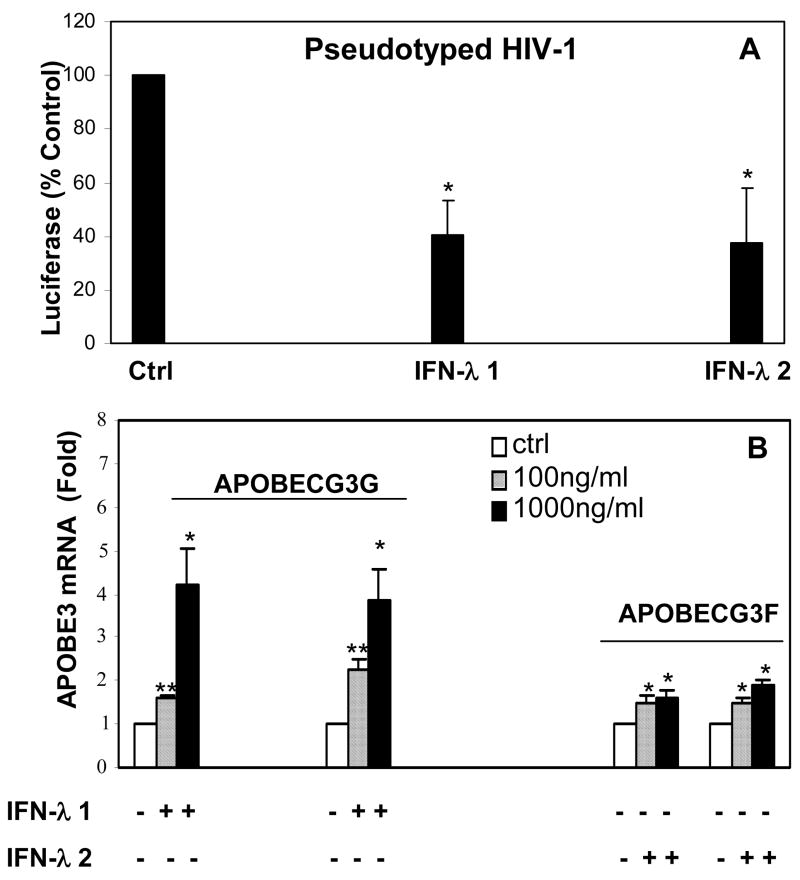
We next examined whether TLR-3 activation can inhibit pseudotyped HIV-1 replication in human neuronal cells. We showed that PolyI:C treatment of NT2-N cells significantly inhibited pseduotyped HIV-1 replication (Fig. 6A). This inhibitory effect of PolyI:C is dose-dependent (Fig. 6A). To investigate whether the induction of endogenous IFN-λ plays a role in TLR3-mediated antiviral activity against pseudotyped HIV-1, we pretreated NT2-N cells with antibody to IL-10Rβ, the receptor for all three IFN-λs. As shown in Fig. 6B, antibody to IL-10Rβ significantly neutralized the antiviral activity of PolyI:C, while control IgG had little effect. IL-10Rβ antibody or control IgG alone had no effect on pseudotyped HIV-1 replication (Fig. 6B). To further confirm IFN-λ has antiviral activity against pseudotyped HIV-1 replication in human neuronal cells, we treated NT2-N cells with recombinant IFN-λ. As demonstrated in Fig. 7A, both IFN-λ1 and IFN-λ2 had the ability to inhibit pseudotyped HIV-1 replication in NT2-N cells. In parallel with their antiviral activity, both IFN-λ1 and IFN-λ2 induced the expression of APOBEC3G and APOBEC3F, the newly identified anti-HIV-1 cellular factors, in the neuronal cells (Fig. 7B).
Fig. 6. Effect of TLR-3 activation on pseudotyped HIV-1 replication.
A. PolyI:C inhibited pseudotyped HIV-1 replication. NT2-N cells were pretreated with PolyI:C at indicated concentration for 24 h prior to infection with pseudotyped HIV-1. B. Effect of antibody to IFN-λ receptor on PolyI:C-mediated anti-pseudotyped HIV-1 activity. NT2-N cells were incubated with or without PolyI:C (10 μg/ml) and/or antibody to IL-10Rβ (5 μg/ml) for 24 h prior to pseudotyped HIV-1 infection. Goat IgG was used as the control. Luciferase activity was measured in the cell lysates 72 h post infection.
Fig. 7. Effect of recombinant IFN-λ on pseudotyped HIV-1 replication and APOBEC3G/3F expression.
A. Effect of IFN-λ on pseduotyped HIV-1 replication. NT2-N cells were pretreated with IFN-λ1 (100 ng/ml), IFN-λ2 (100 ng/ml) for 24 h prior to infection with pseudotyped HIV-1. IFN-λ1 and IFN-λ2 were maintained in the culture post HIV-1 infection. Luciferase activity was measured in the cell lysates 72 h post infection. B. Effect of IFN-λ on APOBEC3G/3F expression. NT2-N cells were treated with IFN-λ1 (100 ng/ml) or IFN-λ2 (100 ng/ml) for 12 h, and total cellular RNA was extracted from cell cultures for the real-time PCR assay. Data is expressed as the increase in induction (n-fold) relative to untreated control and is normalized to GAPDH levels. Value is mean ± SEM of three different experiments (*, P<0.05; **, P<0.01).
DISSCUSSION
Since type III IFN was discovered, the vast majority of research has focused on its expression and biological activity in the immune system, the primary source for the production of cytokines, including IFNs. Some of these cytokines not only have immunoregulatory action but also exhibit neuromodulatory activities (Ernstrom and Soder, 1975, Blalock, 1989, Besedovsky and del Rey, 2002). Recently, CNS cells have been shown to possess their own strategies toward a neuroprotective and less destructive function by expressing these cytokines and intracellular antiviral proteins (Paul et al., 2007). The ability to mount an effective innate immune response in the CNS is likely to be critical in eliminating pathogens, which is vital for the CNS protection (Carpentier et al., 2008). It has been shown that Type I IFNs could be synthesized and released by glial and neuronal cells (Patterson and Nawa, 1993, Wan et al., 2008). We (Wan et al., 2008) and others have reported that human neuronal cells express type I IFNs. In this study, we have for the first time demonstrated there is the expression of type III IFN transcripts in human neuronal cells. This observation, however, disagrees with the study showing infection with Lactate dehydrogenase-elevating virus (LDV) resulted in an absent or very low rate of IFN-λ2/3 expression in brain of mice (Sommereyns et al., 2008). This discrepancy could be due to species-specific difference. Lasfar et al showed that IFN-λ genomes in human and mouse are different. There are three genes encoding human IFN-λ, however there are only two intact mouse genes exsited, representing mouse IFN-λ2 and IFN- λ3 gene orthologues. The mouse IFN-λ1 gene orthologue contains a stop codon in the first exon and is therefore predicted not to encode an intact protein (Lasfar et al., 2006). Interestingly, human neuronal cells constitutively express IFN-λ1, but not IFN-λ2/3 (Fig. 1A). The differential expression pattern of IFN-λ1 and IFN-λ2/3 was also reported by the other group, showing that asthmatic adults had increased sputum IFN-λ2/3 mRNA but similar IFN-λ1 mRNA expression in comparison with healthy subjects (Dominique et al., 2008). The distinct expression of IFN-λ family members in the neuronal cells is also supported by a recent study (Osterlund et al., 2007) demonstrating that similar to IFN-β, IFN-λ1 gene is regulated by virus-activated IRF-3 and IRF-7, whereas IFN-λ2/3 expression is mainly controlled by IRF-7, which resembles IFN-α gene regulation.
IFN-λ expression is tightly controlled and takes place after viral infection (Kotenko et al., 2003, Ank et al., 2006). IFN-λ expression can be also stimulated by the activation of TLRs (Yang et al., 2005). For example, TLR-9 ligand was demonstrated to induce IFN-λ expression in dendritic cells (Coccia et al., 2004). TLR-3, 4, 7, 8 ligands enhanced IFN-λ expression in macrophages (Siren et al., 2005). In this study, we showed that among the TLR ligands tested (PolyI:C, LPS, R837, ssRNA and CpG), only PolyI:C (TLR-3 ligand) significantly enhanced the expression of all the three members of IFN-λ family in the neuronal cells. This PolyI:C-induced IFN-λ expression varied in primary human neurons and NT2-N cells, as the levels of induced IFN- λ expression in NT-2N cells were much higher than those in primary neurons. Although it is unclear what mechanisms are involved in this differential response of primary neurons and NT2-N cells to PolyI:C treatment, it is possible that the restricted response of primary human neurons to PolyI:C stimulation has biological significance in protecting neurons, as over expression of IFN-λ may have detrimental effect on neurons. Further studies are required in order to determine cellular factor(s) that contribute to this restricted response to TLR-3 activation in primary human neurons. Nevertheless, this distinct TLR-3 ligand-mediated IFN-λ expression in human neuronal cells suggests TLR-3 may have a unique role in regulation of biological function of neuronal cells. The modulation of innate immunity by TLR ligands is likely to be relative to the presence of TLR expression in host cells. To date, it has been demonstrated TLR expression is widespread and varies among cell types examined (Paul et al., 2007). The CNS, as a vital organ, expresses all the known TLRs, mainly involving glial cells (microglia, astrocytes and oligodentrocytes) (Olson and Miller, 2004, Jack et al., 2005). However, conflicting data regarding TLR expression in neurons were recently reported (Lehnardt et al., 2003, Ma et al., 2006). Our finding that primary human neuron cells, as well as human neuronal cell lines, constitutively expressed TLR-3 at transcriptional levels (Fig. 2) supports the observation that NT2-N cells expressed TLR-3 at both mRNA and protein levels (Prehaud et al., 2005).
TLR-3 responds to viral dsRNA and induces type I IFN expression, thus playing a key role in antiviral immune responses. TLR-3-mediated antiviral activity in the CNS is also highlighted by a recent report showing TLR-3-deficient patients appear to be specifically prone to herpes simplex virus 1-induced encephalitis (Zhang et al., 2007). In this study, we provided direct experimental evidence that activation of TLR-3 by PolyI:C, an artificial mimic of viral RNA, inhibited pseudotyped HIV-1 replication in human neuronal cells, suggesting TLR-3 signaling has a role in neuronal protection. This TLR-3-mediated antiviral activity in human neuronal cells is partially mediated by the induction of endogenous IFN-λ, which was evidenced by the observation that antibody to IFN-λ receptor could compromise neutralize the inhibitory effect of PolyI:C on pseudotyped HIV-1 replication. The anti-HIV ability of IFN-λ in human neuronal cells was also confirmed by our finding that human recombinant IFN-λ, when added to NT2-N cell cultures, inhibits pseudotyped HIV-1 infection. The antiviral ability of intracellular IFN-λ system is also demonstrated by a recent report that the strong antiviral activity evoked by treatment of mice with TLR-3 ligand was significantly reduced in IFN-λ receptor knockout mice (Ank et al., 2008).
IFN-λ receptor complex was composed of two subunits: IL-28Rα and IL10Rβ. The expression of both IL-28Rα and IFN-10Rβ was detected in the human brain specimens, neuronal cells (primary neurons, NT2-N) and neuronal cell lines (CHP212 and SH-SY5Y) (Fig. 1C). This observation is highly significant, as the coexpression of both IL-28Rα and IL-10Rβ is essential for the biological functions of IFN-λ. It has been reported that IL-10Rβ alone is not efficient to render cells responsive to IFN-λ. Similarly, IL-28Rα alone has little effect (Kotenko et al., 2003). IFN-λ functionally resembles type I IFNs, inducing antiviral protection in vitro (Kotenko et al., 2003, Sheppard et al., 2003, Robek et al., 2005) as well as in vivo (Ank et al., 2006). Our finding that IFN-λ1 and IFN-λ2 had the ability to inhibit pseudotyped HIV-1 replication in human neuronal cells supports the concept that IFN-λ is indeed involved in host innate immunity against viral infection. Although the precise mechanisms by which IFN-λ exerts their effect on HIV-1 replication remained to be determined, our observations that IFN-λ had the ability to induce intracellular expression of anti-HIV-1 factors (APOBEC3G/3F) provide a sound explanation for the IFN-λ action (Fig. 7).
CONCLUSION
Taken together, given the essential functions of IFNs in the host cell-mediated innate immunity, the finding that human neuronal cells possess IFN-λ-mediated intracellular antiviral immunity is highly significant. Although the precise mechanisms underlying the regulation of IFN-λ and antiviral activity of IFN-λ in human neuronal cells remain to be determined, the induction of antiviral IFN-λ by the TLR-3 activation in human neuronal cells, indicates that TLR-3/IFN-λ system may have a crucial role in innate neuronal protection against viral infections in the CNS. Further studies are needed to determine the cellular and molecular mechanisms by which IFN-λ is regulated by viral and/or other cellular factors in the CNS. In addition, it is of a great interest to determine whether IFN-λ has other not yet known biological functions in the CNS.
Acknowledgments
This work was supported by the National Institutes of Health grants DA025477, DA12815 and DA022177 (to WZH) and the Foerderer Fund from the Children’s Hospital of Philadelphia. Lin Zhou is the Scholarship recipient of the China Scholarship Council.
Abbreviation
- APOBEC3G
apolipoprotein B mRNA-editing enzyme, catalytic polypeptide-like 3G
- APOBEC3F
apolipoprotein B mRNA-editing enzyme, catalytic polypeptide-like 3F
- CNS
central nerve system
- GAPDH
glyceraldehydes-3-phosphate dehydrogenase
- HIV
human immunodeficiency virus
- IFN
interferon
- IL-10Rβ
interleukin-10 receptor β subunit
- IL-28Rα
interleukin-28 receptor α subunit
- PolyI
C, polyriboinosinic polyribocytidylic acid
- TLR
toll-like receptor
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Akira S, Uematsu S, Takeuchi O. Pathogen recognition and innate immunity. Cell. 2006;124:783–801. doi: 10.1016/j.cell.2006.02.015. [DOI] [PubMed] [Google Scholar]
- Andrews PW. Retinoic acid induces neuronal differentiation of a cloned human embryonal carcinoma cell line in vitro. Dev Biol. 1984;103:285–293. doi: 10.1016/0012-1606(84)90316-6. [DOI] [PubMed] [Google Scholar]
- Ank N, Iversen MB, Bartholdy C, Staeheli P, Hartmann R, Jensen UB, Dagnaes-Hansen F, Thomsen AR, Chen Z, Haugen H, Klucher K, Paludan SR. An important role for type III Interferon (IFN-lambda/IL-28) in TLR-induced antiviral activity. J Immunol. 2008;180:2474–2485. doi: 10.4049/jimmunol.180.4.2474. [DOI] [PubMed] [Google Scholar]
- Ank N, West H, Paludan SR. IFN-lambda: novel antiviral cytokines. J Interferon Cytokine Res. 2006;26:373–379. doi: 10.1089/jir.2006.26.373. [DOI] [PubMed] [Google Scholar]
- Aravalli RN, Peterson PK, Lokensgard JR. Toll-like receptors in defense and damage of the central nervous system. J Neuroimmune Pharmacol. 2007;2:297–312. doi: 10.1007/s11481-007-9071-5. [DOI] [PubMed] [Google Scholar]
- Besedovsky HO, del Rey A. Introduction: immune-neuroendocrine network. Front Horm Res. 2002;29:1–14. doi: 10.1159/000061055. [DOI] [PubMed] [Google Scholar]
- Blalock JE. A molecular basis for bidirectional communication between the immune and neuroendocrine systems. Physiol Rev. 1989;69:1–32. doi: 10.1152/physrev.1989.69.1.1. [DOI] [PubMed] [Google Scholar]
- Brand S, Beigel F, Olszak T, Zitzmann K, Eichhorst ST, Otte JM, Diebold J, Diepolder H, Adler B, Auernhammer CJ, Goke B, Dambacher J. IL-28A and IL-29 mediate antiproliferative and antiviral signals in intestinal epithelial cells and murine CMV infection increases colonic IL-28A expression. Am J Physiol Gastrointest Liver Physiol. 2005;289:G960–968. doi: 10.1152/ajpgi.00126.2005. [DOI] [PubMed] [Google Scholar]
- Carpentier PA, Duncan DS, Miller SD. Glial toll-like receptor signaling in central nervous system infection and autoimmunity. Brain Behav Immun. 2008;22:140–147. doi: 10.1016/j.bbi.2007.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen K, Huang J, Zhang C, Huang S, Nunnari G, Wang FX, Tong X, Gao L, Nikisher K, Zhang H. Alpha interferon potently enhances the anti-human immunodeficiency virus type 1 activity of APOBEC3G in resting primary CD4 T cells. J Virol. 2006;80:7645–7657. doi: 10.1128/JVI.00206-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coccia EM, Severa M, Giacomini E, Monneron D, Remoli ME, Julkunen I, Cella M, Lande R, Uze G. Viral infection and Toll-like receptor agonists induce a differential expression of type I and lambda interferons in human plasmacytoid and monocyte-derived dendritic cells. Eur J Immunol. 2004;34:796–805. doi: 10.1002/eji.200324610. [DOI] [PubMed] [Google Scholar]
- Colonna M. TLR pathways and IFN-regulatory factors: to each its own. Eur J Immunol. 2007;37:306–309. doi: 10.1002/eji.200637009. [DOI] [PubMed] [Google Scholar]
- Connor RI, Chen BK, Choe S, Landau NR. Vpr is required for efficient replication of human immunodeficiency virus type-1 in mononuclear phagocytes. Virology. 1995;206:935–944. doi: 10.1006/viro.1995.1016. [DOI] [PubMed] [Google Scholar]
- Dominique MAB, Decraene A, Dilissen E, Meyts I, De Boeck K, Dupont LJ, Ceuppens JL. Type III IFN-lambda mRNA expression in sputum of adult and school-aged asthmatics. Clin Exp Allergy. 2008 doi: 10.1111/j.1365-2222.2008.03045.x. [DOI] [PubMed] [Google Scholar]
- Donnelly RP, Sheikh F, Kotenko SV, Dickensheets H. The expanded family of class II cytokines that share the IL-10 receptor-2 (IL-10R2) chain. J Leukoc Biol. 2004;76:314–321. doi: 10.1189/jlb.0204117. [DOI] [PubMed] [Google Scholar]
- Doyle SE, Schreckhise H, Khuu-Duong K, Henderson K, Rosler R, Storey H, Yao L, Liu H, Barahmand-pour F, Sivakumar P, Chan C, Birks C, Foster D, Clegg CH, Wietzke-Braun P, Mihm S, Klucher KM. Interleukin-29 uses a type 1 interferon-like program to promote antiviral responses in human hepatocytes. Hepatology. 2006;44:896–906. doi: 10.1002/hep.21312. [DOI] [PubMed] [Google Scholar]
- Dumoutier L, Tounsi A, Michiels T, Sommereyns C, Kotenko SV, Renauld JC. Role of the interleukin (IL)-28 receptor tyrosine residues for antiviral and antiproliferative activity of IL-29/interferon-lambda 1: similarities with type I interferon signaling. J Biol Chem. 2004;279:32269–32274. doi: 10.1074/jbc.M404789200. [DOI] [PubMed] [Google Scholar]
- Ernstrom U, Soder O. Influence of adrenaline on the dissemination of antibody-producing cells from the spleen. Clin Exp Immunol. 1975;21:131–140. [PMC free article] [PubMed] [Google Scholar]
- Ferrari A, Ehler E, Nitsch RM, Gotz J. Immature human NT2 cells grafted into mouse brain differentiate into neuronal and glial cell types. FEBS Lett. 2000;486:121–125. doi: 10.1016/s0014-5793(00)02251-1. [DOI] [PubMed] [Google Scholar]
- Ge Y, Dombkowski AA, LaFiura KM, Tatman D, Yedidi RS, Stout ML, Buck SA, Massey G, Becton DL, Weinstein HJ, Ravindranath Y, Matherly LH, Taub JW. Differential gene expression, GATA1 target genes, and the chemotherapy sensitivity of Down syndrome megakaryocytic leukemia. Blood. 2006;107:1570–1581. doi: 10.1182/blood-2005-06-2219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillemain I, Alonso G, Patey G, Privat A, Chaudieu I. Human NT2 neurons express a large variety of neurotransmission phenotypes in vitro. J Comp Neurol. 2000;422:380–395. doi: 10.1002/1096-9861(20000703)422:3<380::aid-cne5>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- Guo CJ, Douglas SD, Gao Z, Wolf BA, Grinspan J, Lai JP, Riedel E, Ho WZ. Interleukin-1beta upregulates functional expression of neurokinin-1 receptor (NK-1R) via NF-kappaB in astrocytes. Glia. 2004;48:259–266. doi: 10.1002/glia.20079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo CJ, Douglas SD, Lai JP, Pleasure DE, Li Y, Williams M, Bannerman P, Song L, Ho WZ. Interleukin-1beta stimulates macrophage inflammatory protein-1alpha and -1beta expression in human neuronal cells (NT2-N) J Neurochem. 2003;84:997–1005. doi: 10.1046/j.1471-4159.2003.01609.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hohjoh H, Fukushima T. Marked change in microRNA expression during neuronal differentiation of human teratocarcinoma NTera2D1 and mouse embryonal carcinoma P19 cells. Biochem Biophys Res Commun. 2007;362:360–367. doi: 10.1016/j.bbrc.2007.07.189. [DOI] [PubMed] [Google Scholar]
- Hu S, Sheng WS, Lokensgard JR, Peterson PK. Morphine induces apoptosis of human microglia and neurons. Neuropharmacology. 2002;42:829–836. doi: 10.1016/s0028-3908(02)00030-8. [DOI] [PubMed] [Google Scholar]
- Jack CS, Arbour N, Manusow J, Montgrain V, Blain M, McCrea E, Shapiro A, Antel JP. TLR signaling tailors innate immune responses in human microglia and astrocytes. J Immunol. 2005;175:4320–4330. doi: 10.4049/jimmunol.175.7.4320. [DOI] [PubMed] [Google Scholar]
- Kotenko SV, Gallagher G, Baurin VV, Lewis-Antes A, Shen M, Shah NK, Langer JA, Sheikh F, Dickensheets H, Donnelly RP. IFN-lambdas mediate antiviral protection through a distinct class II cytokine receptor complex. Nat Immunol. 2003;4:69–77. doi: 10.1038/ni875. [DOI] [PubMed] [Google Scholar]
- Lai JP, Ho WZ, Zhan GX, Yi Y, Collman RG, Douglas SD. Substance P antagonist (CP-96,345) inhibits HIV-1 replication in human mononuclear phagocytes. Proc Natl Acad Sci U S A. 2001;98:3970–3975. doi: 10.1073/pnas.071052298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lasfar A, Lewis-Antes A, Smirnov SV, Anantha S, Abushahba W, Tian B, Reuhl K, Dickensheets H, Sheikh F, Donnelly RP, Raveche E, Kotenko SV. Characterization of the mouse IFN-lambda ligand-receptor system: IFN-lambdas exhibit antitumor activity against B16 melanoma. Cancer Res. 2006;66:4468–4477. doi: 10.1158/0008-5472.CAN-05-3653. [DOI] [PubMed] [Google Scholar]
- Lehnardt S, Massillon L, Follett P, Jensen FE, Ratan R, Rosenberg PA, Volpe JJ, Vartanian T. Activation of innate immunity in the CNS triggers neurodegeneration through a Toll-like receptor 4-dependent pathway. Proc Natl Acad Sci U S A. 2003;100:8514–8519. doi: 10.1073/pnas.1432609100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S, Gallo DJ, Green AM, Williams DL, Gong X, Shapiro RA, Gambotto AA, Humphris EL, Vodovotz Y, Billiar TR. Role of toll-like receptors in changes in gene expression and NF-kappa B activation in mouse hepatocytes stimulated with lipopolysaccharide. Infect Immun. 2002;70:3433–3442. doi: 10.1128/IAI.70.7.3433-3442.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Y, Li J, Chiu I, Wang Y, Sloane JA, Lu J, Kosaras B, Sidman RL, Volpe JJ, Vartanian T. Toll-like receptor 8 functions as a negative regulator of neurite outgrowth and inducer of neuronal apoptosis. J Cell Biol. 2006;175:209–215. doi: 10.1083/jcb.200606016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson JK, Miller SD. Microglia initiate central nervous system innate and adaptive immune responses through multiple TLRs. J Immunol. 2004;173:3916–3924. doi: 10.4049/jimmunol.173.6.3916. [DOI] [PubMed] [Google Scholar]
- Osterlund PI, Pietila TE, Veckman V, Kotenko SV, Julkunen I. IFN regulatory factor family members differentially regulate the expression of type III IFN (IFN-lambda) genes. J Immunol. 2007;179:3434–3442. doi: 10.4049/jimmunol.179.6.3434. [DOI] [PubMed] [Google Scholar]
- Patterson PH, Nawa H. Neuronal differentiation factors/cytokines and synaptic plasticity. Cell. 1993;72(Suppl):123–137. doi: 10.1016/s0092-8674(05)80032-7. [DOI] [PubMed] [Google Scholar]
- Paul S, Ricour C, Sommereyns C, Sorgeloos F, Michiels T. Type I interferon response in the central nervous system. Biochimie. 2007;89:770–778. doi: 10.1016/j.biochi.2007.02.009. [DOI] [PubMed] [Google Scholar]
- Pekarek V, Srinivas S, Eskdale J, Gallagher G. Interferon lambda-1 (IFN-lambda1/IL-29) induces ELR(-) CXC chemokine mRNA in human peripheral blood mononuclear cells, in an IFN-gamma-independent manner. Genes Immun. 2007;8:177–180. doi: 10.1038/sj.gene.6364372. [DOI] [PubMed] [Google Scholar]
- Prehaud C, Megret F, Lafage M, Lafon M. Virus infection switches TLR-3-positive human neurons to become strong producers of beta interferon. J Virol. 2005;79:12893–12904. doi: 10.1128/JVI.79.20.12893-12904.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robek MD, Boyd BS, Chisari FV. Lambda interferon inhibits hepatitis B and C virus replication. J Virol. 2005;79:3851–3854. doi: 10.1128/JVI.79.6.3851-3854.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheppard P, Kindsvogel W, Xu W, Henderson K, Schlutsmeyer S, Whitmore TE, Kuestner R, Garrigues U, Birks C, Roraback J, Ostrander C, Dong D, Shin J, Presnell S, Fox B, Haldeman B, Cooper E, Taft D, Gilbert T, Grant FJ, Tackett M, Krivan W, McKnight G, Clegg C, Foster D, Klucher KM. IL-28, IL-29 and their class II cytokine receptor IL-28R. Nat Immunol. 2003;4:63–68. doi: 10.1038/ni873. [DOI] [PubMed] [Google Scholar]
- Siren J, Pirhonen J, Julkunen I, Matikainen S. IFN-alpha regulates TLR-dependent gene expression of IFN-alpha, IFN-beta, IL-28, and IL-29. J Immunol. 2005;174:1932–1937. doi: 10.4049/jimmunol.174.4.1932. [DOI] [PubMed] [Google Scholar]
- Sommereyns C, Paul S, Staeheli P, Michiels T. IFN-lambda (IFN-lambda) is expressed in a tissue-dependent fashion and primarily acts on epithelial cells in vivo. PLoS Pathog. 2008;4:e1000017. doi: 10.1371/journal.ppat.1000017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivas S, Dai J, Eskdale J, Gallagher GE, Megjugorac NJ, Gallagher G. Interferon-lambda1 (interleukin-29) preferentially down-regulates interleukin-13 over other T helper type 2 cytokine responses in vitro. Immunology. 2008 doi: 10.1111/j.1365-2567.2008.02862.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka Y, Marusawa H, Seno H, Matsumoto Y, Ueda Y, Kodama Y, Endo Y, Yamauchi J, Matsumoto T, Takaori-Kondo A, Ikai I, Chiba T. Anti-viral protein APOBEC3G is induced by interferon-alpha stimulation in human hepatocytes. Biochem Biophys Res Commun. 2006;341:314–319. doi: 10.1016/j.bbrc.2005.12.192. [DOI] [PubMed] [Google Scholar]
- Uze G, Monneron D. IL-28 and IL-29: newcomers to the interferon family. Biochimie. 2007;89:729–734. doi: 10.1016/j.biochi.2007.01.008. [DOI] [PubMed] [Google Scholar]
- Wan Q, Wang X, Wang YJ, Song L, Wang SH, Ho WZ. Morphine suppresses intracellular interferon-alpha expression in neuronal cells. J Neuroimmunol. 2008;199:1–9. doi: 10.1016/j.jneuroim.2008.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Douglas SD, Peng JS, Metzger DS, O’Brien CP, Zhang T, Ho WZ. Naltrexone inhibits alcohol-mediated enhancement of HIV infection of T lymphocytes. J Leukoc Biol. 2006;79:1166–1172. doi: 10.1189/jlb.1105642. [DOI] [PubMed] [Google Scholar]
- Yang K, Puel A, Zhang S, Eidenschenk C, Ku CL, Casrouge A, Picard C, von Bernuth H, Senechal B, Plancoulaine S, Al-Hajjar S, Al-Ghonaium A, Marodi L, Davidson D, Speert D, Roifman C, Garty BZ, Ozinsky A, Barrat FJ, Coffman RL, Miller RL, Li X, Lebon P, Rodriguez-Gallego C, Chapel H, Geissmann F, Jouanguy E, Casanova JL. Human TLR-7-, -8-, and -9-mediated induction of IFN-alpha/beta and -lambda Is IRAK-4 dependent and redundant for protective immunity to viruses. Immunity. 2005;23:465–478. doi: 10.1016/j.immuni.2005.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Younkin DP, Tang CM, Hardy M, Reddy UR, Shi QY, Pleasure SJ, Lee VM, Pleasure D. Inducible expression of neuronal glutamate receptor channels in the NT2 human cell line. Proc Natl Acad Sci U S A. 1993;90:2174–2178. doi: 10.1073/pnas.90.6.2174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang SY, Jouanguy E, Sancho-Shimizu V, von Bernuth H, Yang K, Abel L, Picard C, Puel A, Casanova JL. Human Toll-like receptor-dependent induction of interferons in protective immunity to viruses. Immunol Rev. 2007;220:225–236. doi: 10.1111/j.1600-065X.2007.00564.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Z, Hamming OJ, Ank N, Paludan SR, Nielsen AL, Hartmann R. Type III interferon (IFN) induces a type I IFN-like response in a restricted subset of cells through signaling pathways involving both the Jak-STAT pathway and the mitogen-activated protein kinases. J Virol. 2007;81:7749–7758. doi: 10.1128/JVI.02438-06. [DOI] [PMC free article] [PubMed] [Google Scholar]