Abstract
This is a descriptive study reporting normative salivary cortisol values and responsivity to a hospital clinic visit and IV procedure in children. The study presented is a sub-project of a primary research study that examined parents coaching their children in the use of distraction for children requiring an IV placement. One measure of child response in the primary study, salivary cortisol, was included to further our understanding of children’s physiologic response to stressful, painful stimuli. Salivary cortisol samples were obtained on 384 children, 4–10 years of age, on arrival to the clinic and 20 minutes after the IV insertion. Baseline samples were collected at home on a typical day for the child. Data from baseline samples were used to establish normative values between the hours of 8:00 am and 3:00 pm on a non-procedural day. Results demonstrated normative cortisol levels in children follow a pattern similar to the circadian pattern in adults, decreasing from early morning to mid-afternoon. Matched samples from control group children were used to evaluate group responsivity. Salivary cortisol levels on the baseline day were lower than levels obtained during the day of the procedure and tapered over time as expected (−8.7% + 6.7%; p=0.43). Cortisol levels on the clinic day were increased from baseline and increased further in response to IV placement (15.7% +6.7%; p=0.023). A location by time interaction was significant (p= 0.019). Findings demonstrate salivary cortisol is a useful measure of stress response that can be used to evaluate intervention effectiveness.
Keywords: salivary, cortisol, stress, children
Salivary cortisol has emerged as a potential biological measure of stress in research with children (Schmidt, 1997). Recent laboratory techniques have made it possible to detect very small concentrations of cortisol in plasma and saliva. Two advantages that salivary cortisol has over plasma measures are that samples can be collected through relatively non-invasive techniques and by non-health care professionals. Cortisol levels increase in response to stressful stimuli in children and adults (Bruce, Davis, & Gunnar, 2002; Gunnar, Bruce, & Hickman, 2001; Kudielka, Buske-Kirschbaum, Hellhammer & Kirschbaum, 2004). However, inconsistent cortisol responses to varied stimuli and interventions have led to concerns about the use of cortisol as a measure of stress response in children (Gunnar & Vazquez, 2001). Further research is needed to establish normative values and to investigate responsivity of salivary cortisol in children.
The physiological response to stress involves the interaction of two systems, the sympathetic adrenomedullary system (SAM) and the hypothalamic-pituitary-adrenal (HPA) axis. The locus ceruleus-norepinephrine (LC-NE) system is located in the brain stem and releases norepinephrine (NE) and epinephrine in response to stress. NE and epinephrine are released by the SAM system and quickly initiate the “fight/flight” response. The HPA axis is activated more slowly and produces a cascade of neurochemicals. When a stressful stimulus presents, the HPA axis stimulates the hypothalamus to increase corticotrophin-releasing hormone (CRH) which stimulates the pituitary to release adrenocorticotropic hormone (ACTH) into circulation. The adrenal cortex is stimulated by circulating ACTH to secrete the glucocorticoid hormone cortisol (Charmandari, Tsigos & Chrousos, 2005; King & Hegadoren, 2002). Cortisol, the end product of the HPA cascade, is hypothesized to increase short-term survival in a stressful situation by increasing gluconeogenesis, lipolysis, proteolysis and immunosuppression. Negative feedback mechanisms activated by cortisol suppress further release of CRH and ACTH (in the hypothalamus and pituitary). The stress response is generally considered adaptive to survival. However, responses to chronic or repeated stressors may overburden and deteriorate physiological systems leading to serious health consequences (Charmandari, et al.).
Cortisol has a predictable pattern of circadian fluctuations and demonstrates diurnal (light and dark) variations in both adults and children (Kiess et al., 1995; Kudielka et al., 2004). Levels generally peak 20 to 30 minutes after the time of awakening, decrease to half of the peak level by mid-afternoon and are lowest by midnight (King & Hegadoren, 2002; Schmidt, 1997). Cortisol is normally secreted in short bursts with 15–30 pulses over the course of the day (King & Hegadoren). Typical cortisol patterns are generally established in early infancy (Groschl, Rauh, & Dorr, 2003; Gunnar & White, 2001) but fluctuations continue to occur. In a review of studies, Gunnar and Donzella (2002) report morning and evening norms for infants up to 36 months of age. However normal time-specific ranges for salivary cortisol levels in older children are not well established (Groschl et al.). Published norms for children are generally reported either by commercial laboratories or on a study-by-study basis, with small or unknown sample sizes, and generalized values for morning, noon and evening hours (Bruce et al., 2002; Davis; Donzella, Krueger, & Gunnar, 1999; Gunnar, Morison, Chisholm, & Schuder, 2001).
Salivary cortisol has been used to measure infant’s and children’s stress response with inconsistent results. Gunnar and Donzella (2002) reviewed developmental studies of children from birth to five years of age in a variety of care and neglect situations. They found elevated cortisol levels were not consistently observed in response to stress. Several studies showed a flattening pattern and others showed lower cortisol levels and less responsivity than expected. A study of 55 children with leukemia, ages 3 to 18, having lumbar punctures reported variability in children’s salivary cortisol response (Chen, Craske, Katz, Schwartz, & Zeltzer, 2000). Investigators found children with lower levels of pain tolerance showed elevations in cortisol after experiencing the lumbar puncture. The ability to interpret this paper was limited since specific salivary cortisol results and types of analyses were not reported.
Cortisol has been used as an outcome measure by some investigators to evaluate the effectiveness of interventions. For example, an early intervention foster care program for maltreated pre-school children demonstrated changes in salivary cortisol levels that suggest decreased stress and physiological arousal over time in response to the intervention (Fisher, Gunnar, Chamberlain, & Reid, 2000). In another study, Pederson, (1995) compared imagery, parental presence, and control conditions during cardiac catheterization procedures in 24 children, ages 9–17 years. No statistical differences between groups on distress behaviors, self-report of pain, or salivary cortisol were found. Plasma cortisol has been used to evaluate surgical interventions for children. Bozkurt and others (2004) compared two pain treatments in 32 children (2–14 years of age) undergoing a major thoracotomy for a noncardiac surgery. They found neither thoracic epidural nor intravenous infusion of morphine provided suppression of cortisol response in the first 24 hours postoperative. Sakellaris and others (2004) compared pain treatments in inguinal hernia repairs of 45 children (6 to 10 years of age) in three randomly assigned groups (ropivicaine infiltration pre- and post-incision and no local anesthetic control). They found increases in plasma cortisol in all three groups but significantly only in the control group.
The seemingly inconsistent findings reported in the above studies may be related to differences in strength and durations of the stressor, the intervention, or a number of extraneous variables. Large variations in baseline levels and the response magnitude of cortisol levels have been observed within and between individuals (Bruce, et al., 2002; Gunnar, Brodersen, Krueger, & Rigatuso, 1996). Cortisol levels may vary in response to a number of factors including genetic differences, age/developmental stage/pubertal stage, gender, weight, temperament, coping style, social competency, and pain sensitivity (Bartels, de Geus, Kirschbaum, Sluyter, & Boomsma, 2003; Chen et al., 2000; Davis, et al., 1999; Dettling, Gunnar, & Donzella, 1999; Kiess et al., 1995; Kudielka, et al., 2004). Researchers need to consider these extraneous variables and control for them when possible.
In summary, existing research has identified salivary cortisol as a potentially valuable, objective biological marker of stress. However, lack of time-specific normative data and inconsistent findings demonstrate the need to further establish normative cortisol levels and responsivity of cortisol to stressors in children. This analysis was undertaken to address these gaps in research and determine the usefulness of salivary cortisol as an outcome measure for a study on distress management in children.
Purpose
The purposes of this study are to: 1) present normative salivary cortisol values obtained during a baseline day in children 4 to 10 years of age and 2) assess stress responsivity to an IV insertion by comparing salivary cortisol levels obtained on a baseline day with levels obtained on the day of the IV insertion.
Methods
Design
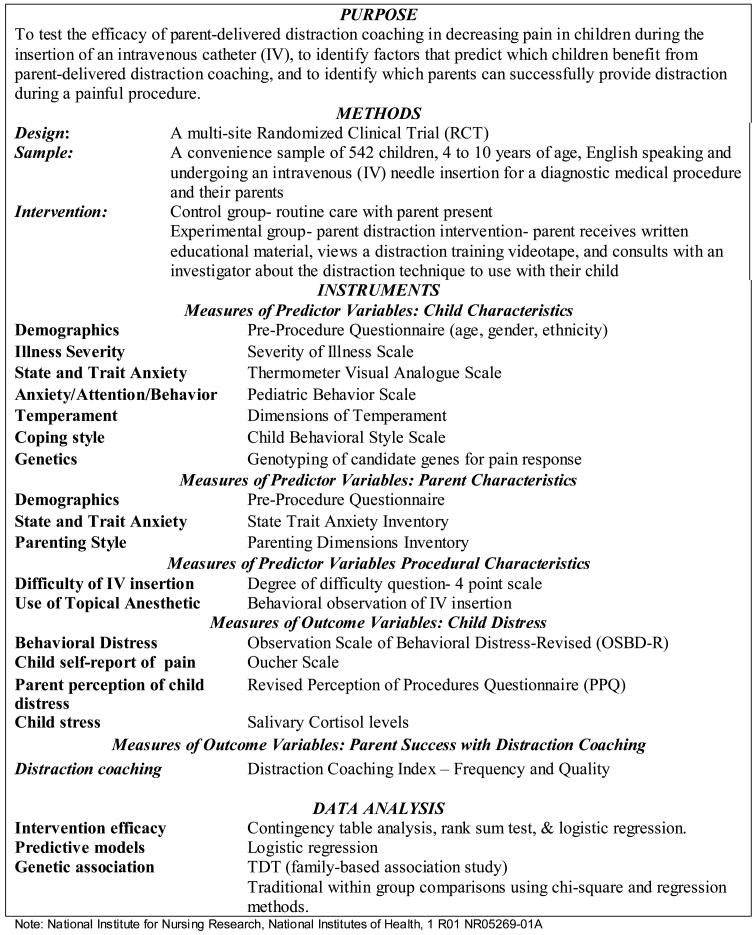
This is a descriptive study reporting normative salivary cortisol values and responsivity to a hospital clinic visit and IV procedure in children. The project presented is a sub-analysis of the primary research study that examined parents coaching their children in the use of distraction for children requiring an IV placement. The primary research study is detailed in Figure 1 to provide context to the current report. In order to use salivary cortisol as an outcome measure of distress and response to the study intervention, establishment of baseline values and response to the stimulus was needed. Baseline day results from children in both the intervention and control groups of the primary research study are used to describe children’s normative salivary cortisol levels, and results from participants in the control group only are used to document cortisol responsivity of children to a hospital clinic visit and an IV insertion. The impact of the intervention on cortisol is a separate issue that will be presented with results from the primary study in different report.
Figure 1.
PrimaryResearch Study: Predicting Children’s Responses to Distraction from Pain
Participants
Following Institutional Review Board approval, families were sent letters from the clinic director informing them of the study and inviting them to participate. Research Assistants met with families in the clinics to obtain informed consent from the parents and assent from children ages 7 and older and to collect data for the study.
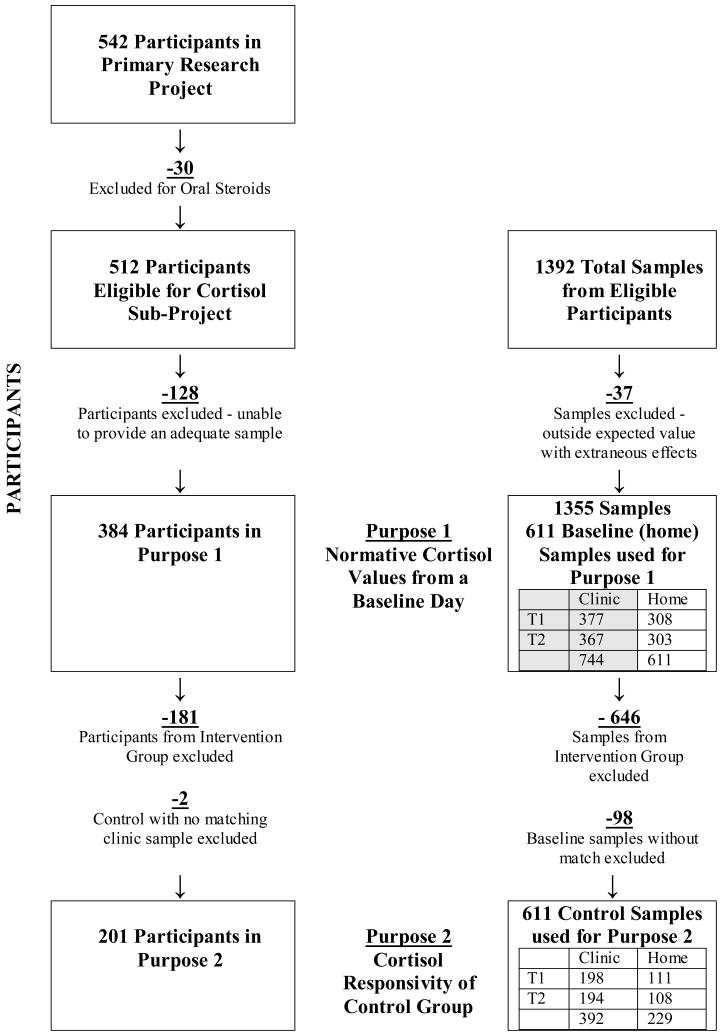
A convenience sample was identified from pediatric specialty clinics (including gastrointestinal, renal, urology and cardiology, but not oncology clinics) at three Midwest Childrens’ Hospitals. Figure 2 summarizes the number of participants in this study and sub-project. The primary research project included 542 children, 4 to 10 years of age, English speaking, and undergoing an IV insertion for a diagnostic medical procedure. Due to the direct effects of steroid medications on cortisol levels, 30 subjects on oral steroids in the 30 days prior to the procedure were excluded from this sub-analysis because there is less systemic absorption with topical and inhaled steroids (Hanrahan, McCarthy, Kleiber, Lutgendorf, & Tsalikian, 2006). Samples from children on these medications were not excluded. Factors such as child refusals, limited time in clinic and inadequate samples decreased the subject pool by 128 to 384 children providing useable cortisol samples. The 384 children participating in this sub-project had a mean age of 7.2 years of age. There were 190 (49%) boys and 194 (51%) girls. Ethnicity of the children was 81% white, 7% African American, 1% Asian, 2% Hispanic or Latino of any race and 9% mixed or unknown race. Children in the control and experimental groups did not differ significantly on these demographic variables.
Figure 2.
Summary of Participants and Cortisol Sample Inclusions and Exclusions
Procedure
Sample Collection
To assure valid results, specific strategies were developed for collecting samples in young children and controlling extraneous variables such as time of day, food intake, activity and medications that may impact salivary cortisol levels and are presented elsewhere (Hanrahan et al., 2006). The primary method for salivary cortisol sample collection was the “Chew and Spit” technique. In adults, salivettes are typically used to collect salivary cortisol. Salivettes are a commercially available collection device consisting of a 2-inch cotton dental roll in a plastic test-tube like container. Pilot testing of the salivettes revealed that some children found the salivettes unpalatable. Therefore, an alternative collection method was identified and adapted. Using the “Chew and Spit” technique, children were offered a piece of Original-flavored Trident® sugarless gum as a salivary stimulant. The gum was discarded and 3–5 minutes was allowed to clear the saliva. The children then spit or passively drool saliva through a short straw into a collection tube. The “Chew and Spit” technique was more child friendly and provided adequate quantities of saliva for analysis in most cases.
A total of four salivary cortisol samples were solicited from each child. The first sample, Time 1-clinic, was collected from the child on the day of the IV placement at the time of arrival at the clinic. This value represents the child’s level of stress at a hospital clinic visit prior to an IV insertion. The second sample, Time 2-clinic, was obtained approximately 20 minutes after the IV was inserted, allowing the HPA axis time to respond. This value represents the child’s stress response to the IV insertion. Two samples were collected at home to determine the child’s baseline cortisol levels. Time 1-baseline and Time 2-baseline were collected by the parents at least 24 hours after the IV placement. Parents were asked to choose a typical day when the child had no unusual stress experiences. To control for fluctuations based on the time of day, baseline samples were obtained at times that matched the times that the samples were obtained in the clinic. For example, if Time 1-clinic sample was obtained at 11:00 am and Time 2-clinic sample was obtained at 12:15 pm, then those same times were used to collect the baseline samples. Home samples were returned by standard mail to the primary research site. Cortisol concentrations remain stable for days if samples are mailed before freezing (Clements & Parker, 1998).
Procedures were developed and followed at each data collection site to ensure consistent data collection and handling. For example, to control for alterations in salivary cortisol related to food and drinks, study participants were asked to refrain from eating or drinking for 30 minutes prior to sample collection. A child who had eaten less than 30 minutes prior to sampling was asked to rinse his or her mouth and wait 3–5 minutes (long enough to re-establish the natural oral environment and a salivary pH of about 6.4 to 7.4) before providing a sample. In addition, to ensure consistent data collection procedures for the cortisol samples collected at home, parents were given written instructions explaining how to obtain accurate salivary samples, a questionnaire asking for information about the child’s activities, food intake, and medications on the baseline day, and information on how to mail the samples back to the investigators. Baseline salivary samples, and those collected in clinic, were stored at the primary site in a −20 degrees Celsius freezer until they were ready to be shipped to the laboratory for analysis.
Sample Inclusion
In addition to summarizing the number of participants included in the analyses, Figure 2 summarizes the number of cortisol samples obtained from the participants and used in the study’s analyses. A total of 1392 samples were provided. Prior to inclusion in analysis, values outside an expected range were reviewed. Since definitive norms for children are not well established, an expected range of values for children’s salivary cortisol was identified by reviewing the literature on studies with healthy children (often a control group) (Bruce et al., 2002; Davis et al., 1999; Groschl et al., 2003; Gunnar, Bruce et al., 2001; Gunnar, Morison et al., 2001; Gunnar & Vazquez, 2001; Kiess et al., 1995). Based on this review, a conservative expected range of values greater than .01mcg/dl (≈ 0 nmole/l) or less than 1.0 mcg/dl (≈ 28 nmole/l) was identified. Cortisol values outside the expected range were reviewed with clinical information. A total of 199 of 1394 samples (54 high and 145 low) were reviewed and 37 samples with values outside the expected range, directly attributable to extraneous variables, were excluded. Samples were excluded for inadequate sample (19), questionable collection techniques (11), or medication interactions (7). In summary, the total 384 participants in this subproject each provided at least one sample, for a total of 1355 useable cortisol samples. For the first purpose in this subproject, normative cortisol levels on a baseline day, 611 baseline samples from 384 children were used. For the second purpose, responsivity to the stress of coming to the clinic and having an IV insertion, only children in the control group were included (because the parent use of distraction coaching would potentially impact cortisol responsivity). Thus 181 children in the experimental group (646 cortisol samples) were excluded. In addition, 98 cortisol samples from children in the control group were not matched for time of collection between baseline and clinic and were excluded. This resulted in 201 children and 611 control cortisol samples available for analyses.
Quality Controls
Measures of cortisol assay reliability for the lab used and for the specific data were reviewed. The lab used in this study routinely monitors and reports intra-assay coefficient of variances (CVs) between 4.0 to 6.7% and inter-assay CVs between 7.1 to 9%. For this specific data set, duplicate analysis, and controls were used to further establish reliability of the data set. The overall CV for 1120 study sample duplicates was 8.18%. Control samples were included in nine of ten batches analyzed for the study. CVs between control samples in each batch were <0.1–8.71 and CVs between batches for control samples were 13.9% (between 4 batches) and 15.2% (between 5 batches).
Analysis of Data
To normalize the cortisol data distribution, the natural log transformation was applied to the cortisol values, and the log transformed data was used in the analyses. Estimates of the mean cortisol levels were calculated by back transformation of the mean of the log transformed values from the fitted linear mixed model to the original scale. Then analyses were done to address the two purposes of this subproject.
Purpose 1
To establish normative salivary cortisol values in children, baseline results from 384 children were used. The range of cortisol levels for children, 4 to 10 years of age, was calculated for the hours between 8:00 am and 3:00 pm. This was done by fitting a linear mixed model that included gender, age (4–6 or 7–10), and hour (8:00 am to 3:00 pm), and all two-factor interactions as the fixed effects. The effect of gender was not significant and was removed from the model. From this fitted model, the mean cortisol levels at each hour were computed.
Purpose 2
To determine salivary cortisol responsivity to a stressor, baseline and clinic results from children in only the control group of the larger multi-site research project were used. Children in the experimental group were excluded from this analysis because the parent use of distraction coaching would potentially impact cortisol responsivity. Changes in cortisol levels, both between the clinic and baseline day and Time 1 and 2, were analyzed using linear mixed model analysis for repeated measures. The fixed effects in the model were location (clinic or home), time (Pre-IV/Time 1 or after IV/Time 2), and the interaction of location-time. From this analysis, a significant interaction effect would indicate that the change in cortisol from Time 1 to Time 2 significantly differs between clinic and home, and that there is a difference in responsivity (clinic-home) between Time 1 and Time 2. Specific comparisons of interest, which included differences in clinic vs. baseline cortisol levels at each time point and change in cortisol levels pre-IV to post-IV and between Time 1 and Time 2 at baseline were performed using test of mean contrast, with the t-test statistic for the mean contrast computed from estimates of the fitted linear mixed model. For each set of tests, p-values were adjusted using Bonferroni’s method to account for the number of tests performed.
Results
Purpose 1 - Normative Values on Baseline Day
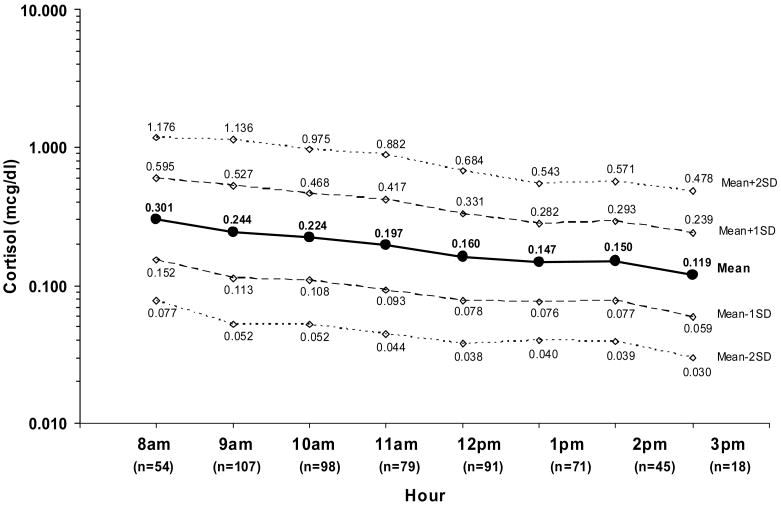
Normal cortisol levels for children based on gender, age and time of day were calculated using 611 samples collected on the baseline day from 384 children (190 males and 194 females). Only samples collected between 8:00 am and 3:00 pm, a total of 563 samples from 291 children, had adequate data points for analyses and were included in the normative values reported. The mean age of participants for the normative values was 7.2 (SD = 1.9) years, with 96 between 4–6 years, and 195 between 7–10 years old. No statistically significant differences were noted based on gender or age. Figure 3 shows cortisol levels for children 4 to 10 years of age from 8:00 am to 3:00 pm on a baseline day, including the mean level ±1 and ±2 standard deviations in the log scale. Little variability is seen in levels across the day. Early morning values are highest and taper to near half by noon. A slight but not significant increase is seen at 2:00 pm.
Figure 3.
Normative Salivary Cortisol: Established by Baseline Group Means
Note: Standard Deviation (SD) refers to SD in the log scale; cortisol values are reported in mcg/dL, n is the number of samples for each time.
Purpose 2- Cortisol Responsivity
As described previously, only data from the control group (n = 201) of the primary study were used to establish cortisol responsivity to the clinic visit and IV insertion. For the analyses of baseline day samples, only matched samples that were obtained within 30 minutes of the specified collection time were included for calculation of the means and standard deviations. There were 111 samples for Time 1 and 108 for Time 2. All clinic samples (392) were included to estimate the clinic day means and standard deviations. Group mean cortisol levels at each of the four data collection times are presented in Table 1.
Table 1.
Cortisol Responsivity: Group Means Pre and Post IV (Time 1–2, Clinic) and Clinic versus Baseline
| Clinic | Baseline (home) | Clinic vs. Baseline %change (clinic-baseline) | |
|---|---|---|---|
| Time 1-Before the IV | 0.243±0.012 | 0.182±0.012 | 33.6%±10.1% p=0.004 |
| Time 2-After the IV | 0.281±0.017 | 0.166±0.011 | 69.3%±14.1% p<0.0001 |
| %change (time2-time1) | 15.7%±6.7% p=0.023 | −8.7%±6.7% p=0.431 | p=0.019 |
Data comparing clinic day salivary cortisol levels indicated a 15.7%±6.7% increase in cortisol from pre-IV Time 1 to IV Time 2 (p=0.023). In contrast, for the baseline samples collected at the corresponding times as the clinic samples, a small decrease (−8.7%±6.7%; p=0.431) in cortisol was observed, which is expected due to the normal circadian pattern of decreasing cortisol levels the during the afternoon hours.
Mean cortisol levels obtained in the clinic were higher than those obtained on the baseline day by 33.6%±10.1% (p=0.004) at the pre-IV time (Clinic Time 1 versus Baseline Time 1) and by 69.3%±14.1% (p<0.0001) at the IV time (Clinic Time 2 versus Baseline Time 2). A location by time interaction was significant (p = 0.019) indicating a significantly greater mean change in cortisol observed at the clinic visit compared to baseline (15.7% vs. −8.7%) and likewise, a greater mean difference in cortisol between clinic and baseline at IV Time 2 (69.3%) compared to pre-IV Time 1 (33.6%).
Discussion
Self-report or observational measures of stress have been problematic, particularly for young children. While other physiological measures exist (including heart rate, oxygen saturation, blood pressure, vagal tone, glucose, catecholamines, and other hormones), they can be highly invasive and may not be specific to the stress response. Salivary cortisol has emerged as a relatively non-invasive and logical measure of stress, since HPA axis activity and cortisol secretion is shown to be increased in individuals in response to stress. However, previous research has been limited by a lack of well established time-specific normative values for cortisol in young children.
This study provides normative ranges in a designated age group over a specified time period (Figure 3). Differences in patterns of cortisol levels were not found based on gender or age, allowing the collapse of the data on these variables. As expected, children’s levels were higher earlier in the day and decreased as the day advanced. Little variability was seen over the day, most likely because early morning awakening (peak) levels are not included. The mean cortisol level at 2:00 pm was slightly higher than that at 1:00 pm, but it then decreased again at 3:00 pm. This transient response may be the result of a small subset of children unique to this data set with high cortisol levels or alternatively, may be a meal-dependent response such as previously reported in adult populations (Gibson et al., 1999).
The normative values provided in this paper may be a valuable resource for investigators using salivary cortisol in research with children. Caution should be used in applying normative ranges or other methods to identify outliers for the exclusion of individual results. Either extremely low or high salivary cortisol levels may be a sign of abnormal HPA activity indicative of a long term or abnormal stress response in the individual child. The normative values provided here are not intended for clinical use.
We examined children’s salivary cortisol responsivity to a clinic visit and IV insertion. Assuming that going to the clinic and having an IV start are stress provoking experiences, and cortisol increases as a result of stress, we expected elevated salivary cortisol response on the day of the IV compared to the normal baseline day and an even greater increase at the time of the actual IV insertion. Our findings did show the expected pattern of salivary cortisol changes. A clinic visit and IV placement were adequate stressors to cause a significant elevation in salivary cortisol from a baseline day. These findings support the use of salivary cortisol levels as a biological measure of stress in children coming to clinic and having an IV placed. While findings support the use of cortisol as a measure of stress for the primary research study described, they may not be generalizable to other procedures or stressors, chronic stress, younger or older children, or evening and night hours. Researchers wanting to use cortisol in future intervention studies may want to use the approach described here, determining the responsivity of cortisol to a specific stressor.
Cortisol secretion is impacted by a wide range of factors that, if not controlled for, can interfere with the interpretation of cortisol responses to stress. In this study, careful consideration was given to addressing all the factors that have the potential for impacting cortisol response in the participating children. Specific strategies to control for extraneous variables included: 1) standardizing the times for sample collection 2) using consistent collection materials and methods, 3) controlling for certain drinks, foods, medications and diagnoses, and 4) establishing procedures and protocols across sites (Hanrahan et al., 2006). Controlling for factors that might impact data collection increased the likelihood that the changes noted in the children’s cortisol levels were a response to the painful medical procedure.
This study has limitations. One limitation is participant non-compliance with the process of collecting baseline samples, reflected in the sample return rate and number of unusable samples. Although careful and detailed verbal and written instructions were provided, some participants were not consistent in returning samples, timing samples correctly or noting the time samples were collected. These indicators of non-compliance suggest other mechanisms for home sampling may be indicated. A limitation for the normative values was that the participants, by nature of needing an IV, may have been different from other healthy children. Further study is needed to determine the normative stress response of special populations (e.g. children with attention deficit hyperactive disorder), to other medical stressors, and the efficacy of specific interventions, such as distraction.
Application
This study establishes normative salivary cortisol values and responsivity to specific stressors in children 4–10 years of age. Findings demonstrate that salivary cortisol is an objective biological measure that can measure child distress. Researchers need reliable measures to document biological stress response in children. The results from research will provide information t clinicians about the complex biological responses of children under stress. This information will contribute to clinicians’ ability to predict children’s individual responses and identify effective interventions that can be readily used to minimize child distress during medical procedures and therefore benefits children, parents and clinicians.
Acknowledgments
This study is funded by R01 grant NR05269-01A2 from the National Institute for Nursing Research.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Ann Marie McCarthy, College of Nursing, NB 430, University of Iowa, Iowa City, IA 52242, Contact: ann-mccarthy@uiowa.edu 319-335-7086, fax 319-335-5326.
Kirsten Hanrahan, College of Nursing, University of Iowa Health Care, 200 Hawkins Drive, University of Iowa, Iowa City, IA 52242, Contact: kirsten-hanrahan@uiowa.edu 319-353-6838, fax 319-356-4541.
Charmaine Kleiber, College of Nursing, NB 364, University of Iowa, Iowa City, IA 52242, Contact: charmaine-kleiber@uiowa.edu 319-355-7057, fax 319-335-5326.
M. Bridget Zimmerman, Biostatistics Consulting Center, Department of Biostatistics, C22-E GH, University of Iowa, Iowa City, IA 52242, Contact: bridget-zimmerman@uiowa.edu 319-384-5022, fax 319-384-5018.
Susan Lutgendorf, Departments of Psychology, Obstetrics and Gynecology, E11 Seashore Hall, University of Iowa, Iowa City IA 52242, Contact: susan-lutgendorf@uiowa.edu 319-335-2432, fax 319-335-0191.
Eva Tsalikian, Department of Pediatrics, Carver College of Medicine, 200 Hawkins Drive, University of Iowa, Iowa City, IA 52242, Contact: eva-tsalikian@uiowa.edu 319-356-1833, fax 319-356-8170.
References
- Bartels M, de Geus EJ, Kirschbaum C, Sluyter F, Boomsma DI. Heritability of daytime cortisol levels in children. Behavior Genetics. 2003;33:421–433. doi: 10.1023/a:1025321609994. [DOI] [PubMed] [Google Scholar]
- Bozkurt P, Kaya G, Yeker Y, AltIntaS F, Bakan M, HacIbekiroglu M, et al. Effectiveness of morphine via thoracic epidural vs intravenous infusion on postthoracotomy pain and stress response in children. Pediatric Anesthesia. 2004;14:748–754. doi: 10.1111/j.1460-9592.2004.01278.x. [DOI] [PubMed] [Google Scholar]
- Bruce J, Davis EP, Gunnar MR. Individual differences in children’s cortisol response to the beginning of a new school year. Psychoneuroendocrinology. 2002;27:635–650. doi: 10.1016/s0306-4530(01)00031-2. [DOI] [PubMed] [Google Scholar]
- Charmandari E, Tsigos C, Chrousos G. Endocrinology of the stress response. Annual Review of Physiology. 2005;67:259–284. doi: 10.1146/annurev.physiol.67.040403.120816. [DOI] [PubMed] [Google Scholar]
- Chen E, Craske MG, Katz ER, Schwartz E, Zeltzer LK. Pain-sensitive temperament: Does it predict procedural distress and response to psychological treatment among children with cancer? Journal of Pediatric Psychology. 2000;25:269–278. doi: 10.1093/jpepsy/25.4.269. [DOI] [PubMed] [Google Scholar]
- Clements AD, Parker CR. The relationship between salivary cortisol concentrations in frozen versus mailed samples. Psychoneuroendocrinology. 1998;23:613–616. doi: 10.1016/s0306-4530(98)00031-6. [DOI] [PubMed] [Google Scholar]
- Davis EP, Donzella B, Krueger WK, Gunnar MR. The start of a new school year: Individual differences in salivary cortisol response in relation to child temperament. Developmental Psychobiology. 1999;35:188–196. doi: 10.1002/(sici)1098-2302(199911)35:3<188::aid-dev3>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- Dettling AC, Gunnar MR, Donzella B. Cortisol levels of young children in full-day childcare centers: Relations with age and temperament. Psychoneuroendocrinology. 1999;24:519–536. doi: 10.1016/s0306-4530(99)00009-8. [DOI] [PubMed] [Google Scholar]
- Fisher PA, Gunnar MR, Chamberlain P, Reid JB. Preventive intervention for maltreated preschool children: Impact on children’s behavior, neuroendocrine activity, and foster parent functioning. Journal of the American Academy of Child & Adolescent Psychiatry. 2000;39:1356–1364. doi: 10.1097/00004583-200011000-00009. [DOI] [PubMed] [Google Scholar]
- Gibson EL, Checkley S, Papadopoulos A, Poon L, Daley S, Wardle J. Increased salivary cortisol reliably induced by a protein-rich midday meal. Psychosomatic Medicine. 1999;61:214–224. doi: 10.1097/00006842-199903000-00014. [DOI] [PubMed] [Google Scholar]
- Groschl M, Rauh M, Dorr HG. Circadian rhythm of salivary cortisol, 17{alpha}-hydroxyprogesterone, and progesterone in healthy children. Clinical Chemistry. 2003;49:1688–1691. doi: 10.1373/49.10.1688. [DOI] [PubMed] [Google Scholar]
- Gunnar MR, Brodersen L, Krueger K, Rigatuso J. Dampening of adrenocortical responses during infancy: Normative changes and individual differences. Child Development. 1996;67:877–889. [PubMed] [Google Scholar]
- Gunnar MR, Bruce J, Hickman SE. Salivary cortisol response to stress in children. Advances in Psychosomatic Medicine. 2001;22:52–60. doi: 10.1159/000059275. [DOI] [PubMed] [Google Scholar]
- Gunnar MR, Donzella B. Social regulation of the cortisol levels in early human development. Psychoneuroendocrinology. 2002;27:199–220. doi: 10.1016/s0306-4530(01)00045-2. [DOI] [PubMed] [Google Scholar]
- Gunnar MR, Morison SJ, Chisholm K, Schuder M. Salivary cortisol levels in children adopted from Romanian orphanages. Development & Psychopathology. 2001;13:611–628. doi: 10.1017/s095457940100311x. [DOI] [PubMed] [Google Scholar]
- Gunnar MR, Vazquez DM. Low cortisol and a flattening of expected daytime rhythm: Potential indices of risk in human development. Development & Psychopathology. 2001;13:515–538. doi: 10.1017/s0954579401003066. [DOI] [PubMed] [Google Scholar]
- Gunnar MR, White BP. Salivary cortisol measures in infant and child assessment. In: Singer LT, Zeskind PS, editors. Biobehavioral assessment of the infant. New York, US: Guilford Press; 2001. pp. 167–189. [Google Scholar]
- Hanrahan K, McCarthy AM, Kleiber C, Lutgendorf S, Tsalikian E. Strategies for salivary cortisol collection and analysis in research with children. Applied Nursing Research. 2006;19:95–101. doi: 10.1016/j.apnr.2006.02.001. [DOI] [PubMed] [Google Scholar]
- Kiess W, Meidert A, Dressendorfer R, Schriever K, Kessler U, Konig A, et al. Salivary cortisol levels throughout childhood and adolescence: Relation with age, pubertal stage, and weight. Pediatric Research. 1995;37:502–506. doi: 10.1203/00006450-199504000-00020. [DOI] [PubMed] [Google Scholar]
- King SL, Hegadoren KM. Stress hormones: How do they measure up? Biological Research for Nursing. 2002;4(2):92–103. doi: 10.1177/1099800402238334. [DOI] [PubMed] [Google Scholar]
- Kudielka B, Buske-Kirschbaum A, Hellhammer D, Kirschbaum C. HPA axis responses to laboratory psychosocial stress in healthy elderly adults, younger adults, and children: Impact of age and gender. Psychoneuroendocrinology. 2004;29:83–98. doi: 10.1016/s0306-4530(02)00146-4. [DOI] [PubMed] [Google Scholar]
- Pederson C. Effect of imagery on children’s pain and anxiety during cardiac catheterization. Journal of Pediatric Nursing. 1995;10:365–374. doi: 10.1016/S0882-5963(05)80034-X. [DOI] [PubMed] [Google Scholar]
- Sakellaris G, Petrakis I, Makatounaki K, Arbiros I, Karkavitsas N, Charissis G. Effects of ropivacaine infiltration on cortisol and prolactin responses to postoperative pain after inguinal hernioraphy in children. Journal of Pediatric Surgery. 2004;39:1400–1403. doi: 10.1016/j.jpedsurg.2004.05.002. [DOI] [PubMed] [Google Scholar]
- Schmidt NA. Salivary cortisol testing in children. Issues in Comprehensive Pediatric Nursing. 1997;20:183–190. doi: 10.3109/01460869709028262. [DOI] [PubMed] [Google Scholar]