Abstract
Recent studies consistently support a hypoxia response in the adipose tissue in obese animals. The observations have led to formation of an exciting concept, adipose tissue hypoxia (ATH), in the understanding of major disorders associated with obesity. ATH may provide cellular mechanisms for chronic inflammation, macrophage infiltration, adiponectin reduction, leptin elevation, adipocyte death, ER stress and mitochondrial dysfunction in white adipose tissue in obesity. The concept suggests that inhibition of adipogenesis and triglyceride synthesis by hypoxia may be a new mechanism for elevated free fatty acids in the circulation in obesity. ATH may represent a unified cellular mechanism for variety of metabolic disorders, and insulin resistance in patients with metabolic syndrome. It suggests a new mechanism of pathogenesis of insulin resistance and inflammation in obstructive sleep apnea. Additionally, it may help us to understand the beneficial effects of caloric restriction, physical exercise, and angiotensin II inhibitors in the improvement of insulin sensitivity. In this review article, literatures are reviewed to summarize the evidence and possible cellular mechanisms of ATH. The directions and road blocks in the future studies are analyzed.
Introduction
Inflammation occurs in adipose tissue in obesity, and has a broad impact on glucose, lipids, and energy metabolism (1–5). Several signaling pathways have been proposed to explain the pathogenesis of obesity-associated inflammation, such as activation of toll-like receptor 4 (TLR4) by fatty acids (6–8), activation of Protein kinase C (PKC) or JNK (c-JUN n-terminal kinase) by fatty acid derivatives (diaglyceride or Ceramide) (9–13), induction of ER (endoplasmic reticulum) stress (14, 15) or increased activities of reactive oxidative species (ROS) (16, 17), and activation of macrophages by adipocyte death (18, 19). Although these theories are able to explain some aspects of inflammation and metabolic disorders in obesity, the linkage between obesity and these factors remains to be identified. It is not clear why free fatty acid (FFA), ER stress, ROS and adipocyte death are increased in obesity. Additionally, there is no unified theory for the metabolic and endocrinological dysfunctions of the white adipose tissue under obesity. Recent reports suggest that hypoxia is a new potential risk factor for the chronic inflammation in obesity (20, 21). The emerging role of adipose tissue hypoxia (ATH) suggests new insights into the mechanisms of pathogenesis of metabolic syndrome.
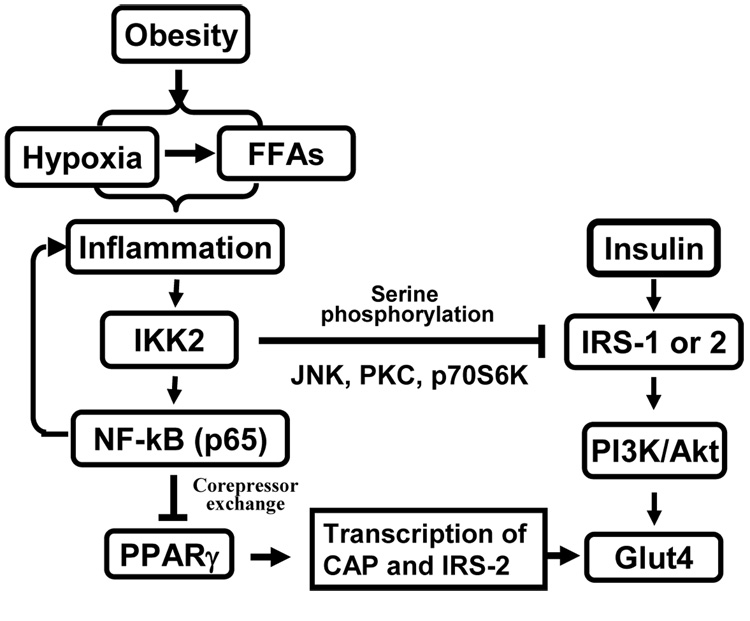
Obesity-associated inflammation is characterized by increased levels of inflammatory mediators in plasma and in adipose tissue (1, 22–25). Macrophage infiltration and activation in the adipose tissue has provided a link between adipose tissue and inflammation (26–28). Inflammation leads to insulin resistance by inhibition of post-receptor signal transduction (Fig. 1), especially inhibition of insulin receptor substrate (IRS) 1 or 2 (IRS-1/2) functions in the insulin signaling pathway (1, 29, 30). Alternatively, inflammation may impair insulin action systemically through increasing FFA and decreasing adiponectin in the blood. Both inflammatory cytokine and FFAs are able to target IRSs proteins for insulin resistance (31–33).
Fig. 1. Mechanisms of inflammation-mediated alteration of insulin signaling.
Hypoxia and FFAs in obesity lead to activation of NF-kB pathway, which induces expression of inflammatory cytokines (TNF-a, IL-1, IL-6, et al) in adipose tissue. Through receptor-mediated signal transduction, these cytokines activates serine kinases including IKK, JNK, PKC and p70S6K to inhibit IRS-1 function. NF-kB inhibits PPARγ function through nuclear corepressor and results in suppression of the gene transcription of CAP and IRS-2, which are signaling molecules in PI(3)K-independent and -dependent signaling pathways for insulin-induced GLUT4 translocation. Hypoxia also induces FFA level through lipolysis.
Serine kinases including IKK (inhibitor kappaB kinase) (34), JNK, PKC and S6K (ribosomal protein S6 kinase) are major signal mediators for inflammation and FFAs in the inhibition of insulin signaling pathway (3, 34–37) (Fig. 1). In addition to IRS proteins, nuclear receptor PPARγ (peroxisome proliferator-activated receptor gamma) is also targeted by inflammation signals for insulin resistance (38–40). The transcriptional activity of PPARγ is required for the maintenance of insulin sensitivity and lipid metabolism (41–43). The insulin-sensitization activity of TZDs (thiazolidinediones) suggests that deficiency in the transcriptional activity of PPARγ may contribute to insulin resistance in obesity. It was reported that IKK/NF-kB (nuclear factor kappa B) signaling pathway might mediate TNF-α (tumor necrosis factor alpha) or IL-1 (interleukine 1) signal in the inhibition of PPARγ function (38, 39). However, the mechanism of IKK/NF-kB action was not clear. A recent study suggests that IKK/NF-kB inhibited PPARγ function by increasing the nuclear corepressor function (Fig. 1) (40). The inhibition involves activation of the histone deacetylase 3 (HDAC3), a component in the nuclear corepresor for PPARg. Therefore, understanding of the events that leads to inflammation or FFA elevation is important in obesity research.
Demonstration of hypoxia in adipose tissue in obesity
Although it is known that hypoxia inhibits differentiation of preadipocytes, and stimulates secretion of leptin and vascular epithelial growth factor (VEGF) from mature adipocytes in vitro (20, 21, 44–47), the biological significance of hypoxia was not clear in the adipose tissue until 2007 (48). Hypoxia was first proposed as a possible cause of inflammation in obesity in 2004 (49). However, the direct evidence for hypoxia in adipose tissue remained missing for three years (48). One reason is that there was no established method for detection of oxygen level in the adipose tissue. Here, four different assays are reviewed for study of ATH (20, 21, 50). Data from these assays provide a strong support to the hypoxia concept (20, 21, 50).
The first is the interstitial partial pressure of oxygen (pO2). In the three recent reports about ATH (20, 21, 50), pO2 was determined in the adipose tissue in two of the studies (20, 50). In our study, pO2 was measured in the epididymal fat with an oxygen meter (20). In ob/ob mice, the interstitial pO2 is about one third of that in the control mice at 12 weeks in age (15.2 mmHg in ob/ob versus 47.9 mmHg in the lean control mice) (20). The 70% reduction in the interstitial pO2 brings the oxygen level to about 2 % in the adipose tissue. In the air, oxygen is 21%. This low level of oxygen concentration suggests hypoxia in the adipose tissue. pO2 in the vein blood at about 23 mmHg (or 3% oxygen) was not reduced in the obese mice, suggesting that there was no systemic hypoxia in the ob/ob mice. The pO2 assay is able to provide definite information about oxygen level in the tissue, but the assay requires special equipment (oxygen meter) and involves surgical operation. The cost is relative high as the optical oxygen probe is fragile and needs to be replaced frequently. Additionally, the assay is dependent on the sensitivity of the oxygen meter and operation skill of the investigator. The observation in mice is consistent with reduced oxygen tension in the adipose tissue in human obesity (51, 52). The studies demonstrated that oxygen tension was reduced in the subcutaneous tissue in the obese patients.
The adipose hypoxia was also detected with a chemical hypoxic probe, pimonidazole hydrochloride. This approach was used in two of the three studies (20, 21). The hypoxiaprobe (pimonidazole hydrochloride) reacts with proteins in a low oxygen environment (pO2 < 10 mmHg) leading to generation of new protein adducts (53). The hypoxiaprobe has high water solubility (400 mM; 116 mg/ml) that facilitates intravenous infusion. Hypoxyprobe™-1 diffuses readily into tumors and normal tissues including brain. Pimonidazole concentration is approximately 3 fold above plasma levels in tumors and normal tissues in vivo. The probe was often used to determine tissue hypoxia in immunohistostaining. However, such application is hard to give a precise quantification for comparison of hypoxia. In our study, the probe was used in tissue staining as well as in the Western blot assay for quantification of hypoxia (20). In the Western, the adipose tissue lysate was blotted on the membrane with a monoclonal antibody to the hypoxia probe. The signal was quantified by the strength of signal. The result demonstrated a significant increase in hypoxia in the epididymal fat of ob/ob mice. The advantage of this approach is that the assay does not need any special equipment and can be done in most laboratories. The weakness of this approach is that the chemical probe cannot give a precise number about the degree of oxygen pressure. The probe can be used as an indirect indicator of hypoxia that facilitates diagnosis of hypoxia. Without pO2 data, this assay is limited in the demonstration of hypoxia.
In the third assay, a group of widely-accepted genes for hypoxia response were used to detect the hypoxia (20, 50). The hypoxia response genes are widely used to determine tissue hypoxia in the study of oxygen-sensing in cancer and ischemia (54). These genes include HIF-1α (hypoxia inducible factor 1alpha), VEGF (vascular endothelial growth factor), GLUT1 (glucose transporter 1), Hemox (Heme oxygenase 1) and PDK1 (pyruvate dehydrogenase kinase 1) (54, 55). HIF-1α is a transcription factor that controls expression of the other four genes in response to hypoxia (54). In the epididymal fat, expression of these genes was examined with western blot, and real time quantative RT-PCR (qRT-PCR) in our study (20). In ob/ob mice, HIF-1α and GLUT1 were significantly increased in proteins in comparison to the age- and gender-matched wild type littermates. All of the hypoxia response genes were increased in mRNA except VEGF. The increase was only observed in the adipose tissue, but not in the skeletal muscle of the ob/ob mice, suggesting that hypoxia is specific to the fat tissue in obese mice. Although plasma insulin is increased in the obese mice, the fat-specific expression of hypoxia response genes does not suggest a role of insulin in the induction of HIF-1α and its target genes. Insulin was reported to induce HIF-1α and hypoxia gene expression in cell cultures (56). Our data suggests that insulin may not have the same activity in vivo.
The fourth is lactate assay in the adipose tissue (21). Lactate is a product of oxygen-independent glucose metabolism or glycolysis in cells. In normal condition, ATP is produced in mitochondria through consumption of oxygen. In the cytosol, ATP production is done through glycolic reaction. With oxygen supply, both pathways are active in ATP production. In the hypoxic condition, mitochondrial respiration is reduced and the glycolytic activity is increased in compensation for the mitochondria inhibition (57, 58). As a result, the lactate production is increased, which may lead to a decrease in the interstitial pH. Therefore, lactate concentration in the tissue may be used as an indicator of tissue hypoxia. In obese mice (DOI or KKAy mice), the lactate concentrations were 1.7- and 1.5-fold higher in the adipose tissue than those of lean control mice, respectively (21). In the muscle, lactate concentration was not increased in these obese mice. Since lactate production is also induced by many other factors, such as insulin, a proper control is required to determine the relationship of lactate and hypoxia.
Hypoxia signal transduction in adipose tissue
HIF-1α is a master signal mediator of hypoxia signal (54). In 2005, HIF-1α was shown to be increased in adipose tissue of obese patient and its expression was reduced after surgery-induced weight loss (59). In 2006, the increase in HIF-1α expression was confirmed in adipose tissue by microarray and immunohistostaining (60). In the primary cell culture, HIF-1α protein was induced in human adipocytes by hypoxia (47). However, the role of hypoxia in the induction of HIF-1α was not identified in the adipose tissue until the protein increase was associated with hypoxia in the adipose tissue in obese mice (20). In addition to HIF-1α, NF-kB is also activated by hypoxia (20).
Regulation of HIF-1α activity has been well documented (54). HIF-1α was cloned in 1995 (61, 62). HIF-1α stays in the nucleus and is not detectable in the cytoplasm. The regulation of HIF-1 activity occurs at multiple levels (63). Whereas HIF-1α mRNA is not changed by hypoxia in cultured cells, it is remarkably induced by hypoxia or ischemia in tissues (64, 65). Under hypoxic conditions, HIF-1α protein levels increase dramatically through inhibition of ubiquitination-proteasome mediated degradation of HIF-1α. HIF-1α protein peaks at 4–8 hour in response to hypoxia and returns to the basal level after overnight in hypoxia condition. Under normal oxygen conditions, HIF-1 is targeted for ubiquitination and rapid degradation in the proteasome (54, 66). The half-life of HIF-1a protein is <5 min under normoxia. An increase in HIF-1 activity is resulted from reduced degradation of HIF-1α protein. This process is induced by PI3K–AKT and MAPK-ERK (MEKK) pathways by growth factors including insulin, IGF, and EGF (56, 67–69). After activation, HIF-1a translocates to the nucleus and dimerizes with HIF-1β [hydrocarbon receptor nuclear translocator (ARNT)] to bind to the promoter DNA of target genes, such as VEGF (54, 66). The core DNA sequence of HIF-1 element is 5’-RCGTG-3’ (70). HIF-1a is also up-regulated by certain transition metals (Co2+, Ni2+, Mn2+) and by iron chelation. Nuclear localization of HIF-1a may also be induced by hypoxia. HIF-1α expression is required for embryonic survival in mice [Iyer, 1998 #4074; Kotch, 1999 #6379].
ATH and inflammation response
ATH may provide an answer to the question about the cause of chronic inflammation in adipose tissue in obesity. It may also explain the impact of ischemia/reperfusion in the adipose tissue (71). The hypoxia is able to induce inflammation in adipose tissue by induction of gene expression in adipocytes and macrophages. This possibility was demonstrated using primary cells and cell lines (20, 21). The induced genes include TNF-α, IL-1, IL-6, MCP-1 (Monocyte chemoattractant protein-1), PAI-1 (plasminogen activator inhibitor-1), MIF (macrophage migration inhibition factor), iNOS (inducible nitric oxide synthase), MMP9 (matrix metalloproteinases 9), and MMP2. The molecular mechanism of gene expression is related to activation of NF-kB and HIF-1α. All of these genes are targets of NF-kB, and some of them are also targets of HIF-1 (PAI-1, MIF, and iNOS) (63).
Activation of NF-kB by hypoxia is well-established in the fields of cancer biology, immunology, and cardiovascular research as being reviewed (72–77). In cancer research, it is proposed that activation of NF-kB is responsible for tumor resistance to radiotherapy or chemotherapy in cancer patients (75). NF-kB increases tumor survival through anti-apoptosis effects. In cardiovascular study, activation of NF-kB by hypoxia is proposed to mediate inflammation for lung injury, and tissue damage in ischemia-reperfusion (76). It is known that hypoxia activates NF-kB through IKK-independent pathway as being reviewed (72, 78). In response to hypoxia, NF-kB is disassociated from IkBa in the absence of IkBa degradation. The disassociation leads to nuclear translocation and transcriptional activation of inflammatory cytokines.
Transcription factor NF-kB is a master regulator of inflammation response (78–80). It is formed by two proteins of the Rel family, p65 and p50 (78). NF-kB controls transcription of many pro-inflammatory cytokines (such as TNF-α, IL-1, and IL-6) or inflammation mediator (such as iNOS, and VACM). The signaling pathway for NF-kB activation has been well documented in reviews (81, 82). In the absence of activation, NF-kB is associated with IkBα (inhibitor) and retained in the cytoplasm. When cells are stimulated by extracellular signal, such as TNF-α or LPS, activation of IKK2 leads to phosphorylation, ubiquitination and degradation of IkBα protein in proteasome. In the absence of IkBα, NF-kB will be activated and translocated into the nucleus to initiate transcription of target genes, which include IkBa, TNF-α, IL-1 and IL-6. After the IkBα protein level is restored from this transcription-based process, NF-kB will be associated with IkBα and then excluded from the nucleus. In this classical signaling pathway, activation of NF-kB is dependent on activation of IKK2. In general, inhibition of IKK2 leads to suppression of the transcriptional activity of NF-kB.
ATH in macrophage infiltration into adipose tissue
The discovery of macrophage infiltration into the adipose tissue in obesity has significantly modified our view of chronic inflammation in the fat tissues (26, 27). This finding provided an excellent explanation for some controversy about TNF-α and resistin production by adipose tissue. In vivo, it is clear that macrophages (not adipocytes) in the adipose tissue are the primary source of these cytokines (83). Macrophage infiltration is a new marker of chronic inflammation of adipose tissue (26, 27). The finding led to progress in the mechanistic study of insulin sensitization by TZDs. Macrophage is found as a TZD target for insulin sensitization (84, 85).
The mechanism of macrophage infiltration remains unknown in adipose tissue. It is generally accepted that MCP-1 attracts macrophage to adipose tissue. MCP-1 is produced predominantly by macrophages and endothelial cells, and is a potent chemotactic factor for monocytes (precursor of macrophage). MCP-1, also known as CCL2, belongs to pro-inflammatory cytokine. The role of MCP-1 in macrophage infiltration is supported by several studies using transgenic mice with either global MCP-1 knockout or fat-specific over-expression of MCP-1 (27, 86, 87). However, it has recently been reported that macrophage infiltration was not associated with MCP-1 in both lean and obese mice (88, 89).
Expression of MIF (macrophage migration inhibition factor) in response to hypoxia may provide a clue to a new mechanism of macrophage infiltration (20). Tissue macrophage infiltration is a result of increased arrival or reduced departure of macrophages. Hypoxia is known to inhibit macrophage departure from the hypoxic region in tissue (90). MIF is one of the factors mediating the hypoxia signal in the inhibition of macrophage departure. These facts suggest that in obesity, macrophage infiltration into the adipose tissue may be related to the MIF-mediated inhibition of out-bond migration of macrophages. MIF is a 114 amino acid protein that circulates in homotrimeric, dimeric and monomeric forms (91). MIF expression is increased by hypoxia and glucocorticoids (92, 93). Study of MIF knockout mice suggests that MIF is required for normal function of macrophage (94). MIF is a target gene of HIF-1α that is required for macrophage infiltration into tissues (95). MIF is produced by many types of cells including adipocytes, monocytes/macrophages, and lymphocytes (96, 97). In human adipocytes, MIF expression is increased with BMI, and negatively associated with insulin sensitivity (96, 98). It remains to be understood how MIF is linked to insulin resistance. Our study suggests that MIF may contribute to insulin resistance by promoting inflammation in adipose tissue (20).
Adipocyte death has been proposed to induce macrophage infiltration into adipose tissue (18). This possibility is supported by observations that macrophages are located around dead adipocytes in the adipose tissue (19). A new study suggests that macrophages were predominantly found in hypoxic areas in adipose tissue of obese mice (50). There are two possibilities to explain the association of macrophage and dead cells in the adipose tissue. One is that macrophages act to clean the dead adipocytes. The other is that macrophage is trapped in the hypoxic area by MIF. MIF expression should be very high in the hypoxic area. If cell death contribute to the macrophage infiltration, it is not clear what induces the adipocytes death. Our study suggests that the cell death is likely a result of hypoxia response (99). In vitro, hypoxia induced cell necrosis in 3T3-L1 adipocytes.
ATH inhibits adiponectin expression
Adiponectin is a cytokine produced by adipocytes (100–103). Serum levels of adiponectin protein correlate with systemic insulin sensitivity (104). A reduction in adiponectin expression contributes to insulin resistance in obesity (105–109). However, it was not clear what causes adiponectin reduction in obesity. Inflammation was proposed to inhibit adiponectin expression, but controversy remains (110). It was hypothesized that chronic inflammation associated with visceral obesity may inhibit production of adiponectin. However, adiponectin levels are elevated rather than decreased in classic chronic inflammatory/autoimmune diseases, such as rheumatoid arthritis, SLE, inflammatory bowel disease, type 1 diabetes, and cystic fibrosis. Adipose tissue hypoxia may provide a new answer to the controversy (20, 21).
Hypoxia was shown to reduce adiponectin expression in adipocytes by several independent labs (20, 21, 46, 47). In the adipose tissue, the hypoxia may inhibit adiponectin mRNA, and induce expression of inflammatory cytokines (20, 21). TNF-α is known to inhibit mRNA expression of adiponectin in adipocytes (111). Therefore, the hypoxia may directly inhibit adiponectin expression or indirectly act through TNF-α (20). This may occur locally in the adipose tissue. This may not apply to the systemic inflammation, in which a high level of adiponectin was observed with chronic systemic inflammatory (110).
The molecular mechanism of adiponectin inhibition by hypoxia and inflammation remains to be investigated. The gene promoter activity of adiponectin was reduced by hypoxia and TNF-α (20, 21, 46). It remains to be investigated how the promoter was down-regulated by hypoxia. The promoter contains response elements for several transcriptional activators, such as PPARγ, SREBP (sterol regulatory element-binding protein), FOXO1 (Forkhead bOX-containing protein, O subfamily) and C/EBP (CCAAT/enhancer-binding proteins) (112–114). Hypoxia is known to inhibit PPARγ function in an HIF-1 dependent manner (44). Hypoxia activates PI3K/Akt (115), and this may lead to inhibition of the transcriptional activity of FOXO1 through nuclear exclusion (116). Therefore, inhibition of both PPARγ and FOXO1 by hypoxia may contribute to the molecular mechanism of adiponectin inhibition. HIF-1 may contribute to the adiponectin inhibition through suppression of PPARg expression. It is not clear if there is a HIF-1 binding site in the adiponectin gene promoter. Inhibition of C/EBPβ was proposed to be responsible for the hypoxia-mediated adiponectin reduction (21). However, this possibility is challenged by the fact of C/EBP activation by hypoxia. The transcriptional activity of C/EBP was enhanced by hypoxia (117, 118).
Hypoxia and leptin expression
Leptin is a primary hormone secreted by adipose tissue and has a well-established function in the control of energy balance (119). Expression of leptin from adipocytes is regulated by body weight (or adiposity), food intake, hormones and hypoxia (119–121). At whole body level, leptin is increased in plasma by obesity, and the increase is dependent on mRNA expression. Loss of adiposity leads to a reduction in leptin mRNA in adipose tissue (119). This may explain leptin reduction in response to the high altitude in some studies [Sierra-Johnson, 2008 #6374]. In the environment of high altitude, lack of oxygen induces a high altitude response that includes loss of appetite, less food intake, and reduction in body weight. The hypoxia and reduction in body weight may contribute to elevation of leptin in the plasma [Cabrera de Leon, 2004 #5944; Tschop, 2000 #5943; Vats, 2004 #5942; Zaccaria, 2004 #5941; Sierra-Johnson, 2008 #6374].
ATH may provide a new explanation to the up-regulated leptin expression in obesity. Leptin was reported as a hypoxia response gene whose transcription is induced by transcription factor HIF-1α (hypoxia inducible factor 1 alpha) (121–124). At mRNA level, leptin expression is increased by insulin, and decreased by beta 3-adrenergic receptor signal that activates cAMP signaling pathway (120, 125). Insulin may mediate food intake signal in the induction of leptin. cAMP may mediate physical excise signal in the inhibition of leptin. In obesity, ATH is associated with leptin elevation (20, 21, 50), suggesting a role of hypoxia. In cell culture, leptin expression was induced by hypoxia (1% oxygen) in human preadipocytes (126). However, this activity of hypoxia is controversial as it was not observed when classical hypoxia response genes were induced by hypoxia in adipocytes (127). Therefore, it is subject to debate that hypoxia directly induces leptin gene expression.
ATH in adipocyte cell death and plasma FFA elevation
Hypoxia may be a potential risk factor for adipocyte death in adipose tissue of obese subjects. An increase in adipocyte death was reported in adipose tissue of obese subjects, and was proposed to induce macrophage infiltration (18). In dietary obese mice, adipocyte death was increased with growth of fat pads (19). However, the reason of adipocyte death is not clear. Our study suggests that hypoxia induces necrosis in 3T3-L1 adipocytes (99). This observation provides an underlying mechanism of cell death in adipose tissue. The cell death may promote lipolysis and release of FFA into blood stream under insulin resistance. This will explain the increase in plasma FFA in obesity. In adult rats, plasma FFA in the vein blood is induced by acute hypoxia in an ischemia model (99). In the newborn mice, plasma FFA is induced by systemic hypoxia (128). In ischemia research, hypoxia has been well-documented in the induction of cell death in heart and brain (129). In adipose tissue, ischemia induces damages in several forms, such as edema congestion and bleeding (71).
Elevation of plasma FFAs is associated with increased lipolysis in obese condition (130, 131). The lipolysis was attributed to reduced insulin sensitivity in WAT (132, 133). Hypoxia may contribute to the lipolysis through induction of insulin resistance and stress responses (99). Insulin signaling activity was inhibited by hypoxia (99, 134). Since insulin inhibits lipolysis and stimulates fatty acid synthesis (135), the loss of insulin signal will promote lipolysis. Inhibition of PPARγ activity by hypoxia may be another mechanism of lipolysis. PPARγ activity is inhibited by hypoxia in the model of adipogenesis (44). PPARγ is a master transcription factor for expression of proteins involved in fatty acid uptake, synthesis, and storage (42, 136). In fatty acid uptake, FATP (fatty acid transporter protein) and CD36 are target genes of PPARγ. In triglyceride synthesis, PPARγ controls glycerol synthesis through PEPCK expression. In fatty acid storage, PPARγ induces FABP4 (fatty acid binding protein 4, aP2) expression. A reduced expression in these genes may contribute to the elevation of plasma FFA. Additionally, Activation of AMPK (AMP-activated protein kinase) by hypoxia may promote lipolysis as well. AMPK is a serine kinase that serves as a sensor for energy supply in cells (137). It is known that hypoxia activates AMPK through inhibition of mitochondrial respiration or oxidative stress (138). Activation of AMPK leads to lipolysis in adipocytes and skeletal muscle (137).
Hypoxia and ER stress
ER stress was first observed in adipose tissue of obese mice in 2004 and proposed as a risk factor for insulin resistance (14). JNK (c-JUN N-terminal Kinase) is activated in obese condition (35, 139, 140), and is shown to inhibit IRS-1 function for insulin resistance (31, 141). In search for the mechanism of obesity-associated JNK activation, ER stress was found in the adipose tissue and liver in obese mice (14, 15). Inhibition of ER stress by transgene or chemical inhibitors was found to protect the mice from insulin resistance in obesity (14, 142, 143). However, it is not clear why ER stress occurs in obesity. Hypoxia is known to induce ER stress (144, 145). To test this activity of hypoxia in adipocytes, Hosogai et al treated 3T3-L1 adipocytes with hypoxia (21). They found that ER stress was induced by hypoxia in induction of mRNA expression of CHOP and GRP78, which are ER stress genes. eIF2a phosphorylation is another marker of ER stress. It was induced in 3T3-L1 adipocytes after 2–6 h hypoxia treatment (21). The study supports that ATH may be involved in induction of ER stress in obesity.
Hypoxia and mitochondrial dysfunction
White adipose tissue (WAT) has much less mitochondrial activity compared to the brown adipose tissue (BAT). However, mitochondrial dysfunction may contribute to malfunction in white adipose tissue (146). This possibility is supported by the reduced number of mitochondria in adipose tissue of obese people (146). However, it is not clear why and how the mitochondrial number is reduced in the adipose tissue. ATH may provide an explanation. It is known that mitochondria respiration and biogenesis are inhibited by hypoxia (147, 148). In obese condition, mitochondrial number and function in adipocytes may be decreased gradually by the hypoxia response in adipose tissue. HIF-1α is a major mediator of the hypoxia signal in the inhibition of mitochondrial function (147, 148).
Possible causes of adipose tissue hypoxia
The physiological basis of ATH might be related to reduction in adipose tissue blood flow (ATBF) (ml/min/100g tissue) and capillary density. Reduction in ATBF has been reported in obesity in both humans (149–151) and animals (152–154). The reduction means that blood perfusion is reduced in each unit of adipose tissue in obesity. In obese people, the ATBF rate was 30–40% lower (P < 0.02–0.05) than that of non-obese subjects (153). Although an earlier study suggests that ATBF was not reduced in obesity (155), all of later studies consistently support the reduction of ATBF (149–154). The ATBF reduction was observed only in the obese diabetic rats (obese Zucker rat), but not in the non-obese diabetic GK rats (154), suggesting a role of adipose tissue mass in the control of blood flow. Insulin resistance may not lead to the ATBF reduction since it occurs in both obese Zucker rats and non-obese GK rats. The ATBF reduction is associated with insulin resistance in obesity (150, 151). Although the association has been known in obesity for years, the intermediate events linking the two conditions remains unknown. The adipose tissue hypoxia may be a potential link.
A reduction in capillary density may contribute to the adipose tissue hypoxia (156). Capillary density is determined by angiogenesis that requires proliferation and tube formation of endothelial cells. Endothelial proliferation was driven by growth factors including VEGF, and FGF2. The tube formation and capillary maturation are controlled by a different set of cytokines including PDGF, TGF-β and Angiopoietin. We observed that VEGF expression was not increased in response to hypoxia in the adipose tissue of ob/ob mice although expression of other hypoxia response genes was up-regulated (20). This defect was associated with a reduced endothelial density in the tissue (156). The evidence suggests that angiogenesis is deficient in the adipose tissue of obese mice, and this defect may account for the reduction in adipose tissue blood flow in obesity. The detail molecular events underlying the angiogenic defect remain to be investigated in obese condition.
Blood perfusion is reduced from a decrease in vasodilation or increase in vasoconstriction. An increase in vasoconstriction in obesity is supported by literature. Angiotensin II (Ang II) is a serum peptide with known function to increase vasoconstriction. Ang II is a component in the renin-angiotensin system (RAS), and produced after hydrolysis of Ang I by angiotensin-converting enzyme (ACE). Ang II acts on both the type 1 (AT1) and type 2 (AT2) receptors (157, 158). In obesity, the Ang II activity is increased in adipose tissue and in circulation (159–161). This may contribute to the ATBF reduction through an increase in vasoconstriction (162). Additionally, the Ang II inhibitors are known to enhance blood perfusion in adipose tissue (161). The inhibitors also decrease inflammation in adipose tissue, and increase systemic insulin sensitivity (163, 164). It remains to be tested if the pharmacological inhibitors for Ang II improve oxygen supply in the adipose tissue in obesity.
In addition to the ATBF reduction, the increase in adipocyte size may contribute to the interstitial hypoxia. In tissue, oxygen can only defuse about 120 micron (165, 166). When adipocyte diameter increases to (or above) 120 micron, oxygen will not be able to reach the cells beyond 120 micron from the capillary. The diameter of a large adipocyte can be over 150 micron (167). This distance effect remains to be tested in the adipose tissue in obesity.
Inflammation may serve to stimulate angiogenesis
Although inflammation is induced locally by hypoxia in adipose tissue, the biological significance remains to be established for the inflammation except the side effects on adipocytes. We believe that inflammation may serve as a physiological signal for angiogenesis, and remodeling of extra-cellular matrix in adipose tissue (Fig. 2). This is supported by several recent studies about macrophage function in the adipose tissue (89, 156, 168). Many pro-inflammatory cytokines have angiogenic activity (Fig. 3) (169, 170). The side effects of inflammation may happen in extreme conditions, such as obesity and infections.
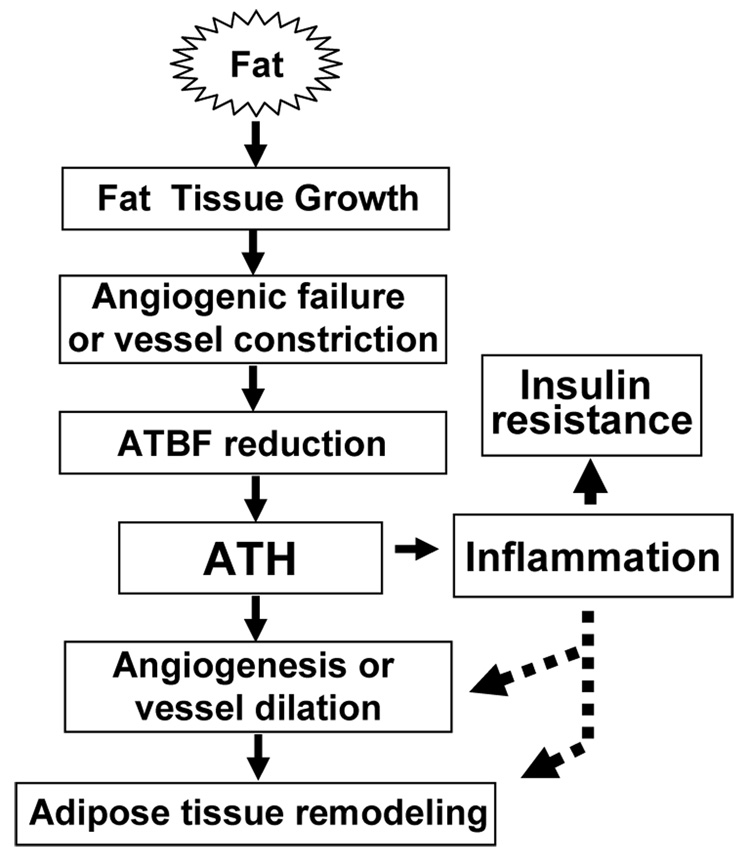
Fig. 2. Up- and down-stream of ATH.
Rapid growth of adipose tissue leads to quick expansion of adipose tissue. When angiogenesis or vessel dilation can not meet the demand for blood supply, reduction in adipose tissue blood flow (ATBF) will happen leading to adipose tissue hypoxia (ATH). ATH will induce angiogenesis and trigger inflammation. Inflammation will promote angiogenesis and vasodilation. When inflammation is out of control, it will promote insulin resistance locally and systemically. ATH is a signal for remodeling of extra-cellular matrix in the adipose tissue. Please refer to Fig. 1 for mechanism of inflammation in insulin resistance.
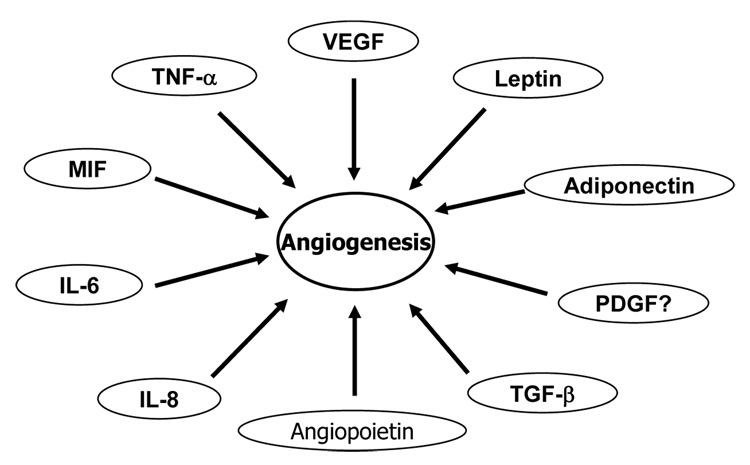
Fig. 3. Cytokines/hormones in angiogenesis.
Cytokines or hormones from adipocytes and macrophages are able to regulate angiogenesis. These include leptin, adiponectin, VEGF (vascular endothelial growth factor), TNF-α (tumor necrosis factor-alpha), MIF (macrophage migration inhibitory factor), IL-6 (interleukin-6), IL-8, PDGF (platelet-derived growth factor), TGF-β (transforming growth factor beta), and angiopoietin.
Angiogenesis (growth of blood vessel) is a physiological response in tissue growth and development (171). It has been extensively studied in cancer biology and developmental biology (171–173). Pathogenic angiogenesis is closely related to cancer and diabetic retinopathy (169, 171). Hypoxia is a primary physiological signal for angiogenesis in both physiological and pathological conditions (174, 175). In the fat tissue, angiogenesis is required for adipocyte differentiation and tissue growth as reviewed (170, 176, 177). Many cytokines produced by adipose tissue have angiogenic activities (23, 160, 169, 170). As shown in Fig. 3, these cytokines include leptin (178), adiponectin (179), VEGF (vascular endothelial growth factor), TNF-α, MIF-1 (macrophage migration inhibitory factor 1), IL-6 (interleukin-6), IL-8, PDGF (platelet-derived growth factor) (180), TGF-β (transforming growth factor beta), and angiopoietin.
Angiogenesis is required for adipose tissue formation. This concept is supported by studies using adipose tissue transplantation (181) and angiogenic inhibitors (168, 177, 182, 183). Inhibition of angiogenesis reduces adipose tissue growth and prevents obesity (168, 177, 182, 183). VEGF is a powerful angiogenic factor and was shown to stimulate adipogenesis in a paracrine manner (177). In the visceral fat, adipocytes have the highest expression of VEGF in a comparative study of multiple cell types (184). The high level expression is pad- or location-specific (185, 186). However, it was not clear how VEGF acts to promote adipogenesis.
Macrophages are much more active than adipocytes in secretion of inflammatory cytokines and pro-angiogenic factors. Angiogenesis is tightly controlled by pro-angiogenic and anti-angiogenic factors. In response to hypoxia, macrophage is able to secrete almost all of the pro-angiogenic factors (73). PDGF is a good example for this activity of macrophages. Although adipocytes have a high basal level of VEGF production (184), it can not produce much PDGF (156). Our data suggests that differentiated 3T3-L1 cells lost its ability in expression of PDGF (156), and gained capacity in VEGF expression. VEGF mainly induces proliferation of endothelial cells. It does not stimulate maturation of capillary. PDGF is able to induce tube formation and recruitment of pericytes for capillary maturation. Therefore, in adipose tissue, pro-angiogenic factors made by adipocytes may not be sufficient for expansion of functional vasculature. Macrophages may facilitate the neovascularation. Without macrophages, angiogenesis was significantly reduced in adipose tissue in lean mice (89).
Hypoxia and insulin resistance
In clinic, obstructive sleep apnoea (OSA) is associated with insulin resistance (187–190). The syndrome of OSA is characterized by recurrent collapse of the upper airway during sleep leading to periods of intermittent hypoxia (IH) and fragmentation of sleep (191). The prevalence of OSA is 40 % to 60 % in obese subjects. OSA increases risk of hypertension, coronary artery disease and stroke. Since systemic hypoxia is associated with insulin resistance and glucose intolerance (192–196), hypoxia was proposed as a risk factor for OSA-associated insulin resistance (187–190, 194).
In a recent study, systemic hypoxia was shown to induce insulin resistance in lean mice (197). The insulin resistance was examined with the hyperinsulinemic euglycemic clamp. The result suggests that hypoxia induced systemic insulin resistance as glucose infusion rate was decreased. A reduction in glucose utilization in the oxidative muscle fibers was observed, suggesting that muscle developed insulin resistance. The role of liver was excluded since hepatic glucose output was not changed by the hypoxia treatment. A role of autonomic nervous system (ANS) was excluded as ANS blocker did not improve the insulin resistance. This study can not exclude the role of adipose tissue response in the muscle insulin resistance as the adipose tissue function and blood FFA were not examined.
Application of ATH
In addition to the role in pathogenesis of insulin resistance, ATH may provide an alternative mechanism for insulin sensitization by several factors, such as physical exercise, fasting, weight loss and Ang II inhibitors. ATBF is increased in response to stress such as exercise (198–200), mental stress (201), fasting (202) and nutrient intake (149, 203–205). ATBF is increased by the Ang II inhibitor (161), epinephrine (206–208), insulin and NO (nitric oxide) (209). Insulin sensitivity is improved by physical exercise, fasting, and the Ang II inhibitors, and ATBF is increased in all of these conditions. An improvement in oxygen supply may contribute to the mechanism of insulin sensitization under these conditions. This possibility needs to be tested in experiment.
Summary
Recent studies have provided compelling evidence for the biological roles of hypoxia response in the control of development, growth and remodeling of adipose tissue. These studies are leading us to test a new hypothesis for the pathogenesis of obesity, type 2 diabetes, and metabolic syndrome. The advance is due to the development of methods for hypoxia detection in adipose tissue, and progress in basic research of oxygen sensing system. The ATH concept may explain many disorders in the adipose tissue in obesity. The biological basis of ATH may be related to reduction in adipose tissue blood flow, which is resulted from over growth of adipose tissue. A failure in compensatory angiogenesis or vasodilation may be the cellular basis of the blood flow reduction. Although these possibilities are supported by increasing number of related studies, they remain to be approved by experiments in vivo. It is expected that transgenic studies in animals, and translational studies in humans will provide more direct evidence in support of the ATH concept.
Acknowledgements
This manuscript was prepared with support by NIH fund (DK68036) and ADA research award (7-07-RA-189) to J. Ye.
Reference
- 1.Hotamisligil GS. Inflammation and metabolic disorders. Nature. 2006;444:860–867. doi: 10.1038/nature05485. [DOI] [PubMed] [Google Scholar]
- 2.Peraldi P, Spiegelman B. TNF-alpha and insulin resistance: summary and future prospects. Mol Cell Biochem. 1998;182:169–175. [PubMed] [Google Scholar]
- 3.Yuan M, Konstantopoulos N, Lee J, Hansen L, Li ZW, Karin M, et al. Reversal of Obesity- and Diet-Induced Insulin Resistance with Salicylates or Targeted Disruption of Ikkbeta. Science. 2001;293:1673–1677. doi: 10.1126/science.1061620. [DOI] [PubMed] [Google Scholar]
- 4.Kim JK, Kim YJ, Fillmore JJ, Chen Y, Moore I, Lee J, et al. Prevention of fat-induced insulin resistance by salicylate. J Clin Invest. 2001;108:437–446. doi: 10.1172/JCI11559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Itani SI, Ruderman NB, Schmieder F, Boden G. Lipid-induced insulin resistance in human muscle is associated with changes in diacylglycerol, protein kinase C, and IkappaB-alpha. Diabetes. 2002;51:2005–2011. doi: 10.2337/diabetes.51.7.2005. [DOI] [PubMed] [Google Scholar]
- 6.Lee JY, Ye J, Gao Z, Youn HS, Lee WH, Zhao L, et al. Reciprocal modulation of Toll-like receptor-4 signaling pathways involving MyD88 and phosphatidylinositol 3-kinases/AKT by saturated and polyunsaturated fatty acids. J Biol Chem. 2003;278:37041–37051. doi: 10.1074/jbc.M305213200. [DOI] [PubMed] [Google Scholar]
- 7.Shi H, Kokoeva MV, Inouye K, Tzameli I, Yin H, Flier JS. TLR4 links innate immunity and fatty acid-induced insulin resistance. J Clin Invest. 2006;116:3015–3025. doi: 10.1172/JCI28898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tsukumo DML, Carvalho-Filho MA, Carvalheira JBC, Prada PO, Hirabara SM, Schenka AA, et al. Loss-of-Function Mutation in Toll-Like Receptor 4 Prevents Diet-Induced Obesity and Insulin Resistance. Diabetes. 2007;56:1986–1998. doi: 10.2337/db06-1595. [DOI] [PubMed] [Google Scholar]
- 9.Yu C, Chen Y, Zong H, Wang Y, Bergeron R, Kim JK, et al. Mechanism by Which Fatty Acids Inhibit Insulin Activation of Insulin Receptor Substrate-1 (IRS-1)-associated Phosphatidylinositol 3-Kinase Activity in Muscle. J Biol Chem. 2002;277:50230–50236. doi: 10.1074/jbc.M200958200. [DOI] [PubMed] [Google Scholar]
- 10.Gao Z, Zuberi A, Quon M, Dong Z, Ye J. Aspirin Inhibits TNF-induced Serine phosphorylation of IRS-1 through Targeting Multiple Serine Kinases. J Biol Chem. 2003;278:24944–24950. doi: 10.1074/jbc.M300423200. [DOI] [PubMed] [Google Scholar]
- 11.Grigsby RJ, Dobrowsky RT. Inhibition of ceramide production reverses TNF-induced insulin resistance. Biochem Biophys Res Commun. 2001;287:1121–1124. doi: 10.1006/bbrc.2001.5694. [DOI] [PubMed] [Google Scholar]
- 12.Teruel T, Hernandez R, Lorenzo M. Ceramide mediates insulin resistance by tumor necrosis factor-alpha in brown adipocytes by maintaining Akt in an inactive dephosphorylated state. Diabetes. 2001;50:2563–2571. doi: 10.2337/diabetes.50.11.2563. [DOI] [PubMed] [Google Scholar]
- 13.Holland WL, Brozinick JT, Wang LP, Hawkins ED, Sargent KM, Liu Y, et al. Inhibition of ceramide synthesis ameliorates glucocorticoid-, saturated-fat-, and obesity-induced insulin resistance. Cell Metab. 2007;5:167–179. doi: 10.1016/j.cmet.2007.01.002. [DOI] [PubMed] [Google Scholar]
- 14.Ozcan U, Cao Q, Yilmaz E, Lee A-H, Iwakoshi NN, Ozdelen E, et al. Endoplasmic Reticulum Stress Links Obesity, Insulin Action, and Type 2 Diabetes. Science. 2004;306:457–461. doi: 10.1126/science.1103160. [DOI] [PubMed] [Google Scholar]
- 15.Nakatani Y, Kaneto H, Kawamori D, Yoshiuchi K, Hatazaki M, Matsuoka T-a, et al. Involvement of Endoplasmic Reticulum Stress in Insulin Resistance and Diabetes. J Biol Chem. 2005;280:847–851. doi: 10.1074/jbc.M411860200. [DOI] [PubMed] [Google Scholar]
- 16.McClung JP, Roneker CA, Mu W, Lisk DJ, Langlais P, Liu F, et al. Development of insulin resistance and obesity in mice overexpressing cellular glutathione peroxidase. Proc Natl Acad Sci U S A. 2004;101:8852–8857. doi: 10.1073/pnas.0308096101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Houstis N, Rosen ED, Lander ES. Reactive oxygen species have a causal role in multiple forms of insulin resistance. Nature. 2006;440:944–948. doi: 10.1038/nature04634. [DOI] [PubMed] [Google Scholar]
- 18.Cinti S, Mitchell G, Barbatelli G, Murano I, Ceresi E, Faloia E, et al. Adipocyte death defines macrophage localization and function in adipose tissue of obese mice and humans. J Lipid Res. 2005;46:2347–2355. doi: 10.1194/jlr.M500294-JLR200. [DOI] [PubMed] [Google Scholar]
- 19.Strissel KJ, Stancheva Z, Miyoshi H, Perfield JW, 2nd, DeFuria J, Jick Z, et al. Adipocyte death, adipose tissue remodeling, and obesity complications. Diabetes. 2007;56:2910–2918. doi: 10.2337/db07-0767. [DOI] [PubMed] [Google Scholar]
- 20.Ye J, Gao Z, Yin J, He H. Hypoxia is a potential risk factor for chronic inflammation and adiponectin reduction in adipose tissue of ob/ob and dietary obese mice. Am J Physiol Endocrinol Metab. 2007;293:E1118–E1128. doi: 10.1152/ajpendo.00435.2007. [DOI] [PubMed] [Google Scholar]
- 21.Hosogai N, Fukuhara A, Oshima K, Miyata Y, Tanaka S, Segawa K, et al. Adipose tissue hypoxia in obesity and its impact on adipocytokine dysregulation. Diabetes. 2007;56:901–911. doi: 10.2337/db06-0911. [DOI] [PubMed] [Google Scholar]
- 22.Lazar MA. How obesity causes diabetes: not a tall tale. Science. 2005;307:373–375. doi: 10.1126/science.1104342. [DOI] [PubMed] [Google Scholar]
- 23.Rajala MW, Scherer PE. Minireview: The adipocyte--at the crossroads of energy homeostasis, inflammation, and atherosclerosis. Endocrinology. 2003;144:3765–3773. doi: 10.1210/en.2003-0580. [DOI] [PubMed] [Google Scholar]
- 24.Kadowaki T, Yamauchi T, Kubota N, Hara K, Ueki K, Tobe K. Adiponectin and adiponectin receptors in insulin resistance, diabetes, and the metabolic syndrome. J Clin Invest. 2006;116:1784–1792. doi: 10.1172/JCI29126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kahn BB, Flier JS. Obesity and insulin resistance. J Clin Invest. 2000;106:473–481. doi: 10.1172/JCI10842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xu H, Barnes GT, Yang Q, Tan G, Yang D, Chou CJ, et al. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J Clin Invest. 2003;112:1821–1830. doi: 10.1172/JCI19451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW., Jr Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest. 2003;112:1796–1808. doi: 10.1172/JCI19246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Takahashi K, Mizuarai S, Araki H, Mashiko S, Ishihara A, Kanatani A, et al. Adiposity elevates plasma MCP-1 levels leading to the increased CD11b-positive monocytes in mice. J Biol Chem. 2003;278:46654–46660. doi: 10.1074/jbc.M309895200. [DOI] [PubMed] [Google Scholar]
- 29.Saltiel AR, Kahn CR. Insulin signalling and the regulation of glucose and lipid metabolism. Nature. 2001;414:799–806. doi: 10.1038/414799a. [DOI] [PubMed] [Google Scholar]
- 30.White MF. IRS proteins and the common path to diabetes. Am J Physiol Endocrinol Metab. 2002;283:E413–E422. doi: 10.1152/ajpendo.00514.2001. [DOI] [PubMed] [Google Scholar]
- 31.Rui L, Aguirre V, Kim JK, Shulman GI, Lee A, Corbould A, et al. Insulin/IGF-1 and TNF-alpha stimulate phosphorylation of IRS-1 at inhibitory Ser307 via distinct pathways. J Clin Invest. 2001;107:181–189. doi: 10.1172/JCI10934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gao Z, Zhang X, Zuberi A, Hwang D, Quon MJ, Lefevre M, et al. Inhibition of Insulin Sensitivity by Free Fatty Acids Requires Activation of Multiple Serine Kinases in 3T3-L1 Adipocytes. Mol Endocrinol. 2004;18:2024–2034. doi: 10.1210/me.2003-0383. [DOI] [PubMed] [Google Scholar]
- 33.Nguyen MT, Satoh H, Favelyukis S, Babendure JL, Imamura T, Sbodio JI, et al. JNK and tumor necrosis factor-alpha mediate free fatty acid-induced insulin resistance in 3T3-L1 adipocytes. J Biol Chem. 2005;280:35361–35371. doi: 10.1074/jbc.M504611200. [DOI] [PubMed] [Google Scholar]
- 34.Gao Z, Hwang D, Bataille F, Lefevre M, York D, Quon MJ, et al. Serine phosphorylation of insulin receptor substrate 1 (IRS-1) by inhibitor KappaB kinase (IKK) complex. J Biol Chem. 2002;277:48115–48121. doi: 10.1074/jbc.M209459200. [DOI] [PubMed] [Google Scholar]
- 35.Hirosumi J, Tuncman G, Chang L, Gorgun C, Uysal K, Maeda K, et al. A central role for JNK in obesity and insulin resistance. Nature. 2002;420:333–336. doi: 10.1038/nature01137. [DOI] [PubMed] [Google Scholar]
- 36.Griffin ME, Marcucci MJ, Cline GW, Bell K, Barucci N, Lee D, et al. Free fatty acid-induced insulin resistance is associated with activation of protein kinase C theta and alterations in the insulin signaling cascade. Diabetes. 1999;48:1270–1274. doi: 10.2337/diabetes.48.6.1270. [DOI] [PubMed] [Google Scholar]
- 37.Tremblay F, Brule S, Hee Um S, Li Y, Masuda K, Roden M, et al. Identification of IRS-1 Ser-1101 as a target of S6K1 in nutrient- and obesity-induced insulin resistance. PNAS. 2007;104:14056–14061. doi: 10.1073/pnas.0706517104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Suzawa M, Takada I, Yanagisawa J, Ohtake F, Ogawa S, Yamauchi T, et al. Cytokines suppress adipogenesis and PPAR-gamma function through the TAK1/TAB1/NIK cascade. Nat Cell Biol. 2003;5:224–230. doi: 10.1038/ncb942. [DOI] [PubMed] [Google Scholar]
- 39.Ruan H, Pownall HJ, Lodish HF. Troglitazone antagonizes TNF-alpha -induced reprogramming of adipocyte gene expression by inhibiting the transcriptional regulatory functions of NF-kB. J Biol Chem. 2003;278:28181–28192. doi: 10.1074/jbc.M303141200. [DOI] [PubMed] [Google Scholar]
- 40.Gao Z, He Q, Peng B, Chiao PJ, Ye J. Regulation of Nuclear Translocation of HDAC3 by I{kappa}B{alpha} Is Required for Tumor Necrosis Factor Inhibition of Peroxisome Proliferator-activated Receptor {gamma} Function. J Biol Chem. 2006;281:4540–4547. doi: 10.1074/jbc.M507784200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Spiegelman BM. PPAR-gamma: adipogenic regulator and thiazolidinedione receptor. Diabetes. 1998;47:507–514. doi: 10.2337/diabetes.47.4.507. [DOI] [PubMed] [Google Scholar]
- 42.Berger J, Moller DE. The mechanisms of action of PPARs. Annu Rev Med. 2002;53:409–435. doi: 10.1146/annurev.med.53.082901.104018. [DOI] [PubMed] [Google Scholar]
- 43.Lazar MA. Progress in cardiovascular biology: PPAR for the course. Nat Med. 2001;7:23–24. doi: 10.1038/83301. [DOI] [PubMed] [Google Scholar]
- 44.Yun Z, Maecker HL, Johnson RS, Giaccia AJ. Inhibition of PPAR gamma 2 gene expression by the HIF-1-regulated gene DEC1/Stra13: a mechanism for regulation of adipogenesis by hypoxia. Dev Cell. 2002;2:331–341. doi: 10.1016/s1534-5807(02)00131-4. [DOI] [PubMed] [Google Scholar]
- 45.Lolmede K, Durand de Saint Front V, Galitzky J, Lafontan M, Bouloumie A. Effects of hypoxia on the expression of proangiogenic factors in differentiated 3T3-F442A adipocytes. Int J Obes Relat Metab Disord. 2003;27:1187–1195. doi: 10.1038/sj.ijo.0802407. [DOI] [PubMed] [Google Scholar]
- 46.Chen B, Lam KS, Wang Y, Wu D, Lam MC, Shen J, et al. Hypoxia dysregulates the production of adiponectin and plasminogen activator inhibitor-1 independent of reactive oxygen species in adipocytes. Biochem Biophys Res Commun. 2006;341:549–556. doi: 10.1016/j.bbrc.2006.01.004. [DOI] [PubMed] [Google Scholar]
- 47.Wang B, Wood IS, Trayhurn P. Dysregulation of the expression and secretion of inflammation-related adipokines by hypoxia in human adipocytes. Pflugers Arch. 2007 doi: 10.1007/s00424-007-0301-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Trayhurn P, Wang B, Wood IS. Hypoxia in adipose tissue: a basis for the dysregulation of tissue function in obesity? Br J Nutr. 2008:1–9. doi: 10.1017/S0007114508971282. [DOI] [PubMed] [Google Scholar]
- 49.Trayhurn P, Wood IS. Adipokines: inflammation and the pleiotropic role of white adipose tissue. Br J Nutr. 2004;92:347–355. doi: 10.1079/bjn20041213. [DOI] [PubMed] [Google Scholar]
- 50.Rausch ME, Weisberg S, Vardhana P, Tortoriello DV. Obesity in C57BL/6J mice is characterized by adipose tissue hypoxia and cytotoxic T-cell infiltration. Int J Obes (Lond) 2008;32:451–463. doi: 10.1038/sj.ijo.0803744. [DOI] [PubMed] [Google Scholar]
- 51.Kabon B, Nagele A, Reddy D, Eagon C, Fleshman JW, Sessler DI, et al. Obesity decreases perioperative tissue oxygenation. Anesthesiology. 2004;100:274–280. doi: 10.1097/00000542-200402000-00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fleischmann E, Kurz A, Niedermayr M, Schebesta K, Kimberger O, Sessler DI, et al. Tissue oxygenation in obese and non-obese patients during laparoscopy. Obes Surg. 2005;15:813–819. doi: 10.1381/0960892054222867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Urtasun RC, Koch CJ, Franko AJ, Raleigh JA, Chapman JD. A novel technique for measuring human tissue pO2 at the cellular level. Br J Cancer. 1986;54:453–457. doi: 10.1038/bjc.1986.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Semenza G. Signal transduction to hypoxia-inducible factor 1. Biochem Pharmacol. 2002;64:993–998. doi: 10.1016/s0006-2952(02)01168-1. [DOI] [PubMed] [Google Scholar]
- 55.Papandreou I, Cairns RA, Fontana L, Lim AL, Denko NC. HIF-1 mediates adaptation to hypoxia by actively downregulating mitochondrial oxygen consumption. Cell Metab. 2006;3:187–197. doi: 10.1016/j.cmet.2006.01.012. [DOI] [PubMed] [Google Scholar]
- 56.Treins C, Giorgetti-Peraldi S, Murdaca J, Semenza GL, Van Obberghen E. Insulin stimulates hypoxia-inducible factor 1 through a phosphatidylinositol 3-kinase/target of rapamycin-dependent signaling pathway. J Biol Chem. 2002;277:27975–27981. doi: 10.1074/jbc.M204152200. [DOI] [PubMed] [Google Scholar]
- 57.Semenza GL, Roth PH, Fang HM, Wang GL. Transcriptional regulation of genes encoding glycolytic enzymes by hypoxia-inducible factor 1. J Biol Chem. 1994;269:23757–23763. [PubMed] [Google Scholar]
- 58.Iyer NV, Kotch LE, Agani F, Leung SW, Laughner E, Wenger RH, et al. Cellular and developmental control of O2 homeostasis by hypoxia-inducible factor 1 alpha. Genes Dev. 1998;12:149–162. doi: 10.1101/gad.12.2.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cancello R, Henegar C, Viguerie N, Taleb S, Poitou C, Rouault C, et al. Reduction of macrophage infiltration and chemoattractant gene expression changes in white adipose tissue of morbidly obese subjects after surgery-induced weight loss. Diabetes. 2005;54:2277–2286. doi: 10.2337/diabetes.54.8.2277. [DOI] [PubMed] [Google Scholar]
- 60.Higami Y, Barger JL, Page GP, Allison DB, Smith SR, Prolla TA, et al. Energy restriction lowers the expression of genes linked to inflammation, the cytoskeleton, the extracellular matrix, and angiogenesis in mouse adipose tissue. J Nutr. 2006;136:343–352. doi: 10.1093/jn/136.2.343. [DOI] [PubMed] [Google Scholar]
- 61.Wang GL, Jiang BH, Rue EA, Semenza GL. Hypoxia-inducible factor 1 is a basic-helix-loop-helix-PAS heterodimer regulated by cellular O2 tension. Proc Natl Acad Sci U S A. 1995;92:5510–5514. doi: 10.1073/pnas.92.12.5510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wang GL, Semenza GL. Purification and characterization of hypoxia-inducible factor 1. J Biol Chem. 1995;270:1230–1237. doi: 10.1074/jbc.270.3.1230. [DOI] [PubMed] [Google Scholar]
- 63.Semenza GL, Agani F, Feldser D, Iyer N, Kotch L, Laughner E, et al. Hypoxia, HIF-1, and the pathophysiology of common human diseases. Adv Exp Med Biol. 2000;475:123–130. doi: 10.1007/0-306-46825-5_12. [DOI] [PubMed] [Google Scholar]
- 64.Yu AY, Frid MG, Shimoda LA, Wiener CM, Stenmark K, Semenza GL. Temporal, spatial, and oxygen-regulated expression of hypoxia-inducible factor-1 in the lung. Am J Physiol. 1998;275:L818–L826. doi: 10.1152/ajplung.1998.275.4.L818. [DOI] [PubMed] [Google Scholar]
- 65.Bergeron M, Yu AY, Solway KE, Semenza GL, Sharp FR. Induction of hypoxia-inducible factor-1 (HIF-1) and its target genes following focal ischaemia in rat brain. Eur J Neurosci. 1999;11:4159–4170. doi: 10.1046/j.1460-9568.1999.00845.x. [DOI] [PubMed] [Google Scholar]
- 66.Minet E, Michel G, Mottet D, Raes M, Michiels C. Transduction pathways involved in Hypoxia-Inducible Factor-1 phosphorylation and activation. Free Radic Biol Med. 2001;31:847–855. doi: 10.1016/s0891-5849(01)00657-8. [DOI] [PubMed] [Google Scholar]
- 67.Fukuda R, Hirota K, Fan F, Jung YD, Ellis LM, Semenza GL. Insulin-like growth factor 1 induces hypoxia-inducible factor 1-mediated vascular endothelial growth factor expression, which is dependent on MAP kinase and phosphatidylinositol 3-kinase signaling in colon cancer cells. J Biol Chem. 2002;277:38205–38211. doi: 10.1074/jbc.M203781200. [DOI] [PubMed] [Google Scholar]
- 68.Richard DE, Berra E, Gothie E, Roux D, Pouyssegur J. p42/p44 mitogen-activated protein kinases phosphorylate hypoxia-inducible factor 1alpha (HIF-1alpha) and enhance the transcriptional activity of HIF-1. J Biol Chem. 1999;274:32631–32637. doi: 10.1074/jbc.274.46.32631. [DOI] [PubMed] [Google Scholar]
- 69.Phillips RJ, Mestas J, Gharaee-Kermani M, Burdick MD, Sica A, Belperio JA, et al. Epidermal Growth Factor and Hypoxia-induced Expression of CXC Chemokine Receptor 4 on Non-small Cell Lung Cancer Cells Is Regulated by the Phosphatidylinositol 3-Kinase/PTEN/AKT/Mammalian Target of Rapamycin Signaling Pathway and Activation of Hypoxia Inducible Factor-1{alpha} J Biol Chem. 2005;280:22473–22481. doi: 10.1074/jbc.M500963200. [DOI] [PubMed] [Google Scholar]
- 70.Semenza GL. HIF-1 and human disease: one highly involved factor. Genes Dev. 2000;14:1983–1991. [PubMed] [Google Scholar]
- 71.Coban YK, Kurutas EB, Ciralik H. Ischemia-reperfusion injury of adipofascial tissue: an experimental study evaluating early histologic and biochemical alterations in rats. Mediators Inflamm. 2005;2005:304–308. doi: 10.1155/MI.2005.304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Michiels C, Minet E, Mottet D, Raes M. Regulation of gene expression by oxygen: NF-kappaB and HIF-1, two extremes. Free Radic Biol Med. 2002;33:1231–1242. doi: 10.1016/s0891-5849(02)01045-6. [DOI] [PubMed] [Google Scholar]
- 73.Murdoch C, Muthana M, Lewis CE. Hypoxia regulates macrophage functions in inflammation. J Immunol. 2005;175:6257–6263. doi: 10.4049/jimmunol.175.10.6257. [DOI] [PubMed] [Google Scholar]
- 74.Lewis JS, Lee JA, Underwood JC, Harris AL, Lewis CE. Macrophage responses to hypoxia: relevance to disease mechanisms. J Leukoc Biol. 1999;66:889–900. doi: 10.1002/jlb.66.6.889. [DOI] [PubMed] [Google Scholar]
- 75.Pouyssegur J, Dayan F, Mazure NM. Hypoxia signalling in cancer and approaches to enforce tumour regression. Nature. 2006;441:437–443. doi: 10.1038/nature04871. [DOI] [PubMed] [Google Scholar]
- 76.Haddad JJ. Science review: redox and oxygen-sensitive transcription factors in the regulation of oxidant-mediated lung injury: role for hypoxia-inducible factor-1alpha. Crit Care. 2003;7:47–54. doi: 10.1186/cc1840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Karin M. Nuclear factor-kappaB in cancer development and progression. Nature. 2006;441:431–436. doi: 10.1038/nature04870. [DOI] [PubMed] [Google Scholar]
- 78.Baeuerle PA, Henkel T. Function and activation of NF-kappa B in the immune system. Annu Rev Immunol. 1994;12:141–179. doi: 10.1146/annurev.iy.12.040194.001041. [DOI] [PubMed] [Google Scholar]
- 79.Baldwin AS., Jr The NF-kappa B and I kappa B proteins: new discoveries and insights. Annu Rev Immunol. 1996;14:649–683. doi: 10.1146/annurev.immunol.14.1.649. [DOI] [PubMed] [Google Scholar]
- 80.Ghosh S, Karin M. Missing pieces in the NF-kappaB puzzle. Cell. 2002;109 Suppl:S81–S96. doi: 10.1016/s0092-8674(02)00703-1. [DOI] [PubMed] [Google Scholar]
- 81.Ghosh S, May MJ, Kopp EB. NF-kappa B and Rel proteins: evolutionarily conserved mediators of immune responses. Ann Rev Immunol. 1998;16:225–260. doi: 10.1146/annurev.immunol.16.1.225. [DOI] [PubMed] [Google Scholar]
- 82.Hayden MS, Ghosh S. Shared Principles in NF-[kappa]B Signaling. Cell. 2008;132:344–362. doi: 10.1016/j.cell.2008.01.020. [DOI] [PubMed] [Google Scholar]
- 83.Rosen ED, Spiegelman BM. Adipocytes as regulators of energy balance and glucose homeostasis. Nature. 2006;444:847–853. doi: 10.1038/nature05483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Odegaard JI, Ricardo-Gonzalez RR, Goforth MH, Morel CR, Subramanian V, Mukundan L, et al. Macrophage-specific PPAR[ggr] controls alternative activation and improves insulin resistance. Nature. 2007;447:1116–1120. doi: 10.1038/nature05894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hevener AL, Olefsky JM, Reichart D, Nguyen MTA, Bandyopadyhay G, Leung H-Y, et al. Macrophage PPAR{gamma} is required for normal skeletal muscle and hepatic insulin sensitivity and full antidiabetic effects of thiazolidinediones. J Clin Invest. 2007;117:1658–1669. doi: 10.1172/JCI31561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kanda H, Tateya S, Tamori Y, Kotani K, Hiasa K-i, Kitazawa R, et al. MCP-1 contributes to macrophage infiltration into adipose tissue, insulin resistance, and hepatic steatosis in obesity. J Clin Invest. 2006;116:1494–1505. doi: 10.1172/JCI26498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kamei N, Tobe K, Suzuki R, Ohsugi M, Watanabe T, Kubota N, et al. Overexpression of Monocyte Chemoattractant Protein-1 in Adipose Tissues Causes Macrophage Recruitment and Insulin Resistance. J Biol Chem. 2006;281:26602–26614. doi: 10.1074/jbc.M601284200. [DOI] [PubMed] [Google Scholar]
- 88.Inouye KE, Shi H, Howard JK, Daly CH, Lord GM, Rollins BJ, et al. Absence of CC chemokine ligand 2 does not limit obesity-associated infiltration of macrophages into adipose tissue. Diabetes. 2007;56:2242–2250. doi: 10.2337/db07-0425. [DOI] [PubMed] [Google Scholar]
- 89.Cho C-H, Jun Koh Y, Han J, Sung H-K, Jong Lee H, Morisada T, et al. Angiogenic Role of LYVE-1-Positive Macrophages in Adipose Tissue. Circ Res. 2007;100:e47–e57. doi: 10.1161/01.RES.0000259564.92792.93. [DOI] [PubMed] [Google Scholar]
- 90.Turner L, Scotton C, Negus R, Balkwill F. Hypoxia inhibits macrophage migration. Eur J Immunol. 1999;29:2280–2287. doi: 10.1002/(SICI)1521-4141(199907)29:07<2280::AID-IMMU2280>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 91.Nishihira J. Novel pathophysiological aspects of macrophage migration inhibitory factor (review) Int J Mol Med. 1998;2:17–28. doi: 10.3892/ijmm.2.1.17. [DOI] [PubMed] [Google Scholar]
- 92.Koong AC, Denko NC, Hudson KM, Schindler C, Swiersz L, Koch C, et al. Candidate genes for the hypoxic tumor phenotype. Cancer Res. 2000;60:883–887. [PubMed] [Google Scholar]
- 93.Calandra T, Bernhagen J, Metz CN, Spiegel LA, Bacher M, Donnelly T, et al. MIF as a glucocorticoid-induced modulator of cytokine production. Nature. 1995;377:68–71. doi: 10.1038/377068a0. [DOI] [PubMed] [Google Scholar]
- 94.Roger T, David J, Glauser MP, Calandra T. MIF regulates innate immune responses through modulation of Toll-like receptor 4. Nature. 2001;414:920–924. doi: 10.1038/414920a. [DOI] [PubMed] [Google Scholar]
- 95.Cramer T, Yamanishi Y, Clausen BE, Forster I, Pawlinski R, Mackman N, et al. HIF-1alpha is essential for myeloid cell-mediated inflammation. Cell. 2003;112:645–657. doi: 10.1016/s0092-8674(03)00154-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Skurk T, Herder C, Kraft I, Muller-Scholze S, Hauner H, Kolb H. Production and release of macrophage migration inhibitory factor from human adipocytes. Endocrinology. 2005;146:1006–1011. doi: 10.1210/en.2004-0924. [DOI] [PubMed] [Google Scholar]
- 97.Hirokawa J, Sakaue S, Furuya Y, Ishii J, Hasegawa A, Tagami S, et al. Tumor necrosis factor-alpha regulates the gene expression of macrophage migration inhibitory factor through tyrosine kinase-dependent pathway in 3T3-L1 adipocytes. J Biochem (Tokyo) 1998;123:733–739. doi: 10.1093/oxfordjournals.jbchem.a021998. [DOI] [PubMed] [Google Scholar]
- 98.Vozarova B, Stefan N, Hanson R, Lindsay RS, Bogardus C, Tataranni PA, et al. Plasma concentrations of macrophage migration inhibitory factor are elevated in Pima Indians compared to Caucasians and are associated with insulin resistance. Diabetologia. 2002;45:1739–1741. doi: 10.1007/s00125-002-0896-4. [DOI] [PubMed] [Google Scholar]
- 99.Yin J, Gao Z, He Q, Ye J. Role of hypoxia in obesity-induced disorders of glucose and lipid metabolism in adipose tissue. AJP-E&M. 2008 doi: 10.1152/ajpendo.90760.2008. (in revision) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Scherer PE, Williams S, Fogliano M, Baldini G, Lodish HF. A Novel Serum Protein Similar to C1q, Produced Exclusively in Adipocytes. J Biol Chem. 1995;270:26746–26749. doi: 10.1074/jbc.270.45.26746. [DOI] [PubMed] [Google Scholar]
- 101.Maeda K, Okubo K, Shimomura I, Funahashi T, Matsuzawa Y, Matsubara K. cDNA cloning and expression of a novel adipose specific collagen-like factor, apM1 (AdiPose Most abundant Gene transcript 1) Biochem Biophys Res Commun. 1996;221:286–289. doi: 10.1006/bbrc.1996.0587. [DOI] [PubMed] [Google Scholar]
- 102.Hu E, Liang P, Spiegelman BM. AdipoQ is a novel adipose-specific gene dysregulated in obesity. J Biol Chem. 1996;271:10697–10703. doi: 10.1074/jbc.271.18.10697. [DOI] [PubMed] [Google Scholar]
- 103.Nakano Y, Tobe T, Choi-Miura NH, Mazda T, Tomita M. Isolation and characterization of GBP28, a novel gelatin-binding protein purified from human plasma. J Biochem (Tokyo) 1996;120:803–812. doi: 10.1093/oxfordjournals.jbchem.a021483. [DOI] [PubMed] [Google Scholar]
- 104.Berg AH, Combs TP, Scherer PE. ACRP30/adiponectin: an adipokine regulating glucose and lipid metabolism. Trends Endocrinol Metab. 2002;13:84–89. doi: 10.1016/s1043-2760(01)00524-0. [DOI] [PubMed] [Google Scholar]
- 105.Hotta K, Funahashi T, Arita Y, Takahashi M, Matsuda M, Okamoto Y, et al. Plasma concentrations of a novel, adipose-specific protein, adiponectin, in type 2 diabetic patients. Arterioscler Thromb Vasc Biol. 2000;20:1595–1599. doi: 10.1161/01.atv.20.6.1595. [DOI] [PubMed] [Google Scholar]
- 106.Lindsay RS, Funahashi T, Hanson RL, Matsuzawa Y, Tanaka S, Tataranni PA, et al. Adiponectin and development of type 2 diabetes in the Pima Indian population. Lancet. 2002;360:57–58. doi: 10.1016/S0140-6736(02)09335-2. [DOI] [PubMed] [Google Scholar]
- 107.Menzaghi C, Ercolino T, Di Paola R, Berg AH, Warram JH, Scherer PE, et al. A haplotype at the adiponectin locus is associated with obesity and other features of the insulin resistance syndrome. Diabetes. 2002;51:2306–2312. doi: 10.2337/diabetes.51.7.2306. [DOI] [PubMed] [Google Scholar]
- 108.Matsubara M, Maruoka S, Katayose S. Decreased plasma adiponectin concentrations in women with dyslipidemia. J Clin Endocrinol Metab. 2002;87:2764–2769. doi: 10.1210/jcem.87.6.8550. [DOI] [PubMed] [Google Scholar]
- 109.Stefan N, Vozarova B, Funahashi T, Matsuzawa Y, Weyer C, Lindsay RS, et al. Plasma adiponectin concentration is associated with skeletal muscle insulin receptor tyrosine phosphorylation, and low plasma concentration precedes a decrease in whole-body insulin sensitivity in humans. Diabetes. 2002;51:1884–1888. doi: 10.2337/diabetes.51.6.1884. [DOI] [PubMed] [Google Scholar]
- 110.Fantuzzi G. Adiponectin and inflammation: consensus and controversy. J Allergy Clin Immunol. 2008;121:326–330. doi: 10.1016/j.jaci.2007.10.018. [DOI] [PubMed] [Google Scholar]
- 111.Fasshauer M, Klein J, Neumann S, Eszlinger M, Paschke R. Hormonal regulation of adiponectin gene expression in 3T3-L1 adipocytes. Biochem Biophys Res Commun. 2002;290:1084–1089. doi: 10.1006/bbrc.2001.6307. [DOI] [PubMed] [Google Scholar]
- 112.Iwaki M, Matsuda M, Maeda N, Funahashi T, Matsuzawa Y, Makishima M, et al. Induction of Adiponectin, a Fat-Derived Antidiabetic and Antiatherogenic Factor, by Nuclear Receptors. Diabetes. 2003;52:1655–1663. doi: 10.2337/diabetes.52.7.1655. [DOI] [PubMed] [Google Scholar]
- 113.Seo JB, Moon HM, Noh MJ, Lee YS, Jeong HW, Yoo EJ, et al. Adipocyte determination- and differentiation-dependent factor 1/sterol regulatory elementbinding protein 1c regulates mouse adiponectin expression. J Biol Chem. 2004;279:22108–22117. doi: 10.1074/jbc.M400238200. [DOI] [PubMed] [Google Scholar]
- 114.Qiao L, Shao J. SIRT1 regulates adiponectin gene expression through Foxo1-C/enhancer-binding protein alpha transcriptional complex. J Biol Chem. 2006;281:39915–39924. doi: 10.1074/jbc.M607215200. [DOI] [PubMed] [Google Scholar]
- 115.Jiang B-H, Jiang G, Zheng JZ, Lu Z, Hunter T, Vogt PK. Phosphatidylinositol 3-Kinase Signaling Controls Levels of Hypoxia-inducible Factor 1. Cell Growth Differ. 2001;12:363–369. [PubMed] [Google Scholar]
- 116.Accili D, Arden KC. FoxOs at the crossroads of cellular metabolism, differentiation, and transformation. Cell. 2004;117:421–426. doi: 10.1016/s0092-8674(04)00452-0. [DOI] [PubMed] [Google Scholar]
- 117.Teng X, Li D, Catravas JD, Johns RA. C/EBP-beta mediates iNOS induction by hypoxia in rat pulmonary microvascular smooth muscle cells. Circ Res. 2002;90:125–127. doi: 10.1161/hh0202.103647. [DOI] [PubMed] [Google Scholar]
- 118.Yan SF, Tritto I, Pinsky D, Liao H, Huang J, Fuller G, et al. Induction of interleukin 6 (IL-6) by hypoxia in vascular cells. Central role of the binding site for nuclear factor-IL-6. J Biol Chem. 1995;270:11463–11471. doi: 10.1074/jbc.270.19.11463. [DOI] [PubMed] [Google Scholar]
- 119.Benoit SC, Clegg DJ, Seeley RJ, Woods SC. Insulin and leptin as adiposity signals. Recent Prog Horm Res. 2004;59:267–285. doi: 10.1210/rp.59.1.267. [DOI] [PubMed] [Google Scholar]
- 120.Gettys TW, Harkness PJ, Watson PM. The beta 3-adrenergic receptor inhibits insulin-stimulated leptin secretion from isolated rat adipocytes. Endocrinology. 1996;137:4054–4057. doi: 10.1210/endo.137.9.8756584. [DOI] [PubMed] [Google Scholar]
- 121.Ambrosini G, Nath AK, Sierra-Honigmann MR, Flores-Riveros J. Transcriptional activation of the human leptin gene in response to hypoxia. Involvement of hypoxia-inducible factor 1. J Biol Chem. 2002;277:34601–34609. doi: 10.1074/jbc.M205172200. [DOI] [PubMed] [Google Scholar]
- 122.Grosfeld A, Andre J, Hauguel-De Mouzon S, Berra E, Pouyssegur J, Guerre-Millo M. Hypoxia-inducible factor 1 transactivates the human leptin gene promoter. J Biol Chem. 2002;277:42953–42957. doi: 10.1074/jbc.M206775200. [DOI] [PubMed] [Google Scholar]
- 123.Grosfeld A, Zilberfarb V, Turban S, Andre J, Guerre-Millo M, Issad T. Hypoxia increases leptin expression in human PAZ6 adipose cells. Diabetologia. 2002;45:527–530. doi: 10.1007/s00125-002-0804-y. [DOI] [PubMed] [Google Scholar]
- 124.Meissner U, Spranger R, Lehner M, Allabauer I, Rascher W, Dotsch J. Hypoxia-induced leptin production in human trophoblasts does not protect from apoptosis. Eur J Endocrinol. 2005;153:455–461. doi: 10.1530/eje.1.01979. [DOI] [PubMed] [Google Scholar]
- 125.Cammisotto PG, Bukowiecki LJ. Mechanisms of leptin secretion from white adipocytes. Am J Physiol Cell Physiol. 2002;283:C244–C250. doi: 10.1152/ajpcell.00033.2002. [DOI] [PubMed] [Google Scholar]
- 126.Wang B, Wood IS, Trayhurn P. Hypoxia induces leptin gene expression and secretion in human preadipocytes: differential effects of hypoxia on adipokine expression by preadipocytes. J Endocrinol. 2008;198:127–134. doi: 10.1677/JOE-08-0156. [DOI] [PubMed] [Google Scholar]
- 127.Yasumasu T, Takahara K, Nakashima Y. Hypoxia inhibits leptin production by cultured rat adipocytes. Obes Res. 2002;10:128. doi: 10.1038/oby.2002.20. [DOI] [PubMed] [Google Scholar]
- 128.Weinberger B, Carbone T, England S, Kleinfeld AM, Hiatt M, Hegyi T. Effects of perinatal hypoxia on serum unbound free fatty acids and lung inflammatory mediators. Biol Neonate. 2001;79:61–66. doi: 10.1159/000047067. [DOI] [PubMed] [Google Scholar]
- 129.Rodrigo J, Fernandez AP, Serrano J, Peinado MA, Martinez A. The role of free radicals in cerebral hypoxia and ischemia. Free Radic Biol Med. 2005;39:26–50. doi: 10.1016/j.freeradbiomed.2005.02.010. [DOI] [PubMed] [Google Scholar]
- 130.Dowling HJ, Fried SK, Pi-Sunyer FX. Insulin resistance in adipocytes of obese women: effects of body fat distribution and race. Metabolism. 1995;44:987–995. doi: 10.1016/0026-0495(95)90094-2. [DOI] [PubMed] [Google Scholar]
- 131.Nicklas BJ, Rogus EM, Colman EG, Goldberg AP. Visceral adiposity, increased adipocyte lipolysis, and metabolic dysfunction in obese postmenopausal women. Am J Physiol. 1996;270:E72–E78. doi: 10.1152/ajpendo.1996.270.1.E72. [DOI] [PubMed] [Google Scholar]
- 132.Zierath JR, Livingston JN, Thorne A, Bolinder J, Reynisdottir S, Lonnqvist F, et al. Regional difference in insulin inhibition of non-esterified fatty acid release from human adipocytes: relation to insulin receptor phosphorylation and intracellular signalling through the insulin receptor substrate-1 pathway. Diabetologia. 1998;41:1343–1354. doi: 10.1007/s001250051075. [DOI] [PubMed] [Google Scholar]
- 133.Mittelman SD, Van Citters GW, Kirkman EL, Bergman RN. Extreme insulin resistance of the central adipose depot in vivo. Diabetes. 2002;51:755–761. doi: 10.2337/diabetes.51.3.755. [DOI] [PubMed] [Google Scholar]
- 134.Kang SG, Brown AL, Chung JH. Oxygen Tension Regulates the Stability of Insulin Receptor Substrate-1 (IRS-1) through Caspase-mediated Cleavage. J Biol Chem. 2007;282:6090–6097. doi: 10.1074/jbc.M610659200. [DOI] [PubMed] [Google Scholar]
- 135.Jaworski K, Sarkadi-Nagy E, Duncan RE, Ahmadian M, Sul HS. Regulation of triglyceride metabolism. IV. Hormonal regulation of lipolysis in adipose tissue. Am J Physiol Gastrointest Liver Physiol. 2007;293:G1–G4. doi: 10.1152/ajpgi.00554.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Evans RM, Barish GD, Wang YX. PPARs and the complex journey to obesity. Nat Med. 2004;10:355–361. doi: 10.1038/nm1025. [DOI] [PubMed] [Google Scholar]
- 137.Kahn BB, Alquier T, Carling D, Hardie DG. AMP-activated protein kinase: Ancient energy gauge provides clues to modern understanding of metabolism. Cell Metabolism. 2005;1:15–25. doi: 10.1016/j.cmet.2004.12.003. [DOI] [PubMed] [Google Scholar]
- 138.Liu L, Cash TP, Jones RG, Keith B, Thompson CB, Simon MC. Hypoxia-induced energy stress regulates mRNA translation and cell growth. Mol Cell. 2006;21:521–531. doi: 10.1016/j.molcel.2006.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Nakatani Y, Kaneto H, Kawamori D, Hatazaki M, Miyatsuka T, Matsuoka T-a, et al. Modulation of the JNK Pathway in Liver Affects Insulin Resistance Status. J Biol Chem. 2004;279:45803–45809. doi: 10.1074/jbc.M406963200. [DOI] [PubMed] [Google Scholar]
- 140.Jaeschke A, Czech MP, Davis RJ. An essential role of the JIP1 scaffold protein for JNK activation in adipose tissue. Genes Dev. 2004;18:1976–1980. doi: 10.1101/gad.1216504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Aguirre V, Uchida T, Yenush L, Davis R, White MF. The c-Jun NH(2)-terminal kinase promotes insulin resistance during association with insulin receptor substrate-1 and phosphorylation of Ser(307) J Biol Chem. 2000;275:9047–9054. doi: 10.1074/jbc.275.12.9047. [DOI] [PubMed] [Google Scholar]
- 142.Ozawa K, Miyazaki M, Matsuhisa M, Takano K, Nakatani Y, Hatazaki M, et al. The Endoplasmic Reticulum Chaperone Improves Insulin Resistance in Type 2 Diabetes. Diabetes. 2005;54:657–663. doi: 10.2337/diabetes.54.3.657. [DOI] [PubMed] [Google Scholar]
- 143.Ozcan U, Yilmaz E, Ozcan L, Furuhashi M, Vaillancourt E, Smith RO, et al. Chemical chaperones reduce ER stress and restore glucose homeostasis in a mouse model of type 2 diabetes. Science. 2006;313:1137–1140. doi: 10.1126/science.1128294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Koumenis C, Naczki C, Koritzinsky M, Rastani S, Diehl A, Sonenberg N, et al. Regulation of protein synthesis by hypoxia via activation of the endoplasmic reticulum kinase PERK and phosphorylation of the translation initiation factor eIF2alpha. Mol Cell Biol. 2002;22:7405–7416. doi: 10.1128/MCB.22.21.7405-7416.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Carriere A, Carmona MC, Fernandez Y, Rigoulet M, Wenger RH, Penicaud L, et al. Mitochondrial reactive oxygen species control the transcription factor CHOP-10/GADD153 and adipocyte differentiation: a mechanism for hypoxia-dependent effect. J Biol Chem. 2004;279:40462–40469. doi: 10.1074/jbc.M407258200. [DOI] [PubMed] [Google Scholar]
- 146.Keijer J, van Schothorst EM. Adipose tissue failure and mitochondria as a possible target for improvement by bioactive food components. Curr Opin Lipidol. 2008;19:4–10. doi: 10.1097/MOL.0b013e3282f39f95. [DOI] [PubMed] [Google Scholar]
- 147.Zhang H, Gao P, Fukuda R, Kumar G, Krishnamachary B, Zeller KI, et al. HIF-1 Inhibits Mitochondrial Biogenesis and Cellular Respiration in VHL-Deficient Renal Cell Carcinoma by Repression of C-MYC Activity. Cancer Cell. 2007;11:407–420. doi: 10.1016/j.ccr.2007.04.001. [DOI] [PubMed] [Google Scholar]
- 148.Kim JW, Tchernyshyov I, Semenza GL, Dang CV. HIF-1-mediated expression of pyruvate dehydrogenase kinase: a metabolic switch required for cellular adaptation to hypoxia. Cell Metab. 2006;3:177–185. doi: 10.1016/j.cmet.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 149.Summers LK, Samra JS, Humphreys SM, Morris RJ, Frayn KN. Subcutaneous abdominal adipose tissue blood flow: variation within and between subjects and relationship to obesity. Clin Sci (Lond) 1996;91:679–683. doi: 10.1042/cs0910679. [DOI] [PubMed] [Google Scholar]
- 150.Jansson PA, Larsson A, Lonnroth PN. Relationship between blood pressure, metabolic variables and blood flow in obese subjects with or without non-insulin-dependent diabetes mellitus. Eur J Clin Invest. 1998;28:813–818. doi: 10.1046/j.1365-2362.1998.00360.x. [DOI] [PubMed] [Google Scholar]
- 151.Karpe F, Fielding BA, Ilic V, Macdonald IA, Summers LK, Frayn KN. Impaired postprandial adipose tissue blood flow response is related to aspects of insulin sensitivity. Diabetes. 2002;51:2467–2473. doi: 10.2337/diabetes.51.8.2467. [DOI] [PubMed] [Google Scholar]
- 152.West DB, Prinz WA, Francendese AA, Greenwood MR. Adipocyte blood flow is decreased in obese Zucker rats. Am J Physiol. 1987;253:R228–R233. doi: 10.1152/ajpregu.1987.253.2.R228. [DOI] [PubMed] [Google Scholar]
- 153.Bolinder J, Kerckhoffs DA, Moberg E, Hagstrom-Toft E, Arner P. Rates of skeletal muscle and adipose tissue glycerol release in nonobese and obese subjects. Diabetes. 2000;49:797–802. doi: 10.2337/diabetes.49.5.797. [DOI] [PubMed] [Google Scholar]
- 154.Kampf C, Bodin B, Kallskog O, Carlsson C, Jansson L. Marked increase in white adipose tissue blood perfusion in the type 2 diabetic GK rat. Diabetes. 2005;54:2620–2627. doi: 10.2337/diabetes.54.9.2620. [DOI] [PubMed] [Google Scholar]
- 155.Crandall DL, Goldstein BM, Huggins F, Cervoni P. Adipocyte blood flow: influence of age, anatomic location, and dietary manipulation. Am J Physiol. 1984;247:R46–R51. doi: 10.1152/ajpregu.1984.247.1.R46. [DOI] [PubMed] [Google Scholar]
- 156.Pang C, Gao Z, Yin J, Zhang J, Jia W, Ye J. Macrophage Infiltration into Adipose Tissue May Promote Angiogenesis for Adipose Tissue Remodeling in Obesity. Am J Physiol Endocrinol Metab. 2008;295:E313–E322. doi: 10.1152/ajpendo.90296.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Paul M, Poyan Mehr A, Kreutz R. Physiology of local renin-angiotensin systems. Physiol Rev. 2006;86:747–803. doi: 10.1152/physrev.00036.2005. [DOI] [PubMed] [Google Scholar]
- 158.Carey RM, Siragy HM. Newly recognized components of the renin-angiotensin system: potential roles in cardiovascular and renal regulation. Endocr Rev. 2003;24:261–271. doi: 10.1210/er.2003-0001. [DOI] [PubMed] [Google Scholar]
- 159.Engeli S, Negrel R, Sharma AM. Physiology and pathophysiology of the adipose tissue renin-angiotensin system. Hypertension. 2000;35:1270–1277. doi: 10.1161/01.hyp.35.6.1270. [DOI] [PubMed] [Google Scholar]
- 160.Kershaw EE, Flier JS. Adipose tissue as an endocrine organ. J Clin Endocrinol Metab. 2004;89:2548–2556. doi: 10.1210/jc.2004-0395. [DOI] [PubMed] [Google Scholar]
- 161.Goossens GH, McQuaid SE, Dennis AL, van Baak MA, Blaak EE, Frayn KN, et al. Angiotensin II: a major regulator of subcutaneous adipose tissue blood flow in humans. J Physiol. 2006;571:451–460. doi: 10.1113/jphysiol.2005.101352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Goossens GH, Blaak EE, Saris WHM, van Baak MA. Angiotensin II-Induced Effects on Adipose and Skeletal Muscle Tissue Blood Flow and Lipolysis in Normal-Weight and Obese Subjects. J Clin Endocrinol Metab. 2004;89:2690–2696. doi: 10.1210/jc.2003-032053. [DOI] [PubMed] [Google Scholar]
- 163.Henriksen EJ, Jacob S, Kinnick TR, Teachey MK, Krekler M. Selective angiotensin II receptor receptor antagonism reduces insulin resistance in obese Zucker rats. Hypertension. 2001;38:884–890. doi: 10.1161/hy1101.092970. [DOI] [PubMed] [Google Scholar]
- 164.Liu Z. The renin-angiotensin system and insulin resistance. Curr Diab Rep. 2007;7:34–42. doi: 10.1007/s11892-007-0007-5. [DOI] [PubMed] [Google Scholar]
- 165.Torres Filho IP, Leunig M, Yuan F, Intaglietta M, Jain RK. Noninvasive measurement of microvascular and interstitial oxygen profiles in a human tumor in SCID mice. Proc Natl Acad Sci U S A. 1994;91:2081–2085. doi: 10.1073/pnas.91.6.2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Helmlinger G, Yuan F, Dellian M, Jain RK. Interstitial pH and pO2 gradients in solid tumors in vivo: high-resolution measurements reveal a lack of correlation. Nat Med. 1997;3:177–182. doi: 10.1038/nm0297-177. [DOI] [PubMed] [Google Scholar]
- 167.Bluher M, Wilson-Fritch L, Leszyk J, Laustsen PG, Corvera S, Kahn CR. Role of insulin action and cell size on protein expression patterns in adipocytes. J Biol Chem. 2004;279:31902–31909. doi: 10.1074/jbc.M404570200. [DOI] [PubMed] [Google Scholar]
- 168.Nishimura S, Manabe I, Nagasaki M, Hosoya Y, Yamashita H, Fujita H, et al. Adipogenesis in obesity requires close interplay between differentiating adipocytes, stromal cells, and blood vessels. Diabetes. 2007;56:1517–1526. doi: 10.2337/db06-1749. [DOI] [PubMed] [Google Scholar]
- 169.Gariano RF, Gardner TW. Retinal angiogenesis in development and disease. Nature. 2005;438:960–966. doi: 10.1038/nature04482. [DOI] [PubMed] [Google Scholar]
- 170.Cao Y. Angiogenesis modulates adipogenesis and obesity. J Clin Invest. 2007;117:2362–2368. doi: 10.1172/JCI32239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Carmeliet P. Angiogenesis in life, disease and medicine. Nature. 2005;438:932–936. doi: 10.1038/nature04478. [DOI] [PubMed] [Google Scholar]
- 172.Carmeliet P, Jain RK. Angiogenesis in cancer and other diseases. Nature. 2000;407:249–257. doi: 10.1038/35025220. [DOI] [PubMed] [Google Scholar]
- 173.Greenberg DA, Jin K. From angiogenesis to neuropathology. Nature. 2005;438:954–959. doi: 10.1038/nature04481. [DOI] [PubMed] [Google Scholar]
- 174.Harris AL. Hypoxia--a key regulatory factor in tumour growth. Nat Rev Cancer. 2002;2:38–47. doi: 10.1038/nrc704. [DOI] [PubMed] [Google Scholar]
- 175.Semenza GL. HIF-1 and tumor progression: pathophysiology and therapeutics. Trends Mol Med. 2002;8:S62–S67. doi: 10.1016/s1471-4914(02)02317-1. [DOI] [PubMed] [Google Scholar]
- 176.Hausman GJ, Richardson RL. Adipose tissue angiogenesis. J Anim Sci. 2004;82:925–934. doi: 10.2527/2004.823925x. [DOI] [PubMed] [Google Scholar]
- 177.Fukumura D, Ushiyama A, Duda DG, Xu L, Tam J, Krishna V, et al. Paracrine regulation of angiogenesis and adipocyte differentiation during in vivo adipogenesis. Circ Res. 2003;93:e88–e97. doi: 10.1161/01.RES.0000099243.20096.FA. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Sierra-Honigmann MR, Nath AK, Murakami C, Garcia-Cardena G, Papapetropoulos A, Sessa WC, et al. Biological action of leptin as an angiogenic factor. Science. 1998;281:1683–1686. doi: 10.1126/science.281.5383.1683. [DOI] [PubMed] [Google Scholar]
- 179.Shibata R, Ouchi N, Kihara S, Sato K, Funahashi T, Walsh K. Adiponectin stimulates angiogenesis in response to tissue ischemia through stimulation of amp-activated protein kinase signaling. J Biol Chem. 2004;279:28670–28674. doi: 10.1074/jbc.M402558200. [DOI] [PubMed] [Google Scholar]
- 180.Ferrara N, Kerbel RS. Angiogenesis as a therapeutic target. Nature. 2005;438:967–974. doi: 10.1038/nature04483. [DOI] [PubMed] [Google Scholar]
- 181.Neels JG, Thinnes T, Loskutoff DJ. Angiogenesis in an in vivo model of adipose tissue development. Faseb J. 2004;18:983–985. doi: 10.1096/fj.03-1101fje. [DOI] [PubMed] [Google Scholar]
- 182.Kolonin MG, Saha PK, Chan L, Pasqualini R, Arap W. Reversal of obesity by targeted ablation of adipose tissue. Nat Med. 2004;10:625–632. doi: 10.1038/nm1048. [DOI] [PubMed] [Google Scholar]
- 183.Rupnick MA, Panigrahy D, Zhang CY, Dallabrida SM, Lowell BB, Langer R, et al. Adipose tissue mass can be regulated through the vasculature. Proc Natl Acad Sci U S A. 2002;99:10730–10735. doi: 10.1073/pnas.162349799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Zhang QX, Magovern CJ, Mack CA, Budenbender KT, Ko W, Rosengart TK. Vascular endothelial growth factor is the major angiogenic factor in omentum: mechanism of the omentum-mediated angiogenesis. J Surg Res. 1997;67:147–154. doi: 10.1006/jsre.1996.4983. [DOI] [PubMed] [Google Scholar]
- 185.Miyazawa-Hoshimoto S, Takahashi K, Bujo H, Hashimoto N, Saito Y. Elevated serum vascular endothelial growth factor is associated with visceral fat accumulation in human obese subjects. Diabetologia. 2003;46:1483–1488. doi: 10.1007/s00125-003-1221-6. [DOI] [PubMed] [Google Scholar]
- 186.Miyazawa-Hoshimoto S, Takahashi K, Bujo H, Hashimoto N, Yagui K, Saito Y. Roles of degree of fat deposition and its localization on VEGF expression in adipocytes. Am J Physiol Endocrinol Metab. 2005;288:E1128–E1136. doi: 10.1152/ajpendo.00003.2004. [DOI] [PubMed] [Google Scholar]
- 187.Strohl KP, Novak RD, Singer W, Cahan C, Boehm KD, Denko CW, et al. Insulin levels, blood pressure and sleep apnea. Sleep. 1994;17:614–618. doi: 10.1093/sleep/17.7.614. [DOI] [PubMed] [Google Scholar]
- 188.Vgontzas AN, Papanicolaou DA, Bixler EO, Hopper K, Lotsikas A, Lin HM, et al. Sleep apnea and daytime sleepiness and fatigue: relation to visceral obesity, insulin resistance, and hypercytokinemia. J Clin Endocrinol Metab. 2000;85:1151–1158. doi: 10.1210/jcem.85.3.6484. [DOI] [PubMed] [Google Scholar]
- 189.Ip MS, Lam B, Ng MM, Lam WK, Tsang KW, Lam KS. Obstructive sleep apnea is independently associated with insulin resistance. Am J Respir Crit Care Med. 2002;165:670–676. doi: 10.1164/ajrccm.165.5.2103001. [DOI] [PubMed] [Google Scholar]
- 190.Punjabi NM, Sorkin JD, Katzel LI, Goldberg AP, Schwartz AR, Smith PL. Sleep-disordered breathing and insulin resistance in middle-aged and overweight men. Am J Respir Crit Care Med. 2002;165:677–682. doi: 10.1164/ajrccm.165.5.2104087. [DOI] [PubMed] [Google Scholar]
- 191.Gastaut H, Tassinari CA, Duron B. Polygraphic study of the episodic diurnal and nocturnal (hypnic and respiratory) manifestations of the Pickwick syndrome. Brain Res. 1966;1:167–186. doi: 10.1016/0006-8993(66)90117-x. [DOI] [PubMed] [Google Scholar]
- 192.Larsen JJ, Hansen JM, Olsen NV, Galbo H, Dela F. The effect of altitude hypoxia on glucose homeostasis in men. J Physiol. 1997;504(Pt 1):241–249. doi: 10.1111/j.1469-7793.1997.241bf.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Braun B, Rock PB, Zamudio S, Wolfel GE, Mazzeo RS, Muza SR, et al. Women at altitude: short-term exposure to hypoxia and/or alpha(1)-adrenergic blockade reduces insulin sensitivity. J Appl Physiol. 2001;91:623–631. doi: 10.1152/jappl.2001.91.2.623. [DOI] [PubMed] [Google Scholar]
- 194.Polotsky VY, Li J, Punjabi NM, Rubin AE, Smith PL, Schwartz AR, et al. Intermittent hypoxia increases insulin resistance in genetically obese mice. J Physiol (Lond) 2003;552:253–264. doi: 10.1113/jphysiol.2003.048173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Calderon R, Llerena LA, Munive L, Kruger F. Intravenous glucose tolerance test in pregnancy in women living in chronic hypoxia. Diabetes. 1966;15:130–132. doi: 10.2337/diab.15.2.130. [DOI] [PubMed] [Google Scholar]
- 196.Davidson MB, Aoki VS. Fasting glucose homeostasis in rats after chronic exposure to hypoxia. Am J Physiol. 1970;219:378–383. doi: 10.1152/ajplegacy.1970.219.2.378. [DOI] [PubMed] [Google Scholar]
- 197.Iiyori N, Alonso LC, Li J, Sanders MH, Garcia-Ocana A, O'Doherty RM, et al. Intermittent Hypoxia Causes Insulin Resistance in Lean Mice Independent of Autonomic Activity. Am J Respir Crit Care Med. 2007 doi: 10.1164/rccm.200610-1527OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Bulow J, Madsen J. Adipose tissue blood flow during prolonged, heavy exercise. Pflugers Arch. 1976;363:231–234. doi: 10.1007/BF00594606. [DOI] [PubMed] [Google Scholar]
- 199.Bulow J. Adipose tissue blood flow during exercise. Dan Med Bull. 1983;30:85–100. [PubMed] [Google Scholar]
- 200.Mulla NA, Simonsen L, Bulow J. Post-exercise adipose tissue and skeletal muscle lipid metabolism in humans: the effects of exercise intensity. J Physiol. 2000;524(Pt 3):919–928. doi: 10.1111/j.1469-7793.2000.00919.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Linde B, Hjemdahl P, Freyschuss U, Juhlin-Dannfelt A. Adipose tissue and skeletal muscle blood flow during mental stress. Am J Physiol. 1989;256:E12–E18. doi: 10.1152/ajpendo.1989.256.1.E12. [DOI] [PubMed] [Google Scholar]
- 202.Engfeldt P, Linde B. Subcutaneous adipose tissue blood flow in the abdominal and femoral regions in obese women: effect of fasting. Int J Obes Relat Metab Disord. 1992;16:875–879. [PubMed] [Google Scholar]
- 203.Coppack SW, Fisher RM, Gibbons GF, Humphreys SM, McDonough MJ, Potts JL, et al. Postprandial substrate deposition in human forearm and adipose tissues in vivo. Clin Sci (Lond) 1990;79:339–348. doi: 10.1042/cs0790339. [DOI] [PubMed] [Google Scholar]
- 204.Bulow J, Astrup A, Christensen NJ, Kastrup J. Blood flow in skin, subcutaneous adipose tissue and skeletal muscle in the forearm of normal man during an oral glucose load. Acta Physiol Scand. 1987;130:657–661. doi: 10.1111/j.1748-1716.1987.tb08189.x. [DOI] [PubMed] [Google Scholar]
- 205.Samra JS, Frayn KN, Giddings JA, Clark ML, Macdonald IA. Modification and validation of a commercially available portable detector for measurement of adipose tissue blood flow. Clin Physiol. 1995;15:241–248. doi: 10.1111/j.1475-097x.1995.tb00515.x. [DOI] [PubMed] [Google Scholar]
- 206.Freyschuss U, Hjemdahl P, Juhlin-Dannfelt A, Linde B. Cardiovascular and metabolic responses to low dose adrenaline infusion: an invasive study in humans. Clin Sci (Lond) 1986;70:199–206. doi: 10.1042/cs0700199. [DOI] [PubMed] [Google Scholar]
- 207.Hjemdahl P, Linde B. Influence of circulating NE and Epi on adipose tissue vascular resistance and lipolysis in humans. Am J Physiol. 1983;245:H447–H452. doi: 10.1152/ajpheart.1983.245.3.H447. [DOI] [PubMed] [Google Scholar]
- 208.Samra JS, Simpson EJ, Clark ML, Forster CD, Humphreys SM, Macdonald IA, et al. Effects of epinephrine infusion on adipose tissue: interactions between blood flow and lipid metabolism. Am J Physiol. 1996;271:E834–E839. doi: 10.1152/ajpendo.1996.271.5.E834. [DOI] [PubMed] [Google Scholar]
- 209.Ardilouze J-L, Fielding BA, Currie JM, Frayn KN, Karpe F. Nitric Oxide and {beta}-Adrenergic Stimulation Are Major Regulators of Preprandial and Postprandial Subcutaneous Adipose Tissue Blood Flow in Humans. Circulation. 2004;109:47–52. doi: 10.1161/01.CIR.0000105681.70455.73. [DOI] [PubMed] [Google Scholar]