Abstract
The Canadian Hypertension Education Program annually appraises data from hypertension research and updates clinical practice recommendation for the diagnosis and management of hypertension. Enormous effort is devoted to disseminating these recommendations to target groups throughout the country and, through the use of institutional databases, to evaluating their effectiveness in improving the health of Canadians by lowering blood pressure in people with hypertension. The mission of the Canadian Hypertension Education Program is to reduce the impact of hypertension on cardiovascular disease in Canada.
Keywords: Clinical practice guidelines, Consensus, Hypertension, Knowledge translation
Abstract
Le Programme éducatif canadien sur l’hypertension évalue tous les ans les données des recherches sur l’hypertension et met à jour les recommandations de pratique clinique pour le diagnostic et la prise en charge de l’hypertension. On consent d’énormes efforts à la diffusion de ces recommandations à des groupes cibles du pays entier et, au moyen de bases de données d’établissements, à l’évaluation de leur efficacité à améliorer la vie des Canadiens en réduisant la tension artérielle des personnes hypertensives. La mission du Programme éducatif canadien sur l’hypertension consiste à réduire les répercussions de l’hypertension sur les maladies cardiovasculaires au Canada.
The Canadian Hypertension Education Program (CHEP) is a unique knowledge translation program. The Canadian Institutes of Health Research has referred to knowledge translation as “the process of supporting the uptake of health research in a manner that improves the health and health care of Canadians through improved understandings, processes, services, products or systems” (1). Thus, the Canadian Institutes of Health Research recognizes that it is through research that hypotheses are tested and new knowledge is created, but that this process alone does not serve the health of Canadians well unless the research findings are able to improve the health of those whom it may benefit. CHEP appraises the best data from clinical hypertension research on an annual basis and updates a set of clinical practice recommendations for the management of hypertension. Furthermore, great effort is devoted to disseminating these recommendations to target groups throughout the country and, through the use of institutional databases, to evaluating their effectiveness in improving the health of Canadians by lowering blood pressure in those with hypertension. The mission of CHEP is to reduce the impact of hypertension on cardiovascular disease in Canada. We believe that CHEP has the world’s most advanced and reliable evidence-based recommendations process.
CHEP was designed to address issues that often prove to be critical shortfalls in national and international hypertension guidelines, as described by McAlister (2). CHEP has broad endorsement from its sponsoring and partner organizations (Table 1). CHEP has an annual process recognizing that among the 15 subgroups, some may have insufficient evidence in a particular year to update the recommendations. The annual consensus conference focuses on the subgroups that identify change substantive enough to potentially alter the recommendations. If no change has been made by a subcommittee over a number of years, the subgroup is asked to conduct a thorough review of its recommendations to generate a position paper. The position paper is then reviewed at the annual consensus conference, along with the associated recommendations and references, to ensure that it remains current.
TABLE 1.
Canadian Hypertension Education Program sponsoring and partner organizations
Sponsoring organizations
|
Partner organizations
|
In 2006, to ensure that different groups did not provide conflicting guidelines and recommendations, CHEP initiated a harmonization process with the Canadian Diabetes Association and the Canadian Society of Nephrology. Subcommittees of the Evidence-Based Recommendations Task Force, with overlap among these guidelines organizations, were populated with members from each and given a mandate to ensure harmonization of their shared recommendations. These members work with each program to ensure that hypertension guidelines among CHEP, the Canadian Diabetes Association and the Canadian Society of Nephrology are identical, and that the wording for the recommendations among the groups is the same. The goal of this effort is to reduce the confusion that may occur in the primary care community if efforts by each separate program to wordsmith the recommendations lead to products that could be interpreted differently.
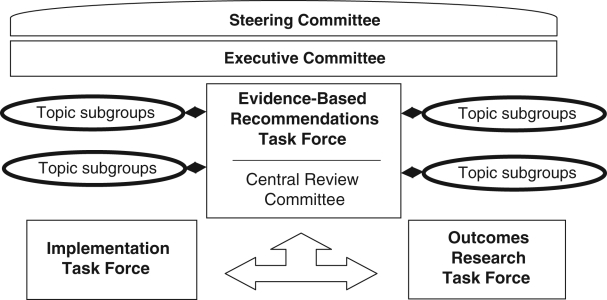
The CHEP Evidence-Based Recommendations Task Force (Figure 1 shows how the task force fits within the CHEP organizational chart) consists of 15 subcommittees with topic-specific content experts (Table 2). To recognize the public health risk posed by the underdiagnosis and undertreatment of hypertension in Canada, the Evidence-Based Recommendations Task Force aims to improve awareness, management and control of hypertension by providing updated recommendations. These are formulated based on ethical, moral, noncommercial and rigorous principles undertaken with transparency. Each subcommittee on this task force annually reviews literature searches conducted by a Cochrane librarian (using standard systematic review search strategies with search terms suggested by the subcommittee) and interprets the evidence arising from similar studies (2). The subcommittees then submit their review, proposed levels of evidence to the recommendations and suggestions for updates to the Central Review Committee (Table 3), a group of clinical epidemiologists with no conflicts of interest. The Central Review Committee applies a standardized set of rules of evidence to ensure that the grading is consistent and that the evidence has been critically appraised in a uniform manner (2). The recommendations are then presented and debated at the annual consensus conference, which is attended by all members of the Evidence-Based Recommendations Task Force and the Central Review Committee. Steering Committee members representing the sponsoring and partnering organizations are invited to observe the process. The recommendations are then finalized and sent to all members of the Evidence-Based Recommendations Task Force for voting. A 70% level of support for each recommendation is required to be included in each year’s finalized recommendations.
Figure 1).
The Canadian Hypertension Education Program organizational chart
TABLE 2.
Members of the Evidence-Based Recommendations Task Force, subcommittees and the Central Review Committee
| Evidence-Based Recommendations Task Force: S Tobe (Chair), R Touyz (Vice-Chair) |
| Accurate Measurement of Blood Pressure: C Abbott (Chair), K Mann, L Cloutier |
| Adherence Strategies for Patients: R Feldman (Chair), A Milot, J Stone, T Campbell |
| Follow-up of Blood Pressure: P Bolli (Chair), G Tremblay |
| Risk Assessment: S Grover (Chair), G Tremblay, A Milot |
| Self-Measurement of Blood Pressure: D McKay (Chair), A Chockalingam |
| Ambulatory Blood Pressure Monitoring: M Myers (Chair), S Rabkin, M Dawes |
| Routine Laboratory Testing: T Wilson (Chair), B Penner, E Burgess |
| Echocardiography: G Honos (Chair) |
| Lifestyle Modification: R Touyz (Chair), N Campbell, N Gledhill, A Logan, R Petrella, L Trudeau |
| Pharmacotherapy of Hypertension in Patients Without Other Compelling Indications: R Lewanczuk (Chair), G Carruthers, J DeChamplain, G Fodor, P Hamet, R Herman, G Pylypchuk |
| Pharmacotherapy for Hypertension in Patients with Cardiovascular Disease: S Rabkin (Chair), M Arnold, G Moe, J-M Boulanger |
| Diabetes: P Larochelle (Chair), L Leiter, R Ogilvie, C Jones, S Tobe, V Woo, P McFarlane |
| Renal and Renovascular Hypertension: S Tobe (Chair), B Culleton, K Burns, M Ruzicka |
| Endocrine Forms of Hypertension: E Schiffrin (Chair) |
| Vascular Protection: R Feldman (Chair), R Hegele, P McFarlane, E Schiffrin |
| Central Review Committee: B Hemmelgarn (Chair), R Padwal, N Khan, F McAlister, M Hill, J Mahon |
TABLE 3.
The Canadian Hypertension Education Program themes for 2007
|
CHEP is sponsored by The Canadian Hypertension Society, the Canadian Coalition for High Blood Pressure Prevention and Control, The College of Family Physicians of Canada, the Heart and Stroke Foundation of Canada, and the Public Health Agency of Canada. Each year, the cardiovascular medication manufacturers provide equal unrestricted educational grants to CHEP to defray the costs of the medical librarian, literature searches, annual consensus conferences and implementation tools. Pharmaceutical company representatives or executives have no input into the literature searches, the interpretation of the evidence, the generation and approval of the recommendations, or the writing and approval of the manuscripts. They also do not attend the consensus conferences or receive copies of the recommendations before their public presentation at the annual Canadian Cardiovascular Congress.
CHEP takes its commitment to reduce the potential for conflicts of interest and bias seriously, and has adopted the following policies:
CHEP has a history of requiring a high level of evidence in terms of patient outcomes for pharmacotherapy recommendations. Thus, one of the unique features of the CHEP recommendations process is that when there is no evidence, CHEP does not advance an opinion-based alternative and leaves the area blank. An example that generates controversy each year is the lack of a recommendation for the use of an angiotensin-converting enzyme inhibitor for the management of isolated systolic hypertension, because no randomized clinical trials have sufficiently addressed this issue (see Treatment Recommendations in the current issue of the Journal [pages 539–550]). This policy has led CHEP to be very consistent in its recommendations over its history.
Each year, the subgroups appraise the quality of any recommendations arising from relevant articles using a standardized scheme as described above (Figures 2 to 5).
Multiple members in most subgroups represent the opportunity for different views. All members of the Evidence-Based Recommendations Task Force are volunteers and academic hypertension specialists from many medical and multiprofessional specialties and backgrounds.
Critical to eliminating bias and to the smooth functioning of the Evidence-Based Recommendations Task Force, the Central Review Committee, which is free of conflicts of interest, oversees the evaluation of evidence and the development of recommendations, and presents the evidence and recommendations at the annual consensus conference. Debates are encouraged and consensus is often achieved. Contentious issues that cannot make it past the consensus conference are reviewed again over the subsequent year and re-evaluated at the next consensus conference, during which, often with new evidence, a consensus can be reached for or against the issue.
Each attendee of the consensus conference has an overt, written disclosure of potential conflicts of interest at the time of development of the recommendations, and this is appended to each member’s consensus conference materials. It is recognized that members of the subcommittees do have some conflicts of interest, and they are instructed not to vote on issues with which they do have conflict.
A consensus approach is used for the drafting of the recommendations, led by the chair of the Central Review Committee.
Following the consensus conference, the recommendations are voted on, and new recommendations are removed if voted against by 30% of members.
Each year, themes, key messages and major implementation tools are developed through consensus of the full executive. Other internal implementation tools require the consensus of two members of the executive. The 2007 themes are found in Table 3.
External implementation tools must be completely consistent with the content and intent of the CHEP recommendations, and require a consensus of three members of the executive. By limiting the endorsement of sponsored implementation tools, such as continuing education activities, CHEP ensures that its content is adhered to rigorously. At its business planning retreat in May 2006, the CHEP executive prioritized minimizing the potential impact of bias, and developed and endorsed these policies.
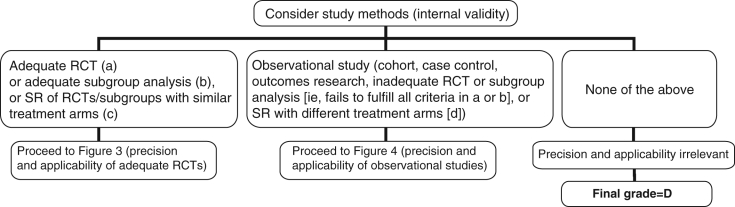
Figure 2).
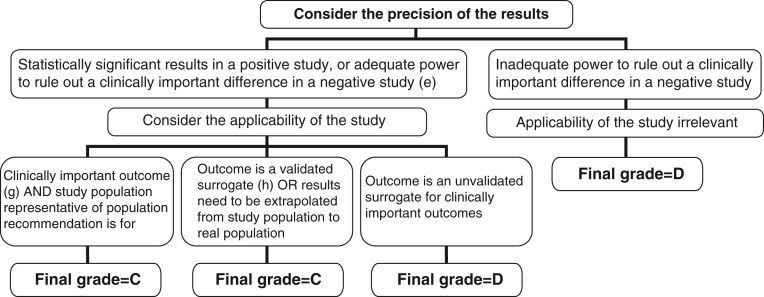
Algorithm for assigning evidence grades to therapy recommendations (step 1); (a) Randomized controlled trial (RCT) with blinded assessment of outcomes, intention-to-treat analysis, adequate follow-up (ie, at least 90%, or losses to follow-up are too few to materially affect the results) and sufficient sample size to detect a clinically important difference with power >80%; (b) Subgroup analysis was a priori, performed within an adequate RCT and one of only a few tested, and there was sufficient sample size within the examined subgroup to detect a clinically important difference with power >80%; (c) Sytematic review (SR), or meta-analysis, in which the comparison arms are derived from head-to-head comparisons within the same RCT; (d) SR in which the comparison arms are derived from different placebo-controlled RCTs and then extrapolations are made across RCTs (continued in Figures 3 and 4)
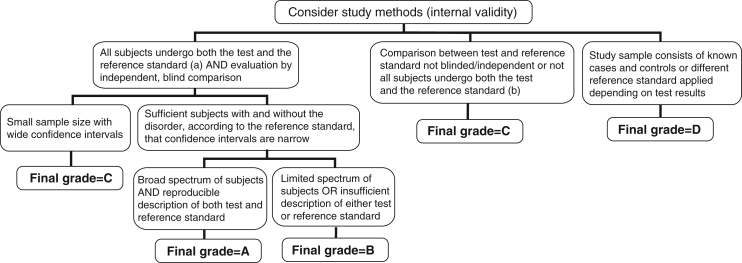
Figure 5).
Algorithm for assigning evidence grades to diagnostic recommendations; (a) The gold standard. This can be either another test that is currently accepted as the gold standard or analysis of a representative cohort of patients who underwent the test of interest and are followed for a sufficient length of time that occurrence of the target outcome is likely if the diagnosis is present (with adjustment for covariates associated with prognosis); (b) Note that if follow-up of a cohort is not sufficiently long or complete enough to rule out diagnostic errors, or if data are not adjusted for covariates, this category would apply
IMPLEMENTATION
The objectives of the CHEP Implementation Task Force are to disseminate the CHEP recommendations through evidence-based implementation and dissemination strategies, which are designed to increase the uptake and use of the recommendations including multifaceted, multimedia and multiaudience strategies. A list of annual Implementation Task Force activities is found in Table 4. To maximize the dissemination process, CHEP uses the following strategies: full scientific manuscripts, short clinical and scientific summaries tailored to different audiences, handouts, posters, pocket cards, advertisements, educational slide kits, text books, slide sets and workshops, online resources, multiple knowledge translation strategies and rigorous evaluation (3).
TABLE 4.
Implementation Task Force dissemination strategies
|
EVALUATION
The Outcomes Task Force
The goals of the Outcomes Task Force, in collaboration with the Public Health Agency of Canada and provincial organizations, are to develop a national surveillance system for hypertension. Ongoing projects include physical measures surveys, analysis of IMS Canada data on prescription drug use among hypertensive patients, involvement in national questionnaire surveys of hypertension management treatment and control, as well as linkage of national hospitalization and mortality data with provincial administrative databases. There is already compelling evidence that prescribing habits in one Canadian province have changed to reflect the CHEP recommendations (4).
In conclusion, CHEP is a volunteer-based, academic, multiprofessional organization. It was designed to translate ongoing research into clinical practice recommendations for the primary care community, to disseminate these recommendations and then to assess whether these recommendation have been effective at improving outcomes.
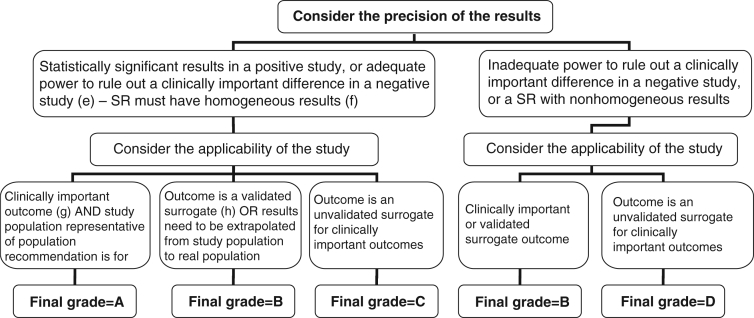
Figure 3).
Algorithm for assigning evidence grades to therapy recommendations (continued from Figure 2 – for adequate randomized controlled trials [RCTs], systematic reviews [SRs] or subgroup analyses); (e) Adequate power in a negative study implies that 95% CI exclude a clinically important difference; (f) Effect estimates in each study included in the systematic review are qualitatively similar (ie, in the same direction); (g) ‘Hard’ end points such as death, stroke, myocardial infarction and hospitalization; (h) End points that have been consistently shown to be associated with the clinical end point in multiple studies (observational or RCT), and RCTs have consistently demonstrated that improvement in the surrogate translates into a consistent and predictable improvement in the clinical end point
Figure 4).
Algorithm for assigning evidence grades to therapy recommendations (continued from Figure 2 – for observational studies); (e) Adequate power in a negative study implies that 95% CI exclude a clinically important difference; (f) Effect estimates in each study included in the systematic review are qualitatively similar (ie, in the same direction); (g) ‘Hard’ end points such as death, stroke, myocardial infarction and hospitalization; (h) End points that have been consistently shown to be associated with the clinical end point in multiple studies (observational or randomized controlled trial [RCT]), and RCTs have consistently demonstrated that improvement in the surrogate translates into a consistent and predictable improvement in the clinical end point
REFERENCES
- 1.Graham ID.Knowledge Translation at CIHR. <www.irsc.gc.ca/e/documents/imha_ktatchir_e.pdf> (Version current at May 8, 2007).
- 2.McAlister FA, for the Canadian Hypertension Education Program The Canadian Hypertension Education Program – a unique Canadian initiative. Can J Cardiol. 2006;22:559–64. doi: 10.1016/s0828-282x(06)70277-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Drouin D, Campbell NR, Kaczorowski J. Implementation of recommendations on hypertension: The Canadian Hypertension Education Program. Can J Cardiol. 2006;22:595–8. doi: 10.1016/s0828-282x(06)70281-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Campbell NR, Tu K, Brant R, Duong-Hua M, McAlister FA, Canadian Hypertension Education Program Outcomes Research Task Force The impact of the Canadian Hypertension Education Program on antihypertensive prescribing trends. Hypertension. 2006;47:22–8. doi: 10.1161/01.HYP.0000196269.98463.fd. [DOI] [PubMed] [Google Scholar]