Abstract
Objectives
To study the effect of exercise and a high fat meal (HFM) on endothelial function.
Background
Postprandial lipemia and exercise oppose each other in terms of cardiovascular risk, however the mechanism of their interaction is not well understood.
Methods
Endothelial function was assessed by brachial artery flow mediated dilation (FMD), in eight healthy men before and after a HFM preceded (16–18 hrs) by rest, a single bout of continuous moderate intensity exercise (CME), and high intensity interval exercise (HIIE).
Results
Before the HFM, initial brachial artery diameters were similar in all trials (0.43±0.04 cm), but after the HFM basal diameter decreased only in the control (0.39±0.03) and CME (0.38±0.04) trials. Prior to the HFM, FMD/shear was improved by a single bout of CME (+20%, p<0.01) and HIIE (+45%, p<0.01, group differences, p<0.01), with no effect in the control trial. After the HFM (30, 120, and 240 mins), FMD decayed to a lesser extent with CME, but in a similar fashion to the control trial. In contrast FMD in the HIIE trial remained elevated following the exercise despite a clear meal-induced lipemia. Although, there were no correlations between vascular function and food-induced markers of cardiovascular risk, antioxidant status was strongly correlated with FMD (r=0.9, p<0.001).
Conclusion
These findings reveal a clinically relevant protective effect of acute exercise upon the vasculature that is clearly exercise intensity dependent and tightly related to exercise-induced antioxidant capacity.
Keywords: Interval training, endothelial function, high-fat meal
INTRODUCTION
Impaired endothelial function is central to the atherosclerotic disease process, and serves as a strong, independent risk factor for future cardiovascular disease and mortality (1,2). The ingestion of a high fat meal (HFM) acutely changes the blood lipid profile and reduces endothelial function for many hours after the meal (3). Thus, as a significant proportion of life is spent in the postprandial state, the factors leading to this transient impairment in endothelial function may well play a key role in the atherosclerotic disease process.
Interestingly, people who perform regular physical activity maintain low lipoprotein levels even after a HFM (4), but this ability is significantly attenuated when a 3 day period of inactivity proceeds the HFM. Recently, Gill and colleagues (3) clearly demonstrated that exercising for 90 minutes at 50% of maximal oxygen uptake (VO2max) 16–18 hours prior to HFM ingestion attenuated the reduction in endothelial function compared to the control situation without exercise. However, this study did not link the changes in endothelial function to either changes in blood lipid profile or a postprandial inflammatory response and only examined CME. Thus, the mechanism responsible for this exercise-induced improvement in postprandial endothelial function as well as the type of exercise that best protects endothelial function in the face of postprandial lipemia, have yet to be defined.
The co-ingestion of antioxidant vitamins with a HFM abolish the postprandial decrement in endothelial function (5), and it may be that exercise acts through a similar mechanism. Indeed, it is known that exercise training improves the antioxidant status in plasma (6) and even acutely there seems to be a mobilisation of antioxidants into muscle itself (7). Recently, we reported that HIIE training was superior to CME training in terms of improving the cardiovascular risk profile, including endothelial function, and total antioxidant status in plasma, of patients with post-infarction heart failure (6). However, whether this link between oxidative stress, vascular function, and exercise intensity is apparent with a single bout of exercise in healthy subjects, as employed by Gill and colleagues (3), is currently unknown.
Therefore this study sought to determine the efficacy of a single bout of two different, but clinically relevant, exercise regimens (6), in terms of their ability to attenuate the endothelial dysfunction induced by postprandial lipemia. It was hypothesized that the two very distinct, but isocaloric, exercise sessions of either HIIE or CME would attenuate the postprandial reduction in endothelial function in an intensity dependent manner and that this would be related to changes in plasma antioxidant status.
METHODS
Subjects
Eight healthy men participated in this study. Exclusion criteria were any known disease, orthopedic and/or neurological limitations to exercise, surgery during the intervention period, drug or alcohol abuse, or participation in another research study. The protocol was approved by the regional ethical committee for medical research, and the study conformed to the Declaration of Helsinki. Written informed consent was obtained from all subjects prior to inclusion to the study. For each individual subject, repeated tests were performed at the same time of day.
Study design
Nine days before the first exercise session, VO2max was determined during uphill treadmill running/walking as previously described (6). Each of the 8 volunteers participated in 3 randomized trials (HIIE, CME, and Control (no exercise)) with one week between each trial and the starting trial was randomized. The timeline for each trial is illustrated on the x-axis of Figure 1. Subjects were provided with standardized meals (Fjordland, Norway), which they consumed for two days prior to the 3 trials. Baseline-1 measurements were made in a rested (>48 hours) and fasted state (>8 hours) before performing either HIIE, CME, or the control (resting) trial on the day preceding the HFM. For the 16–18 hour period post-exercise or the control trial, prior to baseline-2 measurements, subjects abstained from exercise, caffeine, and alcohol. Following baseline-2 measurements subjects ingested the HFM. Endothelial function was then assessed and blood samples taken 30 min, 2 hours and 4 hours after finishing the HFM.
Figure 1. Endothelial function.
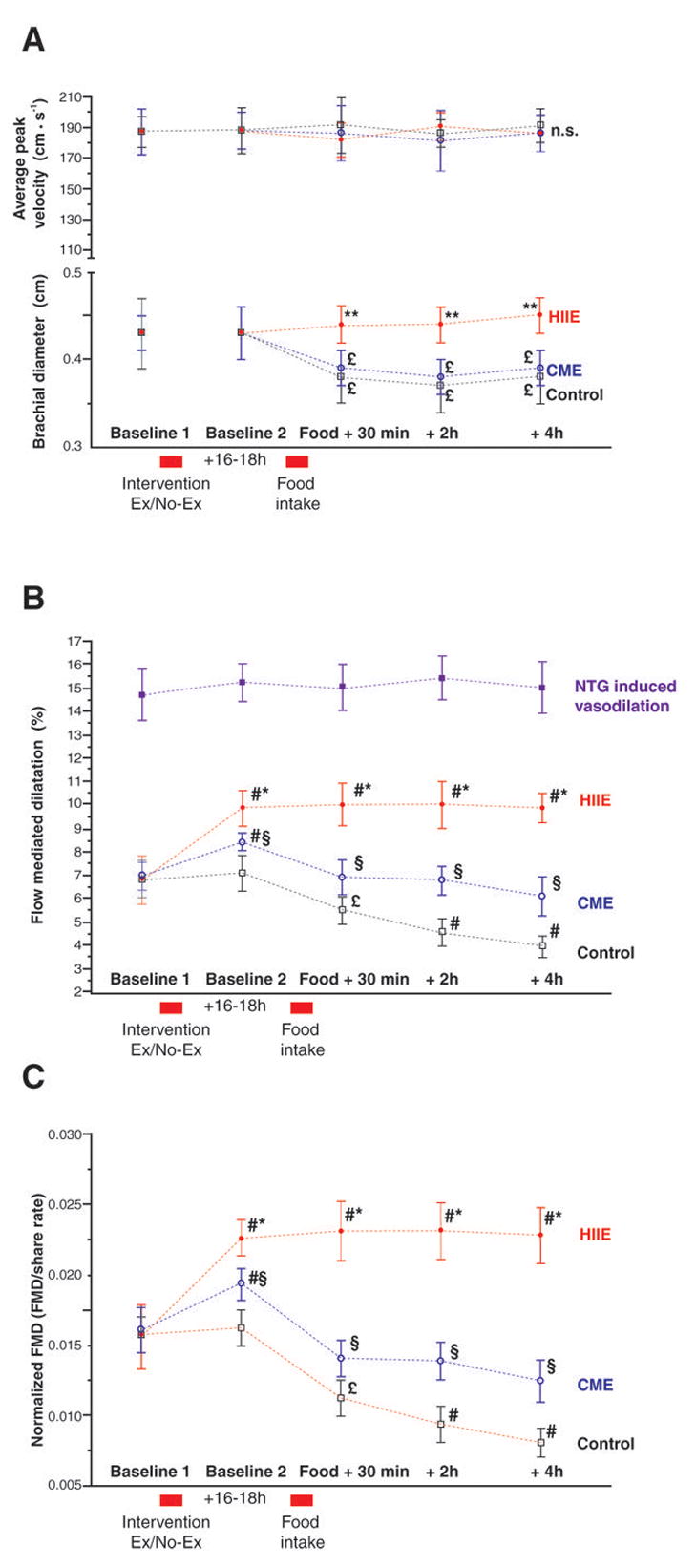
A: Average peak velocity and brachial diameter during the control (no exercise), continuous moderate intensity exercise (CME), high intensity aerobic interval exercise (HIIE), and sublingual glycerol trinitrate (GTN) trials. Baseline-1 is measured before the intervention, baseline-2 16–18 hours post-intervention and immediately before the meal. Thereafter FMD/artery diameter and blood flow were measured 30min, 2 hours and 4 hours after food intake. B: Flow mediated dilatation (FMD) of the brachial artery during the experiment. C: FMD normalized for share rate. # different from baseline 1, p<0.01; * different from moderate and control, p<0.01; § different from control, p<0.01; † different from control, p<0.05; £ different from baseline, p<0.05
Exercise
The HIIE was performed on a treadmill and consisted of a 10 min warm-up period at 50–60% of HRmax followed by 4 intervals of 4 min at an intensity that yielded 85–95 % of HRmax. Between the intervals, the subjects performed 3 min of active recovery at 50–60% of HRmax. The exercise session concluded with a 5 min cool-down period. To achieve an isocaloric protocol, the CME involved walking continuously for 47 minutes on the treadmill at 60–70% of HRmax (8).
HFM
The HFM consisted of a vegetarian mozzarella pizza (Dr. Oetker) with a total weight of 335g and the following nutritional composition: 48.3 g fat, 80.4 g carbohydrate, 38.5 g protein, and a total of 911.2 kcal. Pilot studies confirmed that in a rested state this meal produced a transient impairment in endothelial function.
Endothelial-dependent vascular function
Endothelial function was assessed by FMD of the brachial artery using vascular ultrasound (14 MHz echo Doppler probe, Vivid 7 System, GE Vingmed Ultrasound, Horten, Norway) according to the current guidelines (9). Briefly, measurements were performed on the artery approximately 4.5 cm above the antecubital fossa. After 10 minutes rest in the supine position in a quiet, air-conditioned room with a stable temperature of 22 ± 1°C, the internal diameter of the brachial artery was assessed. Thereafter, a pneumatic cuff (SC10, Hokanson Inc. Bellvue WA) just distal to the elbow on the lower arm was inflated to 250 mmHg for 5 minutes and deflated to create an ischemia-induced hyperemia. Blood velocity spectra were recorded 10 seconds after cuff-release to measure peak blood velocity and thereafter B-mode images were recorded for 5 minutes to assess artery diameter. To avoid confounding effects of arterial compliance and cyclic changes in arterial dimension, all measurements were obtained at the peak of the R-wave in the ECG (diastole). The mean of 3 diameter measurements (intima to intima) was recorded using calipers with a 0.1 mm resolution. Shear rate was calculated as blood velocity (cm · s−1) divided by vessel diameter (cm) according to (10). All ultrasound images were analyzed in random order, using EchoPAC® (GE Vingmed Ultrasound AS, Horten, Norway) by an investigator that was blinded to the treatment.
Endothelial-independent vascular function
In an additional set of subjects (n=8) the complete control protocol (no exercise) was reproduced, but endothelial-independent function was assessed by tracking brachial artery diameter with Doppler ultrasound, as described above, in response to the sublingual administration of 500 μg of glycerol trinitrate (GTN).
Blood profile measurements and analysis
Blood samples were taken in a fasted state (> 8 hours) and plasma triglycerides, HDL cholesterol, total cholesterol, hemoglobin, high sensitive C-reactive protein (hsCRP), glycosylated hemoglobin (HbA1c), glucose and insulin C-peptide and total antioxidant status analyzed as recently described (6).
Statistics
Repeated Measures Analysis of Variance (ANOVA) was performed to examine the differences between each measurement in each of the groups. One Way ANOVA and Mixed Factorial ANOVA was used to examine group differences. The Fisher least significant difference post hoc test was used when appropriate. All data are presented as mean ± SD if not otherwise stated.
RESULTS
Subject characteristics
Table 1 document the subject characteristics and reveals that despite being slightly overweight they exhibited a reasonable level of fitness, as assessed by VO2max. All blood parameters were within the normal range for normal healthy subjects of this age.
Table 1.
Subject characteristics
| Age, year | 42±4 |
| Height, cm | 179±3 |
| Weight, kg | 89.8±3.5 |
| BMI, kg/m2 | 28.8±0.9 |
| HRmax, beats/min | 189±5 |
| VO2max, ml/kg/min | 52.6±2.6 |
| FMD, % | 7.1±0.3 |
| FMD/Shear | 0.016±0.002 |
| Glucose, mmol/L | 5.01±0.24 |
| Cholesterol, mmol/L | 5.61±0.51 |
| Triglyceride, mmol/L | 1.18±0.10 |
| HDL, mmol/L | 1.25±0.08 |
| Hb, g/dL | 15.9±0.14 |
BMI, Body mass index. HRmax, maximal heart frequency. VO2max, maximal oxygen uptake. FMD, Flow mediated vasodilatation. HDL, High density lipoprotein, Hb, Haemoglobin. The table presents baseline characteristics. Data are mean ± SEM.
Basal vessel diameter, blood velocities and shear rates
Initial brachial artery diameters were similar at baseline-1 and baseline-2 in all trials (average 0.43±0.04 cm). However, there was a significant decrease (p<0.05), after the HFM in both the control and CME trial (0.39±0.03 and 0.38±0.04 cm, respectively), with no apparent effect in the HIIE trial (Figure 1A). Consequently, average peak shear rates were similar at baseline-1 and baseline-2, but increased in the control and CME trials and were unchanged in the HIIE trial at all time-points after the HFM (Figure 1A).
Average peak shear rate at baseline-1, baseline-2, in the control and CME trials were 435±29 s−1 and 437±33 s−1, respectively. Average peak shear rate for all time points after the HFM for the control and CME trials was elevated to 490±23 s−1. In contrast average peak shear rate in the HIIE trial was unaltered across all time points, averaging 433±37 s−1, and was significantly lower than the CME and control trial after the HFM (p<0.03).
Endothelial-dependent vascular function
Both a single bout of CME and HIIE improved FMD measured 16–18 hours post-exercise, whereas FMD was unaltered in the control trial (Baseline-2, Figure 1B & C). All subjects achieved the greatest FMD after performing high intensity aerobic interval training (p< 0.01, Figure 1B & C). CME did not completely protect vascular function from the food-induced reduction in FMD, however the postperandial fall in FMD was significantly less than observed in the control trial. In contrast, HIIE not only completely protected the vessel from the lipemia-induced reduction in FMD observed after the HFM in the other 2 trials (CME and control), but actually resulted in greater vascular function than initially assessed at Baseline 1 (Figure 1B & C).
Endothelial-independent vascular function
There was no impact of the HFM on endothelial-independent vascular function and therefore, at all time points, sublingual NTG resulted in approximatley a 15% vasodilation in the rested state (Figure 1B).
Blood analyses
Exercise-induced changes in plasma total antioxidant status revealed a very similar pattern to FMD with a significant increase following both HIIE and CME from Baseline 1 to Baseline 2 (Figure 2A). Then following the HFM, total antioxidant status in the exercise trials remained higher than the control trial at all remaining time points (Figure 2A). Additionally, with all trials pooled, there was strong and significant correlation between total antioxidant status and FMD (Figure 2B).
Figure 2. Total antioxidant status and endothelial function.
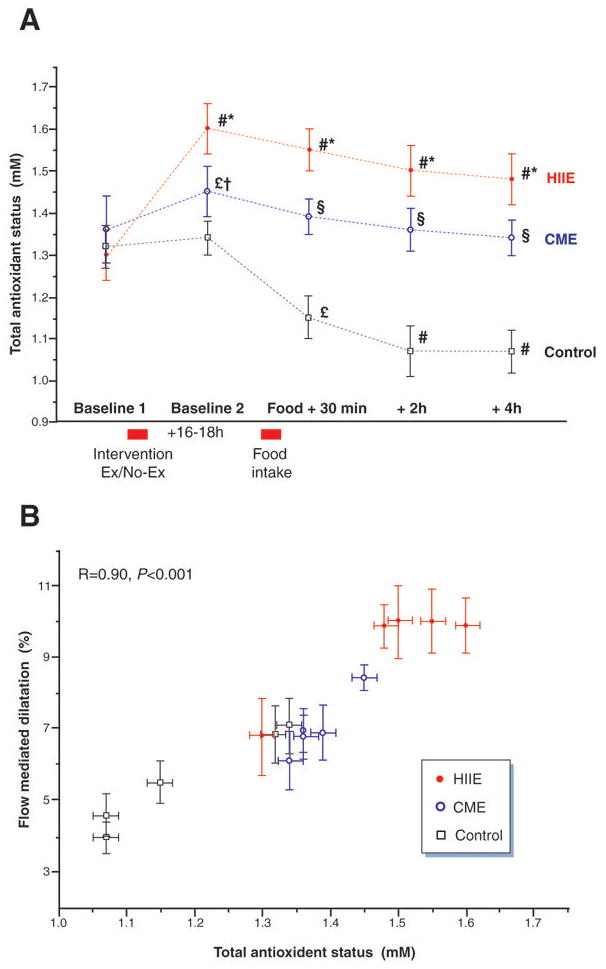
A Total antioxidant status (TAS) during the control (no exercise), continuous moderate intensity exercise (CME) and high intensity aerobic interval exercise (HIIE) trials. B: Correlation between plasma total antioxidant status and flow mediated dilation in the brachial artery. # different from baseline 1, p<0.01; * different from moderate and control, p<0.01; § different from control, p<0.01; † different from control, p<0.05; £ different from baseline, p<0.05
As there were no statistically discernable differences between the 3 trials (HIIE, CME, and control), in terms of the other food ingestion related blood parameters assessed, the group data were pooled to illustrate the time course of the intravascular effects of the HFM. Neither resting nor the performance of HIIE or CME (Baseline 1 to 2) had an effect on the HFM induced changes in glucose, c-peptide, tryglycerides, high density lipoproteins, C-reactive protein, cholesterol, HbA1c and hsCRP, and therefore only the most responsive variables are illustrated in Figure 3. The HFM raised both blood glucose and c-peptide levels within 30 minutes of food ingestion while triglycerides were not elevated until 2 hours post meal (Figure 3A–C). In contrast high density lipoprotein tended to fall after the HFM, achieving significance at the 4 hour post meal time point (Figure 3D).
Figure 3. Effects of acute exercise and food ingestion.
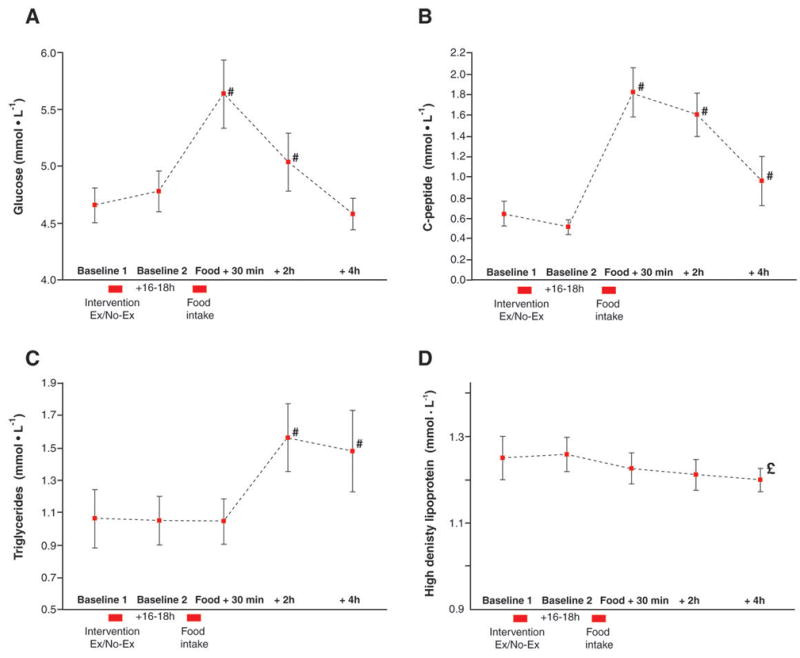
A: glucose, B: C-peptide, C: Triglycerides, and D: High Density Lipoproteins. As there was no difference in these variables across trials the data have been pooled. # different from baseline 1, p<0.01; £ different from baseline, p<0.05.
DISCUSSION
This study examined the efficacy of two forms of acute exercise to attenuate the endothelial dysfunction induced by postprandial lipemia. In agreement with other studies (11), the current data confirm that a single bout of continuous moderate intensity exercise (CME) 16–18 hours prior to the HFM reduced endothelial dysfunction when compared to the control condition, without exercise. The novel finding in the present study was that high intensity aerobic interval exercise (HIIE) not only completely prevented the normal postprandial reduction in endothelial function, but, in fact, augmented flow mediated dilation despite the lipemia. Additionally, the spectrum of plasma total antioxidant status following the HFM, from attenuated in the control to maintained with CME and consistently elevated with HIIE, revealed a strong relationship between the available protection from oxidative stress and vascular function. In combination, these findings reveal a clinically relevant protective effect of exercise upon the vasculature that is clearly exercise intensity dependent and appears to be related to acute exercise-induced antioxidant capacity and therefore suggestive of a link to NO bioavailability.
Exercise, antioxidant status, and vascular function
In terms of the current data, exercise, antioxidants and vascular function, reveal an interesting paradox. Acutely, exercise augments circulating free radical levels in both blood and muscle (7,12–14), which could be presumed to inactivate large amounts of nitric oxide and negatively impact endothelium-mediated vasodilation. However the current findings and studies by others (15,16), reveal an increase in antioxidant capacity and endothelial function following acute exercise. Indeed, it was recently recognized that even during exercise there seems to a be a transfer of antioxidants into muscle itself, presumably from the vasulature (7). Thus, it appears that acute exercise tends to tip the pro and antioxidant balance in favour of increased antioxidant status resulting in similar end result to that previously observed (maintained vascular function) by the co-ingestion of antioxidants and a HFM (5).
Lipemia, oxidative stress, and basal arterial diameter
Although, the HFM utilized in the current study (vegetarian pizza) had similar dietary constituents to a meal commonly selected for this type of research (McDonald’s Corporation breakfast)(17–19) and resulted in parallel changes in blood chemistry markers following such a meal (Figure 3), an unexpected difference was the impact upon basal arterial diameter. Specifically, in the control condition there was a marked reduction (approximately 10%) in basal arterial diameter following the ingestion of the HFM, supportive of the concept that this was purely an effect of the food ingestion. With the concomitant fall in total antioxidant status (Figure 2A), it is tempting to surmise that this reduction in vessel diameter was the consequence of a lipemia-induced increase in oxidative stress and subsequent fall in NO bioavailability. The same reduction in basal vessel diameter was apparent in the CME trial, which utilizing the same potential paradigm could infer that this reduction in basal diameter was apparently not protected by the proceeding exercise intervention. In contrast, in the HIIE trial, which yielded the greatest increase in total antioxidant status and was the only trial to maintain this elevation throughout the study (Figure 2A), there was no such reduction in arterial diameter (Figure 1B). Collectively, these findings support the concept that lipemia elevates oxidative stress and reduces NO bioavailability, as evidenced by reduction in basal vasodilatory tone, while HIIE can not only reverse these effects, but increases NO bioavailability by increasing antioxidant status above and beyond the influence of lipemia. Additional support for the link between NO bioavailability and the current findings are strengthened by the additional trials in which super physiologic NO levels were promoted by sublingual NTG. Here, there was no impact of the HFM on endothelial-independent vascular function (a 15% vasodilation at all time points, Figure 1A) and support the concept that NO bioavailability (alleviated by the NTG) was likely the mechanism responsible for attenuated endothelial-dependent vasodilation instigated by the HFM.
However, although a definitive explanation for this observation, when others have not recognized it, has still to be sought, the current findings of differences in vascular function between the control trial, CME and HIIE can not be explained by these significant changes in baseline diameter. Specifically, the reduction in brachial artery diameter after the HFM in both the control and CME trials mathematically biases the calculation of % FMD toward a greater change from baseline, however even in face of this bias, the HIIE trial (which did not result in a reduction in baseline diameter) yielded a consistently greater % FMD. As shear stress is proportional to share rate times viscosity, one could speculate that a high-fat meal changes viscosity, and thereby share stimulus for FMD. However, this seems unlikely as a study by Cicha et al (20) specifically addressed this issue and found no change in whole blood viscosity after a high-fat meal.
Vascular function, lipemia, and exercise
Although the exact mechanism responsible for the recognized postprandial attenuation in vascular function is not completely understood, the increased levels of plasma lipoproteins are thought to play a major role (21). As already recognized, engagement in regular exercise facilitates the maintenance of lower lipoprotein levels even after a HFM (4), however in the current study the performance of a single bout of either CME or HIIE had no impact on the multiple markers typically used to assess the impact of a meal, suggesting no effect of acute exercise upon how the HFM was handled (Figure 3). The exception to the selected assays was the assessment of total antioxidant status which differentiated the three trials (control, CME, and HIIE). In fact, in the trial that involved no exercise (control), and thus highlights the role of the meal in isolation, there was a precipitous drop in antioxidant status 30 mins following the food intake (Figure 2A). This effect was lessened in the CME trial and ablated following HIIE. Therefore, the current findings suggest that unlike chronic exercise, which may have a significant effect on the direct “handling” of a HFM, acute exercise 16–18 hours prior to the ingestion of the meal appears instead to be related in an exercise intensity dependent manner to the plasma antioxidant status, with a greater protection from the oxidative stress of the HFM afforded by the highest intensity of activity prior to its consumption (Figure 1B and C).
Why HIIE was more effective in improving antioxidant status is not known, but it seems reasonable to speculate that higher shear stress experienced during the exercise bout in the HIIE trial also yields a greater response at the cellular and molecular level. On the other hand, the HIIE response on FMD is similar to that observed when co-ingesting of antioxidant vitamins with a high-fat meal (5). It seems unlikely that ingestion of antioxidants will increase the shear stress of the vessel, and it may, therefore, be that the observed increase of antioxidant is just a marker and not the important underlying mechanism of the observed changes in FMD (5). The option also exist that antioxidant vitamins and exercise training acts on FMD through different signaling pathways. This view is supported by the observation that exercise training, but not necessarily vitamins, improves survival in coronary artery disease. Therefore, the present study indicates, but do not proof, that the effects of different exercise regimens on postprandial FMD is related to different effect on antioxidant status.
Limitations
The number of subjects in our study was small and healthy males only and it is not known whether the present training protocol will give similar adaptations in other populations such as in women and in subjects with cardiovascular disease. Furthermore, the observed relation between antioxidant status and postprandial FMD does not prove causation in explaining the different effects of exercise.
Conclusion
This study has revealed a clinically relevant protective effect of exercise upon the vasculature that is clearly exercise intensity dependent and appears to be tightly related to acute exercise-induced antioxidant capacity and therefore strongly suggestive of a link to NO bioavailability. As much of human life is spent in a postprandial state these findings are of significance for the understanding and reduction of cardiovascular risk and offer insight into the central mechanisms by which exercise reduces these risks.
Supplementary Material
ABBREVIATIONS
- HFM
High Fat Meal consisting of a vegetarian pizza
- HIIE
High Intensity Interval Exercise with intervals at 85–95% of maximal heart rate
- CME
Continuous Moderate Exercise at 60–70% of maximal heart rate
- FMD
Flow Mediated Dilatation of the brachial artery as a measure of endothelial function
- VO2max
Maximal oxygen uptake
Footnotes
Registered clinical trial: NCT00660491
Disclosures
We have no conflicts to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Halcox JP, Schenke WH, Zalos G, et al. Prognostic value of coronary vascular endothelial dysfunction. Circulation. 2002;106:653–8. doi: 10.1161/01.cir.0000025404.78001.d8. [DOI] [PubMed] [Google Scholar]
- 2.Deanfield JE, Halcox JP, Rabelink TJ. Endothelial function and dysfunction: testing and clinical relevance. Circulation. 2007;115:1285–95. doi: 10.1161/CIRCULATIONAHA.106.652859. [DOI] [PubMed] [Google Scholar]
- 3.Gill JM, Al-Mamari A, Ferrell WR, et al. Effects of prior moderate exercise on postprandial metabolism and vascular function in lean and centrally obese men. J Am Coll Cardiol. 2004;44:2375–82. doi: 10.1016/j.jacc.2004.09.035. [DOI] [PubMed] [Google Scholar]
- 4.Merrill JR, Holly RG, Anderson RL, Rifai N, King ME, DeMeersman R. Hyperlipemic response of young trained and untrained men after a high fat meal. Arteriosclerosis. 1989;9:217–23. doi: 10.1161/01.atv.9.2.217. [DOI] [PubMed] [Google Scholar]
- 5.Plotnick GD, Corretti MC, Vogel RA. Effect of antioxidant vitamins on the transient impairment of endothelium-dependent brachial artery vasoactivity following a single high-fat meal. Jama. 1997;278:1682–6. [PubMed] [Google Scholar]
- 6.Wisloff U, Stoylen A, Loennechen JP, et al. Superior cardiovascular effect of aerobic interval training versus moderate continuous training in heart failure patients: a randomized study. Circulation. 2007;115:3086–94. doi: 10.1161/CIRCULATIONAHA.106.675041. [DOI] [PubMed] [Google Scholar]
- 7.Bailey DM, Lawrenson L, McEneny J, et al. Electron paramagnetic spectroscopic evidence of exercise-induced free radical accumulation in human skeletal muscle. Free Radic Res. 2007;41:182–90. doi: 10.1080/10715760601028867. [DOI] [PubMed] [Google Scholar]
- 8.Rognmo O, Hetland E, Helgerud J, Hoff J, Slordahl SA. High intensity aerobic interval exercise is superior to moderate intensity exercise for increasing aerobic capacity in patients with coronary artery disease. Eur J Cardiovasc Prev Rehabil. 2004;11:216–22. doi: 10.1097/01.hjr.0000131677.96762.0c. [DOI] [PubMed] [Google Scholar]
- 9.Corretti MC, Anderson TJ, Benjamin EJ, et al. Guidelines for the ultrasound assessment of endothelial-dependent flow-mediated vasodilation of the brachial artery: a report of the International Brachial Artery Reactivity Task Force. J Am Coll Cardiol. 2002;39:257–65. doi: 10.1016/s0735-1097(01)01746-6. [DOI] [PubMed] [Google Scholar]
- 10.Pyke KE, Tschakovsky ME. The relationship between shear stress and flow-mediated dilatation: implications for the assessment of endothelial function. J Physiol. 2005;568:357–69. doi: 10.1113/jphysiol.2005.089755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gill JM, Hardman AE. Exercise and postprandial lipid metabolism: an update on potential mechanisms and interactions with high-carbohydrate diets (review) J Nutr Biochem. 2003;14:122–32. doi: 10.1016/s0955-2863(02)00275-9. [DOI] [PubMed] [Google Scholar]
- 12.Bailey DM, Davies B, Young IS, et al. Epr spectroscopic evidence of free radical outflow from an isolated muscle bed in exercising humans: functional significance of decreasing intracellular PO2 vs. increasing O2 flux. Adv Exp Med Biol. 2003;540:297–303. doi: 10.1007/978-1-4757-6125-2_42. [DOI] [PubMed] [Google Scholar]
- 13.Bailey DM, Young IS, McEneny J, et al. Regulation of free radical outflow from an isolated muscle bed in exercising humans. Am J Physiol Heart Circ Physiol. 2004;287:H1689–99. doi: 10.1152/ajpheart.00148.2004. [DOI] [PubMed] [Google Scholar]
- 14.Bejma J, Ji LL. Aging and acute exercise enhance free radical generation in rat skeletal muscle. J Appl Physiol. 1999;87:465–70. doi: 10.1152/jappl.1999.87.1.465. [DOI] [PubMed] [Google Scholar]
- 15.Haram PM, Adams V, Kemi OJ, et al. Time-course of endothelial adaptation following acute and regular exercise. Eur J Cardiovasc Prev Rehabil. 2006;13:585–91. doi: 10.1097/01.hjr.0000198920.57685.76. [DOI] [PubMed] [Google Scholar]
- 16.Ji LL. Exercise-induced modulation of antioxidant defense. Ann N Y Acad Sci. 2002;959:82–92. doi: 10.1111/j.1749-6632.2002.tb02085.x. [DOI] [PubMed] [Google Scholar]
- 17.Padilla J, Harris RA, Fly AD, Rink LD, Wallace JP. A comparison between active- and reactive-hyperaemia-induced brachial artery vasodilation. Clin Sci (Lond) 2006;110:387–92. doi: 10.1042/CS20050328. [DOI] [PubMed] [Google Scholar]
- 18.Padilla J, Harris RA, Fly AD, Rink LD, Wallace JP. The effect of acute exercise on endothelial function following a high-fat meal. Eur J Appl Physiol. 2006;98:256–62. doi: 10.1007/s00421-006-0272-z. [DOI] [PubMed] [Google Scholar]
- 19.Vogel RA, Corretti MC, Plotnick GD. Effect of a single high-fat meal on endothelial function in healthy subjects. Am J Cardiol. 1997;79:350–4. doi: 10.1016/s0002-9149(96)00760-6. [DOI] [PubMed] [Google Scholar]
- 20.Cicha I, Suzuki Y, Tateishi N, Maeda N. Enhancement of red blood cell aggregation by plasma triglycerides. Clin Hemorheol Microcirc. 2001;24:247–55. [PubMed] [Google Scholar]
- 21.Westphal S, Taneva E, Kastner S, et al. Endothelial dysfunction induced by postprandial lipemia is neutralized by addition of proteins to the fatty meal. Atherosclerosis. 2006;185:313–9. doi: 10.1016/j.atherosclerosis.2005.06.004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


