Abstract
Ovulation is an essential physiological process in sexual reproduction; however, the underlying cellular mechanisms are poorly understood. We have previously shown that OAMB, a Drosophila G-protein-coupled receptor for octopamine (the insect counterpart of mammalian norepinephrine), is required for ovulation induced upon mating. OAMB is expressed in the nervous and reproductive systems and has two isoforms (OAMB-AS and OAMB-K3) with distinct capacities to increase intracellular Ca2+ or intracellular Ca2+ and cAMP in vitro. Here, we investigated tissue specificity and intracellular signals required for OAMB's function in ovulation. Restricted OAMB expression in the adult oviduct epithelium, but not the nervous system, reinstated ovulation in oamb mutant females, in which either OAMB isoform was sufficient for the rescue. Consistently, strong immunoreactivities for both isoforms were observed in the wild-type oviduct epithelium. To delineate the cellular mechanism by which OAMB regulates ovulation, we explored protein kinases functionally interacting with OAMB by employing a new GAL4 driver with restricted expression in the oviduct epithelium. Conditional inhibition of Ca2+/Calmodulin-dependent protein kinase II (CaMKII), but not protein kinase A or C, in the oviduct epithelium inhibited ovulation. Moreover, constitutively active CaMKII, but not protein kinase A, expressed only in the adult oviduct epithelium fully rescued the oamb female's phenotype, demonstrating CaMKII as a major downstream molecule conveying the OAMB's ovulation signal. This is consistent with the ability of both OAMB isoforms, whose common intracellular signal in vitro is Ca2+, to reinstate ovulation in oamb females. These observations reveal the critical roles of the oviduct epithelium and its cellular components OAMB and CaMKII in ovulation. It is conceivable that the OAMB-mediated cellular activities stimulated upon mating are crucial for secretory activities suitable for egg transfer from the ovary to the uterus.
Introduction
Mating activates highly coordinated physiological processes in the Drosophila female. During copulation, the female receives somatosensory stimulation, sperm and seminal proteins from the male. These mating signals act at multiple sites in the mated female to activate post-mating responses required for successful reproduction [1], [2]. For example, the seminal protein Ovulin stimulates egg-laying for 1 day after mating [3]. Ovulin is not only present in the base of the ovary right after copulation but it also enters the circulatory system, possibly acting at additional sites [4], [5]. Moreover, the seminal sex peptides Acp70A and DUP99B reduce sexual receptivity and stimulate egg-laying [6]. While sex peptides have widespread binding sites in the central nervous system, endocrine glands, and reproductive tissues in the female [7], it is the sex peptide receptor SPR in the neurons expressing the sex determination factor Fruitless that is indispensable for reduced receptivity as well as increased egg-laying [8].
The downstream targets and mechanisms that Ovulin and SPR activate in the mated female are unknown. Several studies, nonetheless, indicate octopamine as a key neuromessenger for ovulation [9]–[13], suggesting it being a downstream signal of Ovulin or SPR for egg-laying. Octopamine is a major monoamine in insects and has similar functions to mammalian norepinephrine. Octopamine is synthesized from tyrosine by sequential actions of tyrosine decarboxylase (dTdc) and tyramine beta-hydroxylase (Tβh). The females defective in dTdc2 encoding neuronal dTdc or tβh are sterile due to defective egg-laying [10], [11]. Octopaminergic neurons innervate numerous brain and thoracico-abdominal ganglion (TAG) areas [14], [15]. In addition, octopaminergic neurons in the TAG project to reproductive tissues such as the ovaries, oviducts, sperm storage organs and uterus [12], [13]. Indeed, the sterility of tβh females is rescued by restored Tβh expression in a subset of neurons including the TAG neurons that innervate the reproductive system [10]. Consistently, octopamine, when applied to the dissected reproductive system, modulates muscle activities in a tissue-specific manner: it enhances muscle contraction in the ovary but inhibits it in the oviduct [12], [13]. This suggests that distinct octopamine receptors present in the ovary and oviduct mediate the opposite actions of octopamine on muscle activity.
Drosophila has four known octopamine receptors: OAMB, Octβ1R, Octβ2R and Octβ3R [16]–[18]. The oamb gene encodes two isoforms OAMB-K3 (K3) and OAMB-AS (AS), which are produced by alternative splicing of the last exon, and differ in the third cytoplasmic loop and downstream sequence. Both K3 and AS transcripts are found in the brain, TAG and reproductive system [9], [16]. When assayed in the heterologous cell lines, both isoforms activate an increase in intracellular Ca2+ [16], [18] while K3 also stimulates a cAMP increase [16]. This implies that the two isoforms may activate distinct combinations of signal transduction pathways in vivo. To investigate OAMB's in vivo functions, we have previously generated several oamb mutants defective in both K3 and AS and their prominent phenotype is female sterility [9]. While oamb mutant females show normal mating, they are impaired in ovulation, causing abnormal retention of mature eggs in the ovary [9]. This raises several important questions regarding mechanism of OAMB activity: where (brain, TAG or reproductive system) does OAMB regulate ovulation? Which isoform is critical for this process and what are the downstream signals? In this report, we show that the critical site for the OAMB's function in ovulation is the oviduct epithelium, in which transgenic expression of either K3 or AS isoform is sufficient to rescue the oamb female's ovulation defect. Moreover, we also demonstrate that OAMB recruits CaMKII as a key downstream effector for this function.
Results
Either OAMB isoform is sufficient to rescue defective ovulation in oamb females
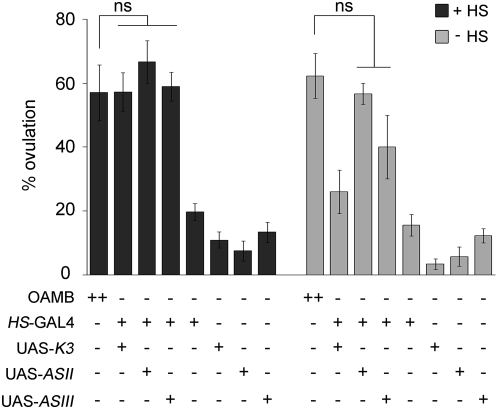
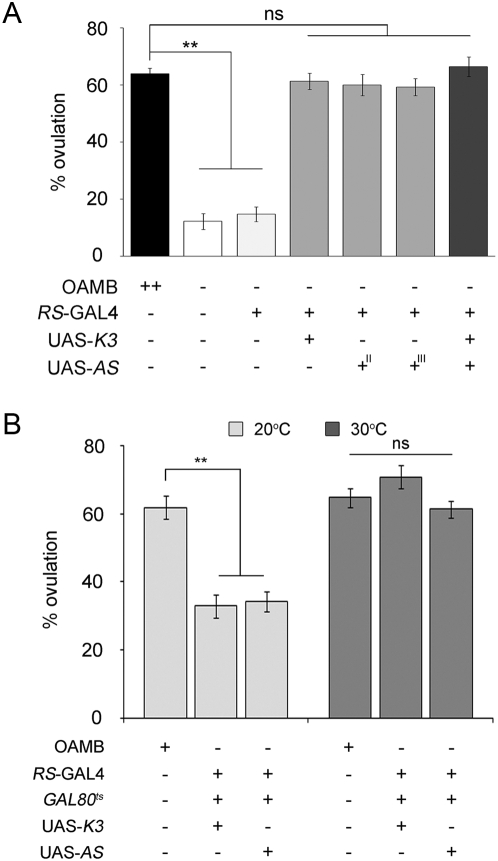
We have previously shown that females lacking both K3 and AS OAMB isoforms are defective in ovulation [9]. To investigate whether K3 or AS or both are crucial for ovulation, we conducted a rescue experiment using the GAL4/UAS binary system, in which the transcription factor GAL4 binds to UAS to activate the downstream gene expression [19]. We employed a HS-GAL4 driver, in which GAL4 expression is controlled by the heat inducible hsp70 promoter [20], for ubiquitous expression of transgenic OAMB in oamb null mutants [9]. The transgenic oamb females carrying HS-GAL4 along with UAS-K3 or UAS-AS were subjected to heat shock at 37°C for 1 h (Figure 1, +HS) then allowed to mate with wild-type Canton S (CS) males at room temperature. To monitor ovulation status, the females were subsequently examined for the presence of an egg in the oviduct or uterus. Another groups of females were tested without heat shock (Figure 1, −HS). The oamb females carrying HS-GAL4, UAS-K3, or UAS-AS alone and CS females were tested with and without heat shock as controls. The oamb females with heat-induced K3 or AS expression showed ovulation levels similar to those of CS females. While the oamb females with HS-GAL4 and UAS-K3 exhibited a heat-shock dependent increase in ovulation, those with HS-GAL4 and UAS-AS showed increased ovulation in the absence of heat shock. This might be due to leaky GAL4 expression since two independent UAS-AS lines with the transgene insertion at different chromosomal locations produced similar results. The oamb females with HS-GAL4, UAS-K3 or UAS-AS alone did not show significant ovulation in the presence or absence of heat shock, indicating that transgene insertions or heat treatments per se do not cause enhanced ovulation. These data demonstrate that both K3 and AS are capable of reinstating normal ovulation in oamb females and suggest that OAMB expressed at the adult stage plays a significant role for this activity.
Figure 1. Rescue of oamb's impaired ovulation by ubiquitous expression of individual OAMB isoforms.
Flies reared at 18°C were treated with heat shock (+HS) to induce HS-GAL4 expression or maintained at 18°C (−HS). Ten virgin females were mated with 30 CS males and a percentage of females with an egg in the oviduct or uterus represents one data point. The bottom panel shows wild-type CS (OAMB++) and the oamb mutant females (OAMB−) carrying various transgenes (HS-GAL4, UAS-K3, UAS-ASII and UAS-ASIII) under test. UAS-ASII and UAS-ASIII are independent transgenic lines of UAS-AS. The ovulation levels of the oamb females expressing K3 or AS driven by HS-GAL4 were similar to that of CS females (OAMB, ++) but significantly different from those of the oamb females carrying HS-GAL4, UAS-K3, or UAS-AS alone or no transgene (ANOVA, F 7,67 = 22.82, p<0.0001; ns, p>0.05 by post hoc Tukey Kramer tests, n = 3–12). Without heat shock, the oamb females carrying HS-GAL4 and UAS-AS had ovulation levels similar to that of CS females, but the ovulation levels of other lines were significantly different from that of CS (F 7,50 = 23.94, p<0.0001; ns, p>0.05 by Tukey Kramer; n = 3–9). ns, not significant.
OAMB is not required in the nervous system for ovulation
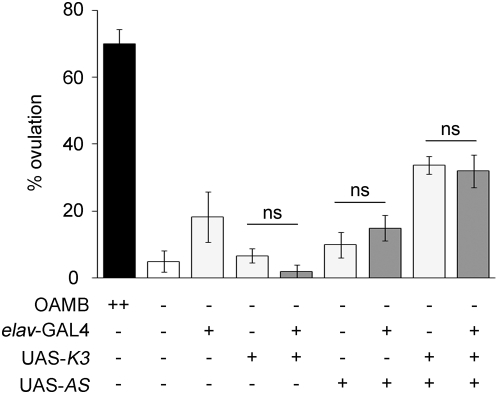
Endogenous OAMB is expressed in the nervous and reproductive systems. HS-GAL4 is ubiquitously expressed upon heat shock and thus activates transgenic OAMB expression in all cells. To direct transgenic OAMB expression in selective tissue types in oamb mutants, we first used elav-GAL4, in which GAL4 is expressed in all neurons [20]. The oamb females carrying elav-GAL4 along with UAS-K3 or UAS-AS displayed ovulation levels comparable to those of the oamb females without any transgene or with elav-GAL4 and UAS-OAMB alone (Figure 2). The oamb females carrying both UAS-OAMB isoforms showed a small yet significant increase in the ovulation level; however, it was not further increased with elav-GAL4 (Figure 2). The oamb females carrying either UAS-K3 or UAS-AS used in this study have a single copy of UAS-OAMB. Thus, the oamb females carrying both UAS-OAMB isoforms may have higher levels of GAL4-independent OAMB expression compared to those with a single copy, leading to partial rescue of defective ovulation. We also tested another neuronal driver Cha-GAL4, which is expressed in cholinergic neurons in the central and peripheral nervous systems [21]. Similar to elav-GAL4, Cha-GAL4-driven K3 or AS expression did not rescue the ovulation phenotype of oamb females (data not shown). Therefore, OAMB expressed in neurons is likely dispensable or insufficient for stimulating ovulation.
Figure 2. Failed rescue of the oamb's phenotype with neuronal OAMB expression.
Pan neuronal elav-GAL4 was used to induce K3 or AS expression in oamb mutant females. The ovulation levels of all oamb females with or without transgenes were significantly different from that of CS females (F 8,46 = 23.42, p<0.0001; n = 5–8) and the ovulation levels of the oamb females carrying UAS-OAMB alone or UAS-OAMB and elav-GAL4 were comparable (ns, p>0.05 by Student t-tests).
OAMB is expressed in the oviduct epithelium
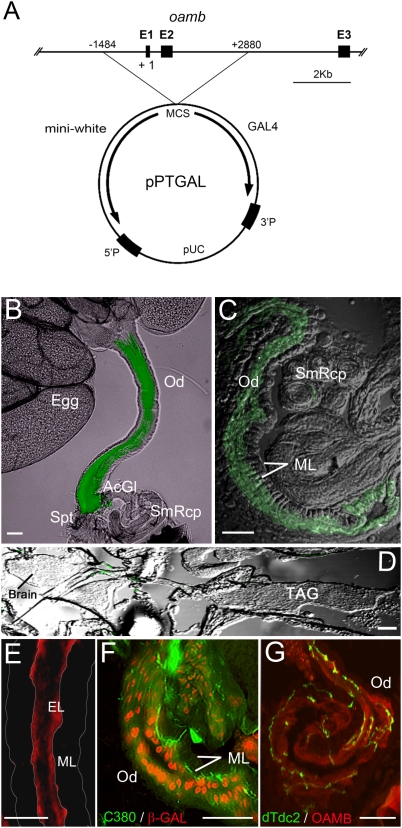
To investigate whether the major site of OAMB's function in ovulation is the reproductive system, we first explored the reproductive system-specific GAL4 drivers. We screened 67 enhancer lines selected based on the reported GAL4 expression in the reproductive system; however, none of those lines showed restricted GAL4 expression in the reproductive system. Thus, we turned our attention to the oamb locus for a reproductive tissue enhancer element. Our previous analysis of hypomorphic oamb mutants suggests that the second intron in the oamb gene may have a transcriptional enhancer element crucial for egg-laying [9]. We generated GAL4 enhancer lines by cloning a 4.4 Kb fragment containing this region and a putative oamb promoter upstream of the promoterless GAL4 gene in pPTGAL [22] (Figure 3A). The oamb-4.4-GAL4 in all six independent lines showed prominent enhancer activities in the female reproductive system [named RS (reproductive system)-GAL4] and images of one representative line crossed with UAS-mCD8::GFP (membrane tethered GFP) are shown in Figure 3. Notably, the oviduct but not the nervous system showed RS-GAL4-driven GFP expression (Figures 3B–3D). The oviduct is a tubular tissue delivering mature eggs from two ovaries to the uterus and consists of two layers, circular muscles and the underlying epithelium [13]. Strong GFP expression was visible only in the epithelial cells (Figures. 3B & 3C). Consistently, immunostaining with isoform-specific OAMB antibodies revealed localization of endogenous K3 and AS proteins in the oviduct epithelium of wild-type CS females (Figures 3E, 4A & 4B).
Figure 3. Reproductive system-specific GAL4.
(A) Scheme. The 4.4 Kb oamb genomic region containing oamb promoter region and part of the second intron was cloned upstream of promoterless-GAL4 in the pPTGAL vector. E, exon; +1, transcription initiation site. (B–D) The RS-GAL4 expression pattern was visualized with UAS-mCD8::GFP. Strong GFP expression was detectable in the oviduct epithelium in the dissected reproduction system (B) and a sagittal section (C), but not in the central nervous system (D). Images were captured under fluorescent as well as dim light illumination to outline tissue morphologies. (E–G) Sagittal sections of the CS (E), C380-GAL4/UAS-mCD8::GFP;P5391/+ (F) or dTdc2-GAL4/UAS-mCD8::GFP (G) female body were immunostained with anti-K3 (E, G) or anti-β-Galactosidase (F) followed by Alexa 555-labeled secondary antibodies. P5391 contains the enhancer P-element P[lArB] in the first oamb intron. P[lArB] has a LacZ reporter gene, which is expressed in the oviduct epithelial cell nuclei (F). Membrane-bound GFP induced by C380- or dTdc2-GAL4 demarcates motor or tyramine/octopamine neuronal processes, respectively, both of which project into the oviduct epithelium where OAMB-K3 immunoreactivities were evident (E, G). Od, oviduct; AcGl, accessory gland; Spt, spermatheca; SmRcp, seminal receptacle; TAG, thoracico-abdominal ganglion; ML, muscle layer. Scale bars, 50 µm. The experiments were conducted on one (F), two (G) or more than three sets (B–E) of females (5–6 females/set) generated by independent crosses, which showed consistent expression patterns of GFP, K3 and β-Galactosidase.
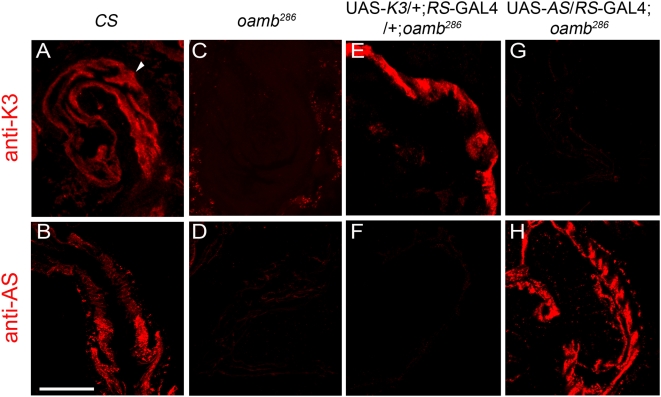
Figure 4. Transgenic OAMB expression driven by RS-GAL4 in the oviduct.
Sagittal sections of CS (A, B), oamb286 (C, D) and the transgenic oamb286 females carrying RS-GAL4 along with UAS-K3 (E, F) or UAS-AS (G, H) were immunostained with anti-K3 (A, C, E, G) or anti-AS (B, D, F, H) followed by Alexa 555-labeled secondary antibodies. Scale bar, 50 µm. The experiments were performed on more than three sets of females (5–6 females/set) generated by independent crosses, which showed consistent K3 and AS expression patterns.
The oviduct receives input from motor neurons and modulatory octopaminergic neurons in the TAG, and those neurons are shown to project to the oviduct circular muscle [10], [11]. Strong OAMB expression in the oviduct epithelium, but not the muscle, raises a question as to whether the epithelium receives input directly from the TAG neurons. To address this, we examined the transgenic females expressing membrane-tethered GFP in motor neurons via C380-GAL4 [23] or in octopaminergic neurons via dTdc2-GAL4 [11]. We also employed the P5391 enhancer trap line containing a nuclear localization signal-tagged LacZ reporter gene in the first oamb intron [9]. In this line, β-Galactosidase encoded by LacZ reflects the endogenous OAMB expression pattern and thus demarcates oviduct epithelial cells. When the cryosections of the P5391 female carrying C380-GAL4 and UAS-mCD8::GFP were examined for β-Galactosidase immunoreactivity and GFP, the β-Galactosidase-positive epithelial cell layer was extensively innervated by GFP-labeled motor neuronal processes (Figure 3F). Similarly, immunohistochemical analysis of the female carrying dTdc2-GAL4 and UAS-mCD8::GFP with anti-K3 antibody revealed octopamine neuronal processes projecting through the muscle layer and making contacts with the OAMB-expressing epithelium (Figure 3G). As previously reported [10], [11], GFP-labeled motor and octopamine neuronal processes were also visible in close contacts with the oviduct circular muscle (data not shown). These observations suggest that the TAG motor and octopaminergic neurons directly regulate oviduct muscle and epithelial cell activities, and OAMB serves as a receptor processing the TAG octopamine input to the epithelium.
OAMB is required in the oviduct epithelium for ovulation
We next investigated whether the oamb female's impaired ovulation could be rescued by restored OAMB expression in the oviduct epithelial cells. OAMB expression was monitored by immunostaining with anti-K3 and anti-AS antibodies in the transgenic oamb null mutants carrying RS-GAL4 and individual OAMB isoforms in which CS and oamb females served as controls. Both K3 and AS immunoreactivities were clearly visible in the CS oviduct epithelium (Figures 4A & 4B) but absent in the oamb oviduct epithelium (Figures 4C & 4D) and in the oviduct muscle and other reproductive tissues of both genotypes (Figure 4 and data not shown). Likewise, distinct isoform-specific immunoreactivities were evident in the oviduct epithelium of the transgenic oamb females carrying RS-GAL4 and UAS-K3 (Figures 4E & 4F) or RS-GAL4 and UAS-AS (Figures 4G & 4H). This demonstrates that RS-GAL4 effectively induces K3 and AS expression in the oviduct epithelium of oamb females.
When the transgenic oamb females carrying RS-GAL4 along with UAS-K3 or UAS-AS were subjected to ovulation tests, they showed ovulation levels similar to those of CS females (Figure 5). Interestingly, transgenic expression of both isoforms together in the oamb female oviduct did not further increase ovulation levels. These results indicate that OAMB expressed in the oviduct epithelium is sufficient to rescue the oamb female's impaired ovulation and both OAMB isoforms have similar capacities for this activity. We employed the TARGET (GAL80ts/GAL4/UAS) system [24] for temporally and spatially regulated transgenic OAMB expression to determine whether OAMB is required during a developmental or physiological process. In the TARGET system, GAL80ts binds to GAL4 to sequester it from activating UAS. At 30°C, the temperature sensitive GAL80ts no longer binds to GAL4, allowing it to act on UAS to induce the downstream gene expression and thus enabling transgene expression in a tissue- and time-dependent manner. To induce OAMB expression only in adult oviduct epithelial cells, the oamb females carrying tubP-GAL80ts (GAL80ts under the tublulin promoter for ubiquitous expression), RS-GAL4 and UAS-OAMB (either -K3 or -AS) were reared at 30°C for 3 days prior to mating. They showed ovulation levels comparable to those of CS females whereas ovulation levels of the oamb females with the same transgenes kept at 20°C remained low (Figure 5B). This indicates the significant role of OAMB during a physiological, but not developmental, process for ovulation. Taken together, the critical site for OAMB's physiological function in ovulation is the oviduct epithelium, in which transgenic expression of either isoform is sufficient to rescue the oamb female's phenotype.
Figure 5. Rescue of the oamb's ovulation defect by RS-GAL4-induced OAMB expression.
(A) The females of various genotypes reared at 25°C were subjected to ovulation tests. The transgenic oamb females carrying RS-GAL4 along with UAS- K3 or UAS-AS or both show ovulation levels comparable to that of CS females (F6,89 = 58.40; **p<0.0001 by Tukey-Kramer; ns, not significant p>0.05; n = 9–15). (B) The oamb females carrying GAL80ts and RS-GAL4 along with UAS-K3 or UAS-AS reared at 30°C for 3 days (OAMB induction), but not at 20°C (no induction), showed similar ovulation levels to the control females (20°C, F2,56 = 26.8, p<0.0001, n = 19–20; 30°C, F2,60 = 2.27, p>0.05, n = 20–22).
CaMKII is a major downstream signaling molecule of OAMB in ovulation
To delineate the cellular mechanism by which OAMB regulates ovulation, we first explored protein kinases that functionally interact with OAMB in the oviduct epithelium. OAMB activates cAMP and Ca2+ increases in vitro [16], [18]. Potential cellular molecules activated by cAMP or Ca2+ include protein kinase A (PKA) for cAMP and protein kinase C (PKC) and CaMKII for Ca2+. Since mutations in these kinases cause developmental lethality, their functions in ovulation are unknown. To assess physiological roles of PKA, PKC and CaMKII in ovulation, we employed the TARGET system with UAS-PK inhibitors or variants. A dominant negative PKAr*, which is defective in binding cAMP [25], and inhibitory peptides of PKC [PKCi; [26]] and CaMKII [ala; [27]] have been shown to effectively inhibit PKA, PKC and CaMKII activities, respectively, and thus were used to manipulate the corresponding protein kinase activities in adult oviduct epithelial cells. In an attempt to sensitize physiological effects of inhibited protein kinase activities, the experiments were conducted in the oamb heterozygous background.
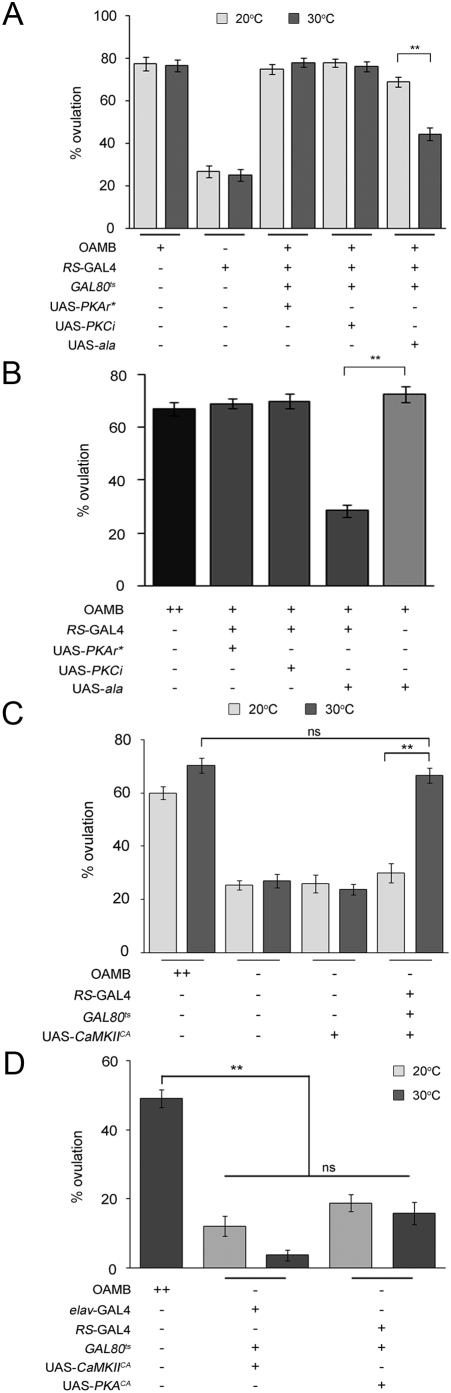
The oamb heterozygous females carrying tubP-GAL80ts and RS-GAL4 along with UAS- PKAr*, UAS-PKCi or UAS-ala showed normal ovulation when reared at permissive temperature 20°C (Figure 6A). However, when they were raised at 30°C for 3 days to inhibit the targeted protein kinase activity, the females with the CaMKII inhibitory peptide ala, but not PKAr* or PKCi, showed significantly decreased ovulation levels (Figure 6A). We also examined the females without GAL80ts, which may have increased inhibitor expression and thus enhanced protein kinase inhibition. Similar to temporal induction, the CaMKII inhibitory peptide ala constantly induced with RS-GAL4 inhibited ovulation; however, persistent induction of PKAr* or PKCi expression had no effect (Figure 6B). These results suggest that CaMKII, but not PKA or PKC, is involved in a physiological process for ovulation and may function as a downstream effector of OAMB. This is consistent with the aforementioned observations that the oamb female's impaired ovulation was rescued by both OAMB isoforms, whose common intracellular signal is Ca2+.
Figure 6. Potential protein kinases activated by OAMB in the oviduct epithelium.
(A) Temporarily induced CaMKII inhibitor peptides (ala; 30°C), but not dominant negative PKA (PKA*) and PKC inhibitor peptides (PKCi), in the oviduct epithelium inhibited ovulation in the heterozygous oamb females (**p<0.0001 by Student t-test, n = 18–19; other pairs, p>0.05, n = 10–20). (B) Persistent induction of ala, but not PKA* and PKCi, driven by RS-GAL4 inhibited ovulation (ANOVA: F4,98 = 53.5, ** p<0.0001 by Tukey-Kramer, n = 20–21). (C) The transgenic oamb females carrying RS-GAL4, GAL80ts and UAS-CaMKIICA had the ovulation level comparable to that of CS females after temperature shift (ns, p>0.05 by Student t-test), indicating full rescue of the oamb's impaired ovulation by constitutively active CaMKII induced in the oviduct epithelium. n = 21–23, ** p<0.0001 by Student t-test. (D) The transgenic oamb females with neuronal CaMKIICA or oviduct epithelial PKACA induced by elav-GAL4 or RS-GAL4, respectively, exhibited ovulation levels significantly lower than that of CS females (F4,75 = 36.8, ** p<0.0001, post hoc Tukey Kramer tests; n = 11 for elav-GAL4 lines, n = 19–21 for the rest). Temperature shift in the transgenic oamb females carrying elav-GAL4, GAL80ts and UAS-CaMKIICA or those carrying RS-GAL4, GAL80ts and UAS-PKACA had no effects on ovulation levels (ns, p>0.05).
If CaMKII indeed functions downstream of OAMB, we reasoned that ligand-independent activation of CaMKII would bypass OAMB for stimulating ovulation and thus rescue the oamb's ovulation phenotype. To test this, we used constitutively active CaMKII (CaMKIICA) whose activity is independent of the upstream signals Ca2+ and calmodulin [28]. The oamb homozygous females carrying tubP-GAL80ts, RS-GAL4 and UAS-CaMKIICA were impaired in ovulation similar to oamb females when reared at 20°C (uninduced CaMKIICA); however, they exhibited ovulation levels comparable to those of CS females upon temperature shift (induced CaMKIICA; Figure 6C). On the contrary, the oamb females carrying tubP-GAL80ts, elav-GAL4 and UAS-CaMKIICA or those carrying tubP-GAL80ts, RS-GAL4 and UAS-constitutively active PKA [PKACA, [29]] did not exhibit enhanced ovulation when subjected to the same temperature shift (Figure 6D). Therefore, constitutively active CaMKII, but not PKA, expressed in the adult oviduct epithelium was sufficient to reinstate ovulation in oamb females whereas constitutively active CaMKII induced in the nervous system failed to rescue the ovulation defect, corroborating the unsuccessful rescue with neuronal OAMB expression (Figure 2). These data point to CaMKII as a major downstream signaling molecule that is activated by OAMB in the oviduct epithelium for ovulation. If OAMB or CaMKII represent the sole ovulation signal activated by mating, CaMKIICA would induce ovulation in the absence of copulation. However, the virgin oamb females expressing CaMKIICA in the oviduct epithelium showed negligible ovulation (data not shown). This suggests additional factors or processes mediating a mating signal, which may include those acting in the ovary or oviduct muscle.
Discussion
Octopamine, as a major neurotransmitter, neuromodulator and neurohormone, regulates diverse physiological processes in invertebrates that include sensory information processing, egg-laying, fight or flight responses, and complex neural functions such as learning and memory [30]. These astonishingly diverse effects of octopamine are initiated by the binding of octopamine to G-protein-coupled receptors expressed in distinct tissue or cell types; however, very little is known about relevant octopamine receptors and underlying cellular mechanisms that mediate octopamine's physiological functions. In this report, we have shown that OAMB regulates ovulation in the oviduct epithelium and recruits CaMKII for this function. This role of OAMB is physiological, as opposed to developmental, since restored OAMB expression in the oviduct epithelium at the adult stage is sufficient for reinstating ovulation in oamb females. This is consistent with the findings observed in the octopamine-less dTdc2 and tβh females, in which feeding octopamine only at the adult stage rescues the sterility phenotype of both mutants [11], [31].
Sex peptides transferred to the female during copulation enhance egg-laying upon binding to the receptor SPR expressed in the Fruitless neurons [8]. The mechanism by which the Fruitless neurons stimulate egg-laying is unknown; however, octopaminergic neurons in the TAG likely represent a downstream target since the egg-laying phenotype of tβh females is rescued by restored TβH expression in these neurons [10]. The TAG octopaminergic neurons project axons to various areas in the reproductive track including the ovary, lateral and common oviducts, sperm storage organs and the uterus [12], [13]. Mating induces distinctive changes in vesicle release at the nerve terminals in different areas of the reproductive track [32], some of which may represent the TAG octopamine neuronal activities. In the dissected reproductive system, octopamine application augments the amplitude of myogenic contractions of the peritoneal sheath in the ovary while it inhibits stimulated muscle contractions of the oviduct [12], [13]. These opposite effects of octopamine may be crucial for coordinated constriction and relaxation of the ovary and oviduct, respectively, in transferring a mature egg to the uterus. OAMB may serve as a receptor processing the octopamine's input in the oviduct while another octopamine receptor may mediate the constriction signal in the ovarian peritoneal sheath, which lacks OAMB expression.
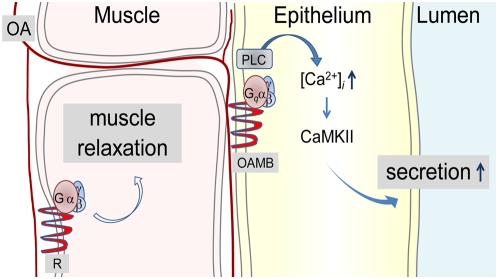
Remarkably, OAMB's activity is required in the epithelium rather than the muscle for normal ovulation. Consistent with this, the histochemical analysis reported here reveals extensive innervation of the TAG octopamine neuronal processes into the oviduct epithelial layer where both OAMB isoforms are enriched, in addition to the muscle. This raises an important question regarding the nature of an OAMB's role in ovulation. While no information is available on the oviduct epithelium in Drosophila or other insects, studies of the mammalian oviduct indicate active roles of the epithelium in fluid secretion and ciliary activity for gamete and embryo transport [33]. Similarly, it is possible that OAMB in the Drosophila oviduct epithelium is involved in regulating fluid secretion to establish proper luminal environment and possibly ciliary action for egg transport. The capacity of either OAMB-K3 or OAMB-AS to reinstate ovulation in oamb females strongly implicates intracellular Ca2+ rather than cAMP as a downstream effector. This is corroborated by our findings demonstrating CaMKII as a key epithelial component downstream of OAMB. It is uncertain whether individual isoforms or two isoforms together have comparable efficacies in activating CaMKII and ovulation. Future studies employing quantitative manipulation of transgenic OAMB expression may clarify this issue. Taken together, the epithelial OAMB stimulated upon mating likely activates CaMKII via increased intracellular Ca2+, which may in turn trigger biochemical changes necessary for fluid secretion (Figure 7). Potential molecules involved in this process may include transporters, ion channels, Na+-K+-ATPase and the molecules involved in cilia movements [33]. In the absence of OAMB, epithelial cell activities and fluid may be inadequate for egg movement, leading to ovulation failure. Since octopamine induces relaxation in the dissected oviduct, relaxation may involve another octopamine receptor in the muscle, and concerted activities of OAMB and a muscle receptor may be crucial for successful egg transport (Figure 7). This working model is currently under test.
Figure 7. Working model for OAMB's roles in the oviduct epithelium.
Mating activates the TAG octopaminergic neurons projecting to the oviduct epithelium where OAMB, upon receiving octopamine input, stimulates fluid secretion via signal transduction pathways involving CaMKII for egg transport from the ovary to the uterus. In addition, the octopamine input may be processed by another octopamine receptor in the oviduct muscle for relaxation. Concerted activities of an epithelial OAMB and a muscle receptor may be crucial for egg transfer through the oviduct.
Octopamine regulates oviduct activities in other insects as well. In the locust oviduct, octopamine inhibits the basal tonus and neurally evoked muscle contractions, which are mediated by cAMP-dependent mechanisms [34], [35]. These effects of octopamine may be mediated by an OAMB-like receptor with the different intracellular effector cAMP. Alternatively, they may involve another octopamine receptor(s) present in the muscle. Drosophila has three octopamine receptors (OctβR1, 2 and 3) that can also stimulate cAMP increases [36]. Spatial expression patterns of three OctβRs are as yet unknown. It is conceivable that an OctβR or OctβR-like receptor, possibly present in the Drosophila or locust oviduct muscle, respectively, is additionally involved in ovulation by inducing muscle relaxation through a cAMP signaling pathway (Figure 7). At present, molecular components and cellular pathways controlling ovulation are largely unknown and likewise very little is known about the oviduct functions and mechanisms. Our findings reported here uncover the critical roles of the oviduct epithelium and its cellular components OAMB and CaMKII in ovulation. Future studies to identify additional downstream effectors of OAMB and their functions should help further our understanding of the important reproductive process ovulation and provide novel insights into the development of effective insecticides. Typically, intracellular signals activated by G-protein-coupled receptors are characterized in in vitro cell lines. This study has identified the intracellular signal activated by the G-protein-coupled receptor OAMB in vivo that has functional significance. Similar approaches could be applied to other receptors to investigate rather poorly defined cellular mechanisms that G-protein-coupled receptors activate for their in vivo functions.
Norepinephrine, a mammalian counterpart of octopamine, also plays profound roles in female reproduction by acting on the reproductive and nervous systems. Sympathetic nerve terminals containing norepinephrine innervate the ovaries, oviducts, and uterus. Moreover, norepinephrine levels in the human fallopian tube vary in a region- and estrous cycle-dependent manner being the highest in the isthmus and the fimbriated end at the time of ovulation [37]. When assayed in vitro, adrenergic receptor agonists not only modulate oviduct muscle activities [38], [39] but they also stimulate fluid secretion possibly via Ca2+-dependent mechanisms [33], [40]. Oviduct fluid in mammals is critical for egg transport, maturation and fertilization; however, the cellular process regulating its secretion is largely unknown. Damage in the oviduct epithelium is associated with pelvic inflammatory disorder, leading to hydrosalpinx formation and reduced fertility [33], [41], [42]. Thus, enhanced understanding of physiological and cellular factors and processes controlling oviduct fluid will provide significant insights into healthy reproduction as well as impaired fertility associated with pelvic inflammatory disorder and other related disorders.
Materials and Methods
Drosophila strains and culture
The oamb mutants used in this study are the null alleles oamb286 and oamb96 [9]. The transgenic lines C380-GAL4, dTdc2-GAL4, elav-GAL4, tubP-GAL80ts, UAS-PKAr*, UAS-PKACA (also known as UAS-mc*), UAS-CaMKIICA and UAS-ala were kindly provided by Drs. M. Ramaswami, J. Hirsh, M. Heisenberg, R. Davis, J. Kiger, Jr., D. Kalderon and L. Griffith, respectively. In addition, heat shock (HS)-GAL4, UAS-PKCi, and UAS-mCD8::GFP were obtained from the Bloomington Stock Center. All fly stocks were reared on standard cornmeal/sugar/yeast medium at 25°C, unless otherwise stated, with approximately 50% relative humidity under a 12 h light/12 h dark cycle.
UAS-OAMB cDNA and RS-GAL4 constructs
For transgenic expression of OAMB, K3 [16] or AS cDNA [9] was cloned under UAS in pPBretU vector [43]. Four independent transgenic lines UAS-K3-I, UAS-K3-III, UAS-AS-II, and UAS-AS-III were established by germ line transformation in the ry506 genetic background and the transgene insertion sites were identified by inverse PCR and sequencing [20]. To generate transgenic lines expressing GAL4 in the oviduct, the endogenous oamb regulatory region (−1484 to +2880) was amplified by PCR using primers 5′-CACTAGTCCACGTCAGCCCATTC-3′ and 5′-GAAGATCTCCCTGTGCTTGGC-3′, cloned in the pPTGAL vector containing the promoterless GAL4 gene [22], and germ-line transformed in the isogenic w1118 to generate six independent lines. The GAL4 expression patterns were visualized after crossing with UAS-mCD8::GFP. Since all six lines showed similar expression patterns, one line (RS-GAL4) with the GAL4 insertion on the second chromosome was used for all experiments described here after backcrossed with the isogenic w1118 for six generations. The individual transgenes (HS-GAL4, elav-GAL4, RS-GAL4, UAS-OAMB, and UAS-CaMKIICA) were placed in the oamb286 or oamb96 null mutant background for rescue experiments.
Immunohistochemistry
A 315 bp fragment of AS cDNA corresponding to the putative third intracellular loop specific to the AS isoform was amplified by PCR using 5′-TGAATTCGCATACATAGAGGACGA-3′ and 5′-TCTCGAGCGTGCTGCTGCTGCTGT-3′ primers and cloned in pGEX-4T-2 (Pharmacia, Piscataway, NJ) to generate a fusion protein with glutathione S-transferase. As previously described [16], a 542 bp fragment corresponding to the putative third intracellular loop of the K3 isoform was also used to generate the fusion protein. After the third injection of the fusion proteins into Swiss Webster mice, ascites fluid was collected and used for immunostaining. Flies were fixed in 4% paraformaldehyde and 40 mM lysine in phosphate buffered saline, pH 7.2, for 3 h at 4°C and then kept in 25% sucrose overnight. Ten-micron sagittal cryosections of the whole fly were subjected to immunostaining as previously described [44] using rabbit anti-β-Galactosidase antibody (1∶1000, Cappel Lab., Cochranville, PA), mouse anti-K3 antibody (1∶200), mouse anti-AS antibody (1∶50), and Alexa 555-conjugated anti-rabbit or anti-mouse IgG antibody (1∶1000; Invitrogen, Eugene, OR) [9]. The antigen specificities of anti-K3 and anti-AS antibodies were established based on deficient K3 and AS immunoreactivities in oamb286 null mutants and negligible cross-reactivities in the oamb mutants expressing transgenic K3 or AS isoform as shown in the results section. Images were acquired with the DMR epifluorescent (Leica, Heidelberg, Germany) or FluoView confocal (Olympus, Melville, NY) microscope. All chemicals were purchased from Sigma-Aldrich (St. Louis, MO) and Fisher Science (Fair Lawn, NJ).
Ovulation test
Virgin females were collected within 12 h after eclosion. Ten 5 to 7 day-old females were housed with 30 wild-type CS males in a food vial for 3 h for mating and then the female reproductive system was dissected to examine the presence of an egg in the oviduct or uterus. The percentage of females with an egg per vial was used to represent one data point. To induce transgenic oamb gene expression at the adult stage, the oamb mutant females carrying HS-GAL4 along with UAS-K3 or UAS-AS reared at 18° to 20°C were treated with heat shock at 37°C for 1 h twice a day for 3 days and then tested for ovulation. For the experiments involving tubP-GAL80ts, 2 to 3 day-old virgin females were reared at 30°C for 3 days and then subjected to mating and ovulation tests at room temperature. The females kept at 20°C were used as an uninduced control. Statistical analyses were performed using Minitab Release 14.1 (Minitab, State College, PA). All data are presented as mean±SEM and were analyzed using ANOVA and post hoc Tukey–Kramer or Student's t-tests.
Acknowledgments
We thank Drs. M. Ramaswami (Trinity College Dublin, Ireland), J. Hirsh (U. Virginia), M. Heisenberg (U. Würzburg, Germany), R. Davis (BCM), D. Amstrong (U. Edinburgh, UK), J. Kiger, Jr. (UC, Davis), D. Kalderon (Columbia U) and L. Griffith (Brandeis U) for providing fly lines and Drs. G. Roman (UH) and D. Eberl (U. Iowa) for pPBretU and pPTGAL, respectively. We are also very grateful to Drs. Michael Heien (PSU) and Esther Siegfried (UPJ) for critical reading and comments on the manuscript and the undergraduate research assistants Erin Stover and Satbyol Kang for technical assistance.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: This work was supported by NIH (HD048766) and NSF (IOB-0620056) grants to K.-A.H. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Bloch Qazi MC, Heifetz Y, Wolfner MF. The developments between gametogenesis and fertilization: ovulation and female sperm storage in Drosophila melanogaster. Dev Biol. 2003;256:195–211. doi: 10.1016/s0012-1606(02)00125-2. [DOI] [PubMed] [Google Scholar]
- 2.Wolfner MF. “S.P.E.R.M.” (seminal proteins (are) essential reproductive modulators): the view from Drosophila. Soc Reprod Fertil Suppl. 2007;65:183–199. [PubMed] [Google Scholar]
- 3.Herndon LA, Wolfner MF. A Drosophila seminal fluid protein, Acp26Aa, stimulates egg laying in females for 1 day after mating. Proc Natl Acad Sci U S A. 1995;92:10114–10118. doi: 10.1073/pnas.92.22.10114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Heifetz Y, Lung O, Frongillo EA, Jr, Wolfner MF. The Drosophila seminal fluid protein Acp26Aa stimulates release of oocytes by the ovary. Curr Biol. 2000;10:99–102. doi: 10.1016/s0960-9822(00)00288-8. [DOI] [PubMed] [Google Scholar]
- 5.Lung O, Wolfner MF. Drosophila seminal fluid proteins enter the circulatory system of the mated female fly by crossing the posterior vaginal wall. Insect Biochem Mol Biol. 1999;29:1043–1052. doi: 10.1016/s0965-1748(99)00078-8. [DOI] [PubMed] [Google Scholar]
- 6.Aigaki T, Fleischmann I, Chen PS, Kubli E. Ectopic expression of sex peptide alters reproductive behavior of female D. melanogaster. Neuron. 1991;7:557–563. doi: 10.1016/0896-6273(91)90368-a. [DOI] [PubMed] [Google Scholar]
- 7.Ottiger M, Soller M, Stocker RF, Kubli E. Binding sites of Drosophila melanogaster sex peptide pheromones. J Neurobiol. 2000;44:57–71. [PubMed] [Google Scholar]
- 8.Yapici N, Kim YJ, Ribeiro C, Dickson BJ. A receptor that mediates the post-mating switch in Drosophila reproductive behaviour. Nature. 2008;451:33–37. doi: 10.1038/nature06483. [DOI] [PubMed] [Google Scholar]
- 9.Lee HG, Seong CS, Kim YC, Davis RL, Han KA. Octopamine receptor OAMB is required for ovulation in Drosophila melanogaster. Dev Biol. 2003;264:179–190. doi: 10.1016/j.ydbio.2003.07.018. [DOI] [PubMed] [Google Scholar]
- 10.Monastirioti M. Distinct octopamine cell population residing in the CNS abdominal ganglion controls ovulation in Drosophila melanogaster. Dev Biol. 2003;264:38–49. doi: 10.1016/j.ydbio.2003.07.019. [DOI] [PubMed] [Google Scholar]
- 11.Cole SH, Carney GE, McClung CA, Willard SS, Taylor BJ, et al. Two functional but noncomplementing Drosophila tyrosine decarboxylase genes: distinct roles for neural tyramine and octopamine in female fertility. J Biol Chem. 2005;280:14948–14955. doi: 10.1074/jbc.M414197200. [DOI] [PubMed] [Google Scholar]
- 12.Rodriguez-Valentin R, Lopez-Gonzalez I, Jorquera R, Labarca P, Zurita M, et al. Oviduct contraction in Drosophila is modulated by a neural network that is both, octopaminergic and glutamatergic. J Cell Physiol. 2006;209:183–198. doi: 10.1002/jcp.20722. [DOI] [PubMed] [Google Scholar]
- 13.Middleton CA, Nongthomba U, Parry K, Sweeney ST, Sparrow JC, et al. Neuromuscular organization and aminergic modulation of contractions in the Drosophila ovary. BMC Biol. 2006;4:17. doi: 10.1186/1741-7007-4-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Monastirioti M, Gorczyca M, Rapus J, Eckert M, White K, et al. Octopamine immunoreactivity in the fruit fly Drosophila melanogaster. J Comp Neurol. 1995;356:275–287. doi: 10.1002/cne.903560210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sinakevitch I, Strausfeld NJ. Comparison of octopamine-like immunoreactivity in the brains of the fruit fly and blow fly. J Comp Neurol. 2006;494:460–475. doi: 10.1002/cne.20799. [DOI] [PubMed] [Google Scholar]
- 16.Han KA, Millar NS, Davis RL. A novel octopamine receptor with preferential expression in Drosophila mushroom bodies. J Neurosci. 1998;18:3650–3658. doi: 10.1523/JNEUROSCI.18-10-03650.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Maqueira B, Chatwin H, Evans PD. Identification and characterization of a novel family of Drosophila beta-adrenergic-like octopamine G-protein coupled receptors. J Neurochem. 2005;94:547–560. doi: 10.1111/j.1471-4159.2005.03251.x. [DOI] [PubMed] [Google Scholar]
- 18.Balfanz S, Strunker T, Frings S, Baumann A. A family of octopamine [corrected] receptors that specifically induce cyclic AMP production or Ca2+ release in Drosophila melanogaster. J Neurochem. 2005;93:440–451. doi: 10.1111/j.1471-4159.2005.03034.x. [DOI] [PubMed] [Google Scholar]
- 19.Brand AH, Perrimon N. Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development. 1993;118:401–415. doi: 10.1242/dev.118.2.401. [DOI] [PubMed] [Google Scholar]
- 20.FlyBase-Consortium. The FlyBase database of the Drosophila genome projects and community literature. Nucleic Acids Res. 2003;31:172–175. doi: 10.1093/nar/gkg094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Salvaterra PM, Kitamoto T. Drosophila cholinergic neurons and processes visualized with Gal4/UAS-GFP. Brain Res Gene Expr Patterns. 2001;1:73–82. doi: 10.1016/s1567-133x(01)00011-4. [DOI] [PubMed] [Google Scholar]
- 22.Sharma Y, Cheung U, Larsen EW, Eberl DF. PPTGAL, a convenient Gal4 P-element vector for testing expression of enhancer fragments in drosophila. Genesis. 2002;34:115–118. doi: 10.1002/gene.10127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Koh YH, Popova E, Thomas U, Griffith LC, Budnik V. Regulation of DLG localization at synapses by CaMKII-dependent phosphorylation. Cell. 1999;98:353–363. doi: 10.1016/s0092-8674(00)81964-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McGuire SE, Roman G, Davis RL. Gene expression systems in Drosophila: a synthesis of time and space. Trends Genet. 2004;20:384–391. doi: 10.1016/j.tig.2004.06.012. [DOI] [PubMed] [Google Scholar]
- 25.Kiger JA, Jr, Eklund JL, Younger SH, O'Kane CJ. Transgenic inhibitors identify two roles for protein kinase A in Drosophila development. Genetics. 1999;152:281–290. doi: 10.1093/genetics/152.1.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kane NS, Robichon A, Dickinson JA, Greenspan RJ. Learning without performance in PKC-deficient Drosophila. Neuron. 1997;18:307–314. doi: 10.1016/s0896-6273(00)80270-6. [DOI] [PubMed] [Google Scholar]
- 27.Griffith LC, Verselis LM, Aitken KM, Kyriacou CP, Danho W, et al. Inhibition of calcium/calmodulin-dependent protein kinase in Drosophila disrupts behavioral plasticity. Neuron. 1993;10:501–509. doi: 10.1016/0896-6273(93)90337-q. [DOI] [PubMed] [Google Scholar]
- 28.Park D, Coleman MJ, Hodge JJ, Budnik V, Griffith LC. Regulation of neuronal excitability in Drosophila by constitutively active CaMKII. J Neurobiol. 2002;52:24–42. doi: 10.1002/neu.10066. [DOI] [PubMed] [Google Scholar]
- 29.Li W, Ohlmeyer JT, Lane ME, Kalderon D. Function of protein kinase A in hedgehog signal transduction and Drosophila imaginal disc development. Cell. 1995;80:553–562. doi: 10.1016/0092-8674(95)90509-x. [DOI] [PubMed] [Google Scholar]
- 30.Roeder T. Tyramine and octopamine: ruling behavior and metabolism. Annu Rev Entomol. 2005;50:447–477. doi: 10.1146/annurev.ento.50.071803.130404. [DOI] [PubMed] [Google Scholar]
- 31.Monastirioti M, Linn CE, Jr, White K. Characterization of Drosophila tyramine beta-hydroxylase gene and isolation of mutant flies lacking octopamine. J Neurosci. 1996;16:3900–3911. doi: 10.1523/JNEUROSCI.16-12-03900.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Heifetz Y, Wolfner MF. Mating, seminal fluid components, and sperm cause changes in vesicle release in the Drosophila female reproductive tract. Proc Natl Acad Sci U S A. 2004;101:6261–6266. doi: 10.1073/pnas.0401337101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Leese HJ, Tay JI, Reischl J, Downing SJ. Formation of Fallopian tubal fluid: role of a neglected epithelium. Reproduction. 2001;121:339–346. doi: 10.1530/rep.0.1210339. [DOI] [PubMed] [Google Scholar]
- 34.Nykamp DA, Lange AB. Interaction between octopamine and protolin on the oviducts of Locusta migratoria. j Insect Physiol. 2000;46:809–816. doi: 10.1016/s0022-1910(99)00170-5. [DOI] [PubMed] [Google Scholar]
- 35.Orchard I, Lange AB. Neuromuscular transmission in an insect visceral muscle. J Neurobiol. 1986;17:359–372. doi: 10.1002/neu.480170502. [DOI] [PubMed] [Google Scholar]
- 36.Evans PD, Maqueira B. Insect octopamine receptors: a new classification scheme based on studies of cloned Drosophila G-protein coupled receptors. Invert Neurosci. 2005;5:111–118. doi: 10.1007/s10158-005-0001-z. [DOI] [PubMed] [Google Scholar]
- 37.Helm G, Owman C, Rosengren E, Sjoberg NO. Regional and cyclic variations in catecholamine concentration of the human fallopian tube. Biol Reprod. 1982;26:553–558. doi: 10.1095/biolreprod26.4.553. [DOI] [PubMed] [Google Scholar]
- 38.Helm G, Owman C, Sjoberg NO, Walles B. Motor activity of the human Fallopian tube in vitro in relation to plasma concentration of oestradiol and progesterone, and the influence of noradrenaline. J Reprod Fertil. 1982;64:233–242. doi: 10.1530/jrf.0.0640233. [DOI] [PubMed] [Google Scholar]
- 39.Helm G, Owman C, Sjoberg NO, Walles B. Quantitative pharmacological characterization of beta-receptors and two types of alpha-receptors mediating sympathomimetic smooth muscle response in the human Fallopian tube at various cyclic stages. Acta Physiol Scand. 1982;114:425–432. doi: 10.1111/j.1748-1716.1982.tb07005.x. [DOI] [PubMed] [Google Scholar]
- 40.Strom C, Dahlstrom A, Lindblom B, Ahlman H. Adrenoceptor modulated flow through the rabbit ampulloisthmic region studied in vivo and in vitro. J Neural Transm. 1986;66:209–219. doi: 10.1007/BF01260915. [DOI] [PubMed] [Google Scholar]
- 41.Ajonuma LC, Ng EH, Chan HC. New insights into the mechanisms underlying hydrosalpinx fluid formation and its adverse effect on IVF outcome. Hum Reprod Update. 2002;8:255–264. doi: 10.1093/humupd/8.3.255. [DOI] [PubMed] [Google Scholar]
- 42.Mardh PA. Tubal factor infertility, with special regard to chlamydial salpingitis. Curr Opin Infect Dis. 2004;17:49–52. doi: 10.1097/00001432-200402000-00010. [DOI] [PubMed] [Google Scholar]
- 43.Roman G, He J, Davis RL. New series of Drosophila expression vectors suitable for behavioral rescue. Biotechniques. 1999;27:54–56. doi: 10.2144/99271bm09. [DOI] [PubMed] [Google Scholar]
- 44.Han KA, Millar NS, Grotewiel MS, Davis RL. DAMB, a novel dopamine receptor expressed specifically in Drosophila mushroom bodies. Neuron. 1996;16:1127–1135. doi: 10.1016/s0896-6273(00)80139-7. [DOI] [PubMed] [Google Scholar]