Abstract
Gender differences in tobacco withdrawal are of considerable clinical importance, but research findings on this topic have been mixed. Methodological variation in samples sizes, experimental design, and measures across studies may explain the inconsistent results. The current study examined whether male (n = 101) and female (n = 102) smokers (≥15 cigarettes/day) differed in abstinence-induced changes on a battery of self-report measures (withdrawal, affect, craving), cognitive performance tasks (attention, psychomotor performance), and physiological responses (heart rate, blood pressure, brain electroencephalogram). Participants attended 2 counterbalanced laboratory sessions, 1 following 12 hr of abstinence and the other following ad libitum smoking. Results showed that women reported greater abstinence-induced increases in negative affect, withdrawal-related distress, and urge to smoke to relieve withdrawal distress. In contrast, both genders reported similar abstinence-induced changes in positive affect and urge to smoke for pleasure. Men and women exhibited generally similar abstinence-induced changes in physiological and cognitive performance measures. In addition, gender did not moderate the association between withdrawal symptoms and baseline measures of smoking behavior and dependence. Abstinence-induced changes in withdrawal distress mediated the effect of gender on latency until the 1st cigarette of the day at trend levels (p < .10). These findings suggest that there are qualitative gender differences in the acute tobacco withdrawal syndrome that may underlie gender-specific smoking patterns.
Keywords: gender differences, nicotine withdrawal, affect, craving, smoking
Research suggests that women are less likely than men to successfully quit smoking. Recent meta-analyses of smoking cessation trials showed lower abstinence rates for women than men, regardless of whether individuals received group or individual counseling (Wetter, Kenford, et al., 1999), nicotine replacement therapy (Cepeda-Benito, Reynoso, & Erath, 2004; Wetter, Kenford, et al., 1999) or placebo nicotine patch (Wetter, Kenford, et al., 1999), or bupropion sustained release or placebo (Collins et al., 2004; Scharf & Shiffman, 2004). Although there is debate over whether women’s quit rates are lower than men’s at the population level (Gritz, Nielsen, & Brooks, 1996) or whether differences are large enough to be clinically significant (Killen, Fortmann, Varady, & Kraemer, 2002), it is important to identify gender-specific processes underlying tobacco addiction so that interventions can be tailored to gender.
In recent years, researchers have investigated several variables that might differentially impact smoking behavior in men and women. These include attitudes toward cessation (Etter, Prokhorov, & Perneger, 2002), concerns about weight gain (Borrelli, Spring, Niaura, Hitsman, & Papandonatos, 2001), depressive and anxious symptomatology (Borrelli, Bock, King, & Pinto, 1996), nicotine reinforcement (Perkins, Donny, & Caggiula, 1999), menstrual cycle effects (Craig, Parrott, & Coomber, 1992), social support during cessation (Murray, Johnston, Dolce, & Lee, 1995), and cue reactivity (Field & Duka, 2004). In addition, examination of gender differences in the tobacco withdrawal syndrome is an especially fruitful area because of the presumed association between withdrawal and smoking behavior (e.g., al’Absi, Hatsukami, Davis, & Wittmers, 2004; Hughes, 1992).
There are a number of ways in which gender, withdrawal, and smoking can interrelate. Men and women may differ in the severity or quality of withdrawal. If so, withdrawal may mediate relationships between gender and smoking behavior, such as cessation. If withdrawal does indeed mediate the gender–smoking relationship to some degree, it would suggest that women would require more treatment of withdrawal symptoms to achieve cessation rates comparable with men’s. In addition, withdrawal symptoms may have a greater or lesser impact on smoking behavior in men versus women, suggesting that the association between withdrawal and smoking behavior may be moderated by gender. For example, in a community sample of smokers enrolled in smoking cessation clinics, Gunn (1986) reported that severity of withdrawal symptoms predicted cessation outcomes in women but not men. If the influence of withdrawal on smoking is indeed greater in women, it would suggest that treatments that alleviate withdrawal would have a larger impact on smoking outcomes in women than men. Thus, the manner in which gender, withdrawal, and smoking interrelate has important implications for gender-specific treatments of nicotine dependence.
Indeed, the gender–withdrawal relationship has been well studied, but findings have been inconsistent. For example, some studies have reported greater withdrawal in women than men on some or all measures (al’Absi, Amunrud, & Wittmers, 2002; al’Absi et al., 2004; Field & Duka, 2004; C. S. Pomerleau, Tate, Lumley, & Pomerleau, 1994; Strong, Kahler, Ramsey, Abrantes, & Brown, 2004; Wetter, Fiore, et al., 1999). Other studies have reported no differences on some or all measures (al’Absi et al., 2002, 2004; Evans, Blank, Sams, Weaver, & Eissenberg, 2006; Field & Duka, 2004; Gunn, 1986; Hatsukami, LaBounty, Hughes, & Laine, 1993; Hughes, Gust, Skoog, & Keenan, 1991; C. S. Pomerleau et al., 1994; Strong et al., 2004; Svikis, Hatsukami, Hughes, & Carroll, 1986; Wetter, Fiore, et al., 1999), and still others have reported more severe withdrawal in men than in women on some or all measures (al’Absi et al., 2004; Hatsukami et al., 1993; O. F. Pomerleau et al., 2005). There have also been discrepant findings in those studies that have examined whether gender moderates the effect of withdrawal on smoking outcomes (Gunn, 1986; Wetter, Kenford, et al., 1999). In contrast to the findings of Gunn (1986) noted earlier, Wetter, Fiore, et al. (1999) examined male and female smokers participating in three randomized trials of the nicotine patch and found that gender did not moderate the effect of any withdrawal measure on cessation outcomes. Wetter, Fiore, et al.’s study also examined whether postcessation variables, such as perceived stress, mediated the effect of gender on cessation outcomes. Although women demonstrated lower quit rates than men across the three trials, there was no evidence of mediation.
Inconsistency across studies might be caused by differences in methodology. First, studies have used different designs. Some studies compared men and women only in abstinent states (Gunn, 1986), whereas others examined differences in abstinence-induced changes (Hughes, Hatsukami, Mitchell, & Dahlgren, 1986). Interpretation of findings from studies comparing men and women in abstinent states only is difficult because women tend to have more anxiety, dysphoria, and irritability (American Psychiatric Association, 1994), making it unclear whether differences in some symptoms are due to baseline differences or to the effects of abstinence. Second, studies have differed in the mode of assessment (i.e., retrospective vs. prospective; Hughes, Higgins, & Hatsukami, 1990). One study compared these two assessment approaches and found women to report greater withdrawal severity only with retrospective assessment methods (C. S. Pomerleau et al., 1994). Third, studies have had very different sample sizes, and thus, some studies may not have had sufficient statistical power to detect small to medium effect sizes. Finally, studies have used different withdrawal measures. Some studies have relied primarily on subjective assessments, such as withdrawal and craving questionnaires (e.g., Gunn, 1986). Others have also used objective assessments, such as physiological and cognitive performance measures (al’Absi et al., 2002). This is an important distinction because gender differences may be apparent with subjective but not objective measures, or differences may be present for only a subset of subjective, physiological, or cognitive performance measures. Another benefit of using multiple measures is that it permits an examination of qualitative differences in withdrawal phenomenology. That is, one can examine whether men and women experience different types of withdrawal syndromes.
Many studies have focused on protracted abstinence, measuring withdrawal after the first 24 hr of abstinence and during the next several weeks (e.g., Hughes, 1992). Relatively few studies, however, have focused on gender differences in withdrawal during the first 24 hr of abstinence (e.g., al’Absi et al., 2002 e.g., al’Absi et al., 2004; Evans et al., 2006). Withdrawal in acute abstinence may be especially important because many relapses occur during the first few days of cessation (Garvey, Bliss, Hitchcock, & Heinold, 1992), with a significant portion of individuals lapsing on their planned quit date (Brown, Herman, Ramsey, & Stout, 1998). In addition, acute (but not protracted) abstinence simulates the type of withdrawal typically experienced by smokers not attempting to quit (e.g., withdrawal experienced before the first cigarette of the day).
Overall, the gender–withdrawal relationship is of considerable scientific and clinical interest, but findings have been mixed. However, if the interrelation between gender, withdrawal, and smoking can be clarified, gender-specific cessation interventions could be developed. To address these issues, we examined gender differences in acute tobacco withdrawal, using the following methods: (a) We included multiple subjective and objective measures, including physiological measures, brain electroencephalogram (EEG) measures, and measures of cognitive performance; (b) we used a relatively large sample (N = 203), allowing power to detect small to medium effect sizes; (c) we conducted assessments in both abstinent and nonabstinent states (order counterbalanced); and (d) we used a sample that was not attempting to quit smoking. Gender differences among smokers not wishing to quit are of interest because such differences may elucidate processes that maintain typical day-to-day smoking patterns as opposed to those involved in cessation and relapse.
In summary, the primary aim of this study was to investigate gender differences in withdrawal effects across a wide range of subjective and objective measures. We also examined whether withdrawal effects mediate the influence of gender on smoking behavior. Finally, to clarify the mixed findings regarding the differential effect of withdrawal on smoking in men and women (Gunn, 1986; Wetter, Kenford, et al., 1999), we evaluated whether the relationships between withdrawal and smoking behavior were moderated by gender.
Methods
Participants
Participants were 203 smokers recruited from the Baltimore metropolitan area by means of newspaper and radio advertisements. The sample was recruited to be balanced in gender (49.8% men, 50.2% women) and race (51.7% Black, 48.3% White). Participants were included in the study if they were 18 years or older, reported being a current smoker of at least 15 cigarettes per day, reported having smoked for at least 2 years, smoked a brand of cigarettes that delivers at least 11.0 mg tar and 0.7 mg nicotine as rated by the Federal Trade Commission method, and had a score of 3 or more on the Fagerström Test for Nicotine Dependence (FTND; Heatherton, Kozlowski, Frecker, & Fagerström, 1991). Participants were excluded (a) if they reported a recent history of certain diseases, including myocardial infarction, heart failure, angina, stroke, diabetes, and hypertension; (b) if they were treated with nicotine replacement products in the past 6 months or with antidepressants in the past year; (c) if they reported using any smoking cessation treatments in the past 6 months; or (d) if their estimated IQ on the Shipley Institute on Living Scale was less than 78 (Shipley, 1940). Women who were pregnant or nursing were also excluded. The study was approved by the Institutional Review Board of the National Institute on Drug Abuse–Intramural Research Program.
In total, 858 participants completed the medical screening visit, of whom 521 were disqualified because of inclusion–exclusion criteria. Out of the 337 eligible participants, 230 attended a subsequent orientation session. Of these, 209 completed the two experimental sessions (i.e., abstinent and nonabstinent sessions); however 6 did not meet criteria for biochemical confirmation of smoking.
At the abstinent session, participants were considered nonabstinent if they reported having smoked on that day or had high carbon monoxide (CO) levels (>11 ppm).1 Under these conditions, the abstinent session was rescheduled. At the nonabstinent session, 6 participants had low CO levels (<10 ppm; for these 6 participants, mean CO at nonabstinent session = 6.3 ppm, range = 3–9 ppm; mean CO at abstinent session = 4.3 ppm, range = 3–8 ppm). These participants were excluded from analyses because of considerable doubt about (a) whether they were 15+ cigarettes per day smokers or (b) whether they complied with the instructions to smoke normally before the nonabstinent session. The final sample of participants (N = 203) had mean CO levels of 30.0 ppm at the nonabstinent session (SD = 12.1, range = 10–69) and had mean CO levels of 6.9 ppm at the abstinent session (SD = 2.5, range = 3–11). The mean change in CO levels between the two sessions was 23.1 ppm (SD = 11.4, range = 6–61), and change in CO did not differ by gender (men: M = 23.1, SD = 11.5; women: M = 23.0, SD = 11.4), t(201) = 0.03, p = .98. Baseline demographic, psychiatric, and smoking characteristics of the sample are reported in Table 1.
Table 1.
Baseline Sample Characteristics
| Men (n = 101) |
Women (n = 102) |
Total sample |
|||||
|---|---|---|---|---|---|---|---|
| Characteristic | M | SD | M | SD | M | SD | t or χ2 |
| Age | 36.8 | 10.0 | 36.6 | 10.2 | 36.7 | 10.1 | ns |
| Race (%) | ns | ||||||
| Black | 48.5 | 54.9 | 51.7 | ||||
| White | 51.5 | 45.1 | 48.3 | ||||
| SCL-90-R | 48.9 | 11.0 | 47.4 | 11.7 | 48.2 | 11.3 | ns |
| Cigarettes/day | 22.5 | 6.48 | 21.9 | 6.76 | 22.2 | 6.61 | ns |
| FTND | 6.26 | 1.62 | 6.69 | 1.75 | 6.47 | 1.70 | ns |
| Years smoking | 19.6 | 10.4 | 19.8 | 10.4 | 19.7 | 10.3 | ns |
| Min until first cigarette (%) | 8.37* | ||||||
| 0–5 | 47.5 | 66.7 | 57.1 | ||||
| 6–30 | 59.8 | 32.4 | 40.4 | ||||
| 31–60 | 4.0 | 1.0 | 2.5 | ||||
| Previous quit attempts | 3.56 | 3.49 | 3.00 | 3.74 | 3.28 | 3.75 | ns |
Note. SCL-90-R = Symptom Check List–90–Revised global severity index; FTND = Fagerström Test of Nicotine Dependence.
p < .05.
Procedure
A preliminary telephone screening (which surveyed tobacco use, recent heart attack, angina, current upper respiratory infection, etc.) was conducted. Eligible participants were invited to attend a screening visit conducted at the National Institute on Drug Abuse—Intramural Research Program. At this screening visit, participants completed a number of psychological assessments, including the Addiction Severity Index (McLellan, Kushner, Metzger, & Peters, 1992), the Shipley Institute on Living Scale (Shipley, 1940), and the Symptom Checklist–90–Revised (SCL-90-R; Derogatis, 1992). Race was obtained from the Addiction Severity Index item asking what race participants considered themselves to be. Options included White (not Hispanic) and Black (not Hispanic); participants could endorse only one option. Participants also completed a number of medical assessments, including a medical history, a physical examination, and routine laboratory chemistries. These data were reviewed by a medically responsible physician (Susan Boyd or Eric T. Moolchan) who determined whether the participant was eligible to participate further in the study.
Eligible participants attended a 90-min orientation session followed by two counterbalanced experimental sessions lasting 60 min each. The three sessions occurred on different days. Participants were instructed to smoke normally before the orientation session. During the orientation session, participants completed a demographics questionnaire. Participants also completed several questionnaires assessing tobacco use and dependence, including the FTND and an author-constructed questionnaire assessing smoking history. Participants received practice on the cognitive performance tasks. During the experimental sessions, participants completed a battery of physiological, subjective, and cognitive measures (detailed in the Measures section). For the nonabstinent session, participants were asked to smoke ad libitum before the session and to smoke a cigarette within 20 min of the beginning of the nonabstinent session. Participants reported that they smoked their last cigarette on average 15.7 min (SD = 10.7) before the nonabstinent session. This time period did not differ by gender (men: M = 16.2, SD = 9.99; women: M = 15.2, SD = 11.5), t(201) = 0.67, p = .51. For the abstinent session, participants were asked to refrain from smoking for at least 12 hr before the session. The order of completion of the abstinent and nonabstinent sessions was counterbalanced. The mean number of days between the experimental sessions was 2.88 (SD = 3.93), which did not differ by gender (men: M = 2.75, SD = 2.33; women: M = 3.02, SD = 5.05), t(201) = −0.48, p = .63. Experimental sessions were scheduled to occur in the afternoons, and we attempted to schedule both experimental sessions at the same time of day for each participant.
Measures
During the experimental sessions, participants completed the following measures. Details on the order in which assessments were completed and the timing of those assessments are shown in Table 2.
Table 2.
Procedure and Timeline for Experimental Sessions
| Procedure | Assessment | Time (min) | Questionnaire wording |
|---|---|---|---|
| Exhaled CO | CO | 2 | — |
| Heart rate/blood pressure (T1) | HR, SBP/DBP | 1 | — |
| Questionnaires (T1) | PANAS, HQ, HHWQ, WSWS, SBQ | 10 | “so far today” |
| TCQ, QSU | 4 | “right now” | |
| Cognitive performance tasks | ST, MT, DSST, RIPT | 16 | — |
| Questionnaires (T2) | PANAS, HHWQ | 4 | “in the last few minutes” |
| QSU | 2 | “right now” | |
| Attentional bias tasksa | Smoking Stroop Task, SSQ | 6 | — |
| Visual Probe Task | 10 | — | |
| EEG | Electrode placement | 10 | |
| Eyes closed | 1 | — | |
| Eyes open | 1 | — | |
| Heart rate/blood pressure (T2) | HR, SBP/DBP | 1 | — |
Note. Assessments are listed in the order in which they were completed. CO = carbon monoxide; T1 = Time 1; T2 = Time 2; HR = heart rate; SBP = systolic blood pressure; DBP = diastolic blood pressure; PANAS = Positive and Negative Affect Schedule; HQ = Hunger Questionnaire; HHWQ = Hughes Hatsukami Withdrawal Questionnaire; WSWS = Wisconsin Smoking Withdrawal Scale; SBQ = Subjective Bias Questionnaire; TCQ = 12-item Tobacco Craving Questionnaire; QSU = Brief Questionnaire of Smoking Urges; ST = Two-Letter Search Task; MT = Serial Math Test; DSST = Digit Symbol Substitution Task; RIPT = Rapid Information Processing Task; SSQ = Subjective Stroop Questionnaire; EEG = electroencephalogram.
Order of completion of the Smoking Stroop Task and Picture Probe Task was counterbalanced across participants.
Physiological assessments
Heart rate, systolic blood pressure, and diastolic blood pressure were measured with an electronic Datascope machine. EEG recordings were collected from Cz, Fz, and Pz electrodes (monopolar, linked ear reference) with an automated EEG collection and analysis system, the Pathfinder (Biologic Instruments, Chicago, IL; described in Pickworth, O’Hare, Fant, & Moolchan, 2003). Gold plated scalp electrodes were used. Participants were assessed for 1 min with their eyes closed and for 1 min with their eyes open. Two-second epochs were continuously acquired from each of the EEG electrodes. The EEG was digitized at 128 Hz, and samples with artifacts were automatically rejected. The digitized EEG was converted to the frequency domain with a fast Fourier transform. For each epoch, the computer printed the power (μV2). EEG frequency was computed as the frequency (Hz) at which 80% of the power of the band had accumulated (resolution, 0.5 Hz) in each of the usual clinical frequency bands: delta = 0.5–3.5 Hz; theta = 4.5–7.5 Hz; alpha = 8.5–12.5 Hz; Beta 1 = 14.5–23.5 Hz; Beta 2 = 25.0–31.5 Hz. To simplify analyses, we report EEG data averaged over the three electrodes and use this as the dependent variable for analyses.2
Subjective assessments
The Hughes Hatsukami Tobacco Withdrawal Questionnaire (HHWQ; Hughes & Hatsukami, 1986) is a widely used measure that assesses 11 symptoms and signs of tobacco withdrawal on 6-point Likert-type scales.
The Wisconsin Smoking Withdrawal Scale (WSWS; Welsch et al., 1999) is a multidimensional measure of tobacco withdrawal. The WSWS contains 23 items that load onto six subscales of tobacco withdrawal (i.e., Anxiety, Anger, Hunger, Concentration Problems, Craving, Sadness) and an Overall Severity scale. (We excluded the items that assess sleep disturbance because participants would not have been abstinent for a long period before going to sleep).
The Brief Questionnaire of Smoking Urges (QSU; Cox, Tiffany, & Christen, 2001) assesses desire for the positive effects of smoking (Factor 1, 5 items) and desire for relief of negative affect and an urgent need to smoke (Factor 2, 5 items).
The 12-item Tobacco Craving Questionnaire (TCQ; Heishman, Singleton, & Moolchan, 2003) assesses four dimensions of craving: (a) anticipation of relief from withdrawal and negative mood by smoking (Emotionality subscale); (b) anticipation of positive outcomes from smoking (Expectancy subscale); (c) inability to control smoking (Compulsivity subscale); and (d) intention to smoke for positive outcomes (Purposefulness subscale).
The Positive and Negative Affect Schedule (PANAS; Watson, Clark, & Tellegen, 1988) includes a Positive Affect scale (10 items; e.g., enthusiastic, strong) and a Negative Affect scale (10 items; e.g., distressed, upset). These scales have been shown to represent orthogonal dimensions of affect (Watson et al., 1988).
Hunger was measured using the methodology of Hill and Blundell (1982). Participants rated their feelings of hunger, fullness, and desire to eat on eight separate 10-point Likert-type scales (1 = not at all, 10 = extremely).
The Subjective Attentional Bias Questionnaire is an eight-item questionnaire that assesses the extent to which participants feel or notice that their attention is captured by cigarettes and smoking cues. Anecdotally, we have noted that smokers often report that they notice cigarettes and people smoking much more frequently when abstaining than when smoking normally (Waters & Sayette, 2005). For example, one item asks how often participants found themselves staring at cigarettes and cigarette smoke. In the current study, the abstinence-induced change scores on the eight items were strongly intercorrelated (α = .88). The total score for this questionnaire (mean of the eight items) was greatly increased by abstinence (effect size d = 1.23, p < .001), and abstinence-induced increases in the total score was reliably correlated with FTND scores (r = .24, p < .001).
Cognitive assessments
The Two-Letter Search Task and Serial Math Test, drawn from the Walter Reed Performance Assessment Battery, were used to assess cognitive performance (Thorne, Genser, Sing, & Hegge, 1985). The Two-Letter Search Task assesses visual scanning, recognition, and attentional abilities. In the Two-Letter Search Task, two target letters appeared at the top of a computer screen, and a string of 20 letters appeared below. The 20-letter series could contain neither, one, or both of the target letters. Participants responded by pressing one key if both target letters were in the 20-letter string and by pressing a second key if one or neither target was in the string. The task ended after 20 trials or after 120 s, whichever came sooner. The Serial Math Test assesses mathematical reasoning. In the Serial Math Test, two random digits were rapidly presented on the screen for 250 ms, followed by a plus or a minus sign and a question mark as a prompt. Participants were required to perform the mathematical operation and to enter the final digit of the response into the keyboard as quickly as possible by pressing a numerical key (e.g., the correct response to 6 + 7 is 3, because the 3 is the final digit of 13). The task ended after 50 trials or after 240 s, whichever came sooner (Pickworth, Rohrer, & Fant, 1997). The mean reaction time (on correct responses) and the percentage error rate were obtained for the Two-Letter Search Task and the Serial Math Test.
The Digit Symbol Substitution Task was used to assess psychomotor performance. A randomly generated digit appeared on a computer monitor, and the participant was required to reproduce the symbol as quickly as possible by pressing the corresponding keys on the number pad of the computer keyboard. The task lasted for 90 s. The total number of correct symbols reproduced within the 90-s period and the percentage of incorrect responses (error rate) were determined.
The Rapid Information Processing Task is a task widely used to assess sustained attention (Foulds et al., 1996). In this task, a series of single digits was presented on the computer screen for 10 min at a rate of 100 digits per minute. Targets were defined as three consecutive odd digits (e.g., 7, 9, 3) or three consecutive even digits (e.g., 2, 8, 6). There were eight targets per minute, making a total of 80 targets in 10 min, with 5–30 digits between each target. Participants were required to press the spacebar as quickly as possible after detecting a target. There was a response window of 1,500 ms. The percentage of targets correctly detected (hit rate) and the mean reaction time on targets (in milliseconds) were computed. The number of false positives per minute (i.e., depressing the spacebar when a target was not present) was also determined.
The Smoking Stroop Task assesses the attention-grabbing properties of smoking cues (Waters et al., 2003). Participants were told that words written in different colors would be presented one after the other on a computer monitor and that their task was to indicate as quickly and as accurately as possible the color the word was written in by pressing one of three colored buttons on a keyboard. They were instructed to ignore the meaning of the word itself and just to respond to the color. We used the same task and scoring methods used by Waters et al. (2003). Participants responded to a block of neutral words (33 trials) followed by a block of smoking words (33 trials). The smoking Stroop effect (the ability of the smoking words to capture attention) is the difference in reaction times on the smoking words and the neutral words. After completing the task, participants were asked to report the extent to which they felt distracted by (a) the nonsmoking (neutral) words and (b) the smoking words while performing the Smoking Stroop Task (Subjective Stroop Questionnaire). A difference score (reported distraction on smoking words minus reported distraction on neutral words) was computed to index subjective distraction by smoking cues (vs. neutral cues). In the current data set, subjective distraction by smoking cues (vs. neutral cues) was increased by abstinence (p < .005) and was correlated with FTND scores at the abstinence session (p < .05).
As noted in Table 2, heart rate, systolic blood pressure, diastolic blood pressure, and some of the questionnaire assessments (HHWQ, PANAS, QSU) were assessed at two time points. With the exception of the TCQ and QSU (which can only assess craving “right now”), questionnaires completed at Time 1 (T1) required participants to report on experiences “so far today” to capture the totality of experience during the course of the abstinent and nonabstinent days. Questionnaires completed at Time 2 (T2) required participants to report on experiences “in the past few minutes” to assess experiences immediately following a demanding task (the cognitive assessments). In addition to the battery of measures just described, participants were randomly assigned to perform one of two different versions of the Picture Probe Task (a measure of the allocation of spatial attention; Waters & Sayette, 2005). Data from this task will be reported in a later article.
Data Analysis
To examine gender differences in abstinence effects, we first conducted separate Gender (male vs. female) × State (nonabstinent vs. abstinent) repeated measures multivariate analyses of variance (MANOVAs), using the proc GLM procedure in the SAS program (SAS Institute, 2003). Separate MANOVAs were conducted for sets of subjective, cognitive, and physiological variables. For measures that had multiple subscales (QSU, TCQ, PANAS), we omitted the total score from the set of dependent variables to avoid analytic redundancy. We also conducted univariate analyses of gender effects on each outcome measure at each assessment point (T1, T2), using t tests on data from the nonabstinent session, the abstinent session, and abstinence-induced change (abstinent − nonabstinent) scores. A significant t statistic for a test on an abstinence-induced change score indicates that men and women differed in the degree of withdrawal for that measure and is equivalent to a Gender (male vs. female) × State (nonabstinent vs. abstinent) interaction in an analysis of variance (ANOVA). The univariate tests allowed for a more comprehensive characterization of gender differences in abstinence effects. At the same time, this approach increased the probability for Type I error. Thus, the results were interpreted in the context of the results of the initial MANOVA analyses. In addition, we also report Cohen’s d statistics, which are effect size estimates (Cohen, 1977) that can be used to interpret the robustness of each effect (small d = .20, medium d = .50, large d = .80). Descriptive statistics and analyses of subjective measures were based on mean score per item, rather than traditional summed scores, to facilitate interpretation across measures.
For some measures with multiple subscales (i.e., QSU, PANAS, TCQ), we conducted follow-up Gender × State × Subscale ANOVAs with T1 data. In these analyses, the presence of the Gender × State × Subscale interaction indicates that gender differences in abstinence-induced changes differed across subscales. For measures that were assessed multiple times (HHWQ, QSU, PANAS, heart rate, systolic blood pressure, diastolic blood pressure), we also conducted Gender × State × Time (T1 vs. T2) ANOVAs. In these analyses, a Gender × State interaction indicates that gender differences in abstinence-induced changes were evident when incorporating data from both T1 and T2 assessments, and a Gender × State × Time interaction indicates that gender differences in abstinence-induced changes differed across T1 and T2 assessments. It should be noted that (a) cognitive performance tasks were completed in between the T1 and T2 assessments, and (b) participants might have shown a natural increase in withdrawal during the nonabstinent session generated by a brief period of nicotine deprivation. Therefore, it is difficult to interpret effects involving time as reactions to a stressor (i.e., completing cognitive performance tasks).
To examine whether abstinence effects mediated any association between gender and smoking, we used the Sobel test (Sobel, 1982). This test evaluates the significance of the mediating variable effect by dividing the estimate of the intervening variable effect, αβ, by its standard error and comparing this value to a standard normal distribution. This method has been shown to demonstrate accurate Type I error rates in comparison with other methods (MacKinnon, Lockwood, Hoffman, West, & Sheets, 2002). In mediational models, gender was the independent variable, abstinence-induced change scores were potential mediating variables, and five smoking characteristics were potential dependent variables (FTND scores, cigarettes smoked per day, time to first cigarette, number of previous quit attempts, and years of regular smoking). Only abstinence-induced change scores that differed between men and women were selected as mediating variables, and only smoking characteristics that were associated with gender were selected as dependent variables for mediation analyses.
To examine whether gender moderated the association between abstinence effects and smoking characteristics, we used the proc GLM procedure in the SAS program and tested whether the interaction between gender and abstinence-induced changes predicted smoking characteristics. With the five smoking characteristics listed earlier, separate moderation analyses were conducted for all T1 measures. Thus, five models were tested for each T1 measure, and we used Bonferroni-corrected analyses (α/5 dependent variables = .01).
Results
The sociodemographic, smoking, and psychiatric characteristics of the sample are presented in Table 1. Men and women did not differ in reported cigarettes smoked per day, FTND scores, years of smoking, or number of previous quit attempts. However, a greater proportion of women than men reported smoking within 5-min of waking up (67% vs. 48% respectively).
Subjective Measures
Multivariate repeated measures analyses of T1 subjective measures yielded a significant main effect of state, F(1, 187) = 479.43, Wilks’s λ = .29, p < .0001, and a significant Gender × State interaction, F(1, 187) = 10.20, Wilks’s λ = .95, p < .005. The main effect of gender did not reach significance, F(1, 187) = 2.90, p = .09.
Table 3 (T1 assessments) and Table 4 (T2 assessments) show the means of each subjective measure by gender for nonabstinent, abstinent, and abstinence-induced change scores. These tables also show the results of t tests examining gender differences in these scores. There were no significant gender effects in the nonabstinent state. However, many gender differences were found in the abstinent state, resulting in several significant gender differences in abstinence-induced changes (see Tables 3 and 4). In particular, women reported more severe abstinence-induced increases in (a) negative affect (i.e., HHWQ Irritable/Angry and Impatient; WSWS Anger, Anxiety, and Sadness; and PANAS Negative Affect) and (b) urge to relieve withdrawal-related distress by smoking (QSU Factor 2, TCQ Emotionality).
Table 3.
Gender Differences in Acute Abstinence (Subjective Measures, Time 1)
| Nonabstinent |
Abstinent |
Abstinence-induced changeb |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Measure | Men | Women | t | d | Men | Women | t | d | Men | Women | t | d |
| Withdrawal | ||||||||||||
| HHWQ (0–5) | ||||||||||||
| Total | 0.73 | 0.75 | ns | — | 1.66 | 2.05 | 3.09** | 0.44 | 0.93 | 1.30 | 2.86** | 0.40 |
| Craving | 2.21 | 2.31 | ns | — | 3.98 | 4.14 | ns | — | 1.77 | 1.82 | ns | — |
| Irritable/angry | 0.53 | 0.54 | ns | — | 2.07 | 2.59 | 2.34* | 0.33 | 1.53 | 2.05 | 2.21* | 0.31 |
| Anxious/tense | 0.75 | 0.77 | ns | — | 2.19 | 2.62 | ns | — | 1.44 | 1.86 | ns | — |
| Concentration | 0.56 | 0.62 | ns | — | 1.47 | 1.95 | 2.20* | 0.31 | 0.90 | 1.33 | nsa | |
| Restlessness | 0.74 | 0.72 | ns | — | 1.73 | 2.14 | nsa | — | 0.98 | 1.42 | ns | — |
| Impatient | 0.82 | 0.76 | ns | — | 2.13 | 2.55 | ns | — | 1.28 | 1.82 | 2.20* | 0.31 |
| Hunger | 0.93 | 0.90 | ns | — | 1.86 | 2.29 | ns | — | 0.92 | 1.39 | ns | — |
| Autonomic | 0.09 | 0.18 | nsa | — | 0.25 | 0.57 | 2.44a* | 0.35 | 0.16 | 0.39 | nsa | — |
| Eating | 0.56 | 0.59 | ns | — | 1.57 | 1.85 | ns | — | 1.01 | 1.26 | ns | — |
| Drowsiness | 0.48 | 0.43 | ns | — | 0.58 | 0.85 | ns | — | 0.09 | 0.42 | ns | — |
| Headaches | 0.15 | 0.23 | nsa | — | 0.34 | 0.72 | 2.48a* | 0.35 | 0.19 | 0.49 | 2.07a* | 0.29 |
| WSWS (0–4) | ||||||||||||
| Total | 1.31 | 1.22 | ns | — | 1.98 | 2.23 | 2.61** | 0.37 | 0.67 | 1.01 | 3.37*** | 0.48 |
| Anger | 0.95 | 0.83 | ns | — | 1.69 | 2.10 | 2.22* | 0.31 | 0.74 | 1.26 | 2.73** | 0.39 |
| Anxiety | 1.25 | 1.15 | ns | — | 1.90 | 2.24 | 2.76** | 0.39 | 0.66 | 1.09 | 3.14** | 0.44 |
| Concentration | 0.95 | 0.97 | ns | — | 1.51 | 1.93 | 2.96** | 0.42 | 0.56 | 0.96 | 2.65** | 0.37 |
| Craving | 1.75 | 1.66 | ns | — | 3.08 | 3.23 | ns | — | 1.34 | 1.57 | ns | — |
| Hunger | 1.59 | 1.46 | ns | — | 2.05 | 2.20 | ns | — | 0.46 | 0.73 | ns | — |
| Sadness | 1.16 | 1.04 | ns | — | 1.46 | 1.60 | ns | — | 0.30 | 0.56 | 2.30* | 0.33 |
|
| ||||||||||||
| Craving | ||||||||||||
| QSU (0–5) | ||||||||||||
| Total | 1.60 | 1.61 | ns | — | 3.21 | 3.59 | 2.67** | 0.38 | 1.61 | 1.97 | 2.27* | 0.32 |
| Factor 1 | 2.24 | 2.21 | ns | — | 4.25 | 4.43 | ns | — | 2.01 | 2.22 | ns | — |
| Factor 2 | 0.96 | 1.01 | ns | — | 2.16 | 2.74 | 3.00** | 0.42 | 1.21 | 1.73 | 3.16** | 0.45 |
| TCQ (1–7) | ||||||||||||
| Total | 3.15 | 3.22 | ns | — | 4.84 | 5.19 | 2.08* | 0.29 | 1.71 | 1.96 | ns | — |
| Emotionality | 2.54 | 2.55 | ns | — | 4.07 | 4.77 | 2.77** | 0.39 | 1.55 | 2.22 | 2.63** | 0.37 |
| Expectancy | 4.05 | 4.05 | ns | — | 6.25 | 6.33 | ns | — | 2.20 | 2.29 | ns | — |
| Compulsivity | 2.30 | 2.54 | ns | — | 3.67 | 4.15 | ns | — | 1.38 | 1.62 | ns | — |
| Purposefulness | 3.71 | 3.76 | ns | — | 5.39 | 5.49 | ns | — | 1.70 | 1.73 | ns | — |
|
| ||||||||||||
| Attentional bias | ||||||||||||
| SBQ (0–4) | 1.27 | 1.26 | ns | — | 2.35 | 2.57 | ns | — | 1.10 | 1.31 | ns | — |
| SSQ (34–4) | 0.53 | 0.80 | ns | — | 0.85 | 1.03 | ns | — | 0.32 | 0.23 | ns | — |
|
| ||||||||||||
| Affect | ||||||||||||
| PANAS (1–5) | ||||||||||||
| PA | 3.07 | 3.15 | ns | — | 2.84 | 2.93 | ns | — | −0.23 | −0.22 | ns | — |
| NA | 1.26 | 1.21 | ns | — | 1.64 | 1.87 | 2.92a** | 0.41 | 0.38 | 0.66 | 3.85*** | 0.54 |
|
| ||||||||||||
| Hunger | ||||||||||||
| HQ (1–10) | 3.02 | 2.88 | ns | — | 3.81 | 4.18 | nsa | — | 0.78 | 1.31 | nsa | — |
Note. Mean scores per item displayed. Only Time 1 assessments are shown. For nonsignificant effects, d and t values are not shown. Because of missing data, sample sizes vary across analyses (Ns = 186–203). HHWQ = Hughes Hatsukami Withdrawal Questionnaire (scale: 0–5); WSWS = Wisconsin Smoking Withdrawal Scale (scale: 0–4); QSU = Brief Questionnaire of Smoking Urges (scale: 0–5); TCQ = Tobacco Craving Questionnaire (scale: 1–7); SBQ = Subjective Bias Questionnaire (scale: 0–4); SSQ = Subjective Stroop Questionnaire (scale: − 4–4); PANAS = Positive and Negative Affect Schedule (scale: 1–5); HQ = Hunger Questionnaire (scale: 1–10).
Satterthwaite test was used because of unequal variances (p < .05).
Abstinence-induced change = abstinent − nonabstinent.
p < .05.
p < .01.
p < .001.
Table 4.
Gender Differences in Acute Abstinence (Subjective Measures, Time 2)
| Nonabstinent |
Abstinent |
Abstinence-induced changeb |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Measure | Men | Women | t | d | Men | Women | t | d | Men | Women | t | d |
| Withdrawal | ||||||||||||
| HHWQ (0–5) | ||||||||||||
| Total | 0.95 | 0.87 | ns | — | 1.61 | 2.06 | 3.24** | 0.44 | 0.65 | 1.19 | 4.26** | 0.58 |
| Craving | 2.03 | 1.98 | ns | — | 3.73 | 3.97 | ns | — | 1.70 | 1.99 | ns | — |
| Irritable/angry | 0.78 | 0.80 | ns | — | 1.90 | 2.52 | 2.52* | 0.35 | 1.10 | 1.72 | 2.58* | 0.36 |
| Anxious/tense | 1.21 | 1.10 | ns | — | 2.14 | 2.60 | ns | — | 0.92 | 1.49 | 2.40* | 0.33 |
| Concentration | 1.54 | 1.33 | ns | — | 2.08 | 2.86 | 3.37* | 0.46 | 0.52 | 1.53 | 4.30** | 0.58 |
| Restlessness | 1.04 | 0.88 | ns | — | 1.77 | 2.20 | nsa | — | 0.72 | 1.31 | 2.58* | 0.36 |
| Impatient | 1.19 | 1.09 | ns | — | 2.07 | 2.72 | 2.67** | 0.37 | 0.86 | 1.63 | 3.42* | 0.47 |
| Hunger | 0.87 | 0.77 | ns | — | 1.50 | 1.71 | ns | — | 0.62 | 0.96 | ns | — |
| Autonomic | 0.17 | 0.11 | nsa | — | 0.20 | 0.58 | 2.63a* | 0.37 | 0.03 | 0.47 | 2.96** | 0.41 |
| Eatingc | — | — | — | — | — | — | — | — | — | — | — | — |
| Drowsiness | 0.42 | 0.36 | ns | — | 0.43 | 0.77 | 2.16* | 0.30 | 0.01 | 0.41 | 2.29* | 0.32 |
| Headaches | 0.22 | 0.266 | nsa | — | 0.26 | 0.70 | 2.66a* | 0.37 | 0.04 | 0.43 | 2.57a* | 0.35 |
|
| ||||||||||||
| Craving | ||||||||||||
| QSU (0–5) | ||||||||||||
| Total | 1.93 | 1.95 | ns | — | 3.22 | 3.54 | 2.03* | 0.29 | 1.29 | 1.58 | ns | — |
| Factor 1 | 2.69 | 2.64 | ns | — | 4.24 | 4.35 | ns | — | 1.56 | 1.71 | ns | — |
| Factor 2 | 1.17 | 1.26 | ns | — | 2.21 | 2.72 | 2.46* | 0.34 | 1.03 | 1.46 | 2.44* | 0.34 |
|
| ||||||||||||
| Affect | ||||||||||||
| PANAS (1–5) | ||||||||||||
| PA | 3.04 | 3.01 | ns | — | 2.77 | 2.60 | ns | — | −0.26 | −0.41 | ns | — |
| NA | 1.44 | 1.45 | ns | — | 1.62 | 1.88 | 2.76a** | 0.39 | 0.19 | 0.43 | 2.88** | 0.39 |
Note. Mean scores per item displayed. Only Time 2 assessments are shown. For nonsignificant effects, d and t values are not displayed. Because of missing data, sample sizes vary across analyses (Ns = 202–203). HHWQ = Hughes Hatsukami Withdrawal Questionnaire (scale: 0–5); QSU = Brief Questionnaire of Smoking Urges (scale: 0–5); PANAS = Positive and Negative Affect Schedule (scale: 1–5).
Satterthwaite test was used because of unequal variances (p < .05).
Abstinence-induced change = abstinent − nonabstinent.
Eating item was omitted because there was no opportunity to eat in between T1 and T2.
p < .05.
p < .01.
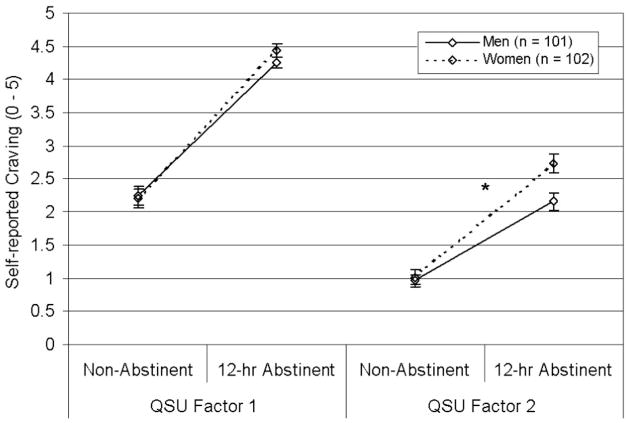
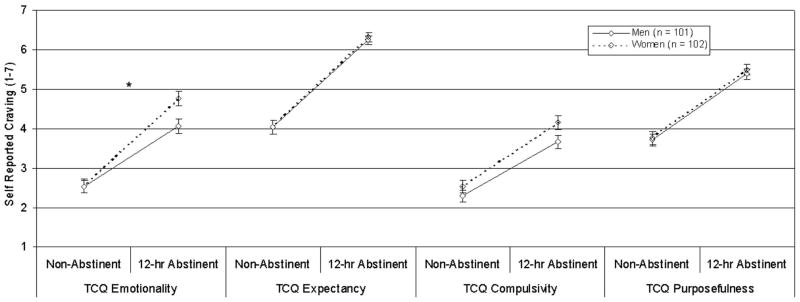
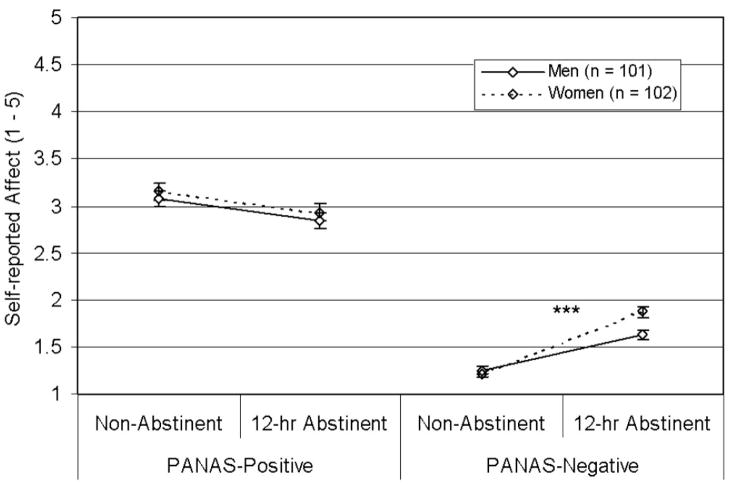
Separate Gender × State (nonabstinent vs. abstinent) × Subscale ANOVAs were conducted for measures with multiple subscales (QSU, TCQ, PANAS). Significant (or near significant) Gender × State × Subscale interactions were found for the QSU, F(1, 200) = 3.18, p = .08 (Figure 1), the TCQ, F(3, 597) = 2.89, p = .04 (Figure 2), and the PANAS, F(1, 201) = 4.94, p = .03 (Figure 3). The three-way interactions indicated that gender differences in abstinence-induced increases in craving/affect varied over sub-scales. Specifically, women reported greater abstinence-induced increases in negative affect and urge to smoke to relieve negative affect than men, but women did not differ in abstinence-induced changes in positive affect, and urge to smoke for pleasure (see Table 3 and Figures 1–3).
Figure 1.
Effects of gender on QSU ratings. Mean scores per item (±SEM) by state and gender are displayed. This graph illustrates a trend-level Gender × State (abstinent vs. nonabstinent) × Subscale (Factor 1 vs. Factor 2) interaction, F(1, 200) = 3.18, p = .08. Women reported greater abstinence-induced increases in urge to relieve negative affect by smoking (Factor 2) than men; however, there were no gender differences in abstinence-induced changes in urge to attain positive effects of smoking (Factor 1). QSU = Brief Questionnaire of Smoking Urges. *p < .01, Gender × State interaction.
Figure 2.
Effects of gender on TCQ ratings. Mean scores per item (±SEM) by state and gender are displayed. This graph illustrates a significant Gender × State (abstinent vs. nonabstinent) × Subscale (emotionality vs. expectancy vs. compulsivity vs. purposefulness) interaction, F(3, 597) = 2.89, p = .04. Women reported greater abstinence-induced increases in anticipation of relief from withdrawal and negative mood by smoking (TCQ Emotionality) than men; however, there were no gender differences in abstinence-induced changes in the other domains of craving. TCQ = Tobacco Craving Questionnaire. *p < .01, Gender × State interaction.
Figure 3.
Effects of gender on PANAS ratings. Mean scores per item (±SEM) by state and gender are displayed. This graph illustrates a significant Gender × State (abstinent vs. nonabstinent) × Affect Type (positive vs. negative) interaction, F(1, 201) = 4.94, p = .03. Women reported greater abstinence-induced increases in negative affect than men; however, there were no gender differences in abstinence-induced changes in positive affect. PANAS = Positive and Negative Affect Schedule. ***p < .0001, Gender × State interaction.
Gender × State × Time (T1 vs. T2) ANOVAs were conducted for measures that were assessed multiple times (HHWQ, QSU, PANAS). These analyses revealed significant Gender × State interactions for HHWQ Total Withdrawal, F(1, 200) = 15.41, p < .0001; HHWQ Irritable/Angry, F(1, 199) = 7.47, p < .01; HHWQ Headaches, F(1, 200) = 6.70, p < .05; QSU total score, F(1, 200) = 4.37, p < .05; QSU Factor 2, F(1, 200) = 8.78, p < .01; and PANAS Negative Affect, F(1, 200) = 15.23, p < .001. Also paralleling T1 findings, Gender × State effects were non-significant for HHWQ Craving, HHWQ Hunger, QSU Factor 1, and PANAS Positive Affect. However, Gender × State interactions that took both T1 and T2 assessments into account were significant for the following HHWQ withdrawal symptoms: Anxiety/Tense, F(1, 193) = 6.50, p < .05; Concentration, F(1, 200) = 13.56, p < .001; Restlessness, F(1, 200) = 6.18, p < .05; Impatient, F(1, 196) = 9.73, p < .01; Autonomic, F(1, 200) = 7.13, p < .01; and Drowsiness, F(1, 200) = 8.97, p < .01. For each of these HHWQ symptoms, women reported larger abstinence-induced increases than men at both T1 and T2; however, differences were statistically significant only for T2 (Table 4). All Gender × State × Time interactions were nonsignificant, indicating that gender differences in abstinence effects did not differ over the T1 and T2 assessments.
Objective Measures
Table 5 shows the means of each objective measure by gender.
Table 5.
Gender Differences in Acute Abstinence (Objective Measures)
| Nonabstinent |
Abstinent |
Abstinence-induced changeb |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Measure | Men | Women | t | d | Men | Women | t | d | Men | Women | t | d |
| Physiological measures | ||||||||||||
| HR (bpm)c | 77.78 | 82.26 | 2.85** | 0.40 | 68.68 | 72.14 | 2.19* | 0.31 | −8.96 | −10.12 | ns | — |
| SBP (mmHg)c | 125.38 | 122.04 | ns | — | 124.85 | 121.92 | ns | — | −0.56 | −0.09 | ns | — |
| DBP (mmHg)c | 75.85 | 74.27 | ns | — | 73.48 | 74.18 | nsa | — | −2.52 | −0.09 | ns | — |
| EEG eyes closed (μV2) | ||||||||||||
| Δ power | 81.76 | 118.51 | 3.73a*** | 0.53 | 109.18 | 149.31 | 3.78*** | 0.53 | 26.53 | 29.01 | ns | — |
| θ power | 49.86 | 84.22 | 4.17a*** | 0.59 | 64.03 | 103.06 | 4.02a*** | 0.57 | 14.18 | 17.91 | nsa | — |
| α power | 145.65 | 191.45 | 2.22* | 0.31 | 140.91 | 192.39 | 2.60a** | 0.37 | −7.36 | −3.83 | ns | — |
| β1 power | 32.09 | 50.14 | 5.29a*** | 0.75 | 36.69 | 55.63 | 4.71a*** | 0.66 | 4.05 | 6.28 | nsa | — |
| β2 power | 5.95 | 8.51 | 3.58*** | 0.51 | 6.58 | 9.16 | 3.57*** | 0.50 | 0.56 | 0.70 | ns | — |
| Δ frequency | 2.66 | 2.71 | ns | — | 2.71 | 2.66 | nsa | — | 0.06 | −0.05 | 2.78** | 0.39 |
| θ frequency | 6.95 | 6.97 | ns | — | 6.97 | 6.98 | ns | — | 0.02 | 0.01 | ns | — |
| α frequency | 10.67 | 10.64 | ns | — | 10.54 | 10.58 | ns | — | −0.13 | −0.05 | ns | — |
| β1 frequency | 19.96 | 19.93 | ns | — | 19.81 | 19.83 | ns | — | −0.15 | −0.11 | ns | — |
| β2 frequency | 29.15 | 29.14 | ns | — | 29.20 | 29.25 | ns | — | 0.06 | 0.11 | ns | — |
| EEG eyes open (μV2) | ||||||||||||
| Δ power | 113.38 | 158.04 | 3.45a*** | 0.49 | 135.57 | 165.31 | 2.00* | 0.28 | 21.94 | 7.27 | ns | — |
| θ power | 43.65 | 65.12 | 3.62a*** | 0.51 | 50.40 | 70.38 | 3.26a** | 0.46 | 6.94 | 6.31 | nsa | — |
| α power | 59.08 | 80.59 | 2.02a* | 0.29 | 64.22 | 87.16 | 2.26a* | 0.32 | 5.41 | 6.57 | ns | — |
| β1 power | 26.29 | 37.26 | 3.87a*** | 0.55 | 29.51 | 41.98 | 4.08a*** | 0.58 | −.57 | 4.76 | nsa | — |
| β2 power | 6.89 | 8.86 | 2.17* | 0.31 | 4.64 | 5.86 | 2.01** | 0.28 | −2.35 | −3.00 | ns | — |
| Δ frequency | 2.65 | 2.64 | ns | — | 2.69 | 2.67 | ns | — | 0.04 | 0.04 | ns | — |
| θ frequency | 6.77 | 6.79 | ns | — | 6.79 | 6.82 | ns | — | 0.01 | 0.03 | ns | — |
| α frequency | 11.11 | 11.03 | ns | — | 10.98 | 10.93 | ns | — | −0.13 | −0.10 | ns | — |
| β1 frequency | 20.24 | 20.22 | ns | — | 20.08 | 20.01 | ns | — | −0.18 | −0.21 | ns | — |
| β2 frequency | 29.26 | 29.28 | ns | — | 29.35 | 29.35 | ns | — | 0.09 | 0.07 | ns | — |
|
| ||||||||||||
| Cognitive measures | ||||||||||||
| RIPT | ||||||||||||
| Hit rate (%) | 51.6 | 50.6 | ns | — | 48.6 | 48.7 | ns | — | −3.30 | −1.97 | ns | — |
| RT (ms) | 537 | 540 | ns | — | 551 | 574 | ns | — | 14.7 | 33.7 | ns | — |
| False positives (per min) | 3.83 | 4.57 | ns | — | 3.83 | 4.79 | ns | — | −0.01 | 0.22 | ns | — |
| ST | ||||||||||||
| RT (s) | 5.42 | 4.93 | 2.09* | 0.30 | 5.93 | 5.40 | ns | — | 0.54 | 0.47 | nsa | — |
| Errors (%) | 7.85 | 5.19 | nsa | — | 6.25 | 4.44 | nsa | — | 32.13 | 30.75 | ns | — |
| MT | ||||||||||||
| RT (s) | 1.97 | 1.91 | nsa | — | 2.06 | 2.04 | ns | — | 0.09 | 0.12 | ns | — |
| Errors (%) | 20.99 | 20.31 | ns | — | 22.45 | 21.31 | ns | — | 1.46 | 1.06 | ns | — |
| DSST | ||||||||||||
| Total correct | 22.93 | 24.09 | ns | — | 22.47 | 22.50 | ns | — | −0.63 | 31.59 | ns | — |
| Errors (%) | 15.34 | 15.68 | ns | — | 15.14 | 21.23 | nsa | — | −0.02 | 5.55 | nsa | — |
| Smoking Stroop Task | ||||||||||||
| Standard effect (RT) | 35.9 | 50.1 | nsa | — | 41.7 | 63.0 | ns | — | 4.31 | 10.1 | ns | — |
| Acute effect (RT) | 42.4 | 87.8 | nsa | — | 95.0 | 103 | ns | — | 47.4 | 13.0 | ns | — |
Note. Mean scores displayed. For nonsignificant effects, d and t values are not shown. Because of missing data, sample sizes vary across analyses (Ns = 183–203). EEGs for eyes closed and eyes open were averaged over Fz, Cz, and Pz electrodes. HR = heart rate; SBP = systolic blood pressure; DBP = diastolic blood pressure; EEG = electroencephalogram; RIPT = Rapid Information Processing Task; ST = Two-Letter Search Task; MT = Serial Math Task; DSST = Digit Symbol Substitution Task; RT = reaction time.
Satterthwaite test was used because of unequal variances (p < .05).
Abstinence–induced change = abstinent − nonabstinent.
Time 1 data reported.
p < .05.
p < .01.
p < .001.
Physiological measures
Multivariate analyses with all T1 physiological measures as dependent variables yielded a significant effect of state, F(1, 189) = 49.19, Wilks’s λ = .79 p < .0001. There was no significant Gender × State interaction, F(1, 189) = 0.02, Wilks’s λ = 1.00 p = .90, and no significant main effect of gender, F(1, 189) = 0.09, p = .77.
Univariate analyses showed that EEG frequency, systolic blood pressure, and diastolic blood pressure levels did not differ between men and women in either state. However, in both the nonabstinent and abstinent states, women exhibited faster heart rate and greater EEG power across all bands than men (Table 5). There were no gender differences in abstinence-induced changes, except that women exhibited greater abstinence-induced reductions in delta frequency than men in the eyes-closed condition; the effect size was moderate (d = .39). Gender × State × Time ANOVAs were conducted for physiological measures that were assessed multiple times (heart rate, systolic blood pressure, diastolic blood pressure). There were no significant Gender × State or Gender × State × Time interactions for any of these measures.
Cognitive measures
Multivariate analyses of T1 cognitive measures yielded a significant effect of state, F(1, 185) = 6.88, Wilks’s λ = .96, p < .01. We found no significant Gender × State interaction, F(1, 185) = 0.03, Wilks’s λ = 1.00, p = .87, and no significant effect of gender, F(1, 189) = 1.47, p = .23.
We found no significant univariate gender effects on the cognitive assessments across the abstinent, nonabstinent, and abstinence-induced change scores (Table 5), with the exception that women were faster than men on the Two-Letter Search Task in the nonabstinent state.
Mediation Analyses
FTND score, cigarettes smoked per day, number of previous quit attempts, and years of regular smoking were not significantly related to gender. However, women did report smoking their first cigarette of the day earlier than men (Table 1). Given that time to first cigarette is considered an important marker of tobacco dependence (e.g., de Leon et al., 2003; Diaz et al., 2005), we examined whether abstinence-induced change scores mediated the relationship between gender (independent variable) and time to first cigarette (dependent variable, coded as follows: 3 = within 5 minutes; 2 = 6 to 30 minutes; and 1 = 31 to 60 minutes). None of the T1 abstinence-induced change scores that showed a statistically significant gender effect mediated the relationship between gender and time to first cigarette when tested at traditional levels of Type I error (p < .05). However, increases in HHWQ total scale, QSU Factor 2, and TCQ Emotionality all demonstrated evidence of mediation at a trend level (p < .10). The indirect effect (through these mediating variables) accounted for 14%, 17%, and 14%, respectively, of the total effect of gender on time to first cigarette.
Moderation Analyses
There were no significant interaction effects between gender and abstinence-induced changes in subjective, physiological, or cognitive measures on any dependent variable.
Discussion
We examined gender differences in the acute tobacco withdrawal syndrome over a range of subjective, physiological, and cognitive measures. The primary aims were to examine whether men and women showed differences in the severity and quality of withdrawal. The data showed that gender differences were found in some domains of tobacco withdrawal but not others.
Gender Effects on Subjective Measures
Women consistently reported larger abstinence-induced increases in negative affect (i.e., anger, anxiety, sadness, irritability, tension, restlessness, impatience) and greater desire to relieve withdrawal distress by smoking than men. Women also tended to report greater abstinence-induced problems in concentrating and unpleasant physical symptoms (i.e., headaches, autonomic symptoms, and drowsiness) as demonstrated by analyses of the HHWQ scores from different times. In contrast, women and men did not differ in abstinence-induced changes in hunger, positive affect, anticipation of positive consequences of smoking, and the subjective attention-grabbing properties of smoking-related cues. Men and women also did not differ on a number of measures of tobacco urges, such as the “craving for nicotine” item on the HHWQ, the WSWS Craving subscale, and the TCQ Compulsivity subscale (i.e., inability to control smoking). Thus, the gender effects on abstinence-induced craving were specific to the desire to reduce distress (see Figures 1 and 2) and did not extend to the desire to attain pleasure or other components of tobacco craving.
One possible explanation for these findings is that women were simply more likely to report withdrawal-related distress than men. Indeed, previous studies in the health literature suggest that men are less likely than women to report their symptoms (e.g., Ritchey, la Gory, & Mullis, 1991). However, this does not easily explain why women reported more severe symptoms than men while abstinent but did not differ from men in the nonabstinent state (see Tables 3 and 4). If a reporting bias were present, one might expect the pattern of gender differences to be present across both abstinent and nonabstinent states. It is possible that a reporting bias is not evident in the nonabstinent state because symptoms are minimal or less severe in that state. However, there was no evidence that women reported more severe symptoms on any measure in the nonabstinent state, even on those symptoms for which the reports were well above floor (e.g., WSWS Hunger and Anxiety subscales).
It is also possible that the pattern of data was influenced by the measures’ scale properties. For example, QSU Factor 1 scores during abstinence were close to ceiling (M = 4.3, range = 0–5; see Figure 1). Many men and women might have selected the most extreme option (a rating of 5, strongly agree) even though women may have actually been experiencing these symptoms to a greater degree. On the other hand, QSU Factor 2 scores during abstinence were near the middle of the range (see Figure 1), perhaps allowing more room for gender effects to emerge. Nevertheless, gender effects were absent on measures for which there was no evidence of range restrictions (e.g., TCQ Compulsivity scale; PANAS Positive Affect scale; see Figures 2 and 3). Thus, the properties of the scales are unlikely to wholly account for the pattern of gender effects.
A third potential explanation is that gender differences in baseline psychiatric symptomatology and/or nicotine dependence explain the pattern of withdrawal effects. Indeed, women tend to have more psychopathology than men (American Psychiatric Association, 1994), and baseline psychopathology and nicotine dependence are associated with more severe nicotine withdrawal (O. F. Pomerleau et al., 2005; Strong et al., 2004). In the current study, however, there was no evidence of gender differences in baseline psychiatric distress (i.e., SCL-90-R Global Severity Index scores; see Table 1), and additional analyses of gender effects, controlling for SCL-90-R Global Severity, did not substantially change any of the findings. In contrast, there were significant gender differences in one index of nicotine dependence (time to first cigarette). However, analyses of gender differences, controlling for time to first cigarette, did not substantially change any of the findings with the exception of T1 HHWQ Anxious/Tense, which fell below statistical significance (p = .12). In summary, the pattern of gender effects on self-report measures most likely reflects gender differences in the subjective experience of withdrawal and is unlikely to be secondary to reporting biases, scale limitations, or differences in psychiatric symptomatology or level of nicotine dependence.
More theoretically, some researchers have suggested that drug use motivation is underpinned by (a) aversive features involving unpleasant withdrawal symptoms, negative affect, and the desire to use drugs for negative reinforcement purposes and (b) appetitive features linked to the appetitive, positively reinforcing effects of drugs (Baker, Morse, & Sherman, 1987; Tiffany & Drobes, 1991). Gender differences appeared to emerge on measures that indexed aversive features of drug use motivation (e.g., increases in negative affect, craving to reduce negative affect), but differences tended to be absent on measures that tapped appetitive features (e.g., craving for pleasure, attention-grabbing properties of cues, decreases in positive affect). The dissociation is consistent with findings from several previous studies. Cook, Spring, McChargue, and Hedeker (2004) showed that gender was associated with abstinence-induced changes in negative affect but was not associated with abstinence-induced changes in craving or positive affect during a 24-hr period of abstinence. In addition, previous studies have reported that women experience greater abstinence-induced increases in fatigue and anxiety (al’Absi et al., 2004; Jacobsen et al., 2005). Similarly, a laboratory smoking study showed that women reported larger reductions in QSU Factor 2 scores—but not QSU Factor 1 scores—after smoking (Eissenberg, Adams, Riggins, & Likness, 1999).
Gender Effects on Objective Measures
In contrast to the findings on subjective measures, there was little evidence of gender differences on abstinence-induced changes on objective measures. This is consistent with previous studies that have reported no gender differences on abstinence-induced decrements in cognitive performance (al’Absi et al., 2002; Jacobsen et al., 2005). For example, al’Absi et al. (2002) reported that there were no gender differences on abstinence-induced decrements on the Paced Auditory Serial Addition Task or on a mental arithmetic task. In addition, the lack of gender differences in smoking Stroop effects is consistent with previous data showing the absence of gender differences in cue-provoked reactivity on objective measures (Field & Duka, 2004; Waters et al., 2004). To our knowledge, this was the first study to evaluate gender differences in attentional bias toward smoking cues.
Women showed a significantly greater abstinence-induced reduction in EEG delta frequency when assessed with their eyes closed (but not with their eyes open). This finding has not been previously reported and may be a chance finding caused by the large number of significance tests performed. Nevertheless, the effect was moderately sized (d = .39), which leaves open the possibility that there are gender-specific differences across modes of assessment. Women also exhibited significantly greater EEG power in both abstinent and nonabstinent states (main effect of gender) and across all bands (Table 5). This finding is consistent with the literature. A number of studies have reported that women exhibit greater EEG power than men over multiple cortical recording sites (e.g., Brière, Forest, Chouinard, & Godbout, 2003; Duffy, Albert, McAnulty, & Garvey, 1984; Veldhuizen, Jonkman, & Poortvliet, 1993).
Gender and the Subjective–Objective Distinction
In this study, women showed more severe withdrawal on some subjective measures but generally not on objective measures.3 Of note, women reported greater abstinence-induced increases in concentration difficulties; however, men and women showed similar decrements on cognitive performance measures. The differential effect of gender on subjective versus objective measures has been reported before in the addictions literature. In the cue-reactivity literature, women have reported greater cue-provoked craving than men (Field & Duka, 2004; Waters et al., 2004) but have exhibited the same degree of cue-provoked change in physiological and cognitive reactivity to smoking and cocaine cues (Field & Duka, 2004; Robbins, Ehrman, Childress, & O’Brien, 1999; Waters et al., 2004). Back, Brady, Jackson, Salstrom, and Zinzow (2005) found that stressor tasks induced greater subjective distress in women but similar levels of physiological stress in a sample of cocaine-dependent individuals. In a laboratory smoking study, Eissenberg et al. (1999) showed that women reported greater subjective effects but not greater physiological effects from smoking. Although the meaning of these dissociations is not clear, it appears that there may be important gender differences in the processes that underlie subjective versus physiological responses and that these differences may generalize across a range of addiction phenomena, including withdrawal responses, cue-reactivity responses, stress responses, and responses to cigarette smoking.
Mediation of the Gender–Smoking Relationship by Withdrawal
There was evidence at the trend level that abstinence-induced increases in desire to relieve withdrawal distress and total withdrawal severity (measured by the HHWQ) mediated the relationship between gender and time to first cigarette of the day. A previous study showed that postcessation perceived stress does not mediate the effect of gender on long-term cessation outcomes (e.g., Wetter, Kenford, et al., 1999). It is possible that gender differences in day-today smoking patterns may be explained by differences in acute withdrawal, but gender differences in long-term cessation may not be driven by acute withdrawal. Further research is required to more fully evaluate whether protracted withdrawal symptoms mediate the associations between gender and other important smoking outcomes.
Moderation of the Withdrawal–Smoking Relationship by Gender
We found no evidence that withdrawal–smoking relationships were different in men and women. It should be noted that the current sample had moderate-to-severe nicotine dependence, which may have limited the variance of outcome variables in moderational analyses. Wetter, Kenford, et al. (1999) examined whether the effect of stress, withdrawal, and negative affect on cessation outcomes was moderated by gender and found no evidence of moderation. Although Gunn (1986) reported that retrospectively assessed withdrawal symptoms predicted cessation outcomes in women but not men, the poor validity of retrospective recall suggests that these findings must be treated with caution. Taken together, it appears that the impact of withdrawal symptoms on smoking behaviors is broadly similar in men and women.
Limitations, Implications, and Conclusions
The current study has several limitations. First, we did not assess menopausal stage, phase of menstrual cycle (for women who were menstruating), or use of hormone therapy.4 Some research has shown that withdrawal is more severe during the luteal than the follicular phase in menstruating women (e.g., Perkins et al., 2000), whereas other research has suggested that menstrual cycle phase has little influence on abstinence effects (e.g., Marks, Pomerleau, & Pomerleau, 1999; Masson & Gilbert, 1999; C. S. Pomerleau et al., 1994; C. S. Pomerleau, Mehringer, Marks, Downey, & Pomerleau, 2000). Data from short-term studies have suggested that there is no need to control for menstrual cycle phase (Allen, Hatsukami, Christianson, & Nelson, 2000). Nonetheless, future studies examining abstinence in women during their early follicular phase, when endogenous hormone levels are low, would clarify this question. In addition, given the potential effects of hormone therapy on abstinence effects, future studies might exclude women on hormone therapy.
Second, because we did not have the facilities for clearing smoke in our laboratory, we did not have participants smoke at the beginning of the nonabstinent session. Thus, we were not able to directly validate participants’ reports of recent smoking at that session. However, as noted earlier, the reported time since last cigarette was very similar in men and women, and we excluded participants with low CO levels (<10 ppm) at the nonabstinent session from analysis. Third, we do not know whether the abstinence effects observed in the study were due to the reduction in levels of nicotine (offset effect) or were the expression of nicotine-induced adaptations (withdrawal effect; Hughes, 1991). The absence of the sensorimotor aspects of cigarette smoking during the abstinence condition may also play a role, given the evidence that women and men differ in their responsiveness to the reinforcing properties of nicotine versus the sensorimotor aspects of cigarette smoking (Perkins et al., 1999). Fourth, we conducted a large number of univariate analytic comparisons without lowering the alpha level for each analysis, which generally increases the likelihood of committing a Type I error. However, we note that the pattern of effects was consistent across multiple measures of similar constructs (i.e., gender effects were consistently found for subjective measures of distress and for desire to smoke to reduce distress), was consistent across assessments (T1 and T2), and was robust to control of potential confounds (e.g., SCL-90-R scores). Fifth, the analyses of the mediation/moderation effects had lower power than the analyses examining the main effects of gender and should be replicated with larger samples. These analyses would also benefit if cessation outcome were available as the dependent variable. Finally, the study was limited to individuals who were not attempting to quit. Therefore, it is unclear whether these findings generalize to smokers attempting to quit.
In summary, the current study indicates that there are qualitative gender differences in acute tobacco withdrawal. These findings have several implications. Gender differences in particular domains of withdrawal (e.g., subjective distress) may mediate relationships between gender and smoking behavior, such as ability to quit smoking. For example, we found preliminary evidence that abstinence-induced increases in craving to smoke for negative reinforcement purposes mediated the effect of gender on time to first cigarette of the day. It is possible that distress and desire to counteract unpleasant symptoms immediately after quitting might partly explain the observed gender differences in smoking cessation (Cepeda-Benito et al., 2004; Scharf & Shiffman, 2004; Wetter, Kenford, et al., 1999). In addition, the current findings point to the need for gender-specific treatments. Interventions designed to reduce the unpleasantness of acute withdrawal symptoms might be especially useful in improving the short-term quality of life of female smokers attempting to abstain (as well as potentially helping them quit smoking). Studies examining the relationship between gender and treatment-induced suppression of abstinence symptoms may clarify which interventions are most effective for women (e.g., Evans et al., 2006).
Acknowledgments
This research was supported by Transdisciplinary Tobacco Use Research Center Grant P5084718 from the National Cancer Institute and the National Institute on Drug Abuse. We thank Eun Lee, Nicole Eid, and Adrienne Heinz for assistance in project management.
Footnotes
It has been proposed that CO levels of 8 to 10 ppm be used as cutoffs to verify abstinence (SRNT Subcommittee on Biochemical Verification, 2002, p. 151). Key results reported in this article remained robust if lower cutoffs were used (<8 ppm to <10 ppm).
We also conducted separate Gender × Electrode (Cz, Fz, Pz) × State (abstinent vs. nonabstinent) × Eyes (open vs. closed) ANOVAs for each band’s power and frequency. With Bonferroni-corrected analyses (α/5 bands = .01), there were no significant interactions involving both gender and electrode, thereby supporting the decision to average over electrodes.
In an ancillary study (Eid, al’Absi, Moolchan, Boyd, & Pick-worth, 2005), we assessed salivary cortisol levels at the abstinent and nonabstinent sessions in a subset of the participants (17 men, 58 women). There were no significant gender differences in cortisol levels while abstinent or nonabstinent and no significant gender differences in abstinence-induced changes in cortisol levels.
To estimate the effects of menopausal stage in the current study, we examined whether age moderated the effects of gender on abstinence-induced changes. Age was coded as a dichotomous variable (<47 vs. 47+; the average age of menopause in female smokers has been estimated as 47.1; Di Prospero, Luzi, & Iacopini, 2004). MANOVAs with gender, age, and their interaction as independent variables revealed no significant Age × Gender effects for subjective, F(28, 158) = 0.64, Wilks’s λ = .90, p = .91, physiological, F(23, 162) = 1.18, Wilks’s λ = .84, p = .17, or cognitive, F(11, 173) = 0.65, Wilks’s λ = .96, p = .78, abstinence-induced change variables. Thus, we infer that menopausal stage is unlikely to greatly influence the pattern of abstinence effects reported here.
Contributor Information
Adam M. Leventhal, The University of Texas M. D. Anderson Cancer Center and University of Houston
Andrew J. Waters, The University of Texas M. D. Anderson Cancer Center
Caryn Lerman, University of Pennsylvania.
Wallace B. Pickworth, National Institute on Drug Abuse
References
- al’Absi M, Amunrud T, Wittmers LE. Psychophysiological effects of nicotine abstinence and behavioral challenges in habitual smokers. Pharmacology, Biochemistry and Behavior. 2002;72:707–716. doi: 10.1016/s0091-3057(02)00739-6. [DOI] [PubMed] [Google Scholar]
- al’Absi M, Hatsukami D, Davis GL, Wittmers LE. Prospective examination of effects of smoking abstinence on cortisol and withdrawal symptoms as predictors of early smoking relapse. Drug and Alcohol Dependence. 2004;73:267–278. doi: 10.1016/j.drugalcdep.2003.10.014. [DOI] [PubMed] [Google Scholar]
- Allen SS, Hatsukami DK, Christianson D, Nelson D. Effects of transdermal nicotine on craving, withdrawal, and premenstrual symptomatology in short-term smoking abstinence during different phases of the menstrual cycle. Nicotine and Tobacco Research. 2000;2:231–241. doi: 10.1080/14622200050147493. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4. Washington, DC: Author; 1994. [Google Scholar]
- Back SE, Brady KT, Jackson JL, Salstrom S, Zinzow H. Gender differences in stress reactivity among cocaine-dependent individuals. Psychopharmacology. 2005;180:169–176. doi: 10.1007/s00213-004-2129-7. [DOI] [PubMed] [Google Scholar]
- Baker TB, Morse E, Sherman J. The motivation to use drugs: A psychobiological analysis of urges. In: Rivers PC, editor. The Nebraska symposium on motivation: Alcohol use and abuse. Lincoln: University of Nebraska Press; 1987. pp. 257–323. [PubMed] [Google Scholar]
- Borrelli B, Bock B, King T, Pinto B. The impact of depression on smoking cessation in women. American Journal of Preventive Medicine. 1996;12:378–387. [PubMed] [Google Scholar]
- Borrelli B, Spring B, Niaura R, Hitsman B, Papandonatos G. Influences of gender and weight gain on short-term relapse to smoking in a cessation trial. Journal of Consulting and Clinical Psychology. 2001;69:511–515. doi: 10.1037//0022-006x.69.3.511. [DOI] [PubMed] [Google Scholar]
- Brière M-È, Forest G, Chouinard S, Godbout R. Evening and morning EEG differences between young men and women adults. Brain and Cognition. 2003;53:145–148. doi: 10.1016/s0278-2626(03)00097-6. [DOI] [PubMed] [Google Scholar]
- Brown RA, Herman KC, Ramsey SE, Stout RL. Characteristics of smoking cessation participants who lapse on quit date; Paper presented at the First International Conference for the Society for Research on Nicotine and Tobacco; Copenhagen, Denmark. 1998. Aug, [Google Scholar]
- Cepeda-Benito A, Reynoso JT, Erath S. Meta-analysis of the efficacy of nicotine replacement therapy for smoking cessation: Differences between men and women. Journal of Consulting and Clinical Psychology. 2004;72:712–722. doi: 10.1037/0022-006X.72.4.712. [DOI] [PubMed] [Google Scholar]
- Cohen J. Statistical power analysis for the behavioral sciences. San Diego, CA: Academic Press; 1977. [Google Scholar]
- Collins BN, Wileyto PE, Patterson F, Rukstalis M, Audrain-McGovern J, Kaufmann V, et al. Gender differences in smoking cessation in a placebo-controlled trial of bupropion with behavioral counseling. Nicotine & Tobacco Research. 2004;6:27–37. doi: 10.1080/14622200310001656830. [DOI] [PubMed] [Google Scholar]
- Cook JW, Spring B, McChargue D, Hedeker D. Hedonic capacity, cigarette craving, and diminished positive mood. Nicotine & Tobacco Research. 2004;6:39–47. doi: 10.1080/14622200310001656849. [DOI] [PubMed] [Google Scholar]
- Cox LS, Tiffany ST, Christen AG. Evaluation of the Brief Questionnaire of Smoking Urges (QSU–Brief) in laboratory and clinical settings. Nicotine & Tobacco Research. 2001;3:7–16. doi: 10.1080/14622200020032051. [DOI] [PubMed] [Google Scholar]
- Craig D, Parrott AC, Coomber JA. Smoking cessation in women: Effects of the menstrual cycle. International Journal of the Addictions. 1992;27:697–706. doi: 10.3109/10826089209068761. [DOI] [PubMed] [Google Scholar]
- de Leon J, Diaz FJ, Becona E, Gurpegui M, Jurado D, Gonzalez-Pinto A. Exploring brief measures of nicotine dependence for epidemiological surveys. Addictive Behaviors. 2003;28:1481–1486. doi: 10.1016/s0306-4603(02)00264-2. [DOI] [PubMed] [Google Scholar]
- Derogatis LR. Symptom Checklist–90–Revised: Administration, scoring and procedures manual. Baltimore, MD: Clinical Psychometric Research; 1992. [Google Scholar]
- Diaz FJ, Jane M, Salto E, Pardell H, Salleras L, Pinet C, de Leon J. A brief measure of high nicotine dependence for busy clinicians and large epidemiological surveys. Australian and New Zealand Journal of Psychiatry. 2005;39(3):161–168. doi: 10.1080/j.1440-1614.2005.01538.x. [DOI] [PubMed] [Google Scholar]
- Di Prospero F, Luzi S, Iacopini Z. Cigarette smoking damages women’s reproductive life. Reproductive BioMedicine Online. 2004;8(2):246–247. doi: 10.1016/s1472-6483(10)60525-1. [DOI] [PubMed] [Google Scholar]
- Duffy FH, Albert MS, McAnulty G, Garvey AJ. Age-related differences in brain electrical activity of healthy subjects. Annals of Neurology. 1984;16:430–438. doi: 10.1002/ana.410160403. [DOI] [PubMed] [Google Scholar]
- Eid NC, al’Absi M, Moolchan ET, Boyd S, Pickworth WB. Reduced salivary cortisol after overnight tobacco deprivation; Paper presented at the annual meeting of the College on Problems of Drug Dependence; Orlando, FL. 2005. Jun, [Google Scholar]
- Eissenberg T, Adams C, Riggins ECI, Likness M. Smokers’ sex and the effects of tobacco cigarettes: Subject-rated and physiological measures. Nicotine & Tobacco Research. 1999;1:317–324. doi: 10.1080/14622299050011441. [DOI] [PubMed] [Google Scholar]
- Etter JF, Prokhorov AV, Perneger TV. Gender differences in the psychological determinants of cigarette smoking. Addiction. 2002;97:733–743. doi: 10.1046/j.1360-0443.2002.00135.x. [DOI] [PubMed] [Google Scholar]
- Evans SE, Blank M, Sams C, Weaver MF, Eissenberg T. Transdermal nicotine-induced tobacco abstinence symptom suppression: Nicotine dose and smokers’ gender. Experimental and Clinical Psychopharmacology. 2006;14:121–135. doi: 10.1037/1064-1297.14.2.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field M, Duka T. Cue reactivity in smokers: The effects of perceived cigarette availability and gender. Pharmacology, Biochemistry and Behavior. 2004;78:647–652. doi: 10.1016/j.pbb.2004.03.026. [DOI] [PubMed] [Google Scholar]
- Foulds J, Stapleton J, Swettenham J, Bell N, McSorley K, Russell MAH. Cognitive performance effects of subcutaneous nicotine in smokers and never-smokers. Psychopharmacology. 1996;127:31–38. doi: 10.1007/BF02805972. [DOI] [PubMed] [Google Scholar]
- Garvey AJ, Bliss RE, Hitchcock JL, Heinold JW. Predictors of smoking relapse among self-quitters: A report from the Normative Aging Study. Addictive Behaviors. 1992;17:367–377. doi: 10.1016/0306-4603(92)90042-t. [DOI] [PubMed] [Google Scholar]
- Gritz ER, Nielsen IR, Brooks LA. Smoking cessation and gender: The influence of physiological, psychological, and behavioral factors. Journal of the American Medical Women’s Association. 1996;51:35–42. [PubMed] [Google Scholar]
- Gunn RC. Reactions to withdrawal symptoms and success in smoking cessation clinics. Addictive Behaviors. 1986;11:49–53. doi: 10.1016/0306-4603(86)90008-0. [DOI] [PubMed] [Google Scholar]
- Hatsukami D, LaBounty L, Hughes J, Laine D. Effects of tobacco abstinence on food intake among cigarette smokers. Health Psychology. 1993;12:499–502. doi: 10.1037//0278-6133.12.6.499. [DOI] [PubMed] [Google Scholar]
- Heatherton TF, Kozlowski LT, Frecker RC, Fagerström KO. The Fagerström Test for Nicotine Dependence: A revision of the Fagerström Tolerance Questionnaire. British Journal of Addiction. 1991;86:1119–1127. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- Heishman SJ, Singleton EG, Moolchan ET. Tobacco Craving Questionnaire: Reliability and validity of a new multifactorial instrument. Nicotine & Tobacco Research. 2003;5:645–654. doi: 10.1080/1462220031000158681. [DOI] [PubMed] [Google Scholar]
- Hill AJ, Blundell JE. Nutrients and behaviour: Research strategies for the investigation of taste characteristics, food preferences, hunger sensations and eating patterns in man. Journal of Psychiatric Research. 1982;17:203–212. doi: 10.1016/0022-3956(82)90023-1. [DOI] [PubMed] [Google Scholar]
- Hughes JR. Distinguishing withdrawal relief and direct effects of smoking. Psychopharmacology. 1991;104:409–410. doi: 10.1007/BF02246044. [DOI] [PubMed] [Google Scholar]
- Hughes JR. Tobacco withdrawal in self-quitters. Journal of Consulting and Clinical Psychology. 1992;60:689–697. doi: 10.1037//0022-006x.60.5.689. [DOI] [PubMed] [Google Scholar]
- Hughes JR, Gust SW, Skoog K, Keenan RM. Symptoms of tobacco withdrawal: A replication and extension. Archives of General Psychiatry. 1991;48:52–59. doi: 10.1001/archpsyc.1991.01810250054007. [DOI] [PubMed] [Google Scholar]
- Hughes JR, Hatsukami D. Signs and symptoms of tobacco withdrawal. Archives of General Psychiatry. 1986;43:289–294. doi: 10.1001/archpsyc.1986.01800030107013. [DOI] [PubMed] [Google Scholar]
- Hughes JR, Hatsukami DK, Mitchell JE, Dahlgren LA. Prevalence of smoking among psychiatric outpatients. American Journal of Psychiatry. 1986;143:993–997. doi: 10.1176/ajp.143.8.993. [DOI] [PubMed] [Google Scholar]
- Hughes JR, Higgins ST, Hatsukami D. Effects of abstinence from tobacco: A critical review. In: Kozlowski LT, Annis HM, Cappell HD, Glaser FB, Goodstadt MS, editors. Research advances in alcohol and drug problems. Vol. 10. New York: Plenum Press; 1990. pp. 317–398. [Google Scholar]
- Jacobsen LK, Krystal JH, Mencl WE, Westerveld M, Frost SJ, Pugh KR. Effects of smoking and smoking abstinence on cognition in adolescent tobacco smokers. Biological Psychiatry. 2005;57:56–66. doi: 10.1016/j.biopsych.2004.10.022. [DOI] [PubMed] [Google Scholar]
- Killen JD, Fortmann SP, Varady A, Kraemer HC. Do men outperform women in smoking cessation trials? Maybe, but not by much. Experimental and Clinical Psychopharmacology. 2002;10:295–301. doi: 10.1037//1064-1297.10.3.295. [DOI] [PubMed] [Google Scholar]
- MacKinnon DP, Lockwood CM, Hoffman JM, West SG, Sheets V. A comparison of methods to test the significance of the mediated effect. Psychological Methods. 2002;7:83–104. doi: 10.1037/1082-989x.7.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marks JL, Pomerleau CS, Pomerleau OF. Effects of menstrual cycle phase on reactivity to nicotine. Addictive Behaviors. 1999;24:127–134. doi: 10.1016/s0306-4603(98)00033-1. [DOI] [PubMed] [Google Scholar]
- Masson CL, Gilbert DG. Cardiovascular and mood responses to quantified doses of cigarette smoke in oral contraceptive users and nonusers. Journal of Behavioral Medicine. 1999;22:589–604. doi: 10.1023/a:1018793729594. [DOI] [PubMed] [Google Scholar]
- McLellan AT, Kushner H, Metzger D, Peters R. The fifth edition of the Addiction Severity Index. Journal of Substance Abuse Treatment. 1992;9:199–213. doi: 10.1016/0740-5472(92)90062-s. [DOI] [PubMed] [Google Scholar]
- Murray RP, Johnston JJ, Dolce JJ, Lee WW. Social support for smoking cessation and abstinence: The Lung Health Study. Addictive Behaviors. 1995;20:159–170. doi: 10.1016/s0306-4603(99)80001-x. [DOI] [PubMed] [Google Scholar]
- Perkins KA, Donny E, Caggiula AR. Sex differences in nicotine effects and self-administration: Review of human and animal evidence. Nicotine & Tobacco Research. 1999;1:301–315. doi: 10.1080/14622299050011431. [DOI] [PubMed] [Google Scholar]
- Perkins KA, Levine M, Marcus M, Shiffman S, D’Amico D, Miller A, et al. Tobacco withdrawal in women and menstrual cycle phase. Journal of Consulting and Clinical Psychology. 2000;68:176–180. doi: 10.1037/0022-006X.68.1.176. [DOI] [PubMed] [Google Scholar]
- Pickworth WB, O’Hare ED, Fant RV, Moolchan ET. EEG effects of conventional and denicotinized cigarettes in spaced smoking paradigm. Brain and Cognition. 2003;53:75–81. doi: 10.1016/s0278-2626(03)00205-7. [DOI] [PubMed] [Google Scholar]
- Pickworth WB, Rohrer MS, Fant RV. Effects of abused drugs on psychomotor performance. Experimental and Clinical Psychopharmacology. 1997;5:235–241. doi: 10.1037//1064-1297.5.3.235. [DOI] [PubMed] [Google Scholar]
- Pomerleau CS, Mehringer A, Marks JL, Downey K, Pomerleau OF. Effects of menstrual phase and smoking abstinence in smokers with and without a history of major depressive disorder. Addictive Behaviors. 2000;25:483–497. doi: 10.1016/s0306-4603(99)00075-1. [DOI] [PubMed] [Google Scholar]
- Pomerleau CS, Tate JC, Lumley MA, Pomerleau OF. Gender differences in prospectively versus retrospectively assessed smoking withdrawal symptoms. Journal of Substance Abuse. 1994;6:433–440. doi: 10.1016/s0899-3289(94)90376-x. [DOI] [PubMed] [Google Scholar]
- Pomerleau OF, Pomerleau CS, Mehringer AM, Snedecor SM, Ninowski R, Sen A. Nicotine dependence, depression, and gender: Characterizing phenotypes based on withdrawal discomfort, response to smoking, and ability to abstain. Nicotine & Tobacco Research. 2005;7:91–102. doi: 10.1080/14622200412331328466. [DOI] [PubMed] [Google Scholar]
- Ritchey FJ, la Gory M, Mullis J. Gender differences in health risks and physical symptoms among the homeless. Journal of Health and Social Behavior. 1991;32:33–48. [PubMed] [Google Scholar]
- Robbins SJ, Ehrman RN, Childress AR, O’Brien CP. Comparing levels of cocaine cue reactivity in male and female outpatients. Drug and Alcohol Dependence. 1999;53:223–230. doi: 10.1016/s0376-8716(98)00135-5. [DOI] [PubMed] [Google Scholar]
- SAS Institute. The SAS System for Windows (Version 8.2) [Computer software] Cary, NC: Author; 2003. [Google Scholar]
- Scharf D, Shiffman S. Are there gender differences in smoking cessation, with and without bupropion? Pooled- and meta-analyses of clinical trials of Bupropion SR. Addiction. 2004;99:1462–1469. doi: 10.1111/j.1360-0443.2004.00845.x. [DOI] [PubMed] [Google Scholar]
- Shipley WC. A self-administering scale for measuring intellectual impairment and deterioration. Journal of Psychology: Interdisciplinary and Applied. 1940;9:371–377. [Google Scholar]
- Sobel ME. Asymptotic confidence intervals for indirect effects in structural equation models. In: Leinhardt S, editor. Sociological methodology 1982. Washington DC: American Sociological Association; 1982. pp. 290–312. [Google Scholar]
- SRNT Subcommittee on Biochemical Verification. Biochemical verification of tobacco use and cessation. Nicotine and Tobacco Research. 2002;4:149–159. doi: 10.1080/14622200210123581. [DOI] [PubMed] [Google Scholar]
- Strong DR, Kahler CW, Ramsey SE, Abrantes A, Brown RA. Nicotine withdrawal among adolescents with acute psychopathology: An item response analysis. Nicotine & Tobacco Research. 2004;6:547–557. doi: 10.1080/14622200410001696484. [DOI] [PubMed] [Google Scholar]
- Svikis DS, Hatsukami DK, Hughes JR, Carroll KM. Sex differences in tobacco withdrawal syndrome. Addictive Behaviors. 1986;11:459–462. doi: 10.1016/0306-4603(86)90028-6. [DOI] [PubMed] [Google Scholar]
- Thorne DR, Genser SG, Sing HC, Hegge FW. The Walter Reed performance assessment battery. Neurobehavioral Toxicology & Teratology. 1985;7:415–418. [PubMed] [Google Scholar]
- Tiffany ST, Drobes DJ. The development and initial validation of a questionnaire on smoking urges. British Journal of Addiction. 1991;86:1467–1476. doi: 10.1111/j.1360-0443.1991.tb01732.x. [DOI] [PubMed] [Google Scholar]
- Veldhuizen RJ, Jonkman EJ, Poortvliet DC. Sex differences in age regression parameters of healthy adults—Normative data practical implications. Electroencephalography and Clinical Neurophysiology. 1993;86:377–384. doi: 10.1016/0013-4694(93)90133-g. [DOI] [PubMed] [Google Scholar]
- Waters AJ, Sayette MA. Implicit cognition and tobacco addiction. In: Wiers RW, Stacy AW, editors. Handbook on implicit cognition and addiction. Thousand Oaks, CA: Sage; 2005. pp. 477–481. [Google Scholar]
- Waters AJ, Shiffman S, Sayette MA, Paty JA, Gwaltney CJ, Balabanis MH. Attentional bias predicts outcome in smoking cessation. Health Psychology. 2003;22:378–387. doi: 10.1037/0278-6133.22.4.378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waters AJ, Shiffman S, Sayette MA, Paty JA, Gwaltney CJ, Balabanis MH. Cue-provoked craving and nicotine replacement therapy in smoking cessation. Journal of Consulting and Clinical Psychology. 2004;72:1136–1143. doi: 10.1037/0022-006X.72.6.1136. [DOI] [PubMed] [Google Scholar]
- Watson D, Clark LA, Tellegen A. Development and validation of brief measures of positive and negative affect: The PANAS scales. Journal of Personality and Social Psychology. 1988;54:1063–1070. doi: 10.1037//0022-3514.54.6.1063. [DOI] [PubMed] [Google Scholar]
- Welsch SK, Smith SS, Wetter DW, Jorenby DE, Fiore MC, Baker TB. Development and validation of the Wisconsin Smoking Withdrawal Scale. Experimental and Clinical Psychopharmacology. 1999;7:354–361. doi: 10.1037//1064-1297.7.4.354. [DOI] [PubMed] [Google Scholar]
- Wetter DW, Fiore MC, Young TB, McClure JB, de Moor CA, Baker TB. Gender differences in response to nicotine replacement therapy: Objective and subjective indexes of tobacco withdrawal. Experimental and Clinical Psychopharmacology. 1999;7:135–144. doi: 10.1037//1064-1297.7.2.135. [DOI] [PubMed] [Google Scholar]
- Wetter DW, Kenford SL, Smith SS, Fiore MC, Jorenby DE, Baker TB. Gender differences in smoking cessation. Journal of Consulting and Clinical Psychology. 1999;67:555–562. doi: 10.1037//0022-006x.67.4.555. [DOI] [PubMed] [Google Scholar]