Abstract
Because different psychopathologic components of depressive symptoms may have distinct etiologies, examining their differential effects on smoking cessation may elucidate mechanisms underlying the smoking-depression relationship. Negative affect (NA), somatic features (SF), low positive affect/anhedonia (PA), and interpersonal disturbance (IP) have been identified as unique dimensions of depression that can be measured using the Center for Epidemiologic Studies Depression Scale (CESD). This study examined common and unique associations between CESD subscales and baseline smoking characteristics, nicotine withdrawal, and relapse in 157 participants enrolled in a smoking cessation trial for heavy social drinkers. Each dimension was univariately associated with negative and positive reinforcement smoking motives. Only SF had unique relations with tolerance smoking motives and univariate associations with nicotine dependence severity. Only PA predicted cessation-related changes in withdrawal symptoms on quit day. Analyses predicting abstinence at 8, 16, and 26 weeks post quit date showed that NA, SF, and PA each univariately predicted relapse, ps≤.0083. Only low PA predicted poorer outcomes incrementally to the other dimensions, even when controlling for level of nicotine dependence, smoking frequency, and history of major depression, p=.0018. Interventions targeting anhedonia and low positive affect may be useful for smokers trying to quit.
Introduction
The association between depression and smoking is well documented. Evidence suggests that smokers are more than twice as likely as nonsmokers to have a history of major depression (Glassman et al., 1990). Researchers have attempted to elucidate the causal basis of this relationship by examining whether a history of depression predicts greater likelihood of relapse during a cessation attempt (Hitsman, Borrelli, McChargue, Spring, & Niaura, 2003). Despite numerous studies, findings generally have been mixed on this issue (Covey, 2004; Covey, Bomback, & Yan, 2006; Hall, 2004; Hitsman et al., 2003; Hitsman, Spring, Borrelli, McChargue, & Niaura, 2004), although evidence suggests that recurrent major depression conveys a greater likelihood of relapse (Brown et al., 2001; Haas et al., 2004).
A related, but separate question is whether higher levels of precessation depressive symptoms have a negative influence on smoking outcomes. Depressive symptoms are associated with greater likelihood of smoking and poorer cessation outcomes at the population level (Anda et al., 1990). In clinical trials, higher levels of pretreatment depressive symptoms are associated with reduced odds of cessation (Anda et al., 1990; Brown et al., 2001; Cinciripini et al., 2003; Ginsberg, Hall, Reus, & Muñoz, 1995; Haas, Muñoz, Humfleet, Reus, & Hall, 2004; Hitsman et al., 1999; Killen, Fortmann, Davis, Strausberg, & Varady, 1999; Kinnunen, Doherty, Militello, & Garvey, 1996; Niaura et al., 2001; Rausch, Nichinson, Lamke, & Matloff, 1990; Swan et al., 2003), although this effect has not been replicated in some investigations (Catley, Ahluwalia, Resnicow, & Nazir, 2003; Catley et al., 2005; Vàzquez & Becoña, 1999). Many studies have shown that depressive symptoms, even at very low levels (e.g., Hamilton Rating Scale for Depression score >2; Niaura et al., 2001), predict poorer outcomes in smokers without current major depression (al’Absi, Hatsukami, & Davis, 2005; al’Absi, Hatsukami, Davis, & Wittmers, 2004; Brown et al., 2001; Cinciripini et al., 2003; Ginsberg et al., 1995; Haas et al., 2004; Killen et al., 1999; Swan et al., 2003).
There are several hypotheses as to why smokers with higher depressive symptoms may be more susceptible to relapse. Smokers with elevated depressive symptoms may develop more severe postcessation nicotine withdrawal or affective distress (Pomerleau, Marks, & Pomerleau, 2000; Pomerleau et al., 2005; Pomerleau et al., 2004), which could undermine quit attempts. They also may be more nicotine dependent or reliant on tobacco’s appetitive and distress-reducing effects (Lerman, Audrain, Orleans, & Boyd, 1996; Niaura et al., 1999), which may in turn influence smoking outcomes. Evidence supporting these explanations is mixed (Cinciripini et al., 2003; Niaura et al., 2001), leaving the precise nature of the relationship between depressive symptoms and smoking cessation unclear.
One potential barrier to understanding this relationship is the heterogeneity of depressive symptoms. Studies examining the effect of depressive symptoms on smoking cessation typically consider depression as a single comprehensive phenotype. However, there is considerable evidence that depression is a complex set of features involving numerous intermediate phenotypes (e.g., anhedonia, vegetative symptoms, negative emotions), rather than a unitary homogenous syndrome (Hasler, Drevets, Manji, & Charney, 2004). Because different psychopathologic forms of depression are associated with distinct biological, psychological, and behavioral correlates and may have unique etiologies (Hasler et al., 2004; Leventhal & Rehm, 2005; Pizzagalli, Jahn, & O’Shea, 2005; Posternak & Zimmerman, 2002), it is difficult to understand mechanisms underlying the depression-smoking relationship by examining associations between smoking outcomes and depressive symptoms as a whole. Accordingly, evaluating whether certain dimensions of depressive symptoms have a greater influence on smoking cessation than others may clarify reasons for depression-smoking comorbidity (Pomerleau et al., 2003).
Negative affect (NA), somatic features (SF), low positive affect/anhedonia (PA), and interpersonal disturbance (IP) have been identified as unique symptom dimensions of depression that are phenomenologically and psychometrically distinct (Devins, Orme, Costello, & Binik, 1988; Knight, Williams, McGee, & Olaman, 1997; Nguyen, Kitner-Triolo, Evans, & Zonderman, 2004; Radloff, 1977; Shafer, 2006; Weissman, Sholomskas, Pottenger, Prusoff, & Locke, 1977). NA is characterized by increased negative emotions (e.g., sadness, anxiety, dysphoria). SF is characterized by vegetative symptoms (appetite and sleep irregularities), concentration difficulties, and anergia. NA and SF are considered nonspecific forms of general distress that commonly occur in various forms of emotional disturbance, including both the mood and anxiety disorders (Clark & Watson, 1991; Watson, 2005). Low PA/anhedonia is characterized by reduced positive emotions and a diminished capacity to experience pleasure (Pizzagalli et al., 2005) and is a key feature of the melancholic subtype of major depression (American Psychiatric Association, 1994). Low PA is considered as a specific symptom of depression that distinguishes mood from anxiety disorders (Clark & Watson, 1991; Watson, 2005). IP is characterized by poor social adjustment. IP may be a consequence of negative self-esteem, social skills deficits, and dysfunctional social behavior and may be a common component of depression and social phobia (Stangier, Esser, Leber, Risch, & Heidenreich, 2006).
The present investigation examines the influence of these psychopathologic components of depressive symptoms on outcomes in a sample of non-clinically depressed social drinkers enrolled in a clinical trial for smoking cessation. We utilized the Center for Epidemiologic Studies Depression Scale (CESD; Radloff, 1977) to examine the differential effects of NA, SF, PA, and IP on smoking cessation. This measure has been shown to predict smoking outcome in numerous studies (al’Absi et al., 2005; al’Absi et al., 2004; Killen et al., 1999; Kinnunen et al., 1996). Its factor structure has been well studied and has been shown to conform to a 4-factor model in which distinguishable dimensions of NA, SF, PA, and IP can be measured using a subscale approach (Shafer, 2006; Blazer & Hybels, 2004; Blazer, Hybels, Fillenbaum, & Piper, 2005). Investigating which psychopathologic components of depressive symptoms have the greatest influence on smoking cessation could potentially (a) clarify which smokers are at the greatest risk of relapse and (b) identify more refined targets for mood management interventions for smokers with depressive symptoms.
We examined the extent to which each CESD subscale univariately and incrementally (to the other subscales) predicted (a) baseline tobacco dependence severity and motives for smoking, (b) abstinence-provoked nicotine withdrawal, and (c) smoking abstinence over the follow-up period (i.e., 8, 16, and 26 weeks after each participant’s quit date). Smokers with high NA and low PA may report greater negative and positive reinforcement smoking motives, respectively, as they may use smoking to regulate affect. Therefore, we hypothesized that NA would be associated with negative reinforcement tobacco dependence motives and PA would be inversely related with positive reinforcement smoking motives. Based on research suggesting that high negative and low positive moods at baseline predict greater withdrawal severity (Cook, Spring, McChargue, & Hedeker, 2004; Ginsberg et al., 1995), we hypothesized that smokers with elevated NA and lower PA scores would be more susceptible to cessation-associated increases in withdrawal symptoms. Finally, given that depressed mood, low positive mood, and anhedonia at pretreatment predict poorer smoking outcomes (Doran et al., 2006; Haas et al., 2004; Niaura et al., 1999), we hypothesized that both NA and PA would associate with abstinence following treatment. We did not have any hypotheses regarding the effects of SF and IP on baseline smoking characteristics, withdrawal, or smoking outcomes because of the paucity of research and theory on these two dimensions. Nor did we have any expectations of which, if any, of the symptom dimensions would predict outcomes incrementally to the other dimensions (i.e., after controlling for the effects of the other dimensions).
Method
Participants
Participants were 157 smokers recruited from the community taking part in a larger, ongoing, randomized clinical trial comparing standard smoking cessation treatment (ST) to standard treatment incorporating a brief alcohol intervention (ST-BI). All participants were classified as heavy drinkers according to guidelines from the National Institute on Alcohol Abuse and Alcoholism (NIAAA, 1995) but did not meet criteria for alcohol dependence. Inclusion criteria were (a) 18 years of age or older, (b) regular cigarette smoking for at least one year, (c) ⩾10 cigarettes smoked per day, and (d) current heavy drinking (>14 drinks per week or ⩾5 drinks per occasion at least once per month over the last 12 months for men; >7 drinks per week or ⩾4 drinks per occasion at least once per month over the past 12 months for women). Participants were excluded if they met full DSM-IV criteria for alcohol dependence or other current psychoactive substance abuse or dependence (excluding nicotine dependence and alcohol abuse) in the past year, had a diagnosis of dysthymia, a major depressive episode, or a manic episode in the past month, were currently psychotic or suicidal, had an unstable medical condition that would suggest caution in the use of the nicotine patch (e.g., unstable angina pectoris, arrhythmia, recent congestive heart failure), were currently pregnant or lactating or intended to become pregnant, or were currently using other tobacco products or nicotine replacement therapy.
The current sample includes the first 157 participants enrolled in the trial who completed follow-up assessments through 26 weeks after their scheduled quit date. Assuming abstinence rates of 40% at post-treatment and using an alpha level of .05, a sample of 157 provides power of .85 for detecting a difference of a medium magnitude (Cohen’s d=.50) between those who are abstinent and those who are not at post-treatment.
Of the 157 participants included in the current report, 49.0% (n=77) were female and 51.0% (n=80) were male, with 30.6% (n=48) married or cohabiting. The mean age of the sample was 41.6 (SD=11.4) years, and the mean education was 14.2 (SD=2.2) years. Most participants (n=147, 93.6%) identified themselves as non-Hispanic White. At baseline, participants smoked an average of 21.2 (SD=8.3) cigarettes per day and had been smoking for an average of 22.8 years (SD=11.3). The sample mean on the Fagerström Test for Nicotine Dependence (FTND; Heatherton, Kozlowski, Frecker, & Fagerström, 1991) was 4.9 (SD=2.0). Participants drank an average of 15.9 (SD=11.1) drinks per week. Ten percent of participants had a lifetime history of alcohol dependence and 33% had a history of major depression.
Procedure
Participants were recruited from the community via postings on community bulletin boards and newspaper and radio advertisements, which asked for social drinkers who wished to quit smoking. Potential participants were first screened by telephone before completing an intake interview, at which they signed a statement of informed consent approved by the Brown University Institutional Review Board.
Treatment consisted of four individual counseling sessions over 3 weeks. The quit date occurred at session 2, one week after session 1. ST (n=80) was based on recent clinical practice guidelines (Fiore, 2000) and focused on problem solving to cope with high-risk situations for smoking relapse, relaxation skills, providing support within the treatment, and encouraging participants to seek support for quitting smoking outside of treatment. In ST-BI (n=77), additional treatment components included discussion of the participant’s alcohol use, which included open-ended discussion of current drinking and smoking patterns, feedback on drinking levels and the risk of smoking relapse associated with drinking, and setting goals to change drinking during smoking cessation. The ST and ST-BI conditions were matched on amount of therapist contact time. All participants received treatment with transdermal nicotine patch with the initial dose starting at 21 mg for four weeks, followed by two weeks of 14 mg patch, and then two weeks of 7 mg patch. Nicotine patch treatment started on participants’ quit day.
Because this trial is not completed, treatment effects are not reported in this paper. However, analyses were run controlling for treatment assignment and testing interactions between treatment condition and each baseline depression subscale score. These analyses indicated that the effect of each depression subscale at baseline was not altered by covarying treatment assignment, nor did any of the depression subscales interact significantly with treatment condition in predicting outcome.
Measures
Baseline measures
Prior to treatment, participants provided demographic information and smoking-related information such as average number of cigarettes smoked per day. Lifetime and current DSM-IV Axis I diagnoses were determined with the SCID (First, Spitzer, Gibbon, & Williams, 1995).
Participants also completed questionnaires assessing nicotine dependence severity, smoking dependence motives, and depression prior to treatment. Severity of nicotine dependence was assessed using the FTND (Heatherton et al., 1991), a well-validated 6-item measure. Smoking dependence motives were assessed using several subscales from the Wisconsin Inventory of Smoking Dependence Motives (WIDSM), which have shown to have adequate psychometric properties (Piper et al., 2004). The WISDM assesses 13 different motives for tobacco dependence. The current study measured three dimensions that were most relevant to depression and smoking cessation: (1) Positive Reinforcement, expectations about the appetitive effects of smoking (e.g., “Smoking brings me a lot of pleasure”); (2) Negative Reinforcement, expectations about the distress-reducing effects of smoking (e.g., “Smoking helps me deal with stress”); and (3) Tolerance, frequent and heavy smoking (e.g., “Other smokers would consider me a heavy smoker”).
Depressive symptoms were assessed using the Center for Epidemiologic Studies Depression Scale (CESD; Radloff, 1977). The CESD is a 20-item, well-validated, self-report scale that has been widely used in depression research and is appropriate for assessment of depressive symptoms in nonclinical samples. Each item is worded as a self statement for which participants indicated how often they felt that way in the past week (e.g., “I had crying spells”). Participants can choose from the following four options: Rarely or none of the time (0–1 days, 0 pts); Some or a little of the time (1–2 days, 1 pt); Occasionally or a moderate amount of the time (3–4 days; 2 pts); or Most or all of the time (5–7 days; 3 pts). A recent meta-analysis of the factor structure of the CESD found a clear four-factor solution (Shafer, 2006) that distinguished somatic, negative affect, positive affect, and interpersonal symptoms. The Shafer (2006) findings were consistent with Radloff’s (1977) original four-factor structure and other exploratory (Devins et al., 1988; Weissman et al., 1977), and confirmatory (Hertzog, Van Alstine, Usala, & Hultsch, 1990; Knight et al., 1997; Nguyen et al., 2004) factor analytic studies that have replicated this structure. Results generally show that items load robustly onto only one of the four factors (i.e., items do not crossload onto multiple factors), suggesting that each item specifically taps one of the four dimensions. Therefore, consistent with previous investigators that have used a subscale approach to the CESD (Blazer & Hybels, 2004; Blazer, Hybels, Fillenbaum, & Pieper, 2005), we computed subscale scores for each dimension by computing their respective items’ average score. The dimensions and their items are as follows: Negative Affect (NA; Felt sad, Crying spells, Could not shake blues, Felt depressed, Felt lonely, Felt fearful, Life is a failure); Somatic Features (SF; Appetite poor, Restless sleep, Could not get going, Can’t keep mind on tasks, Everything an effort, Bothered by things, Talked less than usual); Positive Affect (PA; Hopeful about future, Enjoyed life, Felt as good as others, Was happy); and Interpersonal Disturbance (IP; People dislike me, People were unfriendly). We tested the validity of separating SF, NA, PA, and IP items using Confirmatory Factor Analysis. If an oblique 4-factor model (7-item SF factor, 7-item NA factor, 4-item PA factor and 2-item IP factor, see above) outperformed the standard undifferentiated symptom model with one latent variable defined by all 20 items, the distinction of these subscales would be supported. Results from the factor analytic comparisons indicated that the 4-factor model demonstrated a significantly better fit to the data than the single-factor model, χ2difference (6, N=157)= 189.58, p<.0001, which supported our use of the subscales in the current study. The internal consistency estimates for each of the four subscales were as follows: NA (Cronbach’s α =.89), SF (α =.79), PA (α =.73), and IP (α =.68). A total score was also computed by computing the average score of all twenty items (items on the PA scale are reversed scored because they are positively worded). Internal consistency of the total scale was excellent (α =.90).
Nicotine withdrawal
Nicotine withdrawal symptoms were assessed at each treatment session using the 7-item Minnesota Nicotine Withdrawal Scale (MNWS), which has shown adequate psychometric properties (Hughes & Hatsukami, 1998; Hughes & Hatsukami, 1986). The current report focuses on MNWS scores while non abstinent (session 1, one week before quit day) and during acute abstinence (session 2, on quit day).
Measures of smoking status
Outcome analyses were based on 7-day point prevalence abstinence (i.e., reported abstinence of at least 7 days prior to the assessment day) as assessed at follow-ups occurring 8 (end of treatment with the nicotine patch), 16, and 26 weeks after each participant’s quit date. Self-reported abstinence was verified by breath carbon monoxide (CO) using a Bedfont Scientific Smokelyzer® breath CO monitor. At 16- and 26-week follow-ups, a saliva sample was collected from those who self-reported abstinence. Saliva samples were analyzed for cotinine level determination by enzyme immunoassay. Abstinence was confirmed by a combination of CO ≤10 ppm and cotinine ≤15 ng/ml (SRNT Verification, 2002). Significant other report was used to verify smoking status for those who: (1) did not provide self-report data, or (2) did not provide biochemical verification of abstinence (a total of 4% of assessments). Complete smoking data verified either biochemically or by significant other report was obtained from 93.6%, 91.2%, and 92.4% of participants at the 8-, 16-, and 26-week follow-ups, respectively. Individuals who had missing data were considered smoking (i.e., a worst-case assumption).
Data analyses
We first examined univariate correlations between the CESD total scale, each of the 4 CESD subscales, and baseline smoking characteristics. To examine their incremental effects, we used multiple regression models in which all 4 subscales were simultaneously included as predictors. Separate models were conducted for each baseline smoking characteristic.
Withdrawal effects were examined using multiple regression models in which quit-day (session 2) MNWS scores served as the dependent variable, a CESD scale served as the independent variable, and pre-quit (session 1) MNWS scores served as the covariate. Separate models were conducted for each CESD subscale and the total scale. Including pre-quit withdrawal scores as a covariate allowed prediction of the change in withdrawal symptoms from pre- to post-cessation. Otherwise it would be unclear whether greater tobacco withdrawal severity among more depressed smokers represented a change or a reflection or carryover of baseline levels of affective distress. To examine incremental effects on cessation-provoked withdrawal, a multiple regression model in which all 4 subscales were simultaneously included as predictors (along with session 1 MNWS scores as the covariate) was conducted.
As an initial analysis of smoking outcome, we used ANOVAs at each follow-up point (8, 16, and 26 weeks) to compare CESD total and subscale scores of participants who were abstinent to those who were not abstinent. The primary analysis examining the effect of depression on smoking outcomes involved repeated measures analyses for binomial outcomes using generalized estimating equations (GEE; Zeger & Liang, 1986) in which 7-day point prevalence smoking abstinence at 8, 16, and 26 weeks after quit date served as the dependent variable. GEE allows for inclusion of both categorical and continuous independent variables and for appropriate modeling of covariance structures when observations are correlated across time. Analyses were conducted in SAS using PROC GENMOD (SAS Institute Inc., 2003) with the Logit link function and an exchangeable correlation matrix specified (because the correlations among time points were relatively equal). To examine univariate effects we conducted separate GEEs for each CESD subscale and the total scale. To examine incremental effects, we ran GEEs in which all 4 subscales were simultaneously included as predictors. Finally, we examined whether CESD subscales predicted smoking cessation outcomes over and above the effects of the other subscales and other relevant constructs (i.e., nicotine dependence severity, cigarettes smoked per day, FTND scores, and past major depression) by simultaneously including these variables and all 4 CESD subscales as predictors in a GEE model of cessation outcomes.
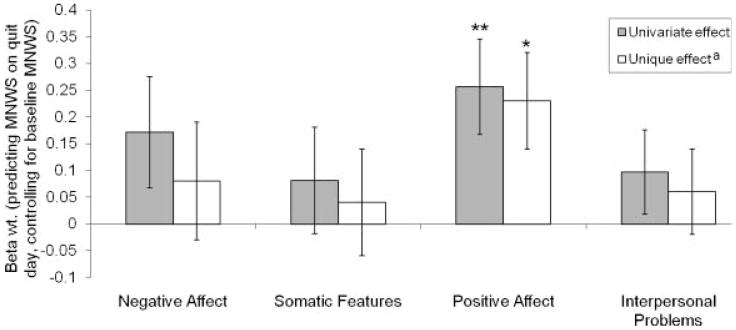
There were no significant relations between the CESD total score or any of the subscale scores and participants’ sex or age. Therefore, these demographic variables were not included as covariates in any analyses. For statistical analyses, the CESD total and subscales were square-root transformed and standardized (z-scored) to correct positive skewness and to ease interpretation of model coefficients. Raw scores were used for presentation of descriptive statistics. In Figure 1, PA was reversed scored to ease comparison to the other scales. All effects were tested using a two-tailed alpha of .05.
Figure 1.
Center for Epidemiologic Studies Depression Scale (CESD) subscales predicting cessation-related changes in nicotine withdrawal. Standardized regression coefficients (Beta wts.) and standard errors of baseline CESD subscales predicting quit-day withdrawal symptoms when controlling for pre-quit withdrawal scores. Positive Affect scores are reversed to allow comparison with other scales. MNWS=Minnesota Nicotine Withdrawal Scale. aAdjusted for the effects of the three other CESD subscales. Effect of subscale: *p<.05, **p<.01.
Results
Preliminary analysis of associations between CESD subscales and baseline smoking characteristics
Associations within and between CESD subscales and baseline smoking characteristics are presented in Table 1. Table 1 also shows each measure’s mean and standard deviation. Consistent with the factor analytic findings, there was a moderate but not substantially large degree of intercorrelation among the CESD subscales (rs .28–.58). SF, NA, and PA showed significant correlations with WISDM-Tolerance. Only SF uniquely associated with tolerance motives in the multiple regression analysis. SF also had a significant but small bivariate correlation with the FTND. None of the subscales were significantly associated with cigarettes smoked per day (see Table 1).
Table 1.
Associations between CESD subscales and baseline smoking characteristics.
| Variable | M | SD | 1. | 2. | 3. | 4. | 5. | 6. | 7. | 8. | 9. | 10. |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Univariate Correlations (r) | ||||||||||||
| 1. CESD-Total | 0.47b | 0.52 | – | |||||||||
| 2. CESD-NA | 0.27b | 0.46 | .80† | – | ||||||||
| 3. CESD-SF | 0.45b | 0.50 | .85† | .58† | – | |||||||
| 4. CESD-PA | 2.47b | 0.60 | −.74† | −.56† | − .49† | – | ||||||
| 5. CESD-IP | 0.16b | 0.37 | .45† | .42† | .31† | − .28*** | – | |||||
| 6. WISDM-Positive Reinforcement | 3.60 | 1.31 | .25† | .20* | .24** | − .19* | .17* | – | ||||
| 7. WISDM-Negative Reinforcement | 4.04 | 1.29 | .34† | .31† | .31† | − .25** | .27*** | .80† | – | |||
| 8. WISDM-Tolerance | 4.54 | 1.47 | .19* | .16* | .24** | − .07 | .12 | .27*** | .37† | – | ||
| 9. FTND | 4.94 | 2.04 | .15 | .11 | .18* | − .07 | .03 | .07 | .11 | .74† | – | |
| 10. Cig/day | 21.21 | 8.34 | .09 | .04 | .10 | − .07 | .05 | −.01 | −.02 | .50† | .62† | – |
| Unique Effects (β)a | ||||||||||||
| CESD-NA | .04 | .10 | .05 | .03 | −.07 | |||||||
| CESD-SF | .16 | .17 | .23* | .18 | .10 | |||||||
| CESD-PA | −.07 | −.08 | .06 | .02 | −.06 | |||||||
| CESD-IP | .08 | .15 | .04 | −.03 | .02 | |||||||
Note. N=157. CESD=Center for Epidemiologic Studies Depression Scale; Total=total scale; NA=Negative Affect Subscale; SF=Somatic Features subscale; PA=Positive affect subscale; IP=Interpersonal Disturbance subscale; WISDM=Wisconsin Inventory of Smoking Dependence Motives; FTND=Fagerström Test for Nicotine Dependence; Cig/day=Average number of cigarettes smoked per day.
Results from multiple regression models in which all four CESD subscales were simultaneously included as predictors of baseline smoking characteristics.
Means based on average response per item (possible range: 0–3).
p<.05;
p<.01;
p<.001;
p<.0001
Associations between CESD subscales and reinforcement smoking motives
Results in Table 1 showed that each dimension had a significant bivariate association with positive and negative reinforcement dependence motives. Multiple regression analyses predicting WISDM-negative reinforcement and WISDM-positive reinforcement with the set of CESD subscales showed no significant unique effects (see Table 1), although the overall models were significant: WISDM-positive reinforcement, F(4, 156)=6.43, R2=.14, p<.0001; WISDM-negative reinforcement, F(4, 156)=3.02, R2=.07, p<.0197. Thus, shared variance among the depressive symptom dimensions contributed to their effect on the tendency to smoke for reinforcement purposes.
Associations between CESD subscales and withdrawal symptoms on quit day
Results showed that the CESD total scale predicted MNWS scores on session 2 (quit day) while controlling for MNWS scores on session 1 (before quit day) at a trend level, β(2, 148)=.21, p=.07. Results broken down by subscales are presented in Figure 1. These analyses showed that PA significantly predicted cessation-induced withdrawal, β=−.26, p=.005; however, the other subscales did not significantly predict withdrawal. When all sub-scales were simultaneously included in the model, only PA significantly predicted quit day MNWS scores (when controlling for pre-quit MNWS scores). Twelve subjects smoked on their quit day. Therefore, we reran the withdrawal analyses in a subsample that excluded these participants (n=145). Results were similar to the original analyses, with PA being the only subscale that significantly predicted postquit withdrawal both univariately, β=−.28, p=.003, and when controlling for the other subscales, β=−.25, p=.009.
Associations between CESD subscales and cessation outcomes
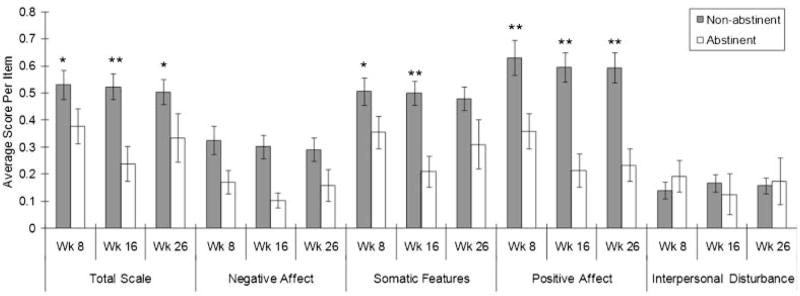
Overall abstinence rates at post-treatment (8 weeks) and at 16 and 26 weeks were 38.2%, 17.8%, and 18.5%, respectively. The correlations of abstinence status at each of these time points were robust (8 and 16 weeks, r=.59, 8 and 26 weeks, r=.54; 16 and 26 weeks, r=.75; all ps<.0001), suggesting that there was consistency in the tendency to remain abstinent among participants. Mean CESD total and subscale scores by abstinence status at each follow-up are shown in Figure 2. At all follow ups, those who were abstinent had higher baseline CESD total scores than those who were not abstinent: week 8, p=.02, Cohen’s d=.37; week 16, p=.002, d=.49; week 26, p=.02, d=.39. Broken down by subscale, those who were abstinent had higher baseline PA scores than those who were not abstinent at all follow ups: week 8, p=.007, d=.44; week 16, p=.003, d=.48; week 26, p=.004, d=.47. Abstinent participants had significantly lower baseline SF scores at week 8, p=.03, d=.35, and week 16, p=.003, d=.48, and trend-level effects at week 26, p=.07, d=.29. Abstinent participants had lower baseline NA scores at all three assessments, but these differences failed to reach significance: week 8, p=.08, d=.28; week 16, p=.08, d=.28; week 26, p=.15, d=.23. There were no differences between abstinent and non-abstinent participants in baseline IP scores at each follow up, Fs≤.68, ps⩾.4123.
Figure 2.
Center for Epidemiologic Studies Depression Scale (CESD) total scale and subscale scores by abstinence status. Mean levels and standard errors of baseline CESD subscale scores comparing those who are abstinent from smoking at each follow-up to those who are not abstinent at that follow-up. Positive Affect scores are reversed to allow comparison with other scales. Means based on average response per item (possible range: 0–3). Abstinent vs. Non-abstinent comparisons: *p<.05, **p<.0.
GEE analyses of CESD total score and subscales predicting abstinence are reported in Table 2. Results showed that higher CESD total scores were associated with significantly lower odds of abstinence, odds ratio (OR)=.92. That is, with each increase in one standard deviation in CESD total scores, participants had 8% lower odds of remaining abstinent. Broken down by subscale, univariate GEE analyses showed that higher NA, higher SF, and lower PA were univariately associated with lower odds of abstinence, whereas IP scores did not predict abstinence (see Table 2). When all four subscales were simultaneously included as predictors, lower PA continued to significantly predict reduced odds of abstinence (or put another way higher PA predicted increased odds of abstinence). In contrast, NA, SF, and IP did not significantly predict abstinence in this model, suggesting that common variance across the subscales accounted for the effects of NA and SF on smoking outcomes previously demonstrated in the univariate analyses.
Table 2.
Generalized estimation equation analyses of CESD scales predicting 7-day point prevalence smoking abstinence at 8, 16, and 26 weeks after quit date
| Univariate effects |
Unique effectsa |
Unique effects beyond other relevant variablesb |
||||
|---|---|---|---|---|---|---|
| OR (95% CI) | p | OR (95% CI) | p | OR (95% CI) | p | |
| Total Scale | .92 (.87–.97) | .001 | – | – | – | – |
| Negative Affect | .95 (.91–.99) | .01 | 1.01 (.94–1.08) | .82 | 1.01 (.94–1.09) | .80 |
| Somatic Features | .93 (.88–.98) | .004 | .95 (.89–1.01) | .11 | .95 (.89–1.02) | .13 |
| Positive Affect | 1.10 (1.06–1.14) | <.0001 | 1.09 (1.03–1.15) | .002 | 1.09 (1.03–1.15) | .002 |
| Interpersonal Disturbance | 1.00 (.94–1.05) | .89 | 1.03 (.98–1.09) | .25 | 1.03 (.97–1.09) | .28 |
Note. Lower ORs indicate that higher scores on the CESD subscale predict reduced odds of abstinence OR=odds ratio of standardized scores (z-scores). OR=1.00 means nonsignificant effect. CI=Confidence Interval. CESD=Center for Epidemiologic Studies Depression Scale. FTND=Fagerström Test for Nicotine Dependence.
Model included all four CESD subscales simultaneously included as predictors.
Model included all four CESD subscales, FTND scores, cigarettes smoked per day, and past history of depression as predictors.
It is possible that overlapping variance among NA and SF that is not shared with PA might restrict the possibility to detect unique effects of NA and SF in our models that included all subscales. That is, NA may contribute incremental variance to the prediction of outcome over PA alone and SF may also predict outcome beyond PA alone. We therefore tested two additional GEE models predicting outcome: (a) a model in which NA and PA were simultaneously included as predictors; and (b) a model in which SF and PA were simultaneously included as predictors. Concordant with results from the original model that included all four subscales, PA significantly predicted outcome on both models (ps≤.0008) and the effects of NA and SF were nonsignificant (ps⩾.15).
In the final GEE model that included all 4 subscales, FTND scores, cigarettes smoked per day, and past history of depression as predictors, PA was the only variable that significantly predicted smoking outcome (see Table 2).
Discussion
This study examined the degree to which different dimensions of depressive symptoms influenced smoking cessation. We hypothesized that high NA and low PA would be associated with negative and positive reinforcement smoking motives, respectively, based on affect regulation principles. However, all 4 subscales and the total scale associated with both forms of reinforcement smoking univariately, and none of them were unique predictors. Studies have shown that depressive symptoms as a whole and a positive history of depression predict both positive and negative reinforcement smoking expectancies (Lerman et al., 1996; Niaura et al., 1999; Pomerleau et al., 2000; Pomerleau et al., 2005). Thus, it appears that depressive disturbance in general explains this relationship and that the association is not affect-specific. However, it should be noted that there was a very high correlation between the positive and negative reinforcement WISDM subscales in the current study (r=.80), which raises doubt regarding the discriminant validity of these scales. In a separate sample of undergraduate smokers (Leventhal et al., 2007), we found that the correlations between WISDM positive and negative reinforcement smoking was also very high (r=.85). Thus, it is unlikely that the unique characteristics of this sample resulted in the strong overlap between these scales.
We also examined the extent to which each depressive dimension predicted cessation-related changes in nicotine withdrawal. Although overall depressive symptoms predicted nicotine withdrawal at a trend level, baseline PA was the only subscale that was significantly associated with withdrawal effects. Gilbert and colleagues (1998) measured withdrawal symptoms both before and after tobacco deprivation and showed that a trait measure of NA (neuroticism) did not predict abstinence-provoked changes in nicotine withdrawal symptoms (Gilbert, et al., 1998). Other studies that have taken into account baseline levels of affect have shown that precessation levels of overall depressive mood and anhedonia predict greater deprivation-induced withdrawal and craving (Cook, Spring, McChargue, & Hedeker, 2004; Ginsberg et al., 1995). Thus, evidence to date is consistent with the notion that overall depressive symptoms or low PA are the most significant precessation affective characteristics that are associated with nicotine withdrawal responses.
The effects of depressive symptoms on outcome were relatively clear. Greater overall depression, NA, SF, and lower PA each predicted poorer cessation outcomes univariately. IP had no effect. Multivariate analyses showed that only PA had an incremental effect, suggesting that common variance among the depressive dimensions as well as unique variance in items tapping positive mood and anhedonia predicted outcome. The effect of PA was robust to controlling for a history of major depression, nicotine dependence severity, and cigarettes smoked per day. Thus, despite the large subgroup of smokers with a history of depression (33%), it is unlikely that these individuals were driving the results. This pattern of findings could be conceptualized as multifaceted influence of depressive symptoms on outcome that includes (a) a general effect of nonspecific affective distress, tapped by NA, SF, and PA items, and (b) a specific effect of low PA, tapped only by PA items. This idea is congruent with the notion that depression scales, such as the CESD, measure multiple specific, lower order factors and a general, higher order depression factor (Shafer, 2006) and that both levels of depression may influence smoking outcomes.
Results from prior investigations corroborate the current findings. Multiple examinations have shown that overall levels of baseline depressive symptoms and depressed mood (i.e., the Profile of Mood States-Depression Scale) predict poorer smoking outcomes (Brown et al., 2001; Cinciripini et al., 2003; Ginsberg et al., 1995; Haas et al., 2004; Hitsman et al., 1999; Killen et al., 1999; Kinnunen et al., 1996; Niaura et al., 2001; Rausch et al., 1990; Swan et al., 2003). Even though the current level of depressive symptomatology in the current study was low (i.e., average scores on CESD scales ranged from .1 to .65 on a 0 to 3 scale), they were still predictive of outcome. This is consistent with Niaura et al.’s (2001) demonstration that very low levels of depressive symptoms (e.g., Hamilton Rating Scale for Depression score >2) predict reduced odds of cessation and decreased relapse latency in smokers without current major depression.
Consistent with our hypothesis, studies that have examined individual psychopathological-affective components of depression have shown anhedonia (loss of interest in normally rewarding activities) and low PA have been associated with relapse (al’Absi et al., 2005; Doran et al., 2006; Niaura et al., 2001). Low-PA smokers might be more susceptible to the effects of tobacco deprivation on hedonic states, and therefore might be more prone to relapse due to their desire to raise hedonic tone with nicotine (Cook et al., 2004; Cook, Spring, & McChargue, 2007).
In contrast, we were surprised that there was no specific effect of baseline NA depressive symptoms on cessation outcomes. In an ecological momentary assessment study of relapse, Shiffman (2005) found that smoking relapses are driven by “local” changes in NA over the course of hours or minutes, rather than background levels of stress and mood over days or weeks. Therefore, assessment of baseline levels of NA depressive symptoms over longer periods may not capture NA in a time frame that is relevant for smoking cessation.
The present study had some limitations. First, the current sample included non-clinically depressed smokers with heavy social drinking patterns because the larger clinical trial from which these data were extracted was interested in this population. Consequently, the sample’s range of drinking severity was restricted such that both non-drinkers and dependent drinkers were excluded. Thus, it is unclear whether the current findings will extend to these populations or to a general sample. However, it should be noted that almost 20% of current smokers consume five or more drinks on one occasion at least once per month, compared to about 6.5% of nonsmokers (Dawson, 2000), suggesting the general population of smokers contains a significant portion of individuals similar to the current sample in their drinking behavior. Second, only a single, self-report measure of depressive symptoms was used. Although the CESD is a well-validated measure, applicable to both clinical and nonclinical populations (Radloff, 1977), using other self-report measures and clinician rating scales would demonstrate that these findings extend to other instruments and are not impacted by method variance. Third, laboratory designs in which withdrawal is assessed during counterbalanced abstinent and non abstinent sessions are the most appropriate method of examining tobacco deprivation while controlling for order effects, which could have impacted the current analyses of cessation-induced withdrawal. Finally, we did not include measures of PA and NA on pre- and post-quit day assessments and therefore could not examine whether abstinence-induced changes in affect mediated the influence of depressive symptoms on smoking outcome.
From a clinical standpoint, these findings suggest that smoking cessation interventions targeting low PA and anhedonia may be beneficial. Studies examining the effects of pharmacotherapies on affect have shown that fluoxetine (Cook, Spring, McChargue, Borrelli et al., 2004), bupropion (Shiffman et al., 2000), and transdermal nicotine (Strasser et al., 2005) elevate (or prevent reductions in) PA in the context of smoking cessation. Given the influence of low PA on outcome, these treatments may be especially useful for smokers with elevated anhedonic-depressive symptoms. Many behavioral treatments have focused on managing NA to improve abstinence rates in depressed smokers (Brown et al., 2001; Hall, Muñoz, Reus, & Sees, 1996). The current findings suggest that psychosocial treatments that raise PA, such as behavioral activation therapy (Hopko, Lejuez, Ruggiero, & Eifert, 2003), may be helpful for anhedonic smokers as an adjunct to standard smoking cessation treatment.
Acknowledgments
This study was supported by grant DA15534 from the National Institute on Drug Abuse to Dr. Christopher W. Kahler. Portions of this work were presented at the Society for Research on Nicotine and Tobacco’s 2007 Annual Scientific Meeting in Austin, TX. The authors gratefully acknowledge Andrea Resendes, Jennifer Larence, Dan Belenky, Catherine Costantino, Cheryl Eaton, Timothy Souza, and Kara Szczesny for their assistance on this project, as well as treatment providers John McGeary, Gail Schilke, James MacKillop, and Jane Metrik. No potential conflicts of interest on the part of any of the authors exist with regards to the research reported in this article.
Footnotes
Publisher's Disclaimer: Full terms and conditions of use: http://www.informaworld.com/terms-and-conditions-of-access.pdf
This article maybe used for research, teaching and private study purposes. Any substantial or systematic reproduction, re-distribution, re-selling, loan or sub-licensing, systematic supply or distribution in any form to anyone is expresslyforbidden.
The publisher does not give any warranty express or implied or make any representation that the contents will be complete or accurate or up to date. The accuracy of any instructions, formulae and drug doses should be independently verified with primary sources. The publisher shall not be liable for any loss, actions, claims, proceedings, demand or costs or damages whatsoever or howsoever caused arising directly or indirectly in connection with or arising out of the use of this material.
References
- al’Absi M, Hatsukami D, Davis GL. Attenuated adrenocorticotropic responses to psychological stress are associated with early smoking relapse. Psychopharmacology. 2005;181:107–117. doi: 10.1007/s00213-005-2225-3. [DOI] [PubMed] [Google Scholar]
- al’Absi M, Hatsukami D, Davis GL, Wittmers LE. Prospective examination of effects of smoking abstinence on cortisol and withdrawal symptoms as predictors of early smoking relapse. Drug and Alcohol Dependence. 2004;73:267–278. doi: 10.1016/j.drugalcdep.2003.10.014. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders: DSM-IV. 4. Washington, DC: American Psychiatric Association; 1994. [Google Scholar]
- Anda RF, Williamson DF, Escobedo LG, Mast EE, Giovino GA, Remington PL. Depression and the dynamics of smoking. A national perspective. Journal of the American Medical Association. 1990;264:1541–1545. [PubMed] [Google Scholar]
- Beck A, Ward C, Mendelson M, Mack J, Erbaugh J. An inventory of measuring depression. Archives of General Psychiatry. 1961;49:599–608. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- Beck AT, Steer RA. Manual for the Beck Depression Inventory-II. San Antonio, TX: Psychological Corporation; 1996. [Google Scholar]
- Blazer DG, Hybels CF. What symptoms of depression predict mortality in community-dwelling elders? Journal of the American Geriatrics Society. 2004;52:2052–2056. doi: 10.1111/j.1532-5415.2004.52564.x. [DOI] [PubMed] [Google Scholar]
- Blazer DG, Hybels CF, Fillenbaum GG, Pieper CF. Predictors of antidepressant use among older adults: Have they changed over time? American Journal of Psychiatry. 2005;162:705–710. doi: 10.1176/appi.ajp.162.4.705. [DOI] [PubMed] [Google Scholar]
- Brown RA, Kahler CW, Niaura R, Abrams DB, Sales SD, Ramsey SE, Goldstein MG, Burgess ES, Miller IW. Cognitive-behavioral treatment for depression in smoking cessation. Journal of Consulting and Clinical Psychology. 2001;69:471–480. doi: 10.1037//0022-006x.69.3.471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catley D, Ahluwalia JS, Resnicow K, Nazir N. Depressive symptoms and smoking cessation among inner-city African Americans using the nicotine patch. Nicotine & Tobacco Research. 2003;5:61–68. [PubMed] [Google Scholar]
- Catley D, Harris KJ, Okuyemi KS, Mayo MS, Evan P, Ahluwalia JS. The influence of depressive symptoms on smoking cessation among African Americans in a randomized trial of bupropion. Nicotine & Tobacco Research. 2005;7:859–870. doi: 10.1080/14622200500330118. [DOI] [PubMed] [Google Scholar]
- Cinciripini PM, Wetter DW, Fouladi RT, Blalock JA, Carter BL, Cinciripini LG, Baile WF. The effects of depressed mood on smoking cessation: Mediation by postcessation self-efficacy. Journal of Consulting and Clinical Psychology. 2003;71:292–301. doi: 10.1037/0022-006x.71.2.292. [DOI] [PubMed] [Google Scholar]
- Clark LA, Watson D. Tripartite model of anxiety and depression: Psychometric evidence and taxonomic implications. Journal of Abnormal Psychology. 1991;100:316–336. doi: 10.1037//0021-843x.100.3.316. [DOI] [PubMed] [Google Scholar]
- Cook JW, Spring B, McChargue D. Influence of nicotine on positive affect in anhedonic smokers. Psychopharmacology. 2007;192:87–95. doi: 10.1007/s00213-006-0688-5. [DOI] [PubMed] [Google Scholar]
- Cook JW, Spring B, McChargue D, Hedeker D. Hedonic capacity, cigarette craving, and diminished positive mood. Nicotine & Tobacco Research. 2004;6:39–47. doi: 10.1080/14622200310001656849. [DOI] [PubMed] [Google Scholar]
- Cook JW, Spring B, McChargue DE, Borrelli B, Hitsman B, Niaura R, Keuthen NJ, Kristeller J. Influence of fluoxetine on positive and negative affect in a clinic-based smoking cessation trial. Psychopharmacology. 2004;173:153–159. doi: 10.1007/s00213-003-1711-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Covey LS. Comments on ‘History of depression and smoking cessation outcome: A meta-analysis’. Nicotine & Tobacco Research. 2004;6:743–745. doi: 10.1080/14622200410001727902. [DOI] [PubMed] [Google Scholar]
- Covey LS, Bomback A, Yan GWY. History of depression and smoking cessation: A rejoinder. Nicotine & Tobacco Research. 2006;8:315–319. doi: 10.1080/14622200500485250. [DOI] [PubMed] [Google Scholar]
- Dawson DA. Drinking as a risk factor for sustained smoking. Drug and Alcohol Dependence. 2000;59:235–249. doi: 10.1016/s0376-8716(99)00130-1. [DOI] [PubMed] [Google Scholar]
- Devins GM, Orme CM, Costello CG, Binik YM. Measuring depressive symptoms in illness populations: Psychometric properties of the Center for Epidemiologic Studies Depression (CES-D) scale. Psychology & Health. 1988;2:139–156. [Google Scholar]
- Doran N, Spring B, Borrelli B, McChargue D, Hitsman B, Niaura R, Hedeker D. Elevated Positive Mood: A Mixed Blessing for Abstinence. Psychology of Addictive Behaviors. 2006;20:36–43. doi: 10.1037/0893-164X.20.1.36. [DOI] [PubMed] [Google Scholar]
- Fiore MC, Bailey WC, Cohen SJ. Treating tobacco use and dependence: clinical practice guideline. Journal of the American Medical Association. 2000;283:3244–3254. [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JB. Structured Clinical Interview for DSM-IV Axis I Disorders- Patient Edition (SCID -I/P, Version 2.0) New York: Biometric Research Department; 1995. [Google Scholar]
- Gilbert DG, McClernon FJ, Rabinovich NE, Plath LC, Jensen RA, Meliska CJ. Effects of smoking abstinence on mood and craving in men: Influences of negative-affect-related personality traits, habitual nicotine intake and repeated measurements. Personality and Individual Differences. 1998;25:399–423. [Google Scholar]
- Ginsberg D, Hall SM, Reus VI, Muñoz RF. Mood and depression diagnosis in smoking cessation. Experimental and Clinical Psychopharmacology. 1995;3:389–395. [Google Scholar]
- Glassman AH, Helzer JE, Covey LS, Cottler LB, Stetner F, Tipp JE, Johnson J. Smoking, smoking cessation, and major depression. Journal of the American Medical Association. 1990;264:1546–1549. [PubMed] [Google Scholar]
- Haas AL, Muñoz RF, Humfleet GL, Reus VI, Hall SM. Influences of mood, depression history, and treatment modality on outcomes in smoking cessation. Journal of Consulting and Clinical Psychology. 2004;72:563–570. doi: 10.1037/0022-006X.72.4.563. [DOI] [PubMed] [Google Scholar]
- Hall SM. ‘History of depression and smoking cessation outcome: A meta-analysis’: The Covey-Hitsman exchange. Nicotine & Tobacco Research. 2004;6:751–752. doi: 10.1080/14622200410001727902. [DOI] [PubMed] [Google Scholar]
- Hall SM, Muñoz RF, Reus VI, Sees KL. Mood management and nicotine gum in smoking treatment: A therapeutic contact and placebo-controlled study. Journal of Consulting and Clinical Psychology. 1996;64:1003–1009. doi: 10.1037//0022-006x.64.5.1003. [DOI] [PubMed] [Google Scholar]
- Hasler G, Drevets WC, Manji HK, Charney DS. Discovering endophenotypes for major depression. Neuropsychopharmacology. 2004;29:1765–1781. doi: 10.1038/sj.npp.1300506. [DOI] [PubMed] [Google Scholar]
- Heatherton TF, Kozlowski LT, Frecker RC, Fagerström KO. The Fagerström Test for Nicotine Dependence: A revision of the Fagerström Tolerance Questionnaire. British Journal of Addiction. 1991;86:1119–1127. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- Hertzog C, Van Alstine J, Usala PD, Hultsch DF. Measurement properties of the Center for Epidemiological Studies Depression Scale (CES-D) in older populations. Psychological Assessment. 1990;2:64–72. [Google Scholar]
- Hitsman B, Borrelli B, McChargue DE, Spring B, Niaura R. History of depression and smoking cessation outcome: A meta-analysis. Journal of Consulting and Clinical Psychology. 2003;71:657–663. doi: 10.1037/0022-006x.71.4.657. [DOI] [PubMed] [Google Scholar]
- Hitsman B, Pingitore R, Spring B, Mahableshwarkar A, Mizes JS, Segraves KA, Kristeller KA, Xu W. Antidepressant pharmacotherapy helps some cigarette smokers more than others. Journal of Consulting and Clinical Psychology. 1999;67:547–554. doi: 10.1037//0022-006x.67.4.547. [DOI] [PubMed] [Google Scholar]
- Hitsman B, Spring B, Borrelli B, McChargue D, Niaura R. ‘History of depression and smoking cessation outcome: A meta-analysis’: Response to Covey. Nicotine & Tobacco Research. 2004;6(4):747–749. doi: 10.1080/14622200410001727902. [DOI] [PubMed] [Google Scholar]
- Hopko DR, Lejuez CW, Ruggiero KJ, Eifert GH. Contemporary behavioral activation treatments for depression: Procedures, principles and progress. Clinical Psychology Review. 2003;23:699–717. doi: 10.1016/s0272-7358(03)00070-9. [DOI] [PubMed] [Google Scholar]
- Hughes J, Hatsukami DK. Errors in using tobacco withdrawal scale. Tobacco Control. 1998;7:92–93. doi: 10.1136/tc.7.1.92a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes JR, Hatsukami D. Signs and symptoms of tobacco withdrawal. Archives of General Psychiatry. 1986;43:289–294. doi: 10.1001/archpsyc.1986.01800030107013. [DOI] [PubMed] [Google Scholar]
- Joiner TE, Brown JS, Metalsky GI. A test of the tripartite model’s prediction of anhedonia’s specificity to depression: Patients with major depression versus patients with schizophrenia. Psychiatry Research. 2003;119:243–250. doi: 10.1016/s0165-1781(03)00131-8. [DOI] [PubMed] [Google Scholar]
- Kashdan TB, Elhai JD, Frueh BC. Anhedonia and emotional numbing in combat veterans with PTSD. Behaviour Research and Therapy. 2006;44:457–467. doi: 10.1016/j.brat.2005.03.001. [DOI] [PubMed] [Google Scholar]
- Killen JD, Fortmann SP, Davis L, Strausberg L, Varady A. Do heavy smokers benefit from higher dose nicotine patch therapy? Experimental and Clinical Psychopharmacology. 1999;7:226–233. doi: 10.1037//1064-1297.7.3.226. [DOI] [PubMed] [Google Scholar]
- Kinnunen T, Doherty K, Militello FS, Garvey AJ. Depression and smoking cessation: Characteristics of depressed smokers and effects of nicotine replacement. Journal of Consulting and Clinical Psychology. 1996;64:791–798. doi: 10.1037//0022-006x.64.4.791. [DOI] [PubMed] [Google Scholar]
- Knight RG, Williams S, McGee R, Olaman S. Psychometric properties of the Centre for Epidemiologic Studies Depression Scale (CES-D) in a sample of women in middle life. Behaviour Research and Therapy. 1997;35:373–380. doi: 10.1016/s0005-7967(96)00107-6. [DOI] [PubMed] [Google Scholar]
- Lerman C, Audrain J, Orleans CT, Boyd R. Investigation of mechanisms linking depressed mood to nicotine dependence. Addictive Behaviors. 1996;21:9–19. doi: 10.1016/0306-4603(95)00032-1. [DOI] [PubMed] [Google Scholar]
- Leventhal AM, Archila MP, Axtell C, Miller EK, Garcia IP, Kahler CW, Waters AJ. Does smoking for positive and negative reinforcement moderate the relationship between affect and desire to smoke?; Paper presented at the Annual Scientific Meeting of the Society for Research on Nicotine and Tobacco; Austin, TX. 2007. http://www.srnt.org/meeting/2007/pdf/onsite/2007SRNTAbstracts-FINAL.pdf. [Google Scholar]
- Leventhal AM, Rehm LP. The empirical status of melancholia: Implications for psychology. Clinical Psychology Review. 2005;25:25–44. doi: 10.1016/j.cpr.2004.09.001. [DOI] [PubMed] [Google Scholar]
- National Institute on Alcohol Abuse and Alcoholism. The Physicians’ Guide to Helping Patients with Alcohol Problems 1995 [Google Scholar]
- Nguyen HT, Kitner-Triolo M, Evans MK, Zonderman AB. Factorial invariance of the CES-D in low socioeconomic status African Americans compared with a nationally representative sample. Psychiatry Research. 2004;126:177–187. doi: 10.1016/j.psychres.2004.02.004. [DOI] [PubMed] [Google Scholar]
- Niaura R, Britt DM, Borrelli B, Shadel WG, Abrams DB, Goldstein MG. History and symptoms of depression among smokers during a self-initiated quit attempt. Nicotine & Tobacco Research. 1999;1:251–257. doi: 10.1080/14622299050011371. [DOI] [PubMed] [Google Scholar]
- Niaura R, Britt DM, Shadel WG, Goldstein M, Abrams D, Brown R. Symptoms of depression and survival experience among three samples of smokers trying to quit. Psychology of Addictive Behaviors. 2001;15:13–17. doi: 10.1037/0893-164x.15.1.13. [DOI] [PubMed] [Google Scholar]
- Piper ME, Piasecki TM, Federman EB, Bolt DM, Smith SS, Fiore MC, Baker TB. A multiple motives approach to tobacco dependence: The Wisconsin Inventory of Smoking Dependence Motives (WISDM-68) Journal of Consulting and Clinical Psychology. 2004;72:139–154. doi: 10.1037/0022-006X.72.2.139. [DOI] [PubMed] [Google Scholar]
- Pizzagalli DA, Jahn AL, O’Shea JP. Toward an objective characterization of an anhedonic phenotype: A signal-detection approach. Biological Psychiatry. 2005;57:319–327. doi: 10.1016/j.biopsych.2004.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pomerleau CS, Marks JL, Pomerleau OF. Who gets what symptom? Effects of psychiatric cofactors and nicotine dependence on patterns of smoking withdrawal symptomatology. Nicotine & Tobacco Research. 2000;2:275–280. doi: 10.1080/14622200050147547. [DOI] [PubMed] [Google Scholar]
- Pomerleau OF, Pomerleau CS, Mehringer AM, Snedecor SM, Ninowski R, Sen A. Nicotine dependence, depression, and gender: Characterizing phenotypes based on withdrawal discomfort, response to smoking, and ability to abstain. Nicotine & Tobacco Research. 2005;7:91–102. doi: 10.1080/14622200412331328466. [DOI] [PubMed] [Google Scholar]
- Pomerleau OF, Pomerleau CS, Snedecor SM, Gaulrapp S, Brouwer RN, Cameron OG. Depression, smoking abstinence and HPA function in women smokers. Human Psychopharmacology: Clinical and Experimental. 2004;19:467–476. doi: 10.1002/hup.623. [DOI] [PubMed] [Google Scholar]
- Posternak MA, Zimmerman M. Partial validation of the atypical features subtype of major depressive disorder. Archives of General Psychiatry. 2002;59:70–76. doi: 10.1001/archpsyc.59.1.70. [DOI] [PubMed] [Google Scholar]
- Radloff LS. The CES-D Scale: A self-report depression scale for research in the general population. Applied Psychological Measurement. 1977;1:385–401. [Google Scholar]
- Rausch JL, Nichinson B, Lamke C, Matloff J. Influence of negative affect on smoking cessation treatment outcome: A pilot study. British Journal of Addiction. 1990;85:929–933. doi: 10.1111/j.1360-0443.1990.tb03723.x. [DOI] [PubMed] [Google Scholar]
- SAS Institute Inc. The SAS System for Windows (Version 8.2) Cary, NC: SAS Institute Inc; 2003. [Google Scholar]
- Shafer AB. Meta-analysis of the factor structures of four depression questionnaires: Beck, CES-D, Hamilton, and Zung. Journal of Clinical Psychology. 2006;62:123–146. doi: 10.1002/jclp.20213. [DOI] [PubMed] [Google Scholar]
- Shiffman S. Dynamic influences on smoking relapse process. Journal of Personality. 2005;73:1–34. doi: 10.1111/j.0022-3506.2005.00364.x. [DOI] [PubMed] [Google Scholar]
- Shiffman S, Johnston JA, Khayrallah M, Elash CA, Gwaltney CJ, Paty JA, Gyns M, Evoniuk G, DeVeaugh-Geiss J. The effect of bupropion on nicotine craving and withdrawal. Psychopharmacology. 2000;148:33–40. doi: 10.1007/s002130050022. [DOI] [PubMed] [Google Scholar]
- Stangier U, Esser F, Leber S, Risch AK, Heidenreich T. Interpersonal problems in social phobia versus unipolar depression. Depression and Anxiety. 2006;23:418–421. doi: 10.1002/da.20190. [DOI] [PubMed] [Google Scholar]
- Strasser AA, Kaufmann V, Jepson C, Perkins KA, Pickworth WB, Wileyto EP, Rukstalis M, Audrain-McGovern J, Lerman C. Effects of different nicotine replacement therapies on postcessation psychological responses. Addictive Behaviors. 2005;30:9–17. doi: 10.1016/j.addbeh.2004.04.005. [DOI] [PubMed] [Google Scholar]
- Swan GE, Jack LM, Chorost M, Javitz H, Curry S, McAfee T, Dacey S. Bupropion SR and counseling for smoking cessation in actual practice: Predictors of outcome. Nicotine & Tobacco Research. 2003;5:911–921. doi: 10.1080/14622200310001646903. [DOI] [PubMed] [Google Scholar]
- Vàzquez FL, Becoña E. Depression and smoking in a smoking cessation programme. Journal of Affective Disorders. 1999;55:125–132. doi: 10.1016/s0165-0327(98)00215-8. [DOI] [PubMed] [Google Scholar]
- SRNT Subcommittee on Biochemical Verification. Biochemical verification of tobacco use and cessation. Nicotine & Tobacco Research. 2002;4:149–159. doi: 10.1080/14622200210123581. [DOI] [PubMed] [Google Scholar]
- Watson D. Rethinking the mood and anxiety disorders: A quantitative hierarchical model for DSM-V. Journal of Abnormal Psychology. 2005;114:522–536. doi: 10.1037/0021-843X.114.4.522. [DOI] [PubMed] [Google Scholar]
- Weissman M, Sholomskas M, Pottenger M, Prusoff B, Locke B. Assessing depressive symptoms in five psychiatric populations: A validation study. American Journal of Epidemiology. 1977;106:203–214. doi: 10.1093/oxfordjournals.aje.a112455. [DOI] [PubMed] [Google Scholar]
- Zeger SL, Liang KY. Longitudinal data analysis for discrete and continuous outcomes. Biometrics. 1986;42:121–130. [PubMed] [Google Scholar]