Abstract
Background
Graft arteriosclerosis (GA) is an important factor limiting long-term outcomes after organ transplantation. We have used a chimeric humanized mouse system to model this arteriopathy in human vessels and found the morphologic and functional changes of experimental GA to be interferon (IFN)-γ-dependent. This study evaluated if HMG-CoA reductase inhibitors, described as inhibitors of IFN-γ production, affect GA in our model.
Methods
C.B-17 SCID/beige mice were transplanted with human artery segments as aortic interposition grafts and inoculated with allogeneic human PBMCs or replication-deficient adenovirus encoding human IFN-γ. Transplant arteries were analyzed from recipients treated with vehicle vs. atorvastatin or simvastatin at different doses. The effects of statins on T cell alloresponses to vascular endothelial cells (ECs) were also investigated in vitro.
Results
PBMC-induced GA-like arteriopathy was reduced by atorvastatin at 30 mg/kg/day or simvastatin at 100 mg/kg/day that correlated with decreased graft-infiltrating CD3+ T cells. Circulating IFN-γ was also reduced, as were graft IFN-γ and IFN-γ-inducible chemokine transcripts and graft HLA-DR expression. GA directly induced by human IFN-γ in the absence of human PBMCs was also reduced by atorvastatin, but only at the highest dose of 100 mg/kg/day. Finally, atorvastatin decreased the clonal expansion and production of IL-2, but not IFN-γ, by human CD4+ T cells in response to allogeneic ECs in co-culture.
Conclusions
Our results suggest a benefit of statin administration in transplantation may include amelioration of GA primarily by inhibiting alloreactive T cell accumulation and consequent IFN-γ production and secondarily through suppression of the arterial response to IFN-γ.
Keywords: transplant arteriosclerosis, allograft arteriopathy, statin, HMG-CoA reductase inhibitors, interferon-γ
INTRODUCTION
Chronic transplant dysfunction, likely caused by ischemia secondary to graft arteriosclerosis (GA), is the most significant factor limiting long-term survival after clinical heart and kidney transplantation. The histopathology of GA includes proliferative intimal expansion, primarily of vascular smooth muscle cells (VSMCs), with inadequate outward vessel remodeling, resulting in luminal narrowing. Evidence supports a possible association of acute graft arterial injury and GA (1–3).
HMG-CoA reductase inhibitors (statins) exhibit immunomodulatory (4–6) and anti-inflammatory (7,8) effects independent of their lipid lowering actions. Specifically relevant to GA, statins were reported to selectively inhibit several pro-inflammatory mediators including interleukin (IL)-2, IL-12, interferon (IFN)-γ, tumor necrosis factor-α, as well as a number of chemokines, adhesion molecules, co-stimulatory molecules and transcription factors (9). Additionally, an association between statins and reduction of endothelial dysfunction (10) and VSMC proliferation (11), both potentially important to the pathobiology of GA, has also been suggested. Also, the incidence of coronary allograft vasculopathy after heart transplantation was reduced (12), and improved functional outcomes after kidney transplantation were reported in association with statin administration (13).
We have established a model of allogeneic arterial injury in severe combined immunodeficiency (SCID)/beige mice bearing transplanted human artery segments, interposed in the infra-renal abdominal aorta, followed by adoptive transfer of human T cells by i.p. inoculation with peripheral blood mononuclear cells (PBMCs) allogeneic to the artery donor. This model has provided an experimental approach for investigating GA with reproducible arterial injury, consisting of allogeneic lymphocyte infiltration, endothelial destruction, intimal proliferation, and vessel remodeling in a pattern reminiscent of the human transplant setting (14–19). Our previous studies suggested that IFN-γ is the primary mediator in this model and that this cytokine can act directly on vessel wall cells to cause dysfunction and arteriosclerosis (20–22). The experiments detailed in this report were designed to evaluate whether two widely used statins, atorvastatin and simvastatin, impact progression of GA-like lesions in human artery, as well as to examine the mechanisms of action in a model of immune-mediated arteriopathy unrelated to lipoprotein abnormalities.
MATERIALS AND METHODS
Animals
C.B-17 SCID/beige mice (Taconic, Germantown, NY) were used at 8 to 12 weeks of age. These animals are severely deficient in T cells, B cells, and natural killer cell function. Animals were housed in micro-isolator cages and fed sterilized water and mouse chow. All experimental animal protocols were approved by Yale’s Institutional Animal Care and Use Committee.
Arterial transplantation
A segment of the infra-renal mouse aorta was replaced with a size-matched human epigastric arterial segment, obtained under protocols approved by Yale’s Human Investigation Committee. The arterial grafts were assessed before implantation under magnification (25X) to ensure the absence of intima or other signs of arteriosclerosis. Only arteries deemed “normal” were chosen for implantation. Adjacent human artery segments, approximating 3 mm each, were serially transplanted in groups of 2–4 mice for each experiment, and then data from individual experiments were pooled to generate sufficient numbers for analysis. The procedure has been described in detail (15), however, briefly under general anesthesia, proximal and distal control of the mouse abdominal aorta was obtained and arterial anastomoses were performed under 18X magnification using 10-0 monofilament nylon suture. Vascular integrity was restored and hemostasis assured. Flow through the grafted artery was confirmed, and the abdomen was flooded with warmed sterile saline before closure. After wound closure, animals were monitored and warmed with a heating pad to avoid hypothermia. Ischemic time approximated 35 min, animals recovered quickly after the procedure, and hind limb function has been a reliable indicator of early graft patency. Mice with hind limb paralysis are re-anesthetized and then euthanized. Technical success rates using this approach have reproducibly exceeded 90%.
Human PBMC isolation and engraftment
PBMCs were isolated from adult volunteer donors by leukapheresis under a protocol approved by Yale’s Human Investigation Committee. PBMCs were then enriched from the apheresis product using Lymphocyte Separation Medium Ficoll-Paque PLUS (Amersham Biosciences Corp, Piscataway, NJ) and discontinuous gradient centrifugation according to the instructions of the manufacturer. The isolated PBMC fraction was suspended in 10% dimethylsulfoxide-fetal bovine serum freeze medium at a concentration of 1–1.5×108 cells/ml, then stored frozen in liquid nitrogen. When needed, generally 7 days after aortic transplantation, cells were thawed, cell numbers and viability quantified by trypan blue exclusion, and then administered to mice i.p. at 3×108 cells in 1–2 ml of Hank’s balanced salt solution.
Experimental agents
Atorvastatin (Lipitor; Pfizer Inc., NY, NY) or simvastatin (Zocor; Merck & Co., Witehouse Station, NJ) was dissolved in 0.5 ml PBS and fed to mice by gavage daily. The vehicle control was PBS alone.
IFN-γ adenovirus
We have recently reported our results using adenoviral transfection of mice to generate sustained, high systemic concentrations of human IFN-γ (22). Briefly, replication-deficient adenovirus from Qbiogene/MP Biomedicals (Solon, OH) encoding the LacZ control transgene (Ad5.CMV-LacZ) or the human transgene encoding IFN-γ (Ad5.CMV-human IFN-γ) were administered i.v. to C.B-17 SCID/beige mice 1 week after receiving a human artery infrarenal interposition graft. Based upon previous experiences, the adenoviral dose was 109 plaque forming units (pfu) which results in IFN-γ plasma levels of 212 ± 12 ng/ml after 1 week, a saturating level of the cytokine.
Determining human lymphocyte engraftment
Human lymphocyte engraftment was assessed at 14 and 28 days after PBMC administration by analysis of heparinized blood using flow cytometry (FACScan, Becton Dickinson, Mountain View, CA). Two-color fluorescence was used to measure the percentage of circulating human CD3+ and murine CD45+ cells. A live, lymphocyte gate was established using forward angle light scatter, and minimally 10,000 events were required for each sample analyzed. Human T cell engraftment was considered successful when a distinct population of human CD3+ cells was demonstrated, typically ranging from 1–10% of the total mononuclear cell population. Although the size of the human CD3+ lymphocyte population does not correlate precisely with arterial injury, animals failing to demonstrate a discrete population of circulating human CD3+ T cells do not develop arterial injury. Accordingly, animals failing to demonstrate CD3+ lymphocyte engraftment were by experimental design excluded from the analysis (success rate, approximately 98%).
Antibodies
Flow cytometry experiments used fluorescein isothiocyanate (FITC)-conjugated anti-human CD3 and phycoerythrin (PE)-conjugated anti-mouse CD45 monoclonal antibodies (Pharmingen, San Diego, CA). Immunohistochemical analyses to identify human lymphocytes used unconjugated anti-CD3 (Pharmingen), anti- platelet-endothelial cell adhesion molecule (PECAM; anti- CD31), and anti-smooth muscle α-actin (Dako, Carpinteria, CA). HLA-DR was detected using mouse anti-human HLA-DR monoclonal antibody (Pharmingen). Secondary antibody and substrate were included in the Vectastain ABC Peroxidase kit and AEC Peroxidase Substrate kit (Vector Laboratories, Burlingame, CA).
Histology and immunohistochemistry
Twenty-eight days after administering PBMCs, allogeneic to human artery bearing SCID/beige mice, animals were anesthetized; arterial grafts were perfused with normal saline, and then excised prior to sacrifice. Arterial grafts were frozen with Tissue Tek, O.C.T. compound. Serial 5-micron transverse sections were cut and stained with hematoxylin and eosin for histologic evaluation, and Elastica-van Gieson (EVG) staining was used for morphometric evaluation. Immunohistochemical peroxidase ABC staining method was used to quantify graft-infiltrating human CD3+ lymphocytes and human CD31+ endothelial cell preservation. Immunohistochemistry was also used to confirm human IFN-γ activity by staining for expression of HLA-DR on human vascular cells.
Morphometry
Elastica-von Gieson (EVG)-stained sections were used for microscopic morphometric evaluation using the Scion software program. For these experiments, data have been reported as the mean intimal area, determined by six experimental measurements under the experimental conditions described. We previously detailed our approach to morphometric analysis (17). However, briefly video microscopy was used to outline the endothelium, internal elastic lamina (IEL), and external elastic lamina (EEL), then area measurements were calculated using an image software program (Scion, Frederick, MD). Luminal area (within the endothelium), intima area (between the endothelium and IEL), media area (between the IEL and EEL), and vessel area (within the EEL) of five cross sections, 150um apart, were assessed and averaged for each vessel.
Plasma assays
Mouse blood was collected by retro-orbital puncture, placed in EDTA-containing tubes (Becton Dickinson, Franklin, NJ), and plasma was obtained by centrifugation. Circulating human IFN-γ was measured using a DuoSet enzyme-linked immunosorbant assay (ELISA) kit (R&D Systems, Minneapolis, MN). Total cholesterol was determined by colorimetric endpoint by a veterinary diagnostic laboratory (Antech Diagnostics, New York, NY). For atorvastatin assays, an extraction solvent, diethyl etherdi-chloromethane (60:40) was added to plasma samples, centrifuged at 3,000 rpm for 10 min, the upper organic layer was dried overnight at 37°C, and reconstituted with water-tetrahydrofuran (75:25). The samples were then analyzed on a Series 200 liquid chromatography system (Perkin Elmer, Shelton, CT) directly coupled to a triple quadrupole mass spectrometer (API5000, Applied Biosystems, Foster City, CA). Untreated mouse plasma spiked with known concentrations of soluble atorvastatin (Pfizer, New London, CT) were analyzed by monitoring the 559.3/440.3 Da parent mass/fragment mass transition to generate a concentration response curve from 0.03 to 30 ng/ml. Experimental samples were analyzed using the same parent mass/fragment mass transition, and the unknown atorvastatin concentration levels in ng/ml were determined using the calibration curve corresponding to the experimentally obtained peak height (counts per seconds) values.
Graft transcript analysis
Frozen tissue sections were air-dried, fixed in 70% ethanol, and then scraped off slides into RNA lysis buffer (Stratagene, La Jolla, CA) and incubated for 30 min at 37°C. Total RNA was isolated and treated with DNase using a nanoprep kit (Stratagene) according to manufacturer’s instructions. Bulk reverse transcription (RT) with random hexamer primers was performed according to the Taqman® Multiscribe RT protocol (Applied Biosystems, Foster City, California). Control reactions excluding reverse transcriptase enzyme were performed in parallel. The reverse transcribed cDNA samples generated, including negative controls, were then amplified by real-time quantitative-polymerase chain reactions (qRT-PCR) using Taqman® PCR reagents and pre-developed primers and probes for human IFN-γ, Mig, IP-10, CD3ε, and GAPDH (Applied Biosystems Assays-on-Demand™) according to the manufacturer’s recommendations for singleplex reactions. Standard curves were constructed to determine the number of transcripts in each sample (copy number). Briefly, standards were first prepared by cloning the purified PCR product of each target into a TOPO TA vector construct (Invitrogen, Carlsbad, CA) according to the manufacturers’ instructions. The plasmids containing cloned target sequences were used in serial dilutions of 10–10000 copies/µl (determined based on mass concentration) as PCR standards in every experiment. The copy number of each target was determined from the standard curve and normalized to the housekeeping gene, GAPDH.
Transplant study design
Each individual experiment used 2–4 mice bearing adjacent human artery segments (the number dependent upon the quantity of vessel available for transplantation), and each experiment was repeated 6 times. When allogeneic PBMCs were administered, mice were randomly assigned to receive atorvastatin (5, 10 or 30 mg/kg/day), simvastatin (10, 30 or 100 mg/kg/day), or a vehicle control at the same time. Grafted animals that received adeno-IFN-γ were treated with atorvastatin (30 or 100 mg/kg/day) vs. vehicle control. The arterial grafts were harvested from mice under general anesthesia 28 days after PBMCs were given, and the mice were euthanized after the vessels were removed. There were no demonstrable differences (e.g. weight loss/gain, agility, feeding or other abnormal behaviors) observed among mice in different treatment groups. For certain experiments human artery-bearing mice were infected with a replication-deficient adenoviral vector encoding human IFN-γ. Previous experiments found this virus selectively infects mouse hepatocytes, not the human artery grafts (22). In immunodeficient animals, the cytokine-encoding virus provides greater than eight weeks of sustained circulating levels of human IFN-γ, and this results in progressive intimal expansion of the graft artery in the absence of mouse or human leukocytic infiltration. Paired animals were treated with statins or vehicle controls following virus infection. Vessels were harvested, as above, 28 days after virus infection.
Co-culture experiments
Human umbilical vein endothelial cells (ECs), isolated as described previously (21), were propagated in gelatin-coated wells of 24-well culture plates, treated with IFN-γ (Invitrogen) at 100 ng/ml for 3 days, washed three times, and confirmed to express HLA-DR molecules by FACS analysis (data not shown). CD4+ T cells were isolated from PBMCs, also as described (21), labeled with 250 nM carboxyfluorescein diacetate succinimidyl ester (CFSE; Molecular Probes, Eugene, OR) for 20 minutes, and 1 × 106 CD4+ T cells in 1 ml of RPMI 1640 medium supplemented with 20% fetal calf serum was added to wells containing 2 × 105 ECs. The cultures were maintained in 5% CO2 at 37°C for 7 days in the absence or presence of soluble atorvastatin (Pfizer, New London, CT) to a maximal concentration of 10 µM (23). T cell proliferation was assessed by FACS (BD Biosciences, San Jose, CA) analysis of CFSE dilution after counterstaining with PE-labeled mouse anti-human CD4 antibody (Beckman Coulter Immunotech, Miami, FL). IL-2 and IFN-γ supernatant levels were measured after 1 day of co-culture by ELISA (Invitrogen) according to the manufacturer’s instructions.
Statistical methods
The statistical validity of experimental observations was determined using Student’s paired t-test for in vivo experiments and One-way analysis of variance (ANOVA) for in vitro experiments (GraphPad Software, San Diego, CA). Comparisons were considered statistically significant when P <0.05.
RESULTS
Effects of statins on intimal expansion
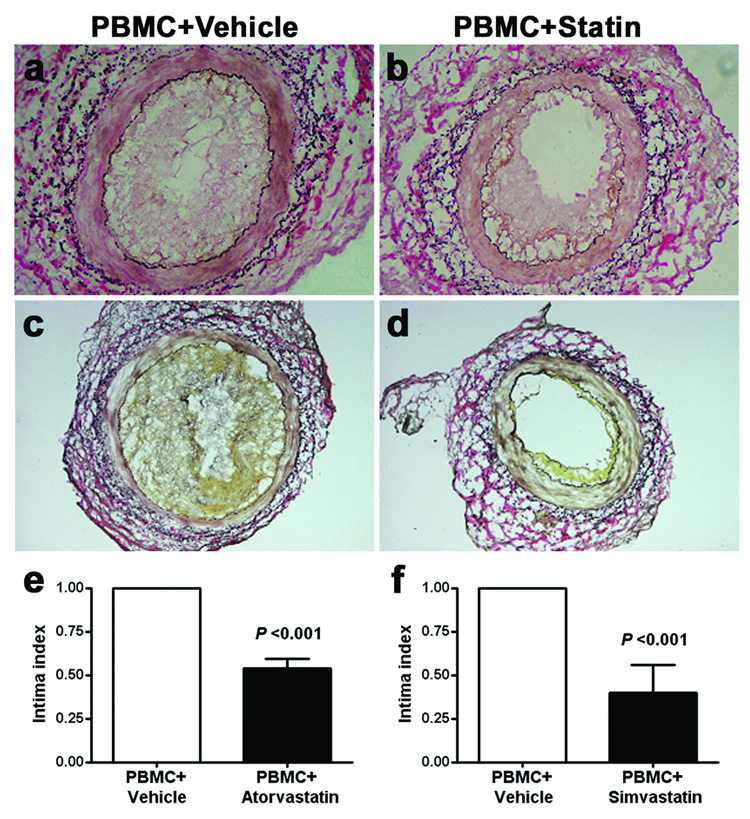
Control animals receiving PBMCs or PBMCs plus vehicle reproducibly demonstrated intimal expansion comprised of VSMCs and infiltrating human CD3+ T cells (Figure 1a, c), as we have previously described (15). When atorvastatin (30 mg/kg/day; Figure 1b) or simvastatin (100 mg/kg/day; Figure 1d) were administered to the recipients, graft intimal expansion was reduced. These results established that statins were capable of ameliorating GA-like lesions in our model.
Figure 1. Atorvastatin and simvastatin effects on intimal expansion.
Panels a and c show representative EVG stains (100X magnification) of human artery grafts from animals that received allogeneic PBMCs plus vehicle control demonstrating the characteristic myointimal proliferative lesions with associated lymphocytic infiltration. Panel b shows a representative result when atorvastatin (30 mg/kg/day) was administered simultaneously with PBMC introduction. Panel d shows similar findings with a higher dose of simvastatin (100 mg/kg/day). The intima area measurements from 6 individual experiments were used to calculate the mean ± SEM. Results from control animals (left columns, Panels e and f) were normalized to “1”, and each treatment group was described as a fraction of that value. Atorvastatin at 30 mg/kg/day (Panel e) and simvastatin at 100 mg/kg/day (Panel f) were associated with a significant reduction in mean intimal area (P <0.001; t-test).
The artery grafts were analyzed by computer-assisted morphometry to provide a quantitative comparison between the various treatment conditions. Individual experiments used adjacent segments from the same artery for each treatment condition, experiments were repeated six times using different arteries, and the results were pooled for aggregate analysis. The mean intimal area for the PBMC plus vehicle control was normalized to “1”, and each treatment group was described as a percentage of that value. The characteristic intimal lesion was significantly reduced by both atorvastatin (30 mg/kg/day; Figure 1e) and simvastatin (100 mg/kg/day; Figure 1f). When artery-bearing animals received lower doses of atorvastatin (5 or 10 mg/kg/day) or simvastatin (10 or 30 mg/kg/day), the changes in intimal expansion were progressively reduced, although the changes were not statistically different from lesions observed in untreated animals until the higher doses, demonstrated in Figure 1, were used.
Effects of statins on CD3+ T cell infiltration of graft arteries
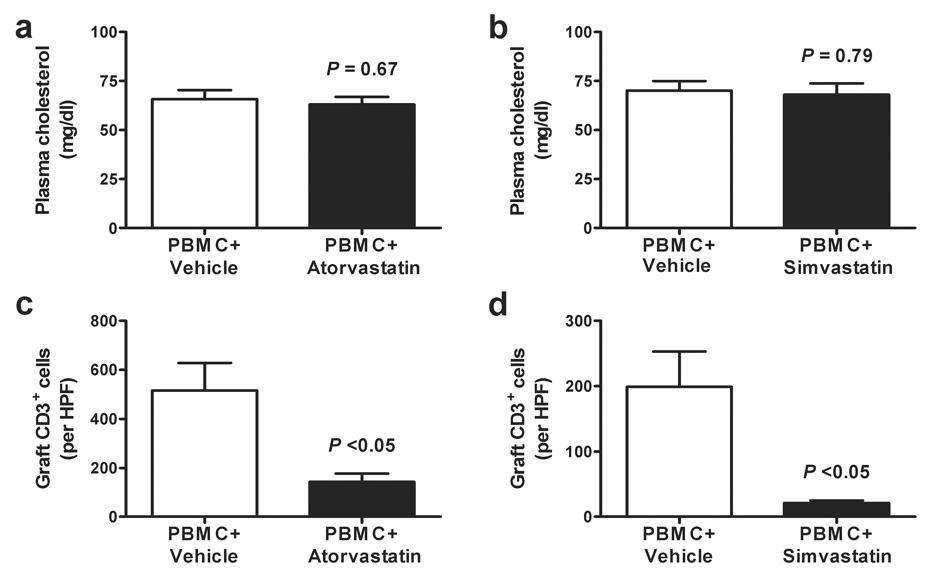
We further investigated the effects of statin treatment in our model. Even at the higher atorvastatin (30 mg/kg/day) and simvastatin (100 mg/kg/day) doses, plasma cholesterol values were not different from control animals receiving vehicle alone (Figure 2a–b).
Figure 2. Effects of atorvastatin and simvastatin on circulating cholesterol levels and human CD3+ lymphocytic infiltration of artery grafts.
Plasma total cholesterol was determined for animals receiving PBMC plus vehicle control vs. atorvastatin at 30 mg/kg/day (Panel a) or simvastatin at 100 mg/kg/day (Panel b). Aggregate analysis has been expressed as the mean ± SEM. At the statin doses used in these normocholesterolemic recipients, there were no differences in plasma cholesterol values comparing control animals with those receiving atorvastatin or simvastatin (P = N.S.; t-test). CD3+ lymphocyte infiltration was also evaluated using immunohistochemistry in sections of artery grafts obtained from control animals receiving PBMC plus vehicle for comparison to those receiving atorvastatin at 30 mg/kg/day (Panel c) or simvastatin at 100 mg/kg/day (Panel d). Aggregate analysis (expressed as the mean ± SEM) of 6 individual experiments found a significant reduction in the number of infiltrating CD3+ cells per high power field (HPF) of grafts from statin-treated animals (P <0.05; t-test).
We have previously described the detailed kinetics of human PBMC engraftment in this model (17), and addition of sirolimus but not cyclosporine prevented lymphocyte engraftment when begun simultaneously with PBMC administration (18). Neither atorvastatin, nor simvastatin affected routine engraftment of the allogeneic PBMC at the doses tested (data not shown). Also, our earlier work found that intimal expansion was observed only when adoptively transferred CD3+ human lymphocytes, allogeneic to the arterial graft, were identified in the mouse circulation. When atorvastatin (30 mg/kg/day) or simvastatin (100 mg/kg/day) were given simultaneously with allogeneic PBMCs, comparable levels of human CD3+ lymphocytes to untreated controls were reliably observed in SCID mouse blood within 2 weeks. However, the relative number of graft-infiltrating cells, assessed by immunostaining, was markedly diminished by treatment with either atorvastatin or simvastatin (Figure 2c–d).
Effects of statins on IFN-γ responses and production in graft arteries
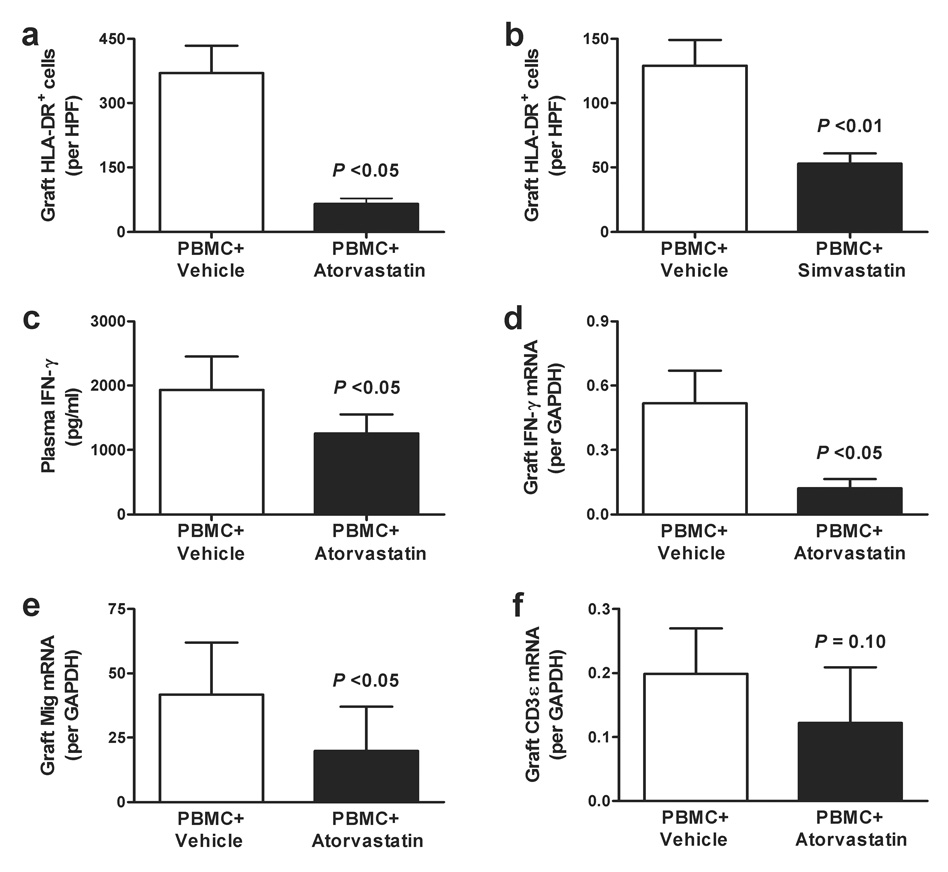
Statin effects on endothelial activation were evaluated by measuring expression of IFN-γ-inducible HLA-DR molecules within the transplanted human vessels. Immunohistochemical analyses of arteries removed from PBMC-inoculated control animals, as well as those receiving each of the experimental agents, were stained with mouse anti-human HLA-DR as well as mouse anti-human CD31 to confirm the presence of human ECs. HLA-DR was reproducibly observed among animals receiving allogeneic PBMCs alone, as well as those receiving only vehicle control. The aggregate analysis (Figure 3a–b) demonstrated that HLA-DR expression was reduced when animals received either atorvastatin (30 mg/kg/day) or simvastatin (100 mg/kg/day), correlating with the observed amelioration of intimal expansion in grafted arteries.
Figure 3. Effects of atorvastatin and simvastatin on IFN-γ production and responses.
Artery-bearing animals received PBMC plus vehicle vs. atorvastatin at 30 mg/kg/day (Panel a) or simvastatin at 100 mg/kg/day (Panel b), and representative sections of the grafts were stained with mouse anti-human HLA-DR antibody and evaluated microscopically. Aggregate analysis (mean ± SEM) of statin-treated animals exhibited a significant reduction in HLA- DR+ graft cells per high power field (HPF) compared to the respective controls (P <0.05; t-test). Panel c describes the concentration of circulating IFN-γ (mean ± SEM) in artery-bearing animals receiving PBMC plus vehicle vs. PBMC plus atorvastatin at 30 mg/kg/day, showing a modest, but significant reduction of IFN-γ plasma levels among atorvastatin-treated animals (P <0.05; t-test). Panel d demonstrates that the amount of IFN-γ mRNA (mean ± SEM) was reduced to an even greater extent within the artery grafts from animals receiving PBMC plus atorvastatin at 30 mg/kg/day (P <0.05; t-test). Panel e demonstrates that the transcript expression (mean ± SEM) of the IFN-γ-inducible chemokine, Mig was decreased to a lesser extent than intra-graft IFN-γ mRNA in the same recipients (P <0.05; t-test). Panel f demonstrates that the expression of IFN-γ mRNA (mean ± SEM) in these artery grafts was not significantly reduced when normalized to the T cell marker, CD3ε (P = 0.10; t-test), suggesting that the effects of atorvastatin on accumulation of allogeneic T cells was greater than that on IFN-γ production.
Previous work found that IFN-γ contributes importantly to arterial injury in this model (17,21). Therefore, we examined the effects of atorvastatin and simvastatin on circulating levels of IFN-γ, as well as IFN-γ transcripts within the transplanted human arteries. Although minimal circulating human IFN-γ was detected during the initial 14 days after introducing allogeneic human PBMCs to artery-bearing animals (data not shown), the concentration was reproducibly increased by 28 days (Figure 3c; left column). The presence of atorvastatin (30 mg/kg/day) was associated with a modest, but significant reduction of human IFN-γ plasma levels (Figure 3c; right column). A similar trend was observed using simvastatin, but the difference did not reach statistical significance (data not shown).
Expression of human IFN-γ mRNA within the artery graft wall was markedly reduced by atorvastatin treatment at 30 mg/kg/day (Figure 3d). There was a lesser effect on the transcript levels for the IFN-γ-inducible chemokines Mig (Figure 3e) and IP-10 (4.78 ± 1.73 vs. 2.46 ± 2.08 IP-10/GAPDH mRNA in control vs. atorvastatin-treated grafts, P = 0.06), perhaps reflecting the biologic activity of circulating IFN-γ in addition to that of locally produced cytokine. However, while the net level of these transcripts normalized to GAPDH was diminished, differences in the expression of IFN-γ and IFN-γ-inducible chemokine transcripts between control and statin-treated recipients did not reach statistical significance when cytokine or chemokine transcripts were normalized to those of CD3ε (data not shown), as there was also a trend to lower CD3ε mRNA in grafts from atorvastatin-treated animals (Figure 3f). These results suggest that although IFN-γ production and responses were suppressed within the statin-treated rejecting human artery grafts, inhibition of allogeneic T cell accumulation may be an additional immunomodulatory effect of atorvastatin.
Effects of statins on direct IFN-γ administration
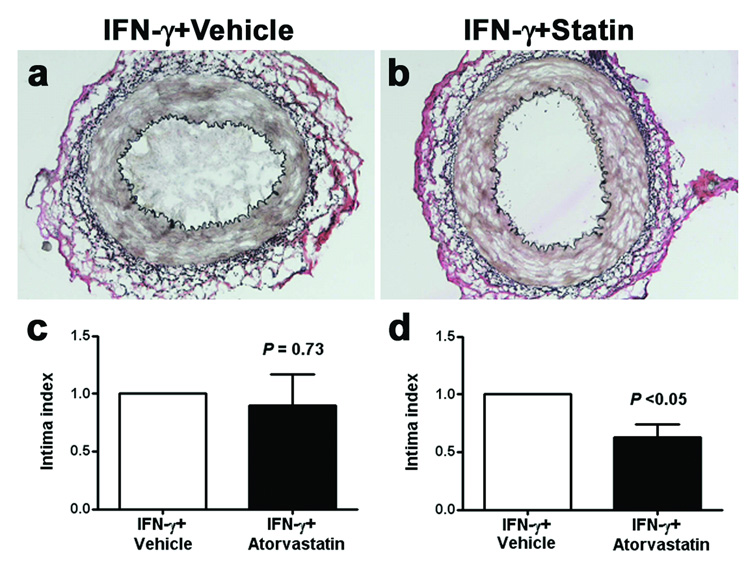
Control animals receiving IFN-γ adenovirus in the absence of allogeneic T cells developed reproducible intimal expansion (Figure 4a) similar to, but more robust than we previously reported using intermittent subcutaneous injection of human IFN-γ (20). The lesion was characterized by an expanded intima, consisting of human smooth muscle α-actin+ cells (suggesting these were VSMCs) and extracellular matrix (22).
Figure 4. Effects of atorvastatin on directly introducing IFN-γ.
A human IFN-γ-encoding, replication-defective adenovirus was used to directly stimulate an inflammatory response in the absence of leukocytes. The resulting, robust intimal proliferative lesion (Panel a) was similar to that observed with allogeneic PBMC and was prevented only at the highest dose of atorvastatin of 100 mg/kg/day (Panel b). Aggregate analysis of 6 experiments (mean ± SEM) with control grafts from vehicle-treated animals indexed as a value of “1” demonstrates in Panel c essentially no response when atorvastatin at 30 mg/kg/day was given (P = N.S.; t-test), in contrast to Panel d where a modest, but significant reduction of intimal expansion was observed if 100 mg/kg/day was given (P <0.05; t-test).
At 30 mg/kg/day, atorvastatin reproducibly reduced the characteristic intimal proliferative lesion induced by allogeneic PBMCs among artery-bearing mice (Figure 1), but this dose of atorvastatin did not reduce the robust intimal lesion associated with IFN-γ adenovirus administration (Figure 4c). However, when the atorvastatin dose was increased to 100 mg/kg/day, (the maximal tolerated dose), intimal expansion was ameliorated (P <0.05; Figure 4b, d). Again, this statin dose did not significantly alter plasma levels of cholesterol or human IFN-γ (data not shown), suggesting the target was proliferating VSMCs of the graft rather than indirect host responses. Atorvastatin plasma levels, even at the highest dose, were within clinical therapeutic values (Table 1). Although the anti-arteriosclerotic effect was recapitulated by dose escalation in the cytokine model, these findings suggest that amelioration of intimal expansion by atorvastatin in the PBMC model may be more dependent on inhibiting T cell accumulation, activation and production of IFN-γ rather than preventing IFN-γ actions on vascular cells.
Table 1.
Plasma levels of atorvastatina
| Dose (mg/kg/day) | 1 hr levels (ng/ml) | 24 hr levels (ng/ml) |
|---|---|---|
| 10 | 7.8±1.8 | 2.7±0.4 |
| 30 | 10.1±2.8 | 2.9±0.5 |
| 100 | 12.8±2.5 | 3.5±1.2 |
SCID/beige mice were treated with various doses of atorvastatin by gavage and plasma samples were collected at 1 and 24 hr after the seventh dose. Data represent means±SEM, n = 3, atorvastatin was undetectable (<1 ng/ml) in untreated SCID/beige mice.
Atorvastatin inhibits IL-2 production and T cell proliferation in response to allogeneic ECs
Finally, to gain further insight into the mechanisms of statin-mediated suppression of T cell alloresponses, we dissected the effects on cellular proliferation vs. cytokine production in vitro. We previously reported that IFN-γ-treated, HLA-DR-expressing ECs induced activation of allogeneic T cells (24) and that a subset of alloreactive CD4+ T cells proliferated in response to IFN-γ-pretreated ECs, as determined by dilution of a cytoplasmic fluorescent label. Consistent with its effects in vivo, atorvastatin inhibited the accumulation of CFSElow T cells after 7 days (Figure 5a–d).
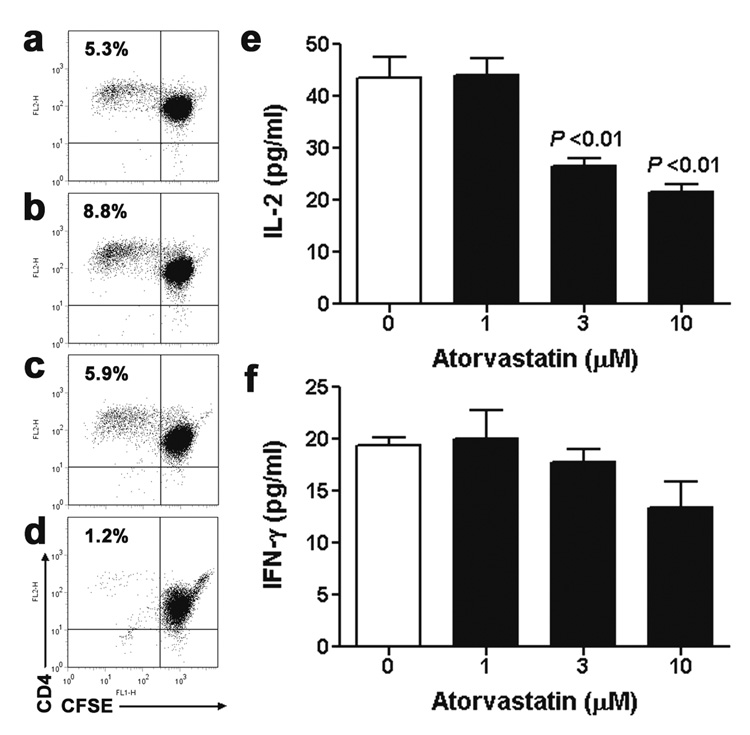
Figure 5. Effects of atorvastatin on allogeneic T cell-EC co-cultures.
CD4+ T cells proliferated in response to allogeneic, IFN-γ-pretreated, HLA-DR-expressing ECs as indicated by CFSE dilution in a subset of cells by 7 days of co-culture (Panel a). Addition of atorvastatin at 1 µM (Panel b) and 3 µM (Panel c) did not prevent T cell alloresponses, but at 10 µM (Panel d) abrogated T cell proliferation and formation of CFSElow cells. Assessment of cytokine levels (n = 6) from the supernatants after 1 day of allogeneic T cell-EC co-culture demonstrated that the higher doses of atorvastatin inhibited the secretion of IL-2 (Panel e), but not of IFN-γ (Panel f) compared to vehicle alone (P <0.01; One-way ANOVA). The data shown are from a single pair of EC and T cell donors and are representative of three independent experiments.
Secretion of IL-2 from CD4+ T cells co-cultured with allogeneic, IFN-γ-pretreated ECs was also markedly inhibited by atorvastatin (Figure 5e). In contrast, atorvastatin at the same doses did not significantly reduce production of IFN-γ by alloresponsive T cells after 1 day (Figure 5f). These data suggest that statin-mediated net reductions of IFN-γ at later times may result from the reduced expansion of IFN-γ-producing T cells.
DISCUSSION
Evidence has continued to support the hypothesis that statins mediate ameliorating effects on GA in experimental systems (7), as well as after clinical transplantation (12). The present report demonstrated amelioration of an experimental GA-like lesion in human arteries by atorvastatin and simvastatin using our established chimeric arterial transplant model (15). Similar to the findings reported by Shimizu et al. (7) using an allogeneic murine model of experimental allograft arteriosclerosis, the effects on GA were demonstrated without affecting circulating cholesterol levels. Infiltration of the transplanted human artery by human CD3+ allogeneic lymphocytes was reproducibly reduced by atorvastatin and simvastatin with associated reduction in HLA-DR+ cells. This was associated with a modest reduction of circulating IFN-γ and a more pronounced reduction of IFN-γ transcripts within the graft arterial wall. Although more than 3-fold higher concentrations of atorvastatin were needed to affect the arterial lesion caused directly by IFN-γ, the findings were consistent in that amelioration of this GA-like lesion was reliably observed. Together, these findings suggest atorvastatin and simvastatin were effective in preventing GA in this model, primarily by inhibiting T cell-mediated allogeneic responses and reducing IFN-γ production.
Examined within the context of our earlier work also using the chimeric human-mouse arterial transplant model, emerging evidence suggests that the Th1 signature cytokine IFN-γ is an important mediator of this process. We have shown that GA-like intimal expansion and outward vascular remodeling of human arteries in the presence of allogeneic PBMCs (17) is most likely a consequence of IFN-γ associated dysregulation of NO synthase (21). The findings have consistently suggested that IFN-γ mediated GA through effects on infiltrating leukocytes and intrinsic vessel wall cells, and it is ameliorated by therapeutic agents, including the mTOR inhibitor, sirolimus (18,22). Statins resemble sirolimus in that (a) they ameliorate GA caused by PBMCs in our model; (b) they also reduce the direct response of the human artery wall to the actions of IFN-γ; (c) the inhibitory effect on IFN-γ action requires a higher drug concentration than the inhibitory effect on IFN-γ synthesis; and (d) the inhibitory effect on IFN-γ synthesis may be indirect, mediated primarily by reducing T cell proliferation and expansion. The plasma levels of statins that we detected in SCID/beige are comparable to clinically-derived values of 20–30 ng/ml at 1 hr and <5 ng/ml at 24 hr after oral administration of atorvastatin to patients at a moderate to high dose of 40 mg/day (25). This suggests that the anti-arteriosclerotic properties of statins may be effective in humans at non-toxic concentrations of the drug. An important caveat to this conclusion is that active metabolites of atorvastatin, whose pharmacokinetics may differ between species, were not measured and metabolism of statins may differ between humans and mice. Our in vitro data supported the interpretation that statins inhibit T cell proliferation by targeting IL-2 production rather than by direct suppression of IFN-γ production or skewing immune responses and cytokine polarization (4). This is consistent with our previous findings that atorvastatin did not modulate IFN-γ secretion by inflammatory or cognate stimuli of autologous T cells infiltrating atherosclerotic human coronary arteries (23).
These observations are likely relevant to the clinical transplant setting, since the hallmark morphological lesion of chronically failing cardiac allografts, as well as kidney allografts, is characterized by stenosis of conduit arteries. Evidence continues to accumulate in support of the hypothesis that late graft failure may result from secondary ischemic injury to the graft parenchyma, distinct from direct immune mediated damage. We acknowledge that the exact etiology of GA, as well as late transplant failure, is not unambiguously linked to this process. However, available results are strongly suggestive of a central role for IFN-γ in GA (19), and that therapeutic strategies focusing on interrupting the consequences of this cytokine may ameliorate the progression of GA and thereby improve long-term outcomes after heart and possibly kidney transplantation.
Abbreviations
- ANOVA
analysis of variance
- CFSE
carboxyfluorescein diacetate succinimidyl ester
- ELISA
enzyme-linked immunosorbant assay
- ECs
endothelial cells
- EVG
elastica-van Gieson
- GA
graft arteriosclerosis
- IFN-γ
interferon-gamma
- IL
interleukin
- PBMCs
peripheral blood mononuclear cells
- qRT-PCR
quantitative reverse transcriptase-polymerase chain reactions
- SCID
severe combined immunodeficiency
- VSMCs
vascular smooth muscle cells
Footnotes
This work was supported by NIH grant PO1 HL 70295. P.C.T. was supported by a Vascular Research Postdoctoral Training Grant from the NIH (T32 HL07950).
REFERENCES
- 1.Libby P, Pober JS. Chronic rejection. Immunity. 2001;14:387. doi: 10.1016/s1074-7613(01)00119-4. [DOI] [PubMed] [Google Scholar]
- 2.Valantine HA. Cardiac allograft vasculopathy: central role of endothelial injury leading to transplant “atheroma”. Transplantation. 2003;76:891. doi: 10.1097/01.TP.0000080981.90718.EB. [DOI] [PubMed] [Google Scholar]
- 3.Eisen HJ, Tuzcu EM, Dorent R, et al. Everolimus for the prevention of allograft rejection and vasculopathy in cardiac-transplant recipients. N Engl J Med. 2003;349:847. doi: 10.1056/NEJMoa022171. [DOI] [PubMed] [Google Scholar]
- 4.Youssef S, Stuve O, Patarroyo JC, et al. The HMG-CoA reductase inhibitor, atorvastatin, promotes Th2 bias and reverses paralysis in central nervous system autoimmune disease. Nature. 2002;420:78. doi: 10.1038/nature01158. [DOI] [PubMed] [Google Scholar]
- 5.Dunn SE, Youssef S, Goldstein MJ, et al. Isoprenoids determine Th1/Th2 fate in pathogenic T cells, providing a mechanism of modulation of autoimmunity by atorvastatin. J Exp Med. 2006;203:401. doi: 10.1084/jem.20051129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kobashigawa JA. Statins in solid organ transplantation: is there an immunosuppressive effect? Am J Transplant. 2004;4:1013. doi: 10.1111/j.1600-6143.2004.00475.x. [DOI] [PubMed] [Google Scholar]
- 7.Shimizu K, Aikawa M, Takayama K, Libby P, Mitchell RN. Direct anti-inflammatory mechanisms contribute to attenuation of experimental allograft arteriosclerosis by statins. Circulation. 2003;108:2113. doi: 10.1161/01.CIR.0000092949.67153.74. [DOI] [PubMed] [Google Scholar]
- 8.Massy ZA, Guijarro C. Statins: effects beyond cholesterol lowering. Nephrol Dial Transplant. 2001;16:1738. doi: 10.1093/ndt/16.9.1738. [DOI] [PubMed] [Google Scholar]
- 9.Steffens S, Mach F. Anti-inflammatory properties of statins. Semin Vasc Med. 2004;4:417. doi: 10.1055/s-2004-869599. [DOI] [PubMed] [Google Scholar]
- 10.Larose E, Ganz P. Statins and endothelial dysfunction. Semin Vasc Med. 2004;4:333. doi: 10.1055/s-2004-869590. [DOI] [PubMed] [Google Scholar]
- 11.Bellosta S, Arnaboldi L, Gerosa L, et al. Statins effect on smooth muscle cell proliferation. Semin Vasc Med. 2004;4:347. doi: 10.1055/s-2004-869591. [DOI] [PubMed] [Google Scholar]
- 12.Kobashigawa JA. Statins and cardiac allograft vasculopathy after heart transplantation. Semin Vasc Med. 2004;4:401. doi: 10.1055/s-2004-869597. [DOI] [PubMed] [Google Scholar]
- 13.Masterson R, Hewitson T, Leikis M, Walker R, Cohney S, Becker G. Impact of statin treatment on 1-year functional and histologic renal allograft outcome. Transplantation. 2005;80:332. doi: 10.1097/01.tp.0000168941.19689.cf. [DOI] [PubMed] [Google Scholar]
- 14.Pober JS, Bothwell AL, Lorber MI, McNiff JM, Schechner JS, Tellides G. Immunopathology of human T cell responses to skin, artery and endothelial cell grafts in the human peripheral blood lymphocyte/severe combined immunodeficient mouse. Springer Semin Immunopathol. 2003;25:167. doi: 10.1007/s00281-003-0135-1. [DOI] [PubMed] [Google Scholar]
- 15.Lorber MI, Wilson JH, Robert ME, et al. Human allogeneic vascular rejection after arterial transplantation and peripheral lymphoid reconstitution in severe combined immunodeficient mice. Transplantation. 1999;67:897. doi: 10.1097/00007890-199903270-00018. [DOI] [PubMed] [Google Scholar]
- 16.Murray AG, Schechner JS, Epperson DE, et al. Dermal microvascular injury in the human peripheral blood lymphocyte reconstituted-severe combined immunodeficient (HuPBL-SCID) mouse/skin allograft model is T cell mediated and inhibited by a combination of cyclosporine and rapamycin. Am J Pathol. 1998;153:627. doi: 10.1016/S0002-9440(10)65604-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang Y, Burns WR, Tang PC, et al. Interferon-gamma plays a nonredundant role in mediating T cell-dependent outward vascular remodeling of allogeneic human coronary arteries. FASEB J. 2004;18:606. doi: 10.1096/fj.03-0840fje. [DOI] [PubMed] [Google Scholar]
- 18.Yi T, Cuchara L, Wang Y, et al. Human allograft arterial injury is ameliorated by sirolimus and cyclosporine and correlates with suppression of interferon-gamma. Transplantation. 2006;81:559. doi: 10.1097/01.tp.0000198737.12507.19. [DOI] [PubMed] [Google Scholar]
- 19.Tellides G, Pober JS. Interferon-gamma axis in graft arteriosclerosis. Circ Res. 2007;100:622. doi: 10.1161/01.RES.0000258861.72279.29. [DOI] [PubMed] [Google Scholar]
- 20.Tellides G, Tereb DA, Kirkiles-Smith NC, et al. Interferon-gamma elicits arteriosclerosis in the absence of leukocytes. Nature. 2000;403:207. doi: 10.1038/35003221. [DOI] [PubMed] [Google Scholar]
- 21.Koh KP, Wang Y, Yi T, et al. T cell-mediated vascular dysfunction of human allografts results from IFN-gamma dysregulation of NO synthase. J Clin Invest. 2004;114:846. doi: 10.1172/JCI21767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang Y, Bai Y, Qin L, et al. Interferon-gamma induces human vascular smooth muscle cell proliferation and intimal expansion by phosphatidylinositol 3-kinase dependent mammalian target of rapamycin raptor complex 1 activation. Circ Res. 2007;101:560. doi: 10.1161/CIRCRESAHA.107.151068. [DOI] [PubMed] [Google Scholar]
- 23.Ranjbaran H, Sokol SI, Gallo A, et al. An inflammatory pathway of IFN-γ production in coronary atherosclerosis. J Immunol. 2007;178:592. doi: 10.4049/jimmunol.178.1.592. [DOI] [PubMed] [Google Scholar]
- 24.Shiao SL, McNiff JM, Pober JS. Memory T cells and their costimulators in human allograft injury. J Immunol. 2005;175:4886. doi: 10.4049/jimmunol.175.8.4886. [DOI] [PubMed] [Google Scholar]
- 25.Lins RL, Matthys KE, Verpooten GA, et al. Pharmacokinetics of atorvastatin and its metabolites after single and multiple dosing in hypercholesterolaemic haemodialysis patients. Nephrol Dial Transplant. 2003;18:967. doi: 10.1093/ndt/gfg048. [DOI] [PubMed] [Google Scholar]