Summary
Background
Increased platelet activation occurs in ischemic heart disease (IHD), but increased platelet activation is also seen in cerebrovascular atherosclerosis and peripheral artery disease. It is not clear therefore whether platelet activation is an indicator of IHD or a marker of generalized atherosclerosis and inflammation. South Asian subjects are at high risk of IHD, but little is known regarding differences in platelet and leukocyte function between European and South Asian subjects.
Methods
Fifty-four male subjects (age 49-79 years) had coronary artery calcification measured by multislice computed tomography (CT), aortic atherosclerosis assessed by measurement of carotid-femoral pulse wave velocity (aortic PWV), and femoral and carotid atherosclerosis measured by B-mode ultrasound. Platelet and leukocyte activation was assessed by flow cytometry of platelet-monocyte complexes (PMC), platelet expression of PAC-1 binding site and CD62P, and expression of L-selectin on leukocytes.
Results
Elevated circulating PMC correlated significantly with elevated aortic PWV and PMC were higher in subjects with femoral plaques. In contrast PMC did not differ by increasing coronary artery calcification category or presence of carotid plaques. Higher numbers of PMC were independently related to elevated levels of C-reactive protein (CRP), higher aortic PWV, hypertension and smoking in a multivariate model. Markers of platelet and leukocyte activation did not differ significantly by ethnicity.
Conclusions
Increased PMC are related to the extent of aortic and femoral atherosclerosis rather than coronary or carotid atherosclerosis. The association between elevated CRP and increased PMC suggests that inflammation in relation to generalized atherosclerosis may play an important role in PMC activation.
Keywords: atherosclerosis, monocyte, platelet, pulse wave velocity
Introduction
Activation of platelets is thought to play a key role in the development, progression and stability of atherosclerosis [1,2]. Previous studies have observed increased platelet activation in patients with coronary artery disease or acute myocardial infarction [3-6]. However, some studies have also reported increased platelet activation in association with carotid atherosclerosis [7,8] and peripheral arterial disease [9,10] and it is not clear whether platelet activation is a consequence of atherosclerosis at a particular location (e.g. the coronary artery) or is due to the impact of generalized atherosclerosis and inflammation.
Despite the evidence linking platelet activation to atherosclerosis, there is limited evidence regarding ethnic differences in platelet activation and its relationship to atherosclerosis, and what evidence exists is inconsistent [11-14]. This relative lack of information is surprising because there are marked differences in risk of cardiovascular disease between different ethnic groups. For example, people of South Asian origin are at markedly elevated risk of ischemic heart disease (IHD) and stroke compared with individuals of European origin in the UK [15] and USA [16].
This study was undertaken to test two hypotheses: (i) that platelet/leukocyte activation would be more closely associated with aortic/femoral atherosclerosis than carotid or coronary atherosclerosis; and (ii) that platelet/leukocyte activation would be increased in subjects of South Asian origin compared with those of European ethnicity across the spectrum of atherosclerotic disease.
Methods
Study design and patient recruitment
The study was conducted as a nested sub-study within the Peripheral ARtery disease in South Asian and European men with Coronary artery disease (PARSEC) study, which was designed to assess relationships between peripheral arterial disease and coronary artery disease in people of European and South Asian descent [17]. The study population was recruited from two sources: the first consisted of a male population sample drawn from a family practice register in north-west London. Over 97% of individuals are registered with such family practices [18]. Men aged 45 years and above (European, n = 41, and South Asian, n = 43), were invited to participate. As levels of atherosclerotic disease were anticipated to be low in a sample drawn from the population, this group was enriched with men who had known coronary artery disease established by angiography. These individuals (European, n = 42, and South Asian, n = 41) were recruited from cardiology clinics at St Mary’s Hospital and Ealing Hospital in London, UK (the referral centres for the participating family practices). Men with coronary stents or atrial fibrillation were excluded, as these interfere with assessment of coronary calcification. Individuals with unstable angina, or other severe co-morbidity that restricted full participation, were also excluded from the study.
From the main study, a total of 64 men agreed to participate in a sub-study examining platelet and leukocyte function. Of these, 10 were excluded from the main analyses as they were receiving clopidogrel at the time of the study and this agent interferes with the measures of platelet activation used in this study ([19] and unpublished data). The data presented therefore pertain to the remaining 54 subjects (30 Europeans and 24 South Asians) whose characteristics are shown in Table 1. Both PARSEC and the sub-study were approved by the St Mary’s Hospital Local Research Ethics committee and all participants gave informed consent to both studies.
Table 1.
Subject characteristics
| Parameter | All subjects (n = 54) | Europeans (n = 30) | South Asians (n = 24) | P-value |
|---|---|---|---|---|
| Age, years | 64.3 ± 7.7 | 65.3 ± 7.2 | 63.0 ± 8.1 | 0.3 |
| BMI, kg m−2 | 27.2 ± 3.8 | 28.7 ± 3.9 | 25.3 ± 2.6 | < 0.001 |
| Systolic blood pressure, mmHg | 140.3 ± 15.1 | 142.6 ± 15.6 | 137.4 ± 14.1 | 0.2 |
| Diastolic blood pressure, mmHg | 83.2 ± 8.4 | 84.3 ± 8.1 | 81.9 ± 8.7 | 0.3 |
| Fasting glucose, mmol L−1 | 5.9 ± 1.4 | 5.9 ± 1.2 | 5.8 ± 1.7 | 0.8 |
| HbA1c, % | 6.0 ± 0.9 | 5.8 ± 0.6 | 6.3 ± 1.1 | 0.048 |
| Total cholesterol, mmol L−1 | 4.3 ± 1.0 | 4.2 ± 1.0 | 4.4 ± 1.1 | 0.7 |
| HDL cholesterol, mmol L−1 | 1.1 ± 0.3 | 1.1 ± 0.3 | 1.1 ± 0.3 | 0.9 |
| Fasting triglyceride, mmol L−1 | 1.2 (0.9–1.7) | 1.2 (0.9–1.8) | 1.2 (1.0–1.7) | 0.7 |
| CRP, mmol L−1 | 1.9 (0.9–3.76) | 2.1 (1.1–4.8) | 1.3 (0.9–3.0) | 0.08 |
| Coronary calcification, Hounsfield Units | 214 (12–768) | 241 (35–697) | 134 (12–768) | 0.3 |
| Aortic PWV, m s−1 | 17.5 ± 5.3 | 16.8 ± 5.5 | 18.3 ± 5.1 | 0.3 |
| Femoral plaque, n (%) | 28 (52) | 18 (60) | 10 (42) | 0.2 |
| Carotid plaque, n (%) | 13 (24) | 9 (30) | 4 (17) | 0.3 |
| Ischemic heart disease, n (%) | 27 (50) | 14 (47) | 13 (54) | 0.6 |
| Diabetes mellitus, n (%) | 9 (17) | 3 (10) | 6 (26) | 0.1 |
| Hypertension, n (%) | 20 (37) | 8 (27) | 12 (50) | 0.08 |
| Smokers, n (%) | 26 (55) | 19 (68) | 7 (37) | 0.04 |
| Subjects on statins, n (%) | 28 (52) | 16 (53) | 12 (50) | 0.8 |
| Subjects on aspirin, n (%) | 30 (56) | 18 (60) | 12 (50) | 0.5 |
BMI, body mass index; HDL, high density lipoprotein; CRP, C-reactive protein; PWV, pulse wave velocity.
Data are means ± SD, medians (interquartile range) or frequency (%). P-value is derived from a Student’s t-test comparing the two ethnic groups.
Study investigations
Angina symptoms were ascertained by patients’ self-completion of the Rose angina questionnaire [20]. Measures of height, weight and other anthropomorphic parameters were performed as previously described [17]. A resting ECG was also performed and sitting brachial systolic and diastolic blood pressure and heart rate (HR) were measured as the average of three readings made using a validated automatic BP device (Omron 705CP; Omron Healthcare Inc., Vernon Hills, IL, USA). Hypertension was defined as blood pressure > 140/90 mmHg or use of antihypertensive agents. Fasting bloods were also taken for quantification of serum glucose, total and high density lipoprotein (HDL) cholesterol, triglycerides and C-reactive protein (CRP). CRP was measured with a highly sensitive sandwich enzyme immunoassay, using rabbit anti-human CRP immunoglobulin as a catching and detecting antibody (Dako, Copenhagen, Denmark).
Assessment of atherosclerosis
Coronary calcification
All subjects underwent coronary computed tomography (CT) scanning using either a Philips M ×8000 4-detector multislice CT scanner, or a Philips M ×8000 IDT 16-detector multislice CT scanner. To ensure similar imaging between machines, identical scanning parameters were used in all subjects. Contiguous 3-mm sections covering the whole heart were acquired using prospective ECG gating during suspended inspiration. Scans were acquired with 120 kV, 140 mAs, field of view of 250 mm, and display matrix 512 × 512. Coronary artery calcification was quantified using proprietary software on a Philips MxView computer workstation, and calcification was defined as an area > 1mm2 of density > 130 Hounsfield Units (HU). The coronary artery calcification score was calculated as the sum of all lesion scores. Coronary calcification scores are highly skewed and therefore for the purposes of analysis subjects were subdivided into tertiles of coronary calcification score using cut-off points of 0-49, 50-400 and > 400 HU. A single observer (ARW) made all coronary calcification measurements. A repeatability study of 20 scans showed a mean difference ± SD of difference between cycles of 1 ± 10 HU.
Ultrasound investigations
B mode, color and pulsed Doppler measurements were performed in all subjects using an HDI 5000 ultrasound machine (Philips, Bothell, WA, USA) equipped with a linear array broad-band 7-4 MHz transducer. Atherosclerotic plaques (defined as focal thickenings of the intima-media > 1.3mm or a distinct area with an IMT > 50% thicker than the adjacent region) [21] were identified in the carotid and femoral artery using B mode ultrasound. Color Doppler and pulsed Doppler were also used to assist in confirmation of possible plaques.
Aortic PWV, an indicator of aortic atherosclerosis, was measured using ECG-gated pulsed Doppler, as previously described [22]. The reproducibility of PWV was studied in six subjects and the mean ± SD intra-observer difference was 0.39 ± 0.9 m s−1.
Assessment of platelet and leukocyte activation
Peripheral venous blood was collected into siliconized Vacutainer tubes containing trisodium citrate (Becton Dickinson, Oxford, UK) by clear venepuncture with minimal stasis according to the European Working Group on Clinical Cell Analysis: Consensus Protocol for the Flow Cytometric Characterization of Platelet Function [23].
Platelet activation was assessed by measurement of platelet-monocyte complexes (PMC) and the platelet activation markers, PAC-1 binding and p-selectin (CD62P) in unstimulated whole blood. PAC-1 binding and CD62P were also measured following activation with adenosine diphosphate (10 μm; ADP) for 2 min. Measurement of L-selectin was performed only on unstimulated samples.
For measurement of PAC-1 binding and CD62P, samples of citrated whole blood were incubated with the following monoclonal antibodies (mAb): peridinin chlorophyll protein (PerCP)-conjugated anti-CD61, fluorescein isothiocyanate (FITC)-conjugated PAC-1 and phycoerythrin (PE)-conjugated anti-CD62P at room temperature for 15 min according to the manufacturers’ recommendations. IgG1-PE and IgG1-FITC were utilized for non-specific binding isotype controls and the specific inhibitor of PAC-1 binding to the platelet surface, Arg-Gly-Asp-Ser peptide (RGDS; 10 mg mL−1) was included in controls for PAC-1. Samples for platelet activation tests were then fixed with 0.1% paraformaldehyde (CellFIX, BD Biosciences, Oxford, UK) and kept in the dark at 4°C prior to analysis.
Samples for PMC were incubated with PE-conjugated anti-CD14 + FITC-conjugated anti-CD42a and samples for leukocyte activation studies were incubated with PE-conjugated anti-CD62L. PE-CD14 + FITC-IgG1 and IgG1-PE acted as controls for PMC studies and leukocyte studies, respectively. After incubation samples were treated with erythrocyte lysis solution (FACS Lysing Solution, Becton Dickinson) at room temperature for 15 min and then centrifuged (900 × g for 4 min) and washed with Cell Wash (BD Biosciences) twice. After the second wash the pellet was resuspended in 0.5 mL of Cell Wash. All samples were stored in the dark at 4°C until analysis, which was performed within 60 min of blood withdrawal.
Flow cytometric analysis
Samples were analyzed in a Becton Dickinson FACScan flow cytometer with cellquest software (Becton Dickinson Immunocytometry Systems, Oxford, UK) by ARW (OD) masked to patient identity. The flow cytometer was calibrated regularly using BD facscomp software with BD CaliBRITE beads. Platelets were identified on the basis of their light scatter characteristics and positive staining for the GPIIIa subunit of the GPIIb/IIIa complex (PerCP-CD61). Gating was set so that only fluorescence positive events were analyzed and platelets positive for PAC-1 binding and CD62P expression were calculated as the percentage of the CD61-positive population. PMC formation was quantified within the monocyte population as the percentage of events positive for both a platelet-specific marker, CD42a, and a monocyte marker, CD14. L-selectin expression, a marker of neutrophil activation, was quantified as the average mean fluorescence intensity (MFI). More than 5000 activation-independent platelet events were acquired for each assay and the coefficients of intra- and inter-assay variability were < 15%.
Reagents
ADP was purchased from Roche Applied Science (Indiana-polis, IN, USA); all mAb were purchased from BD Biosciences; and RGDS and other reagents were purchased from Sigma (Poole, UK).
Statistical analysis
Statistical power calculations were conducted using estimates of effect size based on previously published data [24,25], with a significance level of 5% and 80% power. On this basis, in order to detect a correlation (r ≥ 0.4) between aortic PWV and PMC, CD62P or PAC-1 binding, a minimum total of 46 subjects was required. To detect a difference of ≥ 25% in PMC, CD46P and PAC-1 binding between subjects with and without carotid and femoral plaque, at least 34 subjects were required, and to detect an increase ≥ 15% in PMC, CD62P or PAC-1 binding at each level of coronary artery calcification category, a minimum of 45 subjects were required. All analyses were conducted using stata (version 9.2, Stata Corp LP, College Station, TX, USA). Subject characteristics are presented as means ± SD and results as means (95% CI). Skewed data were log transformed prior to statistical analysis and are presented as medians (interquartile range). Statistical comparisons were made using analysis of covariance with age as a prespecified covariate. Additional exploratory analyses were undertaken using multiple linear regression. A P-value of < 0.05 was considered statistically significant.
Results
Platelet/leukocyte activation and regional atherosclerosis
Circulating PMC correlated positively and significantly with aortic PWV (Fig. 1) and this relationship was also evident after statistical adjustment for age (Table 2). There were no significant relationships between PAC-1 binding, CD62P or L-selectin and aortic PWV (Table 2).
Fig. 1.
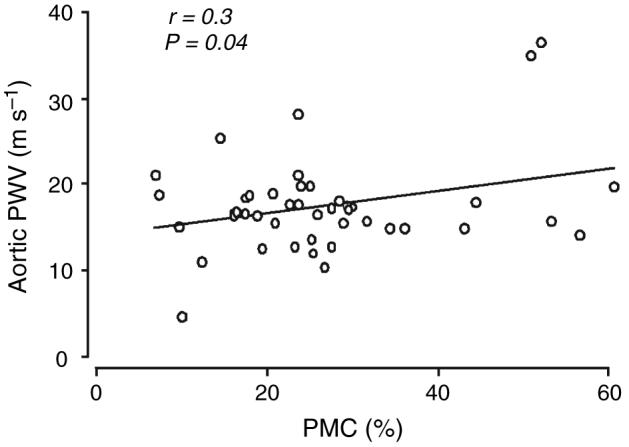
Relationship between platelet-monocyte complexes and carotid-femoral pulse wave velocity (aortic PWV).
Table 2.
Relationship of platelet leukocyte measures to aortic pulse wave velocity
| β coefficient (SE) | P-value | |
|---|---|---|
| PMC | 0.92 (0.39) | 0.02 |
| PAC-1 | 0.40 (0.40) | 0.3 |
| PAC-1 + ADP | 0.26 (3.5) | 0.9 |
| CD62P | −0.01 (0.03) | 0.8 |
| CD62P + ADP | 0.56 (0.31) | 0.08 |
| CD61 | 0.20 (0.14) | 0.14 |
| L-selectin | −2.3 (2.5) | 0.4 |
PMC, platelet-monocyte complexes; PAC-1, PAC-1 binding.
Data are regression (β) coefficients and SE of age-adjusted data.
PMC were also significantly elevated in subjects with femoral plaques compared with subjects without femoral plaques (Table 3). PAC-1 binding, CD62P and L-selectin did not differ significantly between subjects with and without femoral atherosclerosis.
Table 3.
Platelet and leukocyte measures in subjects with and without femoral and carotid plaques
| Femoral (%) | Without plaque (n = 26) | With plaque (n = 28) | P-value |
|---|---|---|---|
| PMC | 21.8 (18.1–25.5) | 30.0 (23.7–36.3) | 0.04 |
| PAC-1 | 14.4 (8.7–20.1) | 18.4 (13.1–23.7) | 0.3 |
| PAC-1 + ADP | 82.0 (75.7–88.3) | 84.3 (78.0–90.6) | 0.6 |
| CD62P | 1.7 (1.3–2.1) | 2.0 (1.6–2.4) | 0.4 |
| CD62P + ADP | 81.4 (77.1–85.7) | 82.9 (78.6–87.2) | 0.8 |
| L-selectin (mean fluorescence intensity) | 201 (169.6–232.4) | 195 (165.6–224.4) | 0.8 |
| Carotid (%) |
Without plaque (n = 41) |
With plaque (n = 13) |
|
|---|---|---|---|
| PMC | 27.1 (22.6–31.6) | 22.2 (14.6–29.8) | 0.3 |
| PAC-1 | 14.3 (10.0–18.6) | 23.1 (15.5–30.7) | 0.06 |
| PAC-1 + ADP | 82.1 (77.0–87.2) | 86.3 (77.7–94.9) | 0.4 |
| CD62P | 2.0 (1.6–2.4) | 1.5 (0.9–2.1) | 0.2 |
| CD62P + ADP | 76.2 (70.1–82.3) | 82.2 (72.0–92.4) | 0.3 |
| L-selectin (mean fluorescence intensity) | 210.6 (187.3–233.9) | 153.5 (112.5–194.5) | 0.02 |
PMC, platelet-monocyte complexes; PAC-1, PAC-1 binding.
Data are means (95% CI) of age-adjusted data. P-values were calculated by ancova.
PMC and other markers of platelet activation did not differ with increasing coronary artery calcification category or with respect to presence of plaques in the carotid artery (Tables 3 and 4). L-selectin did not differ by coronary artery calcification category, but was reduced in subjects with carotid plaques.
Table 4.
Platelet and leukocyte measures by coronary artery calcification category
| Lowest CAC category (17) | Middle CAC category (15) | Highest CAC category (22) | P-value | |
|---|---|---|---|---|
| PMC | 26.8 (20.1–33.5) | 25.9 (22.0–29.8) | 25.0 (18.9–31.1) | 0.7 |
| PAC-1 | 15.2 (8.7–21.7) | 16.4 (12.5–20.3) | 17.7 (11.8–23.6) | 0.6 |
| PAC-1 + ADP | 86.6 (79.2–94.0) | 83.5 (79.2–87.8) | 80.5 (74.0–87.0) | 0.3 |
| CD62P | 1.7 (1.1–2.3) | 1.8 (1.4–2.2) | 2.0 (1.6–2.4) | 0.4 |
| CD62P + ADP | 85.5 (80.2–90.8) | 82.6 (79.7–85.5) | 79.7 (75.4–84.0) | 0.14 |
| CD62L | 181 (146–216) | 197 (175–219) | 212 (181–243) | 0.2 |
PMC, platelet-monocyte complexes; PAC-1, PAC-1 binding.
Data are means (95% CI) of age-adjusted data. P-values were calculated by ancova.
Platelet/leukocyte measures and ethnicity
Subjects of South Asian origin had lower body mass index (BMI), increased HbA1c and were less likely to smoke, otherwise the two groups did not differ significantly (Table 1). Platelet/leukocyte measures did not differ significantly between the two ethnic groups (Table 5), although L-selectin tended to be higher in subjects of South Asian origin. Interethnic differences remained non-significant after further statistical adjustment for BMI, HbA1c and smoking (data not shown).
Table 5.
Platelet and leukocyte measures in European and South Asian men
| European (30) | South Asian (24) | P-value | |
|---|---|---|---|
| PMC | 24.2 (18.9–29.5) | 27.7 (22.0–33.4) | 0.4 |
| PMC + ADP | 34.7 (27.4–42.0) | 38.2 (30.4–46.0) | 0.5 |
| PAC-1 | 16.7 (11.4–22.0) | 16.2 (10.3–22.1) | 0.9 |
| PAC-1 + ADP | 84.4 (78.5–90.3) | 81.6 (75.1–88.1) | 0.5 |
| CD62P | 1.8 (1.4–2.2) | 2.0 (1.6–2.4) | 0.5 |
| CD62 + ADP | 80.5 (76.2–84.8) | 83.9 (79.6–88.2) | 0.3 |
| CD62L | 181 (152–210) | 218 (187–249) | 0.09 |
PMC, platelet-monocyte complexes; PAC-1, PAC-1 binding.
Data are means ± SEM of age adjusted data. P-values were calculated by ancova.
Predictors of elevated PMC
CRP was closely associated with increased PMC (r = 0.44, P < 0.001). Hypertension was also associated with an increase in PMC (normotensive = 28.6 ± 2.6, hypertensive = 39.0 ± 3.4, P = 0.019). Stepwise multivariate regression modeling (including age, total cholesterol, HDL-cholesterol, triglycerides, creatinine, fasting glucose, HbA1c, BMI, CRP, presence of femoral or carotid plaques, coronary artery calcification category, carotid and femoral IMT, known IHD, hypertension and diabetes status) showed that CRP, PWV, hypertension and smoking were independent significant predictors of PMC (Table 6), and after adjustment these accounted for 51% of the variance in PMC.
Table 6.
Independent predictors of platelet-monocyte complexes (PMC)
| Variable | Std. Beta | β coefficient (SE) | P-value |
|---|---|---|---|
| C-reactive protein (CRP) | 0.54 | 0.72 (0.17) | < 0.001 |
| Aortic pulse wave velocity (PWV) | 0.43 | 0.96 (0.28) | 0.002 |
| Hypertension | 0.39 | 10.3 (3.7) | 0.009 |
| Smoking | 0.30 | 7.9 (3.3) | 0.023 |
| Constant | 54.0 (14.5) | 0.001 |
Adjusted r2 = 0.51; P < 0.001.
Age, serum total cholesterol, high density lipoprotein cholesterol, triglycerides, creatinine, fasting glucose, HbA1c, body mass index, CRP, presence of femoral or carotid plaques, coronary artery calcification category, carotid and femoral intima media thickness, aortic PWV, diabetes, hypertension and smoking status were entered into a forward stepwise regression model. The significance level for retention in the model was set at P = 0.05. Data shown are the standardized beta coefficients (Std. beta), the regression coefficient (β coefficient) with SE and the relevant P-value.
Discussion
This study shows that increased PMC, a sensitive measure of platelet activation in vivo, is closely associated with increased aortic and femoral atherosclerosis, but is not significantly related to the extent of carotid and coronary atherosclerosis. We also observed that increased CRP (along with aortic PWV, hypertension and smoking) was an independent predictor of elevated circulating PMC in multivariate analyses, suggesting that enhanced PMC formation could be related to increased inflammation in association with atherosclerosis.
Aortic PWV has been shown to correlate closely with aortic atherosclerosis [26,27] and to predict fatal and non-fatal cardiovascular events, including IHD and strokes[28-30], while the presence of femoral plaques on ultrasound is a good measure of lower limb atherosclerosis [31]. Our observations showing that PMC are related to these measures of aortic and femoral atherosclerosis suggest that formation of PMC may be an indicator of the overall burden of atherosclerosis rather than atherosclerosis in a particular territory. While atherosclerosis is a generalized disease affecting all territories [32], it is also well recognized that the burden of atherosclerosis in an individual varies substantially [33,34], with carotid and coronary atherosclerosis tending to show closer inter-associations. Such regional differences in atherosclerosis may account for the lack of association between PMC and carotid and coronary atherosclerosis seen in our study. The aorta and femoral arteries are likely to make a much greater contribution to total atherosclerosis load than the coronary and carotid arteries, as they have a greater surface area and atherosclerosis develops earlier and more extensively at these sites [35,36]. To explore possible mechanisms linking atherosclerosis to increased PMC we performed multivariate analyses. These analyses have limitations: the sample size is relatively small and therefore may be underpowered to identify weak predictors of PMC or interactions between covariates; the use of hypertension and smoking status as categorical variables is likely to underestimate blood pressure and smoking exposures. However, current blood pressure on treatment is probably an even less satisfactory measure of lifetime blood pressure exposure and other measures of smoking exposure (e.g. pack years and cotinine) were not available to us. Despite their limitations, these exploratory analyses do suggest that inflammation, as assessed by serum CRP, could play a role in mediating the increase in PMC seen in association with atherosclerosis.
Our observation of only weak and non-significant relationships between other markers of platelet activation, PAC-1 binding and CD62P, and atherosclerosis is consistent with previous studies suggesting that PMC may be a more sensitive marker of platelet activation than PAC-1 and CD62P in vivo [4,5,24,37]. Platelet activation is associated with an initial increase in CD62P [38] and this results in rapid binding of platelets to monocytes, neutrophils and endothelium via an interaction with a constitutively expressed receptor, PSGL-1. This process will deplete the number of circulating unbound CD62P-positive platelets. In addition, degranulated platelets rapidly lose surface P-selectin, although they continue to circulate and function [39]. Both these mechanisms are likely to result in a tendency for platelet CD62P to underestimate the extent of platelet activation.
Although some studies have shown that platelet activation is increased in IHD [3,4,6], findings have not been consistent [40,41] and, in keeping with our observations, another study found no relation between the extent of coronary disease and platelet microparticles, although platelet microparticles were increased in patients with IHD compared with healthy controls[6]. Previous studies have also found conflicting evidence of increased platelet activation in subjects with carotid atherosclerosis [8,42,43]. Possible explanations for differences between studies include methodological differences in assessment of platelet activation status, the impact of ischemia or plaque instability on platelet activation and, on the basis of our data, variability in the overall extent of atherosclerotic disease.
It is possible that differential use of medication (e.g. statins, anti-platelet drugs other than clopidogrel) could have influenced our results. Use of aspirin and statins was predictably more common in subjects with carotid, femoral and coronary artery disease, but comparison of PMC in those receiving statin or aspirin showed no statistically significant difference in PMC (data not shown). While we cannot exclude an effect of therapy on PMC, it seems unlikely to account for our findings.
We also found that markers of platelet and leukocyte activation did not differ significantly by ethnicity and while our sample size is relatively small our observations do not suggest that there are major differences in platelet activation that are likely to account for the marked differences in risk of myocardial infarction and stroke in South Asian individuals.
In conclusion, increased circulating platelet-monocyte activation is closely related to the extent of aortic and femoral atherosclerosis rather than coronary or carotid atherosclerosis. The relationship between elevated CRP and increased platelet-monocyte activation suggests that inflammation in the context of generalized atherosclerosis may play an important role in this process.
Acknowledgements
The study was supported by a grant from the British Heart Foundation and OD was in receipt of a Fellowship from the European Society of Cardiology. We would like to thank J Kooner, E Coady and S Raynor, Imperial College London, UK, for their assistance with the study and C Stehouwer, Maastricht University, The Netherlands, for his help with measurement of C-reactive protein.
Footnotes
Disclosure of Conflict of Interests
The authors state that they have no conflict of interest.
References
- 1.Libby P. Changing concepts of atherogenesis. J Intern Med. 2000;247:349–58. doi: 10.1046/j.1365-2796.2000.00654.x. [DOI] [PubMed] [Google Scholar]
- 2.Huo Y, Ley KF. Role of platelets in the development of atherosclerosis. Trends Cardiovasc Med. 2004;14:18–22. doi: 10.1016/j.tcm.2003.09.007. [DOI] [PubMed] [Google Scholar]
- 3.FitzGerald DJ, Roy L, Catella F, Fitzgerald GA. Platelet activation in unstable coronary disease. N Engl J Med. 1986;315:983–9. doi: 10.1056/NEJM198610163151602. [DOI] [PubMed] [Google Scholar]
- 4.Furman MI, Benoit SE, Barnard MR, Valeri CR, Borbone ML, Becker RC, Hechtman HB, Michelson AD. Increased platelet reactivity and circulating monocyte-platelet aggregates in patients with stable coronary artery disease. J Am Coll Cardiol. 1998;31:352–8. doi: 10.1016/s0735-1097(97)00510-x. [DOI] [PubMed] [Google Scholar]
- 5.Furman MI, Barnard MR, Krueger LA, Fox ML, Shilale EA, Lessard DM, Marchese P, Frelinger AL, III, Goldberg RJ, Michelson AD. Circulating monocyte-platelet aggregates are an early marker of acute myocardial infarction. J Am Coll Cardiol. 2001;38:1002–6. doi: 10.1016/s0735-1097(01)01485-1. [DOI] [PubMed] [Google Scholar]
- 6.Tan KT, Tayebjee MH, Macfadyen RJ, Lip GY, Blann AD. Elevated platelet microparticles in stable coronary artery disease are unrelated to disease severity or to indices of inflammation. Platelets. 2005;16:368–71. doi: 10.1080/00207230500120401. [DOI] [PubMed] [Google Scholar]
- 7.Koyama H, Maeno T, Fukumoto S, Shoji T, Yamane T, Yokoyama H, Emoto M, Shoji T, Tahara H, Inaba M, Hino M, Shioi A, Miki T, Nishizawa Y. Platelet P-selectin expression is associated with atherosclerotic wall thickness in carotid artery in humans. Circulation. 2003;108:524–9. doi: 10.1161/01.CIR.0000081765.88440.51. [DOI] [PubMed] [Google Scholar]
- 8.Shoji T, Koyama H, Fukumoto S, Maeno T, Yokoyama H, Shinohara K, Emoto M, Shoji T, Yamane T, Hino M, Shioi A, Nishizawa Y. Platelet activation is associated with hypoadiponectinemia and carotid atherosclerosis. Atherosclerosis. 2006;188:190–5. doi: 10.1016/j.atherosclerosis.2005.10.034. [DOI] [PubMed] [Google Scholar]
- 9.Ruf A, Patscheke H. Flow cytometric detection of activated platelets: comparison of determining shape change, fibrinogen binding, and P-selectin expression. Semin Thromb Hemost. 1995;21:146–51. doi: 10.1055/s-2007-1000389. [DOI] [PubMed] [Google Scholar]
- 10.Gresele P, Catalano M, Giammarresi C, Volpato R, Termini R, Ciabattoni G, Nenci GG, Davi G. Platelet activation markers in patients with peripheral arterial disease - a prospective comparison of different platelet function tests. Thromb Haemost. 1997;78:1434–7. [PubMed] [Google Scholar]
- 11.McKeigue PM, Shah B, Marmot MG. Relation of central obesity and insulin resistance with high diabetes prevalence and cardiovascular risk in South Asians. Lancet. 1991;337:382–6. doi: 10.1016/0140-6736(91)91164-p. [DOI] [PubMed] [Google Scholar]
- 12.Markovitz JH, Kulkarni K, Goldschmidt-Clermont P, Kiefe CI, Rustagi P, Sekar P, Nanda N. Increased platelet activation and fibrinogen in Asian Indians. Potential implications for coronary risk. Eur Heart J. 1998;19:720–6. doi: 10.1053/euhj.1997.0800. [DOI] [PubMed] [Google Scholar]
- 13.Whincup PH, Gilg JA, Papacosta O, Seymour C, Miller GJ, Alberti KG, Cook DG. Early evidence of ethnic differences in cardiovascular risk: cross sectional comparison of British South Asian and white children. BMJ. 2002;324:635. doi: 10.1136/bmj.324.7338.635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McKeigue PM, Marmot MG, Syndercombe Court YD, Cottier DE, Rahman S, Riemersma RA. Diabetes, hyperinsulinaemia and coronary risk factors in Bangladeshis in East London. Br Heart J. 1988;60:390–6. doi: 10.1136/hrt.60.5.390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wild S, McKeigue P. Cross sectional analysis of mortality by country of birth in England and Wales, 1970-92. BMJ. 1997;314:705–10. doi: 10.1136/bmj.314.7082.705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Enas EA, Garg A, Davidson MA, Nair VM, Huet BA, Yusuf S. Coronary heart disease and its risk factors in first-generation immigrant Asian Indians to the United States of America. Indian Heart J. 1996;48:343–53. [PubMed] [Google Scholar]
- 17.Chaturvedi N, Coady E, Mayet J, Wright AR, Shore AC, Byrd S, McG Thom SA, Kooner JS, Schalkwijk CG, Hughes AD. Indian Asian men have less peripheral arterial disease than European men for equivalent levels of coronary disease. Atherosclerosis. 2007;193:204–12. doi: 10.1016/j.atherosclerosis.2006.06.017. [DOI] [PubMed] [Google Scholar]
- 18.Bone M. Registration with General Medical Practitioners in Inner London: A Survey Carried Out on Behalf of the Department of Health and Social Security. HMSO; London: 1984. [Google Scholar]
- 19.Klinkhardt U, Bauersachs R, Adams J, Graff J, Lindhoff-Last E, Harder S. Clopidogrel but not aspirin reduces P-selectin expression and formation of platelet-leukocyte aggregates in patients with atherosclerotic vascular disease. Clin Pharmacol Ther. 2003;73:232–41. doi: 10.1067/mcp.2003.13. [DOI] [PubMed] [Google Scholar]
- 20.Rose GA. Chest pain questionnaire. Milbank Mem Fund Q. 1965;43:32–9. [PubMed] [Google Scholar]
- 21.Wendelhag I, Wiklund O, Wikstrand J. Atherosclerotic changes in the femoral and carotid arteries in familial hypercholesterolemia. Ultrasonographic assessment of intima-media thickness and plaque occurrence. Arterioscler Thromb. 1993;13:1404–11. doi: 10.1161/01.atv.13.10.1404. [DOI] [PubMed] [Google Scholar]
- 22.Tillin T, Chambers J, Malik I, Coady E, Byrd S, Mayet J, Wright AR, Kooner J, Shore A, Thom S, Chaturvedi N, Hughes A. Measurement of pulse wave velocity: site matters. J Hypertens. 2007;25:383–9. doi: 10.1097/HJH.0b013e3280115bea. [DOI] [PubMed] [Google Scholar]
- 23.Schmitz G, Rothe G, Ruf A, Barlage S, Tschope D, Clemetson KJ, Goodall AH, Michelson AD, Nurden AT, Shankey TV. European Working Group on Clinical Cell Analysis: consensus protocol for the flow cytometric characterisation of platelet function. Thromb Haemost. 1998;79:885–96. [PubMed] [Google Scholar]
- 24.Michelson AD, Barnard MR, Krueger LA, Valeri CR, Furman MI. Circulating monocyte-platelet aggregates are a more sensitive marker of in vivo platelet activation than platelet surface P-selectin: studies in baboons, human coronary intervention, and human acute myocardial infarction. Circulation. 2001;104:1533–7. doi: 10.1161/hc3801.095588. [DOI] [PubMed] [Google Scholar]
- 25.Nomura S, Inami N, Iwasaka T, Liu Y. Platelet activation markers, microparticles and soluble adhesion molecules are elevated in patients with arteriosclerosis obliterans: therapeutic effects by cilostazol and potentiation by dipyridamole. Platelets. 2004;15:167–72. doi: 10.1080/09537100410001682779. [DOI] [PubMed] [Google Scholar]
- 26.Farrar DJ, Green HD, Bond MG, Wagner WD, Gobbee RA. Aortic pulse wave velocity, elasticity, and composition in a nonhuman primate model of atherosclerosis. Circ Res. 1978;43:52–62. doi: 10.1161/01.res.43.1.52. [DOI] [PubMed] [Google Scholar]
- 27.Sawabe M, Takahashi R, Matsushita S, Ozawa T, Arai T, Hamamatsu A, Nakahara K, Chida K, Yamanouchi H, Murayama S, Tanaka N. Aortic pulse wave velocity and the degree of atherosclerosis in the elderly: a pathological study based on 304 autopsy cases. Atheroscler. 2005;179:345–51. doi: 10.1016/j.atherosclerosis.2004.09.023. [DOI] [PubMed] [Google Scholar]
- 28.Laurent S, Katsahian S, Fassot C, Tropeano AI, Gautier I, Laloux B, Boutouyrie P. Aortic stiffness is an independent predictor of fatal stroke in essential hypertension. Stroke. 2003;34:1203–6. doi: 10.1161/01.STR.0000065428.03209.64. [DOI] [PubMed] [Google Scholar]
- 29.Willum-Hansen T, Staessen JA, Torp-Pedersen C, Rasmussen S, Thijs L, Ibsen H, Jeppesen J. Prognostic value of aortic pulse wave velocity as index of arterial stiffness in the general population. Circ. 2006;113:664–70. doi: 10.1161/CIRCULATIONAHA.105.579342. [DOI] [PubMed] [Google Scholar]
- 30.Sutton-Tyrrell K, Najjar SS, Boudreau RM, Venkitachalam L, Kupelian V, Simonsick EM, Havlik R, Lakatta EG, Spurgeon H, Krit-chevsky S, Pahor M, Bauer D, Newman A. Elevated aortic pulse wave velocity, a marker of arterial stiffness, predicts cardiovascular events in well-functioning older adults. Circ. 2005;111:3384–90. doi: 10.1161/CIRCULATIONAHA.104.483628. [DOI] [PubMed] [Google Scholar]
- 31.Suurkula M, Fagerberg B, Wendelhag I, Agewall S, Wikstrand J, Risk Intervention Study (RIS) Group Atherosclerotic disease in the femoral artery in hypertensive patients at high cardiovascular risk. The value of ultrasonographic assessment of intima-media thickness and plaque occurrence. Arterioscler Thromb Vasc Biol. 1996;16:971–7. doi: 10.1161/01.atv.16.8.971. [DOI] [PubMed] [Google Scholar]
- 32.Megnien JL, Sene V, Jeannin S, Hernigou A, Plainfosse MC, Merli I, Atger V, Moatti N, Levenson J, Simon A, The PCV METRA Group Coronary calcification and its relation to extracoronary atherosclerosis in asymptomatic hypercholesterolemic men. Circ. 1992;85:1799–807. doi: 10.1161/01.cir.85.5.1799. [DOI] [PubMed] [Google Scholar]
- 33.Hulthe J, Wikstrand J, Emanuelsson H, Wiklund O, de-Feyter PJ, Wendelhag I. Atherosclerotic changes in the carotid artery bulb as measured by B-mode ultrasound are associated with the extent of coronary atherosclerosis. Stroke. 1997;28:1189–94. doi: 10.1161/01.str.28.6.1189. [DOI] [PubMed] [Google Scholar]
- 34.Giral P, Pithois MI, Filitti V, Levenson J, Plainfosse MC, Mainardi C, Simon AC, Prevention Cardio-Vasculaire en Medecine du Travail METRA Group Risk factors and early extracoronary atherosclerotic plaques detected by three-site ultrasound imaging in hypercholesterolemic men. Arch Intern Med. 1991;151:950–6. [PubMed] [Google Scholar]
- 35.McGill HC, Jr, McMahan CA, Pathobiological Determinants of Atherosclerosis in Youth (PDAY) Research Group Determinants of atherosclerosis in the young. Am J Cardiol. 1998;82:30T–6T. doi: 10.1016/s0002-9149(98)00720-6. [DOI] [PubMed] [Google Scholar]
- 36.Kroger K, Kucharczik A, Hirche H, Rudofsky G. Atherosclerotic lesions are more frequent in femoral arteries than in carotid arteries independent of increasing number of risk factors. Angiology. 1999;50:649–54. doi: 10.1177/000331979905000805. [DOI] [PubMed] [Google Scholar]
- 37.McCabe DJ, Harrison P, Mackie IJ, Sidhu PS, Purdy G, Lawrie AS, Watt H, Machin SJ, Brown MM. Increased platelet count and leucocyte-platelet complex formation in acute symptomatic compared with asymptomatic severe carotid stenosis. J Neurol Neurosurg Psychiatry. 2005;76:1249–54. doi: 10.1136/jnnp.2004.051003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stenberg PE, McEver RP, Shuman MA, Jacques YV, Bainton DF. A platelet alpha-granule membrane protein (GMP-140) is expressed on the plasma membrane after activation. J Cell Biol. 1985;101:880–6. doi: 10.1083/jcb.101.3.880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Michelson AD, Barnard MR, Hechtman HB, MacGregor H, Connolly RJ, Loscalzo J, Valeri CR. In vivo tracking of platelets: circulating degranulated platelets rapidly lose surface P-selectin but continue to circulate and function. Proc Natl Acad Sci USA. 1996;93:11877–82. doi: 10.1073/pnas.93.21.11877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Knight CJ, Panesar M, Wright C, Clarke D, Butowski PS, Patel D, Patrineli A, Fox K, Goodall AH. Altered platelet function detected by flow cytometry. Effects of coronary artery disease and age. Arterioscler Thromb Vasc Biol. 1997;17:2044–53. doi: 10.1161/01.atv.17.10.2044. [DOI] [PubMed] [Google Scholar]
- 41.McBane RD, Karnicki K, Tahirkheli N, Miller RS, Owen WG. Platelet characteristics associated with coronary artery disease. J Thromb Haemost. 2003;1:1296–303. doi: 10.1046/j.1538-7836.2003.00183.x. [DOI] [PubMed] [Google Scholar]
- 42.Doruk H, Kaptan K, Saglam M, Ateskan U, Beyan C, Nevruz O, Mas MR, Kutlu M, Kocar IH. The relationship between carotid atherosclerosis and platelet aggregation in elderly. Arch Gerontol Geriatr. 2003;37:235–9. doi: 10.1016/s0167-4943(03)00050-5. [DOI] [PubMed] [Google Scholar]
- 43.Shimizu M, Kohara S, Yamamoto M, Ando Y, Haida M, Shinohara Y. Significant relationship between platelet activation and intra-media thickness of the carotid artery in patients with ischemic cerebrovascular disease. Thromb Res. 2006;117:647–52. doi: 10.1016/j.thromres.2005.05.009. [DOI] [PubMed] [Google Scholar]


