Abstract
Specification of neural crest progenitors begins during gastrulation at the neural plate border, long before migration or differentiation. Neural crest cell fate is acquired by progressive activation of discrete groups of transcription factors that appear to be highly conserved in vertebrates; however, comprehensive analysis of their expression has been lacking in chick, an important model system for neural crest development. To address this, we analyzed expression of ten transcription factors that are known specifiers of neural plate border and neural crest fate and compared them across developmental stages from gastrulation to neural crest migration. Surprisingly, we find that most neural crest specifiers are expressed during gastrulation in chick, concomitant with and in similar domains as neural plate border specifiers. This suggests that interactions between these molecules may occur much earlier than previously thought, an important consideration for interpretation of functional studies.
Keywords: chick neural crest, neural plate border, gene regulatory network, NC-GRN
INTRODUCTION
Neural crest cells are a transient, multipotent population of migratory cells that arise during development in dorsal neural tissue. After emigrating from the neural tube, they travel extensively throughout the body to form diverse derivatives in the periphery (Sauka-Spengler and Bronner-Fraser, 2008). Many of the processes and molecules that govern neural crest induction, commitment, migration and differentiation have been uncovered over the past several decades through experimentation on a number of vertebrate models. In particular, the Xenopus embryo has been invaluable to our understanding of neural crest induction and underlying gene cascades. In addition, genetic studies using mouse and zebrafish have resolved many of the molecular interactions operating during neural crest development. Due to its easy accessibility to manipulation as well as optical clarity, the chick embryo has added much to our knowledge of neural crest formation and migration. In addition, its relatively slow development compared to other vertebrates and recently available genome have been advantageous for resolving early genetic events in chick neural crest development (Le Douarin and Kalcheim, 1999; Le Douarin, 2004).
Neural crest cells acquire their identity early in development, at gastrula stages, and often retain stem cell-like properties during migration (Le Douarin et al., 2004; Basch et al., 2006; Crane and Trainor, 2006). Fate map studies in the chick show that cells originating from the junction of neural and non-neural ectoderm, known as the presumptive “neural plate border” region, are progenitors of dorsal neural folds, dorsal neural tube, and migrating neural crest cells (Ezin et al., manuscript in preparation). Furthermore, when explanted from the embryo, tissue from this region executes a neural crest cell program in the absence of exogenous factors (Basch et al., 2006). Therefore, progenitor cells in the prospective neural plate border have already received instructive signals that specify them as neural crest in the gastrula. However, the nature of these early signals remains largely unknown.
Comparison of molecular data from several model organisms has led to the formulation of a putative neural crest gene regulatory network (NC-GRN) to help explain the signaling and transcriptional events underlying neural crest development (Meulemans and Bronner-Fraser, 2004; Sauka-Spengler and Bronner-Fraser, 2008). The NC-GRN suggests that hierarchical relationships between distinct groups of genes contribute to progressive acquisition of neural crest cell fate. As such, the first signals are received during gastrulation, when diffusible growth factor “induction signals” (BMP, FGF, Wnt) subdivide the ectoderm into neural plate and non-neural ectoderm. A specific, finely tuned combination of such signals at the junction between neural and non-neural tissues induces a cadre of transcription factors that specify this region as the neural plate border (Bang et al., 1997; Suzuki et al., 1997; Pera et al., 1999; Streit and Stern, 1999; Luo et al., 2001a; Tribulo et al., 2003; Monsoro-Burq et al., 2005). These “neural plate border specifiers” (Msx1, Zic1, Pax7, Dlx5, Dlx3) cooperate to delineate the neural plate border, which contains a heterogeneous population of progenitor cells including those fated to become neural crest, placodes, and dorsal neural tube (McLarren et al., 2003; Tribulo et al., 2003; Woda et al., 2003; Sato et al., 2005; Hong and Saint-Jeannet, 2007; Merzdorf, 2007). Acquisition of these distinct cells fates depends on particular combinations of downstream molecules. Specifically in neural crest progenitors, the aforementioned “neural plate border specifiers” induce a group of “neural crest specifiers” (FoxD3, Snail2, c-Myc, N-Myc, AP-2α, Sox9, Sox10, among others) that function to impart bona fide neural crest characteristics, such as migratory ability on these progenitors. In addition, these genes play a crucial role in cell survival and differentiation, and their expression in pre-migratory and migrating neural crest is maintained by extensive cross- and auto-regulation (Wakamatsu et al., 1997; LaBonne and Bronner-Fraser, 2000; Dottori et al., 2001; Sasai et al., 2001; Bellmeyer et al., 2003; Cheung and Briscoe, 2003; Luo et al., 2003; McKeown et al., 2005; Sakai et al., 2006; Stewart et al., 2006; Taneyhill et al., 2007). Finally, the neural crest specifiers activate effector genes that regulate differentiation of distinct neural crest derivative lineages. These include Col2a (chondrocyte), Trp (melanocyte), c-Ret (enteric neuron), and many others (Sauka-Spengler and Bronner-Fraser, 2008).
Comparative studies of NC-GRN in modern vertebrates (zebrafish, frog, chick and mouse), basal vertebrate (lamprey), and non-vertebrate chordates (amphioxus and ascidian) suggest that neural plate border genes are remarkably conserved throughout chordates but that expression of neural crest specifier genes at the neural plate border is unique to vertebrates (Meulemans and Bronner-Fraser, 2004; Sauka-Spengler et al., 2007; Yu et al., 2008). Recent studies in the lamprey suggest that some neural crest specifier genes, such as AP-2 and c-Myc, may be activated much earlier than previously thought, concomitant with neural plate border specifiers during gastrulation (Sauka-Spengler et al., 2007). Likewise in Xenopus, neural crest specifiers Snail-2 and FoxD3 are co-expressed in the neural plate border with Msx1 and Pax3 during gastrulation (Huang and Saint-Jeannet, 2004). Orthologs of these genes have been identified and studied individually, but there is little information regarding their onset, duration, or overlap of expression in the chick. Although expression patterns of chick NC-GRN members have been examined in detail at neurula stages, much less is known about their early deployment. Such information represents a critical backdrop for interpretation of functional perturbation studies.
Here, we characterize and carefully compare the expression patterns of transcription factors of the neural plate border and neural crest specifier category in chick embryos from stages of gastrulation to neural crest migration. Surprisingly, we find that a number of neural crest specifier genes are co-expressed with neural plate border specifiers during gastrulation in remarkably similar expression domains, suggesting possible early regulatory relationships. This suggests that interactions between chick specifier genes in the NC-GRN may be more complex and occur earlier than previously thought.
RESULTS
Here, we present a detailed analysis of the expression of ten transcription factors that are part of the putative neural crest gene regulatory network in chick embryos using whole-mount mRNA in situ hybridization. We compare their expression patterns across each stage of development from gastrulation (Hamburger and Hamilton (HH) stage 4) to cranial neural crest migration (HH stage 10) (Hamburger and Hamilton, 1992).
HH stage 4
The expression patterns of known neural plate border specifiers were compared with early expression domains of neural crest specifiers during chick gastrulation. Surprisingly, the results show that many neural crest specifiers are already present in the gastrula and their distribution patterns are strikingly similar to those of canonical neural plate border specifiers. When compared at stage 4, these markers loosely group into three expression categories: 1- neural plate border and posterior epiblast; 2- neural plate, neural plate border, and anterior epiblast; and 3- non-neural ectoderm adjacent to and including part of the neural plate border.
Msx1 and c-Myc fall into the first category. Similar to observations in Xenopus and zebrafish and previous work in the chick, we find that Msx1 is expressed in posterior epiblast (ventrolateral ectoderm and mesodermal progenitors) and in the posterior and lateral edges of the neural plate (Fig. 1B,B’) (Streit and Stern, 1999; Tribulo et al., 2003; Phillips et al., 2006). c-Myc is also expressed at high levels in prospective mesoderm progenitors in the posterior epiblast. Its anterior boundary of expression encompasses the neural plate border, similar to Msx1 (Fig. 1C,C’).
Fig. 1.

A-JJ: Neural plate border and neural crest specifier gene expression during early development. Hamburger and Hamilton (HH) stage 4 (B-I), HH stage 5 (J-R), HH stage 6 (S-AA) and HH stage 7 (BB-JJ) embryos were analyzed by whole-mount in situ hybridization using digoxigenin (DIG)-labeled RNA probes for chick Pax7 (J, S, BB), Msx1 (B, K, T, CC), c-Myc (C, L, U, DD), Zic1 (D, M, V, EE), N-Myc (E, N, W, FF), FoxD3 (F, O, X, GG), Dlx3 (G, P, Y, HH), Dlx5 (H, Q, Z, II), and AP-2α (I, R, AA, JJ). Transverse sections were performed on HH stage 4 (B’-I’) and HH stage 6 (S’-AA’) embryos as indicated. A rough schematic of a HH stage 4 embryo showing the respective positions of the primitive streak, Hensen’s node, neural plate, neural plate border, pre-placodal region, non-neural ectoderm, and mesodermal progenitors is shown in A. Gene expression at the neural plate border and pre-placodal region is indicated by arrows and arrowheads, respectively. Ec, non-neural ectoderm; hn, Hensen’s node; mes, mesoderm; nf, neural fold; not, notochord; np, neural plate; npb, neural plate border; ppr, pre-placodal region; ps, primitive streak; som, somite.
Zic1, FoxD3, N-Myc, and Dlx3 fall in the second category. Zic1 is a known neural specifier that is also expressed in the neural plate border and in the anterior epiblast region that contains placodal progenitors (Fig. 1D,D’) (Merzdorf, 2007). Interestingly, neural crest specifiers FoxD3 and N-Myc are also expressed at gastrulation in a domain remarkably similar to that of Zic1. Namely, FoxD3 transcripts are present in anterior neural plate, where their rostral-most boundary of expression is adjacent to and slightly overlapping with that of Dlx5, the neural plate border and placodal specifier (Fig. 1F,F’; Fig. 3B). However, FoxD3 does not overlap with Msx1 in the posterior neural plate border (Fig. 3A). Some of the FoxD3-positive cells anterior to the node are likely notochord progenitors, since FoxD3 functions in development of this structure at later stages (Odenthal and Nusslein-Volhard, 1998). However, FoxD3 appears to be mainly restricted to the neural plate, as it does not overlap with ectodermal specifier AP-2 (data not shown). The oncogene N-Myc is expressed at very high levels in the neural plate border, and to a lesser extent in anterior epiblast surrounding the presumptive neural plate (Fig. 1E,E’). It overlaps in the posterior neural plate border with Msx1 (Fig. 3C). Dlx3 is a known specifier of ectoderm and placode fates that also functions indirectly to position the neural plate border by repressing adjacent neural fates (Woda et al., 2003). Therefore, we were surprised to find low levels of chick Dlx3 transcripts in a large swath of epiblast tissue encompassing the presumptive neural plate border and lateral portions of the prospective neural plate, suggesting an additional novel role for Dlx3 in the chick (Fig. 1G,G’). Dlx3 transcripts are concentrated at higher levels in the anterior epiblast, which contains the pre-placodal region. In contrast, Dlx3 in the frog is never expressed in the neural plate border (Luo et al., 2001b).
Fig. 3.

Co-expression of neural crest network genes in the chick gastrula. HH stage 4 or 4+ embryos were analyzed by whole-mount double in situ hybridization using digoxigenin (DIG)- and fluorescein (FITC)-labeled RNA probes for chick Msx1, AP-2α, FoxD3, N-Myc, Dlx5, and Irx1. A. Expression of FoxD3 and Msx1 is complementary. FoxD3 (purple) is expressed in anterior neural plate while Msx1 (blue) is expressed in posterior epiblast and neural plate border. B. FoxD3 expression domain (purple) in the anterior neural plate border (arrow) lies adjacent to and slightly overlaps with pre-placodal marker Dlx5 (blue). C. N-Myc (purple) is co-expressed in the posterior neural plate border (arrow) with Msx1 (blue). D. AP-2 (purple) is co-expressed in posterior neural plate border (arrow) with Msx1 (blue). E. Dlx5 (purple) and AP-2 (blue) are co-expressed in the pre-placodal region (arrowhead). F. Expression of the placodal marker Irx1 at stage 4+ is shown for comparison with E. Npb, neural plate border; ppr, pre-placodal region.
The third category of neural crest gene expression at stage 4 includes Dlx5 and AP-2α (henceforth referred to as AP-2). We find that Dlx5 is expressed during gastrulation in anterior epiblast adjacent to the neural plate border that contains the pre-placodal region, consistent with previously published frog and chick studies (Fig. 1H,H’) (Yang et al., 1998; Luo et al., 2001b; McLarren et al., 2003; Woda et al., 2003; Bhattacharyya et al., 2004). Chick AP-2 is expressed broadly throughout non-neural ectoderm at all axial levels (Fig. 1I,I’). It is co-expressed anteriorly with Dlx5 in the pre-placodal region that is defined by Irx1 expression (Fig. 3E,F). Surprisingly, AP-2 transcripts also overlap in the posterior neural plate border with Msx1 (Fig. 3D).
HH stage 5
At stage 5, when the primitive streak begins to regress and leave notochord tissue in its wake, network genes become more clearly resolved at the neural plate border. Msx1 is maintained in posterior epiblast but its expression at the neural plate border becomes more specific, and also begins to extend anteriorly (Fig. 1K). In the lateral and posterior portions of the neural plate border, Msx1 expression domain is identical to that of the border specifier Pax7, which becomes upregulated at this stage (Fig. 1J,J’). c-Myc is maintained in posterior epiblast and posterior neural plate border, but unlike Msx1, does not extend anteriorly (Fig. 1L). Zic1 persists in anterior neural tissue and begins to accumulate more strongly at the edges of the neural plate (Fig. 1M). Likewise, N-Myc expression is maintained in the neural plate and its border, where it extends posteriorly as the embryo elongates (Fig. 1N). The expression domain of Dlx3 is almost identical to that of N-Myc (Fig. 1P). Conversely, FoxD3 is no longer expressed in the neural plate or its border and is almost exclusively localized to the notochord (Fig. 1O). Dlx5 and AP-2 remain in the non-neural ectoderm, with AP-2 marking ectoderm at all axial levels and Dlx5 being concentrated anteriorly (Fig. 1Q,R).
HH stages 6 and 7
At stages 6 and 7, neural folds begin to thicken and elevate, allowing for better resolution of gene expression at their edges. During this time, Pax7 is expressed exclusively in cells at the neural plate border caudal to the anterior prominence of the neural folds (Fig. 1S,S’,BB). Likewise, Msx1 becomes refined in the neural plate border both anteriorly (similarly to the Dlx genes) and posteriorly, and it is also maintained in ectomesoderm near the tail (Fig. 1T,T’,CC). With progressive development, the neural plate closes anteriorly, while staying open at the caudal end of the embryo where the streak has not yet fully regressed. Gene expression at the open neural plate level recapitulates events that occur earlier at rostral levels. Expression of c-Myc in the caudal open neural plate is markedly similar to Pax7 and Msx1, but it is not expressed more anteriorly (Fig. 1U,U’). However at stage 7, very low levels of c-Myc transcripts begin to accumulate in the anterior-most neural folds (Fig. 1DD). Zic1 and N-Myc are both maintained in the neural plate and upregulated at its edges, although Zic1 becomes primarily restricted to the anterior neural folds (Fig. 1V,V’,W,W’,EE,FF). A stripe of enhanced N-Myc expression is visible in the region of the first forming somite at stage 7 (Fig. 1FF). FoxD3 transcripts continue to mark the notochord, but also begin to accumulate at low levels in the anterior neural folds at stage 6 (Fig. 1X,X’,GG). Dlx3 is expressed most prominently in the anterior neural ridge and placode region where it shares its domain with Dlx5 (Fig. 1Y,Y’,HH). Low levels of Dlx3 remain in neural tissue at all axial levels. Dlx5 and AP-2 are expressed in ectoderm directly adjacent to the neural folds (Fig. 1Z,Z’,II,AA,AA’,JJ).
HH stage 8
At the 3 to 6 somite stage (HH 8), neural folds are markedly elevated and begin to fuse anteriorly. At this stage, most of the genes examined are co-expressed in the dorsal neural folds. Msx1 and Pax7 show almost identical expression in dorsal neural folds and border of the open neural plate (Fig. 2A,A’,B,B’). Strong Snail2 expression is present in the dorsal neural folds at the mid- and hindbrain level (Fig. 2C,C’). Likewise, FoxD3 is recruited to the dorsal neural folds, where it is co-expressed in the mid- and hindbrain with Snail2 and in the forebrain with Zic1, N-Myc, and c-Myc (Fig. 2D,D’). Zic1 and N-Myc have almost identical expression patterns in the anterior neural folds, though N-Myc is also found in heart mesoderm and in posterior lateral plate mesoderm (Fig. 2E,E’,F,F’). At this stage, we observe clear expression of c-Myc in the anterior-most neural folds (Fig. 2G,G’). Intriguingly, its expression seems to be divided into two completely separate domains - anterior neural folds and posterior lateral plate mesoderm. The expression domain of Dlx3 also resolves cleanly at this stage. It has disappeared from the neural folds and is expressed exclusively in the anterior neural ridge and surrounding ectoderm, where it is co-expressed with Dlx5 (Fig. 2H,H’). However, the Dlx5 expression domain extends more caudally to the level of the hindbrain and into the lateral-most edge of the dorsal neural folds (Fig. 2I,I’). At this stage, AP-2 transcripts are also recruited to the lateral edge of the neural folds (Fig. 2J,J’).
Fig. 2.

Neural plate border and neural crest specifier gene expression during neurulation and neural crest migration. HH stage 8 (A-J), HH stage 9 (K-T) and HH stage 10-/10 (U-DD) embryos were analyzed by whole-mount in situ hybridization using digoxigenin (DIG)-labeled RNA probes for chick Pax7 (A, K, U), Msx1, (B, L, V), Snail2 (C, M, W), FoxD3 (D, N, X), Zic1 (E, O, Y), N-Myc (F, P, Z), c-Myc (G, Q, AA), Dlx3 (H, R, BB), Dlx5 (I, S, CC), and AP-2α (J, T, DD). Transverse sections were performed on HH stage 8 (A’-J’) and HH stage 10 (U’-DD’) embryos as indicated. Gene expression in migrating neural crest cells is indicated by arrows. Bl, blood islands; ec, non-neural ectoderm; hb, hindbrain; hm, heart mesoderm; lpm, lateral plate mesoderm; mnc, migrating neural crest; olf, olfactory placode/pit; onp, open neural plate; ot, otic placode; som, somites.
HH stage 9
At the 7 to 9 somite stage (HH 9) the neural folds have completely fused in the head and neural crest precursors that arose from the neural plate border come to lie in the dorsal neural tube and begin to emigrate. Many of the neural plate border and neural crest specifiers now mark pre-migratory neural crest in the dorsal neural tube and emigrating cranial neural crest cells. The expression domains of Msx1 and Pax7 are identical (Fig. 2K,L), marking neural crest progenitors in the head, trunk and tail. Snail2 and FoxD3 also exhibit similar expression patterns in emigrating cranial neural crest and trunk dorsal neural tube (Fig. 2M,N). However, they are not expressed in the open neural plate like Msx1 and Pax7. At this time, Zic1, Dlx3, and Dlx5 expression patterns are similar; their transcripts are almost exclusively restricted to olfactory progenitors in the anterior forebrain (Fig. 2O,R,S). However, Zic1 is additionally expressed in a specific stripe in the hindbrain, and Dlx3 is present in cranial ectoderm and prospective otic placode. N-Myc is maintained in anterior neural tissue at high levels, and is also expressed in heart mesoderm and posterior lateral plate mesoderm (Fig. 2P). c-Myc is expressed in the forebrain and emigrating neural crest cells at the level of the midbrain, where its expression is highly similar to that of Snail2 and FoxD3. It is additionally expressed in the blood islands (Fig. 2Q). At this stage AP-2 is recruited to the dorsal neural tube at the cranial and vagal trunk levels. Its transcripts also persist in non-neural ectoderm adjacent to the open neural plate (Fig. 2T).
HH stage 10
At the 9 to 11 somite stage (HH 10-/10), migrating neural crest cells can be identified by their characteristic “cobra-hood” pattern in the head. Many neural crest specifiers are expressed in these cells, including Snail2, FoxD3, c-Myc, and AP-2 (Fig. 2W,W’,X,X’,AA,AA’,DD,DD’). They are also maintained in premigratory trunk neural crest. Likewise, the neural plate border specifiers Pax7 and Msx1 persist in migrating cranial and pre-migratory trunk neural crest cells, suggesting a role in maintenance of neural crest traits (Fig. 2U,U’,V,V’). However, the other specifier genes are excluded from neural crest at this stage and are instead expressed in other cell types such as placodes (Zic1, Dlx3, Dlx5 (Fig. 2Y,BB,BB’,CC,CC’)) and neural tissue and somites (Zic1 (Fig. 2Y,Y’)). N-Myc is also not expressed by migrating neural crest cells despite transcripts being concentrated in the dorsal neural tube (Fig. 2Z,Z’). It has been shown that N-Myc plays a role in neural crest migration at much later stages (Wakamatsu et al., 1997).
DISCUSSION
Expression patterns of ten transcription factors that are members of the chick NC-GRN were compared between HH stages 4 to 10. We show that transcripts of most neural crest specifiers are already expressed in or around the presumptive neural plate border at gastrulation stages. Thus, this work challenges our current formulation of gene interactions within the chick NC-GRN. The remarkable similarities in expression patterns of neural plate border and neural crest specifiers at gastrulation suggests that regulatory events are occurring much earlier than previously thought. For instance, the shared expression domain of Zic1 and FoxD3 hints at a direct interaction between these two molecules at HH stage 4. In addition, the overall similarity in expression of most of these specifier genes at the neural plate border and in the dorsal neural tube support data from ongoing studies that demonstrate extensive cross- and auto-regulation between these genes. Importantly, comparison of NC-GRN specifier expression patterns as they are resolved throughout development lays crucial groundwork for functional studies aimed at understanding the interactions between these transcription factors. Namely, such comprehensive and comparative expression data provide key information on when and where genes should be inactivated or activated during functional studies. Finally, resolving the overlap between all of these molecules brings us closer to defining the “neural plate border” and the location of neural crest progenitors within it, which is summarized in Fig. 4A. Interestingly, there is strong conservation of early expression patterns of neural crest network genes between chick and Xenopus (Fig. 4B), suggesting that early specification of neural crest progenitors is likely to be conserved across vertebrates.
Fig. 4.
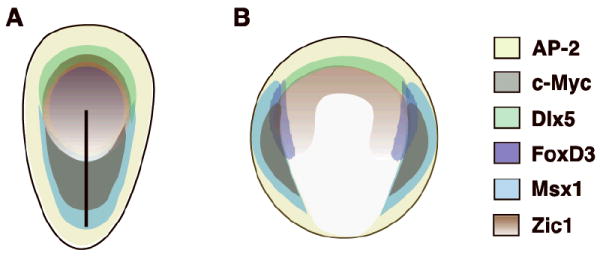
Schematic comparison of neural plate border and neural crest specifier gene expression during gastrulation in chick and Xenopus. A. Expression of AP-2 (yellow), c-Myc (gray), Dlx5 (green), FoxD3 (purple), Msx1 (blue) and Zic1 (brown) in the chick at mid-gastrulation. B. Expression of AP-2 (yellow), c-Myc (gray), Dlx5 (green), FoxD3 (purple), Msx1 (blue) and Zic1 (brown) in Xenopus at mid-gastrulation. During neurulation, expression of these genes overlaps in the neural plate border and dorsal neural tube in both organisms.
While comparing the expression patterns of neural plate border and neural crest specifiers at each stage of early neural crest development, we found some interesting trends in terms of which genes are maintained and which are differentially expressed from stage to stage. For instance, the canonical neural plate border specifiers Msx1 and Pax7 are continuously expressed in neural crest progenitors from gastrulation to migration, suggesting a role in both induction and maintenance of other neural crest genes. In contrast, Zic1, Dlx5, and Dlx3 are expressed in the neural plate border region at early stages but are later recruited to other regions of the embryo, such as neural tissue, placodes, and somites. This suggests that they play several spatio-temporally separable roles during development, only one of which is specific to neural crest.
Changes in expression patterns of neural crest specifiers are even more interesting. N-Myc is maintained in neural crest progenitors throughout development like Msx1 and Pax7, but it is also expressed in other areas of the embryo, such as neural, ectodermal, and mesodermal tissues. Being an oncogene, N-Myc is likely playing a role in proliferation and maintenance of all of these progenitor cell types throughout development. It is surprising, however, that it is not expressed in migrating neural crest cells at HH stage 10 since those cells proliferate extensively. In contrast, although AP-2 is continuously expressed in the gastrula and neurula, it does not appear in neural crest progenitors until HH stage 9, when it is recruited to the dorsal neural tube. Its early role as an ectodermal specifier is highly conserved among chordates, but its recruitment to the dorsal neural tube is a vertebrate-specific event. It is likely that this event in vertebrates has occurred as a result of the evolution of a novel crest-specific AP-2 regulatory element that is distinct from the element(s) driving its expression in the ectoderm. We hypothesize that it may also have an earlier function in neural crest development by maintaining ectodermal fate and repressing neuronal markers, therefore contributing to the placement of the prospective neural plate border. It has been shown that the Dlx3/5 genes function to position the neural plate border in this manner (McLarren et al., 2003; Woda et al., 2003). Likewise, Snail2 is not expressed in the neural plate border at HH stage 4 but instead plays a role in epithelial-to-mesenchymal transition of ingressing mesoderm cells during gastrulation (Nieto et al., 1994). Its transcripts begin to accumulate in dorsal neural folds only around late HH stage 6 (data not shown). Intriguingly, we find two very specific stripes of Snail2-positive mesodermal cells directly under the forming neural plate border at HH stages 5-7 (data not shown). We conjecture that it is possible that Snail2 also plays an early role in neural crest specification by participating in a feedback loop to maintain neural plate border specifier expression. The expression pattern of c-Myc is intriguing in that it is present in the neural plate border and mesoderm progenitors at gastrulation, but is then exclusively expressed in mesoderm until HH stage 8, when it strikingly appears in the anterior neural folds. Likewise, FoxD3 is expressed in the neural plate at stage 4, after which it becomes restricted to the notochord and does not definitively appear in neural crest progenitors until HH stage 8. This suggests that c-Myc and FoxD3 may play temporally distinct roles in specification and maintenance of cell fates. In conclusion, these results represent an important first step in elucidating regulatory interactions between these transcription factors and for comparative analysis with other species.
METHODS
Chick embryo incubation
Fertilized chicken eggs were obtained from AA Enterprises (Ramona, CA) and incubated at 38°C in a humidified incubator (Lyon Electric, Chula Vista, CA). Embryos were staged according to the Hamburger and Hamilton chick staging system (Hamburger and Hamilton, 1992).
Wholemount in situ hybridization
Chick embryos were dissected in Ringer’s solution and fixed in 4% paraformaldehyde at 4°C overnight. Wholemount in situ hybridization was performed as described previously (Nieto et al., 1996; Xu and Wilkinson, 1998), with some modifications involving more extensive washes adapted from a lamprey in situ protocol (Sauka-Spengler et al., 2007). Stained embryos were photographed in 50% glycerol on a Zeiss Stemi SV11 microscope using AxioVision software (Release 4.6) and processed using Photoshop 7.0 (Adobe Systems).
Cryosectioning
To obtain transverse sections for histological analysis, embryos were equilibrated in 15% sucrose (in PBS) for 2 hours at room temperature and subsequently transferred to 30% sucrose and incubated overnight at 4°C. Embryos were embedded in O.C.T. Compound (Tissue-Tek, catalog #4583) and frozen at -80°C. 20 or 25 μm thick sections were obtained by cryosectioning at -23°C on a Microm HM550 cryostat. For imaging, slides were washed twice for 10 minutes in PBS with 0.1% Tween, rinsed in double-distilled water, and mounted with PermaFluor Mountant Medium (Thermo Electron Corporation, catalog #434990). Sections were imaged on a Zeiss Axioskop 2 Plus microscope and processed as described for wholemount images.
In situ mRNA probes
Many of the templates for mRNA probe synthesis were obtained from the BBSRC ChickEST Database (http://www.chick.umist.ac.uk). The clones used were: Msx1 (ChEST900p21), Zic1 (ChEST459n6), c-Myc (ChEST191o11), N-Myc (ChEST895e1), AP-2α (ChEST765g1), and Irx1 (ChEST523e4). The other template plasmids used were: Pax7 (Basch et al., 2006), Dlx5 (Bhattacharyya et al., 2004), Dlx3 a(Brown et al., 2005), Snail2 (Nieto et al., 1994), and FoxD3 (Kos et al., 2001). Linearized DNA was used to synthesize digoxigenin- and fluorescein-labeled antisense probes with Promega buffers and RNA polymerases (Promega Corp). RNA probes were purified with illustra ProbeQuant™ G-50 Micro Columns (GE Healthcare, product code 28-9034-08).
Acknowledgments
We thank Tatjana Sauka-Spengler, Sujata Bhattacharyya, Meyer Barembaum, and Jack Sechrist for their technical advice and discussion. This work was supported by NS36585 to Marianne Bronner-Fraser.
Grant Sponsor: NIH; Grant number NS36585
References
- Bang AG, Papalopulu N, Kintner C, Goulding MD. Expression of Pax-3 is initiated in the early neural plate by posteriorizing signals produced by the organizer and by posterior non-axial mesoderm. Development. 1997;124:2075–2085. doi: 10.1242/dev.124.10.2075. [DOI] [PubMed] [Google Scholar]
- Basch ML, Bronner-Fraser M, Garcia-Castro MI. Specification of the neural crest occurs during gastrulation and requires Pax7. Nature. 2006;441:218–222. doi: 10.1038/nature04684. [DOI] [PubMed] [Google Scholar]
- Bellmeyer A, Krase J, Lindgren J, LaBonne C. The protooncogene c-myc is an essential regulator of neural crest formation in xenopus. Dev Cell. 2003;4:827–839. doi: 10.1016/s1534-5807(03)00160-6. [DOI] [PubMed] [Google Scholar]
- Bhattacharyya S, Bailey AP, Bronner-Fraser M, Streit A. Segregation of lens and olfactory precursors from a common territory: cell sorting and reciprocity of Dlx5 and Pax6 expression. Dev Biol. 2004;271:403–414. doi: 10.1016/j.ydbio.2004.04.010. [DOI] [PubMed] [Google Scholar]
- Brown ST, Wang J, Groves AK. Dlx gene expression during chick inner ear development. J Comp Neurol. 2005;483:48–65. doi: 10.1002/cne.20418. [DOI] [PubMed] [Google Scholar]
- Cheung M, Briscoe J. Neural crest development is regulated by the transcription factor Sox9. Development. 2003;130:5681–5693. doi: 10.1242/dev.00808. [DOI] [PubMed] [Google Scholar]
- Crane JF, Trainor PA. Neural crest stem and progenitor cells. Annu Rev Cell Dev Biol. 2006;22:267–286. doi: 10.1146/annurev.cellbio.22.010305.103814. [DOI] [PubMed] [Google Scholar]
- Dottori M, Gross MK, Labosky P, Goulding M. The winged-helix transcription factor Foxd3 suppresses interneuron differentiation and promotes neural crest cell fate. Development. 2001;128:4127–4138. doi: 10.1242/dev.128.21.4127. [DOI] [PubMed] [Google Scholar]
- Hamburger V, Hamilton HL. A series of normal stages in the development of the chick embryo. 1951. Dev Dyn. 1992;195:231–272. doi: 10.1002/aja.1001950404. [DOI] [PubMed] [Google Scholar]
- Hong CS, Saint-Jeannet JP. The activity of Pax3 and Zic1 regulates three distinct cell fates at the neural plate border. Mol Biol Cell. 2007;18:2192–2202. doi: 10.1091/mbc.E06-11-1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang X, Saint-Jeannet JP. Induction of the neural crest and the opportunities of life on the edge. Dev Biol. 2004;275:1–11. doi: 10.1016/j.ydbio.2004.07.033. [DOI] [PubMed] [Google Scholar]
- Kos R, Reedy MV, Johnson RL, Erickson CA. The winged-helix transcription factor FoxD3 is important for establishing the neural crest lineage and repressing melanogenesis in avian embryos. Development. 2001;128:1467–1479. doi: 10.1242/dev.128.8.1467. [DOI] [PubMed] [Google Scholar]
- LaBonne C, Bronner-Fraser M. Snail-related transcriptional repressors are required in Xenopus for both the induction of the neural crest and its subsequent migration. Dev Biol. 2000;221:195–205. doi: 10.1006/dbio.2000.9609. [DOI] [PubMed] [Google Scholar]
- Le Douarin N. The avian embryo as a model to study the development of the neural crest: a long and still ongoing story. Mech Dev. 2004;121:1089–1102. doi: 10.1016/j.mod.2004.06.003. [DOI] [PubMed] [Google Scholar]
- Le Douarin N, Kalcheim C. The Neural Crest. 2 Cambridge University Press; New York: 1999. [Google Scholar]
- Le Douarin NM, Creuzet S, Couly G, Dupin E. Neural crest cell plasticity and its limits. Development. 2004;131:4637–4650. doi: 10.1242/dev.01350. [DOI] [PubMed] [Google Scholar]
- Luo T, Lee YH, Saint-Jeannet JP, Sargent TD. Induction of neural crest in Xenopus by transcription factor AP2alpha. Proc Natl Acad Sci U S A. 2003;100:532–537. doi: 10.1073/pnas.0237226100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo T, Matsuo-Takasaki M, Lim JH, Sargent TD. Differential regulation of Dlx gene expression by a BMP morphogenetic gradient. Int J Dev Biol. 2001a;45:681–684. [PubMed] [Google Scholar]
- Luo T, Matsuo-Takasaki M, Sargent TD. Distinct roles for Distal-less genes Dlx3 and Dlx5 in regulating ectodermal development in Xenopus. Mol Reprod Dev. 2001b;60:331–337. doi: 10.1002/mrd.1095. [DOI] [PubMed] [Google Scholar]
- McKeown SJ, Lee VM, Bronner-Fraser M, Newgreen DF, Farlie PG. Sox10 overexpression induces neural crest-like cells from all dorsoventral levels of the neural tube but inhibits differentiation. Dev Dyn. 2005;233:430–444. doi: 10.1002/dvdy.20341. [DOI] [PubMed] [Google Scholar]
- McLarren KW, Litsiou A, Streit A. DLX5 positions the neural crest and preplacode region at the border of the neural plate. Dev Biol. 2003;259:34–47. doi: 10.1016/s0012-1606(03)00177-5. [DOI] [PubMed] [Google Scholar]
- Merzdorf CS. Emerging roles for zic genes in early development. Dev Dyn. 2007;236:922–940. doi: 10.1002/dvdy.21098. [DOI] [PubMed] [Google Scholar]
- Meulemans D, Bronner-Fraser M. Gene-regulatory interactions in neural crest evolution and development. Dev Cell. 2004;7:291–299. doi: 10.1016/j.devcel.2004.08.007. [DOI] [PubMed] [Google Scholar]
- Monsoro-Burq AH, Wang E, Harland R. Msx1 and Pax3 cooperate to mediate FGF8 and WNT signals during Xenopus neural crest induction. Dev Cell. 2005;8:167–178. doi: 10.1016/j.devcel.2004.12.017. [DOI] [PubMed] [Google Scholar]
- Nieto MA, Patel K, Wilkinson DG. In situ hybridization analysis of chick embryos in whole mount and tissue sections. Methods Cell Biol. 1996;51:219–235. doi: 10.1016/s0091-679x(08)60630-5. [DOI] [PubMed] [Google Scholar]
- Nieto MA, Sargent MG, Wilkinson DG, Cooke J. Control of cell behavior during vertebrate development by Slug, a zinc finger gene. Science. 1994;264:835–839. doi: 10.1126/science.7513443. [DOI] [PubMed] [Google Scholar]
- Odenthal J, Nusslein-Volhard C. fork head domain genes in zebrafish. Dev Genes Evol. 1998;208:245–258. doi: 10.1007/s004270050179. [DOI] [PubMed] [Google Scholar]
- Pera E, Stein S, Kessel M. Ectodermal patterning in the avian embryo: epidermis versus neural plate. Development. 1999;126:63–73. doi: 10.1242/dev.126.1.63. [DOI] [PubMed] [Google Scholar]
- Phillips BT, Kwon HJ, Melton C, Houghtaling P, Fritz A, Riley BB. Zebrafish msxB, msxC and msxE function together to refine the neural-nonneural border and regulate cranial placodes and neural crest development. Dev Biol. 2006;294:376–390. doi: 10.1016/j.ydbio.2006.03.001. [DOI] [PubMed] [Google Scholar]
- Sakai D, Suzuki T, Osumi N, Wakamatsu Y. Cooperative action of Sox9, Snail2 and PKA signaling in early neural crest development. Development. 2006;133:1323–1333. doi: 10.1242/dev.02297. [DOI] [PubMed] [Google Scholar]
- Sasai N, Mizuseki K, Sasai Y. Requirement of FoxD3-class signaling for neural crest determination in Xenopus. Development. 2001;128:2525–2536. doi: 10.1242/dev.128.13.2525. [DOI] [PubMed] [Google Scholar]
- Sato T, Sasai N, Sasai Y. Neural crest determination by co-activation of Pax3 and Zic1 genes in Xenopus ectoderm. Development. 2005;132:2355–2363. doi: 10.1242/dev.01823. [DOI] [PubMed] [Google Scholar]
- Sauka-Spengler T, Bronner-Fraser M. A gene regulatory network orchestrates neural crest formation. Nat Rev Mol Cell Biol. 2008;9:557–568. doi: 10.1038/nrm2428. [DOI] [PubMed] [Google Scholar]
- Sauka-Spengler T, Meulemans D, Jones M, Bronner-Fraser M. Ancient evolutionary origin of the neural crest gene regulatory network. Dev Cell. 2007;13:405–420. doi: 10.1016/j.devcel.2007.08.005. [DOI] [PubMed] [Google Scholar]
- Stewart RA, Arduini BL, Berghmans S, George RE, Kanki JP, Henion PD, Look AT. Zebrafish foxd3 is selectively required for neural crest specification, migration and survival. Dev Biol. 2006;292:174–188. doi: 10.1016/j.ydbio.2005.12.035. [DOI] [PubMed] [Google Scholar]
- Streit A, Stern CD. Establishment and maintenance of the border of the neural plate in the chick: involvement of FGF and BMP activity. Mech Dev. 1999;82:51–66. doi: 10.1016/s0925-4773(99)00013-1. [DOI] [PubMed] [Google Scholar]
- Suzuki A, Ueno N, Hemmati-Brivanlou A. Xenopus msx1 mediates epidermal induction and neural inhibition by BMP4. Development. 1997;124:3037–3044. doi: 10.1242/dev.124.16.3037. [DOI] [PubMed] [Google Scholar]
- Taneyhill LA, Coles EG, Bronner-Fraser M. Snail2 directly represses cadherin6B during epithelial-to-mesenchymal transitions of the neural crest. Development. 2007;134:1481–1490. doi: 10.1242/dev.02834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tribulo C, Aybar MJ, Nguyen VH, Mullins MC, Mayor R. Regulation of Msx genes by a Bmp gradient is essential for neural crest specification. Development. 2003;130:6441–6452. doi: 10.1242/dev.00878. [DOI] [PubMed] [Google Scholar]
- Wakamatsu Y, Watanabe Y, Nakamura H, Kondoh H. Regulation of the neural crest cell fate by N-myc: promotion of ventral migration and neuronal differentiation. Development. 1997;124:1953–1962. doi: 10.1242/dev.124.10.1953. [DOI] [PubMed] [Google Scholar]
- Woda JM, Pastagia J, Mercola M, Artinger KB. Dlx proteins position the neural plate border and determine adjacent cell fates. Development. 2003;130:331–342. doi: 10.1242/dev.00212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Q, Wilkinson D. In Situ Hybridisation: A Practical Approach. Oxford University Press; Oxford: 1998. In situ hybridisation of mRNA with hapten labeled probes; pp. 87–106. [Google Scholar]
- Yang L, Zhang H, Hu G, Wang H, Abate-Shen C, Shen MM. An early phase of embryonic Dlx5 expression defines the rostral boundary of the neural plate. J Neurosci. 1998;18:8322–8330. doi: 10.1523/JNEUROSCI.18-20-08322.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu JK, Meulemans D, McKeown SJ, Bronner-Fraser M. Insights from the amphioxus genome on the origin of vertebrate neural crest. Genome Res. 2008;18:1127–1132. doi: 10.1101/gr.076208.108. [DOI] [PMC free article] [PubMed] [Google Scholar]


