Abstract
Mammals have single-rowed dentitions whereas many non-mammalian vertebrates have teeth in multiple rows. Neither the molecular mechanism regulating iterative tooth initiation nor that restricting mammalian tooth development in one row is known. We found that mice lacking the transcription factor Osr2 develop supernumerary teeth lingual to their molars due to expansion of the odontogenic field. Osr2 was expressed in a lingual-to-buccal gradient and restricted expression of Bmp4, an essential odontogenic signal, in the developing tooth mesenchyme. Expansion of odontogenic field in Osr2-deficient mice required Msx1, a feedback activator of Bmp4 expression. These findings suggest that the Bmp4-Msx1 pathway propagates mesenchymal activation for sequential tooth induction and that spatial modulation of this pathway provides a potentially general mechanism patterning vertebrate dentition.
Teeth are vertebrate-specific organs and distinct dentition patterns have played critical roles in vertebrate diversification and specialization (1–3). In addition to variations in tooth number, size, and shape, many non-mammalian vertebrates have multi-rowed dentitions whereas mammals develop teeth in a single row. Studies of tooth development in several fish species showed that multi-rowed dentitions result from sequential iterative tooth initiation along the mesial-to-distal and labial-to-lingual directions (3–5). The molecular mechanisms regulating the precise spatiotemporal patterns of sequential tooth initiation are unknown. Development of the single-rowed mammalian dentition likely involves restricting odontogenic field along the buccolingual axis; the mechanism underlying this control is also unknown.
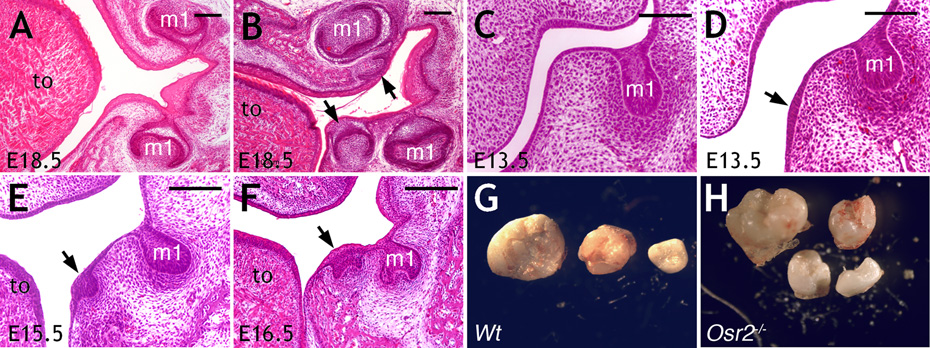
Current understanding of the molecular mechanisms controlling tooth development has come mostly from studies in mice (1, 6). Although supernumerary teeth have been reported in several mutant mouse strains (7–10), the majority developed within the tooth row from vestigial diastemal tooth buds (10, 11). We recently generated mice lacking the Osr2 (odd-skipped related-2) gene (12, 13) and found that they exhibited supernumerary tooth development lingual to their molar teeth (Fig. 1A, B, and Fig. S1). Histological analyses (14) traced initiation of these supernumerary tooth germs to aberrant thickening of oral epithelium lingual to the first molar tooth buds at E13.5 (Fig. 1C, D). By E15.5, as the first molar germs developed to late “cap” stage (1), the ectopically thickened oral epithelia in Osr2−/− embryos invaginated and the underlying mesenchyme condensed (Fig. 1E). These ectopic epithelial invaginations resembled cap stage tooth germs by E16.5 (Fig. 1F). Because Osr2−/− mice die shortly after birth due to cleft palate (13), we transplanted E13.5 mandibular molar tooth germs under renal capsules of adult mice to allow complete tooth morphogenesis (14). After 21 days, wildtype and heterozygous molar tooth germs gave rise to two to three mineralized molar teeth, representing the normal molars (Fig. 1G). In contrast, Osr2−/− mutant molar tooth germs gave rise to four to five mineralized teeth (Fig. 1H, and Fig. S2). These data indicate that a complete odontogenic program is ectopically activated lingual to the molar teeth in Osr2−/− mice.
Fig. 1.
(A, B) Frontal sections of E18.5 wildtype (A) and Osr2−/− (B) littermates. Arrows in B point to supernumerary tooth germs. (C) Frontal section of wildtype first molar region at E13.5. (D–F) Frontal sections of Osr2−/− mutant first molar regions at E13.5, E15.5 and E16.5. Arrows point to supernumerary tooth germs. (G, H) Mineralized teeth from renal capsule cultures of E13.5 wildtype (G) and Osr2−/− (H) molar tooth germs. m1, first molar tooth germ; to, tongue. Scale bar, 100 µm.
To gain insight into supernumerary tooth formation in Osr2−/− mice, we examined expression of selected marker genes during early tooth development. Pitx2 was initially expressed throughout oral epithelium and its expression selectively maintained in dental epithelium after E11 (15). In Osr2−/− embryos, Pitx2 expression abnormally persisted in oral epithelium lingual to the first molar tooth buds (Fig. S3A, B). By E14.5, strong Pitx2 expression marked the supernumerary dental placodes and first molar tooth buds (Fig. S3C, D). At E13.5, Shh was expressed in the enamel knot of developing molar tooth buds (16) (Fig. S3E). In Osr2−/− mutants, Shh was ectopically expressed in a subset of epithelial cells lingual to the first molar buds (Fig. S3F). By E14.5, Shh expression was clearly detected in the supernumerary dental placodes in Osr2−/− embryos (Fig. S3H). In addition, expression of dental mesenchyme markers Msx1 and Lef1 were upregulated and expanded lingually in Osr2−/− mice (Fig. S3, I–L). These data suggest that supernumerary tooth development resulted from lingual expansion of the odontogenic field from the first molar tooth germs.
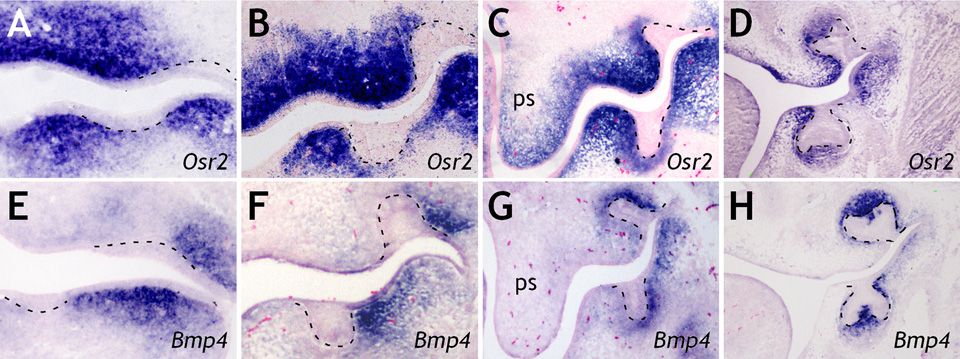
To understand how Osr2 regulates the odontogenic field, we examined Osr2 expression during normal tooth development. At E11.5, Osr2 was strongly expressed in the mesenchyme lingual to the dental lamina in both the maxilla and mandible (Fig. 2A). Osr2 was also highly expressed in the proximal mandibular mesenchyme buccal to the dental lamina (Fig. 2A). As tooth buds developed from E12.5 to E14.5, Osr2 mRNA was expressed in a gradient in the developing tooth mesenchyme, with higher levels lingual and lower levels immediately buccal to the tooth buds (Fig. 2, B–D). Overall, the Osr2 expression pattern is complementary to that of Bmp4 (Fig. 2, E–H), an essential odontogenic signal preferentially expressed on the buccal side in developing molar mesenchyme (17–20).
Fig. 2.
Expression patterns of Osr2 (A–D) and Bmp4 (E–H) mRNAs along the buccolingual axis of mouse molar tooth germs at E11.5 (A, E), E12.5 (B, F), E13.5 (C, G), and E14.5 (D, H). Lingual side is to the left in all panels. Black dashed lines mark the boundary between dental epithelium and mesenchyme. ps, palatal shelf.
The expression pattern and mutant phenotype suggest that Osr2 functions to restrict odontogenic potential in the developing tooth mesenchyme. Consistent with this hypothesis, Bmp4 expression is upregulated and expanded into mesenchyme lingual to first molar buds in Osr2−/− embryos by E13.5, compared with wildtype littermates (Fig. 3A, B, Fig. S4). Moreover, Smad1 activation was enhanced in the molar tooth germ and expanded lingually to the oral epithelium and mesenchyme in Osr2−/− embryos, compared with wildtype littermates (Fig. 3C, D).
Fig. 3.
(A, B) Bmp4 mRNA expression in E13.5 wildtype (A) and Osr2−/− (B) first molar tooth germs. Arrows in B point to lingually expanded Bmp4 expression. (C, D) Increased levels of phospho-Smad1 accompany the supernumerary dental placode (arrow in D) in an E14.5 Osr2−/− embryo, compared with wildtype littermate (C). (E, F) Isolated molar tooth mesenchyme from E13.5 wildtype (E) and Osr2−/− (F) embryos induced tooth formation from E10.5 second branchial arch epithelia. (G, H) Isolated mesenchyme lingual to E13.5 molar tooth germ of Osr2−/− (H), but not that of wildtype (G), induced tooth formation from E10.5 second branchial arch epithelia. White dashed lines in E–H mark the boundary between epithelium and mesenchyme. Insets show sections of renal capsule cultures of corresponding recombinant explants. Numbers in E–H indicate the ratios of corresponding recombinant explants forming teeth in renal capsules. m1, first molar tooth bud; ps, palatal shelf; T, tooth in renal capsule.
To test whether mesenchymal odontogenic field is expanded in Osr2−/− mice, we examined the ability of isolated E13.5 mandibular mesenchyme to induce tooth formation in non-dental epithelia from E10.5 second branchial arches (14). The molar tooth mesenchyme from both wildtype and Osr2−/− embryos induced tooth germ-like structures in non-dental epithelia in recombinant explant cultures (Fig. 3E, F). While mandibular mesenchyme lingual to wildtype molar tooth germs had no odontogenic activity (Fig. 3G), mandibular mesenchyme lingual to Osr2−/− molar tooth germs induced tooth-characteristic changes in non-dental epithelia (Fig. 3H). We next cultured the recombinant tissues in vitro followed by transplanting them under renal capsules to allow complete tooth morphogenesis (14). Histological analyses showed that the mutant, but not wildtype, mandibular mesenchyme lingual to molar tooth germs induced tooth morphogenesis in non-dental epithelium (Fig. 3, E–H), confirming that supernumerary teeth in Osr2−/− mice were induced by lingually expanded mesenchymal signals from the molar tooth germs.
We next investigated whether exogenous Bmp4 was sufficient to induce supernumerary tooth formation lingual to the developing first molars. Implantation of Bmp4-soaked beads lingual to the first molar tooth germs of E13.5 wildtype embryos did not cause supernumerary tooth initiation in any of eighteen mandibular explants examined (Fig. S5), suggesting that supernumerary tooth induction in Osr2−/− mutants involved activation of additional mesenchymal odontogenic signals on the lingual side of the first molars.
Activation of odontogenic potential, including Bmp4 expression, in the dental mesenchyme is mediated by the transcription factor Msx1 (17–20). Mice lacking Msx1 exhibited loss of Bmp4 expression in the dental mesenchyme and molar developmental arrest at the bud stage (17, 21). Since Msx1 is expressed throughout the early tooth mesenchyme (20), the spatially restricted Bmp4 expression in normal dental mesenchyme and the expansion of Bmp4 expression in Osr2−/− tooth mesenchyme suggest that Osr2 repressed Msx1-mediated activation of odontogenic signals. To test this hypothesis, we examined tooth development in mice carrying mutations in both Osr2 and Msx1. In contrast to early tooth developmental arrest in Msx1−/− mice, all five Msx1−/−Osr2−/− mutant pups harvested at E18.5 showed first molar development to late bell stage, with well-patterned ameloblast and odontoblast differentiation (Fig. 4, A–C, and Fig. S6). While Bmp4 expression was down-regulated in Msx1−/− first molar mesenchyme from E12 to E13.5, (Fig. 4D, E, and Fig. S4), it was partially restored in Msx1−/−Osr2−/− littermates (Fig. 4F). By E14.5, both wildtype and Msx1−/−Osr2−/− first molar germs had developed to the cap stage, with similar patterns of Bmp4 expression (Fig. 4G, I) while Msx1−/− first molar germs remained at the bud stage with little Bmp4 expression (Fig. 4H). By E15, both wildtype and Msx1−/−Osr2−/− first molar germs had progressed to late cap stage and strongly expressed Lef1 (Fig. 4J, L), a downstream target of Bmp4 signaling (17), while Msx1−/− first molar germs remained arrested, with little Lef1 expression (Fig. 4K). At all stages examined, however, no supernumerary tooth development was detected in Msx1−/−Osr2−/− mutants. Moreover, the pattern of Osr2 expression during molar tooth development was similar in Msx1−/− and wildtype littermates (Fig. S7). These data indicate that Msx1 and Osr2 act antagonistically to pattern the tooth morphogenetic field by controlling the levels and spatial distribution of mesenchymal odontogenic signals along the buccolingual axis. Disruption of the balance of this antagonistic interaction may underlie supernumerary tooth formation, such as in Osr2−/− mice, and tooth agenesis, such as in Msx1−/− mice and in humans with MSX1 mutations (21, 22).
Fig. 4.
(A–C) Frontal sections through the first molar tooth germs (arrows) of E18.5 wildtype (A), Msx−/− (B), and Msx1−/−Osr2−/− (C) embryos. (D–I) Bmp4 mRNA expression in the first molar tooth mesenchyme (arrows) in wildtype (D, G), Msx1−/− (E, H), and Msx1−/−Osr2−/− (F, I) embryos at E13.5 (D–F) and E14.5 (G-I). (J–L) Lef1 mRNA expression in the first molar tooth mesenchyme (arrows) in E15 wildtype (J), Msx1−/− (K), and Msx1−/−Osr2−/− (L) littermates. ps, palatal shelf; to, tongue. Scale bar, 200 µm.
In Msx1−/−Osr2−/− mutant mice, sufficient levels of Bmp4 were expressed to drive morphogenesis of the first molar teeth but no supernumerary tooth initiated. Remarkably, mandibular second molars also failed to develop in Msx1−/−Osr2−/− mutant mice (Fig. S8). Kavanagh et al. (23) recently showed that mouse mandibular first molar tooth germ inhibited second molar development and proposed an inhibitory cascade model, in which initiation of posterior molars depended on a balance between intermolar inhibition and mesenchymal activation, to account for sequential molar initiation in mammals. Similar activator-inhibitor mechanisms have been proposed for periodic dentition patterning in other vertebrates (3–5, 24), but the molecular underpinnings have not been identified. Bmp4 and Msx1 have been shown to regulate each other in a positive feedback loop in the dental mesenchyme (17–19). Bmp4 also induced expression of ectodin, a secreted Bmp inhibitor whose inactivation caused fusion of first and second molars as well as extra teeth in mice (9, 25). Taken together, these and our finding that Msx1 is required for expansion of the odontogenic field in Osr2−/− mice suggest that the Bmp4-Msx1 pathway is a driving force in the activator-inhibitor network regulating sequential tooth initiation. In mammals, Osr2 suppresses this pathway along the buccolingual axis to restrict molar development to one tooth row. Diversity in dentition patterns in other vertebrates is likely due, at least in part, to evolutionary changes in antagonistic interactions regulating this pathway across the tooth morphogenetic field. In addition, reiterative initiation of other ectodermal organs, such as feather buds and taste papillae, which are also controlled by epithelial-mesenchymal interactions (6, 25, 26), may be driven by propagation of mesenchymal activators through a similar mechanism.
Supplementary Material
Materials and Methods
Figs. S1, S2, S3, S4, S5, S6, S7, S8
Footnotes
This manuscript has been accepted for publication in Science. This version has not undergone final editing. Please refer to the complete version of record at http://www.sciencemag.org/. The manuscript may not be reproduced or used in any manner that does not fall within the fair use provisions of the Copyright Act without the prior, written permission of AAAS.**
References and Notes
- 1.Tucker A, Sharpe PT. Nat. Rev. Genet. 2004;5:499. doi: 10.1038/nrg1380. [DOI] [PubMed] [Google Scholar]
- 2.Streelman JT, Web JF, Albertson RC, Kocher TD. Evol. Dev. 2003;5:600. doi: 10.1046/j.1525-142x.2003.03065.x. [DOI] [PubMed] [Google Scholar]
- 3.Smith MM. Evol. Dev. 2003;5:394. doi: 10.1046/j.1525-142x.2003.03047.x. [DOI] [PubMed] [Google Scholar]
- 4.Fraser GJ, Graham A, Smith MM. J. Exp. Zool. 2006;306B:183. doi: 10.1002/jez.b.21097. [DOI] [PubMed] [Google Scholar]
- 5.Fraser GJ, Bloomquist RF, Streelman JT. BMC Biol. 2008;6:32. doi: 10.1186/1741-7007-6-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pispa J, Thesleff I. Dev. Biol. 2003;262:195. doi: 10.1016/s0012-1606(03)00325-7. [DOI] [PubMed] [Google Scholar]
- 7.Zhang Q, et al. Dev. Dyn. 2003;227:78. doi: 10.1002/dvdy.10289. [DOI] [PubMed] [Google Scholar]
- 8.Mustonen T, et al. Dev. Biol. 2003;259:123. doi: 10.1016/s0012-1606(03)00157-x. [DOI] [PubMed] [Google Scholar]
- 9.Kassai Y, et al. Science. 2005;309:2067. doi: 10.1126/science.1116848. [DOI] [PubMed] [Google Scholar]
- 10.Klein OD, et al. Dev. Cell. 2006;11:181. doi: 10.1016/j.devcel.2006.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Peterkova R, Lesot H, Peterka M. J. Exp. Zool. 2006;306B:234. doi: 10.1002/jez.b.21093. [DOI] [PubMed] [Google Scholar]
- 12.Lan Y, Kingsley PD, Cho E-S, Jiang R. Mech. Dev. 2001;107:175. doi: 10.1016/s0925-4773(01)00457-9. [DOI] [PubMed] [Google Scholar]
- 13.Lan Y, et al. Development. 2004;131:3207. doi: 10.1242/dev.01175. [DOI] [PubMed] [Google Scholar]
- 14.Materials and methods are available as supporting material on Science online.
- 15.Mucchielli ML, et al. Dev. Biol. 1997;189:275. doi: 10.1006/dbio.1997.8672. [DOI] [PubMed] [Google Scholar]
- 16.Dassule HR, McMahon AP. Dev. Biol. 1998;202:215. doi: 10.1006/dbio.1998.8992. [DOI] [PubMed] [Google Scholar]
- 17.Chen YP, Bei M, Woo I, Satokata I, Mass R. Development. 1996;122:3035. doi: 10.1242/dev.122.10.3035. [DOI] [PubMed] [Google Scholar]
- 18.Bei M, Maas R. Development. 1998;125:4325. doi: 10.1242/dev.125.21.4325. [DOI] [PubMed] [Google Scholar]
- 19.Maas R, Bei M. Crit. Rev. Oral Biol. Med. 1997;8:4. doi: 10.1177/10454411970080010101. [DOI] [PubMed] [Google Scholar]
- 20.Keranen SV, Aberg T, Kettunen P, Thesleff I, Jernvall J. Dev. Genes Evol. 1998;208:477. doi: 10.1007/s004270050206. [DOI] [PubMed] [Google Scholar]
- 21.Satokata I, Maas R. Nat. Genet. 1994;6:348. doi: 10.1038/ng0494-348. [DOI] [PubMed] [Google Scholar]
- 22.Vastardis H, Karimbux N, Guthua SW, Seidman JG, Seidman CE. Nat. Genet. 1996;13:417. doi: 10.1038/ng0896-417. [DOI] [PubMed] [Google Scholar]
- 23.Kavanagh KD, Evans AR, Jernvall J. Nature. 2007;449:427. doi: 10.1038/nature06153. [DOI] [PubMed] [Google Scholar]
- 24.Kulesa PM, et al. Theor. Biol. 1996;180:287. [Google Scholar]
- 25.Laurikkala J, Kassai Y, Pakkasjarvi L, Thesleff I, Itoh N. Dev. Biol. 2003;264:91. doi: 10.1016/j.ydbio.2003.08.011. [DOI] [PubMed] [Google Scholar]
- 26.Jung H-S, et al. Dev. Biol. 1998;196:11. doi: 10.1006/dbio.1998.8850. [DOI] [PubMed] [Google Scholar]
- 27.This work was supported by NIH grants R01DE013681 (R.J.) and T32DE007202 (Z.Z.). We thank K. Maltby for technical assistance, K. Ackerman, C.E. Ovitt, and M. Dixon for comments.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Materials and Methods
Figs. S1, S2, S3, S4, S5, S6, S7, S8