Abstract
The use of human neural progenitor cells (hNPC) has been proposed to provide neuronal replacement or astrocytes delivering growth factors for brain disorders such as Parkinson’s and Huntington’s disease. Success in such studies likely requires migration from the site of transplantation and integration into host tissue in the face of ongoing damage. In the current study, hNPC modified to release glial cell line derived neurotrophic factor (hNPCGDNF) were transplanted into either intact or lesioned animals. GDNF release itself had no effect on the survival, migration or differentiation of the cells. The most robust migration and survival was found using a direct lesion of striatum (Huntington’s model) with indirect lesions of the dopamine system (Parkinson’s model) or intact animals showing successively less migration and survival. No lesion affected differentiation patterns. We conclude that the type of brain injury dictates migration and integration of hNPC which has important consequences when considering transplantation of these cells as a therapy for neurodegenerative diseases.
Keywords: Parkinson’s disease, Huntington’s disease, striatum, stem cell transplants
Introduction
The possibility of using transplantation therapies for the treatment of both Parkinson’s disease and Huntington’s disease has been widely discussed (3,9). This may be to either replace damaged neurons which would require neuronal differentiation and integration, or provide supportive glial cells that secrete trophic factors such as GDNF that are known to promote the survival of degenerating neurons in various disease models (27) . Clearly, both the cell source and the host environment will dictate how and if the cells integrate and survive. Early studies where primary rodent fetal brain tissue (which contains a mixture of stem cells, progenitor cells and terminally differentiated cells) was grafted into the striatum have shown that mild to moderate lesions can significantly increase the survival of the transplants (30). This was presumably due to a combination of chemokine and growth factor release at the injury site supporting the new cells, in addition to the creation of more physical space within the brain due to the lesion.
Recent developments in tissue culture methods have allowed the expansion of neural stem and progenitor cells from primary rodent and human brain tissue (1,29). Some studies have shown that transplantation of neural stem or progenitor cells into non neurogenic regions of the adult intact brain leads to the generation of mainly astrocytes (32) unless the cells are first pre-differentiated into post mitotic neurons in vitro (4). Other studies have transplanted neural stem and progenitor cells into areas of the adult brain undergoing active neurogenesis such as the hippocampus and sub ventricular zone, and shown that the cells can identify local cues and follow endogenous patterns of proliferation and differentiation into neurons (8,11,25). In some cases these cells have been shown to survive for extended periods of time and integrate well into the host brain tissue (17). Thus, the environment seems to play a crucial role in the survival and differentiation of both primary fetal tissue and stem or progenitor cell transplants which have the potential to form long term grafts within the brain.
As Parkinson’s and Huntington’s diseases involve the loss of different sets of neurons within the brain, it is likely that the transplanted stem or progenitor cells will react differently to the two environments. Furthermore, modifying the cells to release factors such as GDNF may affect their survival and integration. However, there are no systematic studies that have tested how identical sets of neural stem or progenitor cells survive, migrate, integrate and differentiate when transplanted into these two models, or how GDNF may affect this process. To test this we generated a line of human neural progenitor cells (34) that were also modified to release GDNF (2) and showed that their survival and migration, but not differentiation, were critically dependent on the type of lesion environment they were exposed to, but not on the release of GDNF.
Methods
Cell growth
Human cortical neural progenitor cells were isolated from primary human fetal cortex between 10 and 15 weeks of gestation according to protocols approved by the NIH and local ethics committee at the University of Wisconsin Madison and by the University of Washington Birth Defects Laboratory. Following dissociation in 0.1% trypsin, cells were seeded at 200,000 cells per ml in T75 flasks containing DMEM/Ham’s F12 (Gibco-BRL) supplemented with penicillin/streptomycin (Gibo-BRL,1%), N2 (Gibco-BRL, 1%), epidermal growth factor (Sigma, 20 ng/ml) with heparin (Sigma, 5 µg/ml)and fibroblast growth factor– 2 (R&D, 20 ng/ml). Neurosphere colonies rapidly formed and were expanded by chopping using an automated tissue chopper as described in detail previously (28). At 10 weeks the cells were switched to medium containing LIF (Chemicon, 10 ng/ml) and under these conditions the cultures could be grown for at least another 30 weeks in a relatively stable form based on gene expression patterns and differentiation potential (33).
Lentiviral infection
As described in detail previously (5), the mouse phosphoglycerate kinase 1 (PGK) promoter (strong constitutive promoter) drives the tetracycline transactivator (tTA1) in the lenti-tTA construct. The tTA1 binds to the tetracycline operon that is upstream of a minimal promoter driving GDNF in the lenti-GDNF construct. The lentiviral particles were suspended in 1% fetal bovine serum albumin in phosphate buffered saline. For lentivirus infection, neurospheres were dissociated to single cells and infected with 5 ng of lenti-GDNF and 10 ng of lenti-tTA per 105 cells for 24 hours before the virus was diluted out with fresh medium. New spheres then formed through aggregation and allowed to expand before transplantation. Non-infected wild-type hNPC (hNPCWT) were grown in parallel.
Partial 6-OHDA and QA lesions
Adult male Lewis rats (300–350 g) were injected with a total of 21 µg of 6-hydroxydopamine (6-OHDA) resuspended in 0.9% saline with 0.2 µg ascorbic acid. Injections of 7 µg/3.5 µl were made over 3 sites into the striatum (calculated from bregma): AP +1.0, ML −3.0, DV −5.0; AP −0.1, ML −3.7, DV −5.0; AP −1.2, ML −4.5, DV −5.0. For quinolinic acid (QA) lesions, adult male Lewis rats (300–350 g) received a single 200nM injection of QA into the striatum at AP +0.48, ML −3.0, DV −5.0 from bregma. Animals received cell transplants into the striatum one week following the lesion.
Cell Transplants
Cells were plated as neurospheres for 1 week in B27 differentiation media supplemented with cilliary neurotrophic factor (5 ng/µl, R&D). To prepare cells for transplantation, medium was removed, cells were rinsed, and accutase was added for 15 min at 37°C to detach plated spheres from the flask. hNPCWT or hNPCGDNF were collected, centrifuged at 1000 RPM for 5 minutes, accutase removed, and cells rinsed in plating media. Cells were then incubated in DNAase for 10 minutes at 37°C, centrifuged at 1000 RPM for 5 minutes, DNAase removed, and cells dissociated in Liebovitz/0.6% glucose (1:1) supplemented with B27 (1:50). Cells were then counted, centrifuged at 1000 RPM for 5 minutes, re-suspended at 66,500/µl in fresh Liebovitz/0.6% glucose/B27, and maintained on ice.
Control non-lesioned rats were transplanted with hNPCGDNF at one site (AP −0.3, ML −3.6, DV −5.2). In QA lesioned rats, hNPCWT (n=7) or hNPCGDNF (n=7) were transplanted into one site at AP −0.3, ML −3.6, DV −5.2. Another group received QA lesion but no transplant (n=5). In 6-OHDA lesioned rats, hNPCGDNF (n=6) were transplanted into one site at AP 0.3, ML −3.5, DV − 5.2. A 10 µl Hamilton syringe was lowered from dura, left in place for 1 minute before injecting 3 µl cells over 3 minutes, and retracted after an additional 3 minutes. Animals were injected with cyclosporine (i.p. 10 mg/kg) 1 day before and every day following transplantation. At 7 weeks post-transplantation animals were perfused with chilled 0.9% saline and 4% paraformaldehyde. Brains were cryoprotected in 30% sucrose with 0.1% sodium azide, and sectioned to 40 µm using a sliding microtome.
Immunohistochemistry
Sections were stained for human nuclei (Chemicon, mouse 1:200) or GDNF (R&D, goat 1:250). For human nuclei, sections were rinsed in Tris-HCl, incubated in 2N HCl for 30 minutes at 37°C, quenched in 5% H2O2 and 10% methanol, and blocked in 10% normal horse serum prior to primary antibody. Biotinylated mouse secondary (Vector labs, 1:2000) and ABC (Vectastain kit) were followed by DAB (Sigma tablets) development. For human GDNF, sections were rinsed in TBS containing Triton, quenched in 0.1 M sodium periodate and blocked in 3% NHS and 2% BSA prior to primary antibody. Biotinylated goat secondary (Vector labs, 1:2000) and ABC were followed by DAB development with nickel ammonium sulfate enhancement. For double labeling with human nuclei and nestin, GFAP, or NeuN, sections were processed as above, but primary antibodies for human nuclei, nestin (Chemicon, rabbit 1:200), GFAP (DAKO, rabbit 1:500), and NeuN (Chemicon, mouse 1:100) were followed by mouse AF546- and rabbit AF488-conjugated secondary antibodies (Molecular Probes, 1:500).
Cell counts
All human nuclei positive cells were counted in every 6th section of every rat in each group through the rostral/caudal extent of the striatum beginning in sections containing the anterior portion of the lateral ventricle to the hippocampus. For counts using confocal microscopy, human nuclei positive cells co-labeled with either GFAP or nestin were counted in randomly selected sections for 4 rats in each group. Cell counts ranged from approximately 200–500.
Data analysis
Human nuclei, nestin, and GFAP cell counts were all analyzed by one-way ANOVA or Student’s T-test. Human nuclei positive cell distribution was analyzed by area under the curve calculations performed in Prism. All data are presented ± S.E.M.
Results
Infection of hNPC with lenti GDNF does not affect their survival, migration, or differentiation following transplantation into QA lesioned rats
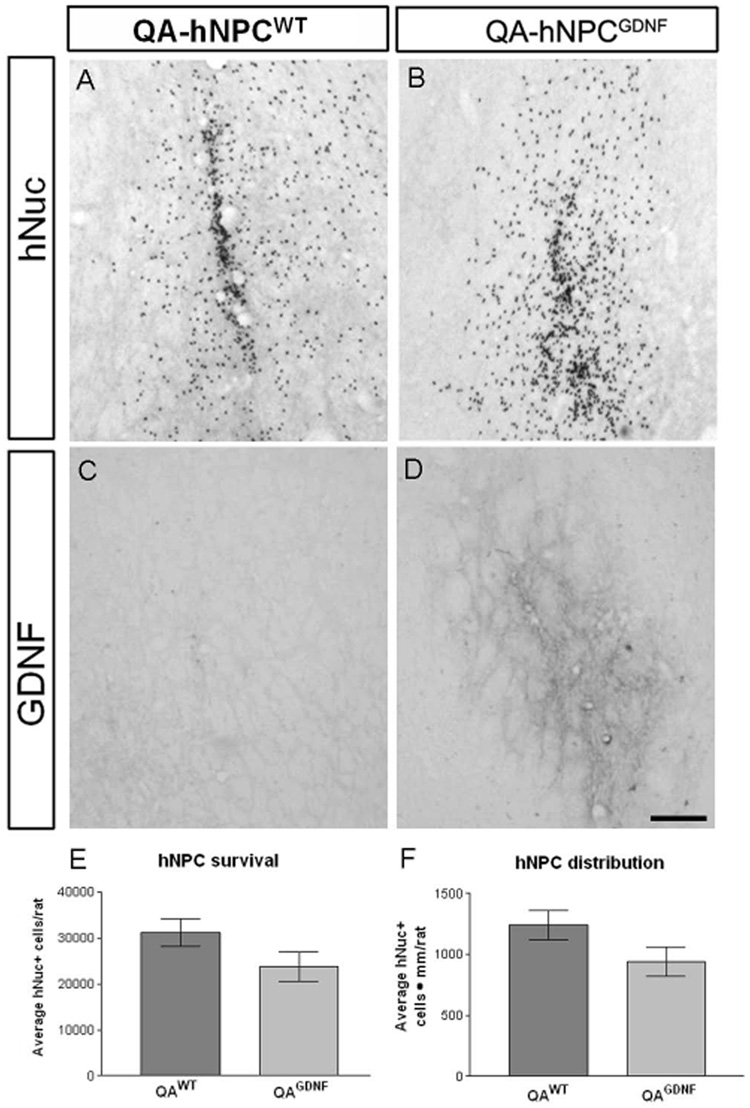
We have previously shown that wild type (WT) hNPC not modified to release GDNF can survive and migrate within the striatum of rats lesioned with either quinolinic acid (18) or 6-hydroxydopamine (6-OHDA; (2)). However, we did not assess the possible effects of GDNF itself on the survival, migration or differentiation following transplantation. To address this question, adult rats (n=19) were lesioned with an intrastriatal injection of QA. Stable lines of hNPC expressing GDNF were generated as described previously (2) and grown for 9 weeks in vitro. One week following the lesion, either hNPCWT (n=7) or hNPCGDNF (n=7) were transplanted into the striatum surrounding the lesion site. Five rats were used as no transplant lesion controls. All animals were treated daily with cyclosporine and sacrificed at 7 weeks post-lesion. Using the specific human nuclear antigen marker (hNuc), substantial transplants were evident in both groups of transplanted rats (Fig. 1A,B), but no cells were seen in the non transplanted controls confirming the specificity of this marker (data not shown). GDNF immunohistochemistry revealed local expression around the transplantation site in the hNPCGDNF group, but not the hNPCWT group (Fig. 1C,D), that correlated exactly with the hNuc staining. This showed the hNPCWT do not release GDNF.
Figure 1.
Infection with a lentivirus encoding GDNF does not affect the survival or migration of transplanted hNPC compared to non-transduced hNPC. hNuc positive cells are observed in the striatum of QA lesioned rats (A and B), but only the cells infected with lenti-GDNF express GDNF in the area immediately surrounding the transplant (C and D). Counts of hNuc positive cells showed no difference in total survival (E) or migration through the striatum (F) between hNPCWT and hNPCGDNF. Scale bar = 50µm
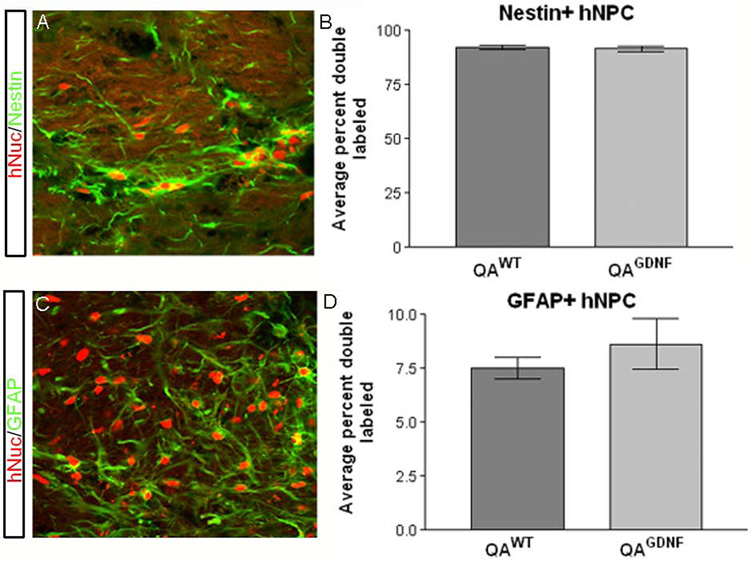
The average number of hNuc positive cells was not significantly different between the transplant groups (31,076 ± 3,002 for hNPCWT, 23,766 ± 3,181 for hNPCGDNF; Fig 1E). Similarly, there was no difference in the anterior-posterior distribution based on area under the curve calculations of the number of hNuc+ cells observed in brain slices (1241.0±119.8 hNPCWT, 942.8±121.0 hNPCGDNF hNuc+ cells · mm; Fig 1F). Double labeling with hNuc and antibodies to either nestin or glial fibrillary acidic protein (GFAP) revealed that the vast majority of hNuc positive cells in both groups remained nestin positive (92.3±0.88% hNPCWT, 91.6±1.4% hNPCGDNF; Fig 2A,B), whereas only a small proportion were positive for both hNuc and GFAP (7.5±0.5% hNPCWT, 8.6±1.2% hNPCGDNF; Fig 2C,D). There were no hNuc positive cells that co-expressed NeuN to label neurons (data not shown). Together these data show that GDNF releasing hNPC behave in an identical fashion to wild type hNPC following transplantation into the lesioned striatum.
Figure 2.
Lenti-GDNF infection does not alter differentiation potential of transplanted hNPC. Confocal microscopy and subsequent quantification showed that the vast majority (92.3±0.88% hNPCWT, 91.6±1.4% hNPCGDNF) of transplanted hNPC remained nestin positive (A and B) and only approximately 8% of hNuc positive cells co-labeled with GFAP (7.5±0.5% hNPCWT, 8.6±1.2% hNPCGDNF, C and D) regardless of infection with a lentivirus encoding GDNF. Only a negligible amount of hNuc positive cells double labeled with NeuN (<1%; data not shown). There is no significant difference for either the nestin or GFAP results.
The lesion environment controls the survival and migration of hNPCGDNF
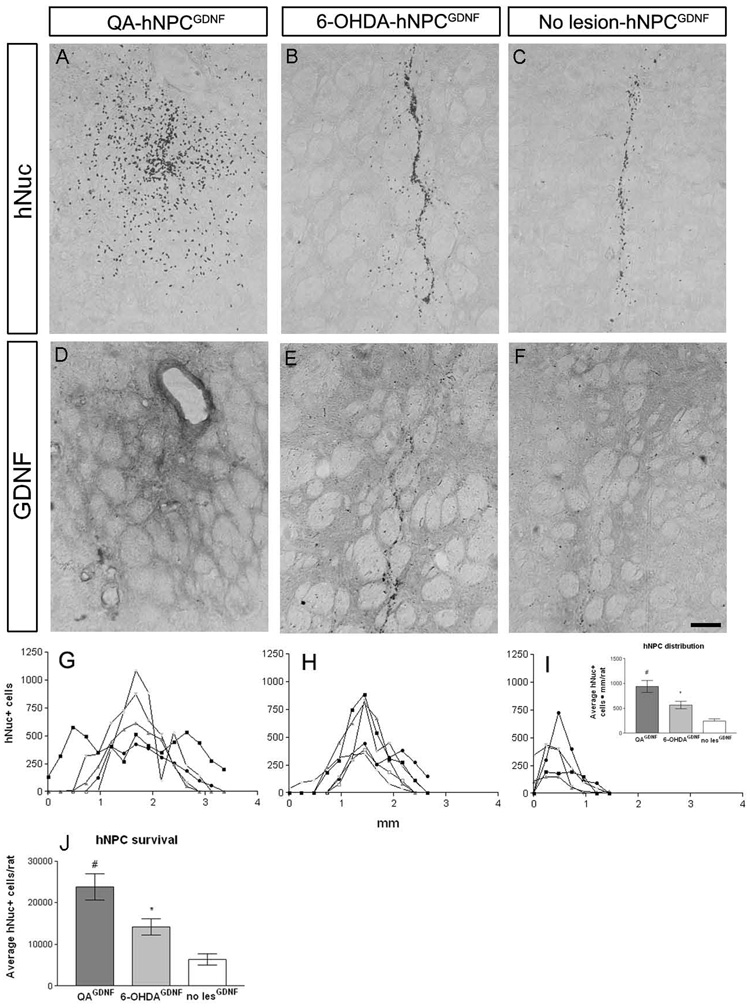
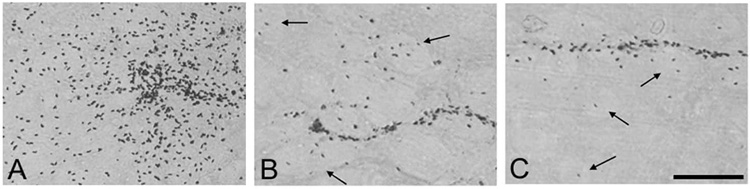
We next addressed how the lesion environment may modify cell survival, migration and differentiation. hNPCGDNF were transplanted into the striatum of intact rats (n=7) and seven days following either 6-OHDA (n=6) or QA (n=7) lesions. All three groups showed surviving cells within the transplant with hNuc immunohistochemistry (Fig. 3A,B and C). Furthermore, GDNF protein was observed in the striatum in the area immediately surrounding the transplant site that decreased in intensity further from the transplant core (Fig. 3 D,E, and F). However, there was considerably more spread of the cells away from the graft site in the QA lesioned animals when compared to the 6-OHDA lesioned animals, and even more so compared to non lesioned animals (Fig. 3G,H, I, and J). This migration of cells was not simply diluting out the total number of cells transplanted as counts revealed that there were significantly more hNuc positive hNPCGDNF in the QA lesioned rats compared to 6-OHDA lesioned rats and intact animals (23,766 ± 3,181 QA, 14,194 ± 1,899 6-OHDA, 6,306±1,326 non lesioned, Fig. 3K). Moreover, cells in the QA lesioned brain showed a preference for the collapsed gray matter within the striatum, whereas cells in the 6-OHDA lesioned and unlesioned brain primarily remained in the transplant core and/or migrated into white matter tracts (Fig 4A,B and C).
Figure 3.
The type of lesion dramatically affected the migration and survival of GDNF expressing hNPC. hNPCGDNF transplanted into the striatum of QA lesioned (A), 6-OHDA lesioned (B) or unlesioned (C) rats survived and expressed GDNF in the area surrounding the transplant core with a gradient of GDNF expression further from the core (D–F). However, hNPCGDNF transplanted into the striatum of QA lesioned rats migrated significantly further in the QA lesioned brain than in either the 6-OHDA lesioned or unlesioned brains (using area under the curve measurements (hNuc+ cells · mm), G–J). Graphs G–I show the number of cells that were counted in each brain section of individual rats within each group. hNuc positive cells were found in 15 consecutive sections in the QA lesioned rats compared to 12 in the 6-OHDA rats and 7 in the unlesioned rats. The graph in J shows the average area under the curve values for the three groups (942.8±121.0QA, 564.3±76.4 6-OHDA, 240.6±51.6 unlesioned; #p<0.05 compared to 6-OHDA and unlesioned; *p<0.05 compared to unlesioned). Furthermore, hNPCGDNF survived significantly more in QA lesioned brain compared to hNPCGDNF in both 6-OHDA and unlesioned brain (#p<0.05, K). hNPCGDNF transplanted into 6-OHDA rats survived better than in unlesioned rats (*p<0.05, K). The hole visible in D (and also present in A) could be necrotic tissue at the site of QA injection. Scale bar = 50µm
Figure 4.
High power images showing hNuc positive cells in the white matter tracts (indicated by arrows) in 6-OHDA lesioned (B) and unlesioned rats (C). There is a complete loss of white matter bundles in the QA lesioned rats (A), so the hNuc positive cells remain in the gray matter. Scale bar = 50µm
The differentiation of hNPCGDNF is not affected by the lesion environment
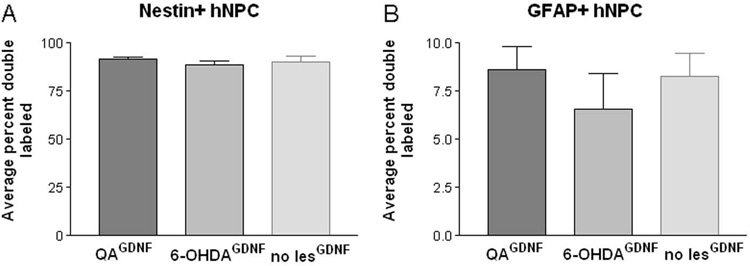
We next asked whether the lesion environment had any effect on the maturation of the cells into neurons or astrocytes. In agreement with our previous studies (2,15,18) very few of the hNPCGDNF matured into neurons under these conditions, and this was not affected by the lesion type (data not shown). Confocal microscopic analysis of immunofluorescent labeling for human nuclei and either GFAP or nestin showed that approximately 90% of the hNuc positive cells remained nestin positive (91.6±1.4% QA, 88.7±2.2% 6-OHDA, 90.4±2.6% unlesioned), and only a small proportion of hNuc positive cells co-labeled with GFAP (8.6±1.2% QA, 6.6±1.9% 6-OHDA, 8.3±1.2% unlesioned, Fig 5A and B). We conclude that the lesion, while affecting the overall survival and migration of the hNPCGDNF, did not affect their default nestin positive status, or enhance their differentiation into neurons or GFAP positive astrocytes.
Figure 5.
The type of lesion did not alter the differentiation properties of hNPCGDNF in vivo. Quantification of confocal microscopic analysis revealed that the majority of cells (91.6±1.4% QA, 88.7±2.2% 6-OHDA, 90.4±2.6% unlesioned) remained nestin positive. Only a small percentage of hNuc positive cells co-labeled with GFAP (8.6±1.2% QA, 6.6±1.9% 6-OHDA, 8.3±1.2% unlesioned). There is no significant difference for either the nestin or GFAP results. Only a negligible amount of hNuc positive cells double labeled with NeuN (<1%; data not shown).
Discussion
In the current study, we show that direct lesions of cell bodies in the striatum provide an enhanced environment for stem cell survival compared to either terminal lesions of dopamine fibers in the striatum or an intact striatum. Neural stem cells have been transplanted into a wide variety of disease models with many different reported outcomes. Interpretation is complicated by the fact that the source of stem cells in each study is often very different with regard to region of origin, age of donor, species of donor and in some cases immortalizing agents that were used. However, in most cases when a non lesion environment is compared to a lesioned environment, the stem cells show better survival and integration into the host tissue when transplanted into a lesioned, but non necrotic, environment. For example, immortalized neural stem cells will migrate towards damage in the brain caused by hypoxia (22), and human neural stem cells migrate towards damage induced by stroke, but do not survive well if placed in the necrotic core (13). Similarly, in diseases of the myelin sheath where there is excessive white matter damage, human neural stem cells isolated from either the adult or developing brain survive and integrate well when transplanted into the shiverer mouse (31). In models of multiple sclerosis, stem cells can migrate in from the periphery to the central nervous system in response to neuronal injury, inflammation and damage to the blood brain barrier (23). We have previously shown that when mutant SOD1 is expressed in rats, the environment around degenerating motor neurons encourages the survival and migration of hNPC releasing GDNF (15). The exact signals released by the damaged brain that encourage stem cell migration and survival remain to be established, although release of growth factors such as IGF-1 or specific chemoattractants from damaged tissues may play a crucial role (35,36). Furthermore, neural stem cells express CXC chemokine receptor 4 (CXCR4) on their surface which may home in to the ligand stromal cell-derived factor 1 alpha that is up regulated in areas of inflammation (10). Further experiments will be required to establish why cell body lesions of the striatum increase stem cell survival. However, it is possible that increasing the lesion size further may not be beneficial because previous reports suggest that there is a threshold above which damage to the striatum or hippocampus leads to a reduction in tissue graft survival (24,30).
While the lesion environment increased survival and migration, it did not affect the maturation of the cells into either neurons or GFAP positive astrocytes. A recent study showed differentiation of neural stem cells derived from human embryonic stem cells at 6 weeks post-transplant in a QA lesioned adult rat striatum (12). Our results could differ from this report because of the cell type and total number transplanted. Furthermore, hNPC require one round of final cell division to produce neurons within a neurogenic environment (20, 21). We hypothesize that the intact or lesioned brain environment does not provide those signals which results in a block on division and differentiation into neurons. Clearly, transplantation of similar cells into a neurogenic environment, such as the neonatal rat brain, sub ventricular zone of the forebrain or the hippocampus, does lead to neuron formation (7,8), perhaps through exposure to the correct proliferation signals and a final round of division within the brain. While not yet established, it also appears that rapid differentiation into GFAP positive astrocytes is also not occurring in any of these environments. We have previously shown that it takes over 20 weeks for GFAP positive astrocytes to develop from hNPC following transplantation into the 6-OHDA lesioned striatum (26), and so perhaps longer time periods are required for full maturation.
GDNF has been shown to protect both dopamine neurons and striatal neurons undergoing degeneration in models of Parkinson’s and Huntington’s disease when delivered directly to the striatum of primates and rodents using in vivo gene therapy approaches (14,16,19) Ex vivo approaches in which stem cells are modified to release GDNF and then transplanted offer an interesting alternative that does not require the injection of live virus to the brain. However, to achieve widespread delivery of GDNF, it will be necessary for the cells to migrate and integrate across wide areas. Our data suggest that the intact, non damaged striatum does not provide a good environment for hNPCGDNF migration and delivery. Modest damage caused by 6-OHDA lesions to the terminals in the striatum, but not the cell bodies, resulted in far greater amounts of migration and survival, which could be further enhanced by direct lesions of cell bodies within the striatum by QA. Although it is impossible to relate this to the exact situation in patients, we suggest that the damage caused by ongoing cell death in Parkinson’s disease may promote the survival and integration of transplanted cells. However, it was also clear that many of the transplanted cells migrated within the white mater tracts of the internal capsule, avoiding the intact gray matter of the striatum. As the human striatum is divided into the gray matter of the caudate nucleus and putamen with all of the white matter tracts bundled into the internal capsule, there could be very different levels of migration than what is seen in the rodent model. Indeed, our preliminary studies transplanting these cells into the rhesus striatum have shown reduced migration and more compact grafts (2,6). This situation may be very different in Huntington’s disease models. While we found migration of the cells to other regions of the brain, they also collected within the striatum in both white and gray matter (Fig. 4; (18)). Although primate studies have not yet been performed using Huntington’s models, we predict better survival and migration of the transplants than seen in our Parkinson’s model studies. This would allow good delivery of GDNF and replacement of glial cells to a wide area of the Huntington’s diseased brain.
In conclusion we have shown that hNPC releasing GDNF are very sensitive to the host environment. They survive and migrate poorly when there is no damage, better when there is minimal direct damage and best when there is greater degeneration at the site of transplantation. This is a remarkable feature of human neural progenitor cells. They do not seem to be damaged by the reactive environment, but rather thrive amongst the degenerating cells. Thus, we postulate that this feature of the lesion environment providing support for cell migration and survival of human cells will be of high significance when moving forward into clinical trials for neurodegenerative diseases. The challenge will be to predict the exact lesion environment prior to transplantation in order to provide the best dosing of cells and estimate of migration. Further studies in this area using non human primates are now essential, and this may be aided by imaging methods such as PET scanning for dopamine metabolism or MRI for striatal atrophy. Appreciating the preference of human neural progenitor cells for certain in vivo environments, and assessing the lesion state prior to transplantation, may provide Critical predictive information to optimize their survival, migration, and therapeutic potential.
Acknowledgements
The authors would like to thank Shengli Xi, Elizabeth Capowski and Jacalyn McHugh for technical assistance. We would also like to thank Patrick Aebischer for supplying the lenti GDNF constructs. This work was supported by the NIH Stem Cell Training Program T32AG027566 (ADE) and the Department of Defense (DAMD17-03-1-0122).
Reference List
- 1.Barker RA, Jain M, Armstrong RJ, Caldwell MA. Stem cells and neurological disease. J. Neurol. Neurosurg. Psychiatry. 2003;74:553–557. doi: 10.1136/jnnp.74.5.553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Behrstock S, Ebert A, McHugh J, Vosberg S, Moore J, Schneider B, Capowski E, Hei D, Kordower J, Aebischer P, Svendsen CN. Human neural progenitors deliver glial cell line-derived neurotrophic factor to parkinsonian rodents and aged primates. Gene Ther. 2006;13:379–388. doi: 10.1038/sj.gt.3302679. [DOI] [PubMed] [Google Scholar]
- 3.Bjorklund A, Lindvall O. Cell replacement therapies for central nervous system disorders. Nat. Neurosci. 2000;3:537–544. doi: 10.1038/75705. [DOI] [PubMed] [Google Scholar]
- 4.Burnstein RM, Foltynie T, He X, Menon DK, Svendsen CN, Caldwell MA. Differentiation and migration of long term expanded human neural progenitors in a partial lesion model of Parkinson's disease. Int. J. Biochem. Cell Biol. 2004;36:702–713. doi: 10.1016/j.biocel.2003.11.011. [DOI] [PubMed] [Google Scholar]
- 5.Deglon N, Tseng JL, Bensadoun JC, Zurn AD, Arsenijevic Y, Pereira dA, Zufferey R, Trono D, Aebischer P. Self-inactivating lentiviral vectors with enhanced transgene expression as potential gene transfer system in Parkinson's disease. Hum. Gene Ther. 2000;11:179–190. doi: 10.1089/10430340050016256. [DOI] [PubMed] [Google Scholar]
- 6.Emborg ME, Ebert AD, Moirano J, Peng S, Suzuki M, Capowski E, Joers V, Roitberg BZ, Aebischer P, Svendsen CN. GDNF-secreting human neural progenitor cells increase tyrosine hydroxylase and VMAT2 expression in MPTP-treated cynomolgus monkeys. Cell Transplant. 2008;17:383–395. [PubMed] [Google Scholar]
- 7.Englund U, Fricker-Gates RA, Lundberg C, Bjorklund A, Wictorin K. Transplantation of human neural progenitor cells into the neonatal rat brain: extensive migration and differentiation with long-distance axonal projections. Exp. Neurol. 2002;173:1–21. doi: 10.1006/exnr.2001.7750. [DOI] [PubMed] [Google Scholar]
- 8.Fricker RA, Carpenter MK, Winkler C, Greco C, Gates MA, Bjorklund A. Site-specific migration and neuronal differentiation of human neural progenitor cells after transplantation in the adult rat brain. J. Neurosci. 1999;19:5990–6005. doi: 10.1523/JNEUROSCI.19-14-05990.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Goldman SA, Windrem MS. Cell replacement therapy in neurological disease. Philos. Trans. R. Soc. B - Biol. Sci. 2006;361:1463–1475. doi: 10.1098/rstb.2006.1886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Imitola J, Raddassi K, Park KI, Mueller FJ, Nieto M, Teng YD, Frenkel D, Li J, Sidman RL, Walsh CA, Snyder EY, Khoury SJ. Directed migration of neural stem cells to sites of CNS injury by the stromal cell-derived factor 1alpha/CXC chemokine receptor 4 pathway. Proc. Natl. Acad. Sci. U. S. A. 2004;101:18117–18122. doi: 10.1073/pnas.0408258102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jain M, Armstrong RJ, Elneil S, Rosser AE, Barker RA. Migration and differentiation of transplanted human neural precursor cells. NeuroReport. 2003;14:1257–1262. doi: 10.1097/00001756-200307010-00014. [DOI] [PubMed] [Google Scholar]
- 12.Joannides AJ, Webber DJ, Raineteau O, Kelly C, Irvine KA, Watts C, Rosser AE, Kemp PJ, Blakemore WF, Compston A, Caldwell MA, Allen ND, Chandran S. Environmental signals regulate lineage choice and temporal maturation of neural stem cells from human embryonic stem cells. Brain. 2007;130:1263–1275. doi: 10.1093/brain/awm070. [DOI] [PubMed] [Google Scholar]
- 13.Kelly S, Bliss TM, Shah AK, Sun GH, Ma M, Foo WC, Masel J, Yenari MA, Weissman IL, Uchida N, Palmer T, Steinberg GK. Transplanted human fetal neural stem cells survive, migrate, and differentiate in ischemic rat cerebral cortex. Proc. Natl. Acad. Sci. U. S. A. 2004;101:11839–11844. doi: 10.1073/pnas.0404474101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kirik D, Georgievska B, Bjorklund A. Localized striatal delivery of GDNF as a treatment for Parkinson disease. Nat. Neurosci. 2004;7:105–110. doi: 10.1038/nn1175. [DOI] [PubMed] [Google Scholar]
- 15.Klein SM, Behrstock S, McHugh J, Hoffmann K, Wallace K, Suzuki M, Aebischer P, Svendsen CN. GDNF delivery using human neural progenitor cells in a rat model of ALS. Hum Gene Ther. 2005;16:509–521. doi: 10.1089/hum.2005.16.509. [DOI] [PubMed] [Google Scholar]
- 16.Kordower JH, Emborg ME, Bloch J, Ma SY, Chu Y, Leventhal L, McBride J, Chen EY, Palfi S, Roitberg BZ, Brown WD, Holden JE, Pyzalski R, Taylor MD, Carvey P, Ling Z, Trono D, Hantraye P, Deglon N, Aebischer P. Neurodegeneration prevented by lentiviral vector delivery of GDNF in primate models of Parkinson's disease. Science. 2000;290:767–773. doi: 10.1126/science.290.5492.767. [DOI] [PubMed] [Google Scholar]
- 17.Lepore AC, Neuhuber B, Connors TM, Han SS, Liu Y, Daniels MP, Rao MS, Fischer I. Long-term fate of neural precursor cells following transplantation into developing and adult CNS. Neuroscience. 2006;139:513–530. doi: 10.1016/j.neuroscience.2005.12.043. [DOI] [PubMed] [Google Scholar]
- 18.McBride JL, Behrstock SP, Chen EY, Jakel RJ, Siegel I, Svendsen CN, Kordower JH. Human neural stem cell transplants improve motor function in a rat model of Huntington's disease. J. Comp. Neurol. 2004;475:211–219. doi: 10.1002/cne.20176. [DOI] [PubMed] [Google Scholar]
- 19.McBride JL, Ramaswamy S, Gasmi M, Bartus RT, Herzog CD, Brandon EP, Zhou L, Pitzer MR, Berry-Kravis EM, Kordower JH. Viral delivery of glial cell line-derived neurotrophic factor improves behavior and protects striatal neurons in a mouse model of Huntington's disease. Proc. Natl. Acad. Sci. U. S. A. 2006;103:9345–9360. doi: 10.1073/pnas.0508875103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nelson AD, Svendsen CN. Low concentrations of extracellular FGF-2 are sufficient but not essential for neurogenesis from human neural progenitor cells. Mol. Cell. Neurosci. 2006;33:29–35. doi: 10.1016/j.mcn.2006.06.003. [DOI] [PubMed] [Google Scholar]
- 21.Ostenfeld T, Svendsen CN. Requirement for Neurogenesis to Proceed through the Division of Neuronal Progenitors following Differentiation of Epidermal Growth Factor and Fibroblast Growth Factor-2-Responsive Human Neural Stem Cells. Stem Cells. 2004;22:798–811. doi: 10.1634/stemcells.22-5-798. [DOI] [PubMed] [Google Scholar]
- 22.Park KI, Hack MA, Ourednik J, Yandava B, Flax JD, Stieg PE, Gullans S, Jensen FE, Sidman RL, Ourednik V, Snyder EY. Acute injury directs the migration, proliferation, and differentiation of solid organ stem cells: evidence from the effect of hypoxia-ischemia in the CNS on clonal "reporter" neural stem cells. Exp. Neurol. 2006;199:156–178. doi: 10.1016/j.expneurol.2006.04.002. [DOI] [PubMed] [Google Scholar]
- 23.Pluchino S, Zanotti L, Rossi B, Brambilla E, Ottoboni L, Salani G, Martinello M, Cattalini A, Bergami A, Furlan R, Comi G, Constantin G, Martino G. Neurosphere-derived multipotent precursors promote neuroprotection by an immunomodulatory mechanism. Nature. 2005;436:266–271. doi: 10.1038/nature03889. [DOI] [PubMed] [Google Scholar]
- 24.Shindo T, Matsumoto Y, Wang Q, Kawai N, Tamiya T, Nagao S. Differences in the neuronal stem cells survival, neuronal differentiation and neurological improvement after transplantation of neural stem cells between mild and severe experimental traumatic brain injury. J. Med. Invest. 2006;53:42–51. doi: 10.2152/jmi.53.42. [DOI] [PubMed] [Google Scholar]
- 25.Song H, Stevens CF, Gage FH. Astroglia induce neurogenesis from adult neural stem cells. Nature. 2002;417:39–44. doi: 10.1038/417039a. [DOI] [PubMed] [Google Scholar]
- 26.Svendsen CN, Caldwell MA, Shen J, ter Borg MG, Rosser AE, Tyers P, Karmiol S, Dunnett SB. Long-term survival of human central nervous system progenitor cells transplanted into a rat model of Parkinson's disease. Exp. Neurol. 1997;148:135–146. doi: 10.1006/exnr.1997.6634. [DOI] [PubMed] [Google Scholar]
- 27.Svendsen CN, Langston JW. Stem cells for Parkinson disease and ALS: replacement or protection? Nat. Med. 2004;10:224–225. doi: 10.1038/nm0304-224. [DOI] [PubMed] [Google Scholar]
- 28.Svendsen CN, ter Borg MG, Armstrong RJ, Rosser AE, Chandran S, Ostenfeld T, Caldwell MA. A new method for the rapid and long term growth of human neural precursor cells. J. Neurosci. Methods. 1998;85:141–152. doi: 10.1016/s0165-0270(98)00126-5. [DOI] [PubMed] [Google Scholar]
- 29.Tai YT, Svendsen CN. Stem cells as a potential treatment of neurological disorders. Curr. Opin. Pharmacol. 2004;4:98–104. doi: 10.1016/j.coph.2003.09.006. [DOI] [PubMed] [Google Scholar]
- 30.Watts C, Dunnett SB. Effects of severity of host striatal damage on the morphological development of intrastriatal transplants in a rodent model of Huntington's disease: implications for timing of surgical intervention. J. Neurosurg. 1998;89:267–274. doi: 10.3171/jns.1998.89.2.0267. [DOI] [PubMed] [Google Scholar]
- 31.Windrem MS, Nunes MC, Rashbaum WK, Schwartz TH, Goodman RA, McKhann G, Roy NS, Goldman SA. Fetal and adult human oligodendrocyte progenitor cell isolates myelinate the congenitally dysmyelinated brain. Nat. Med. 2004;10:93–97. doi: 10.1038/nm974. [DOI] [PubMed] [Google Scholar]
- 32.Winkler C, Fricker RA, Gates MA, Olsson M, Hammang JP, Carpenter MK, Bjorklund A. Incorporation and glial differentiation of mouse EGF-responsive neural progenitor cells after transplantation into the embryonic rat brain. Mol. Cell. Neurosci. 1998;11:99–116. doi: 10.1006/mcne.1998.0674. [DOI] [PubMed] [Google Scholar]
- 33.Wright LS, Li J, Caldwell MA, Wallace K, Johnson JA, Svendsen CN. Gene expression in human neural stem cells: effects of leukemia inhibitory factor. J. Neurochem. 2003;86:179–195. doi: 10.1046/j.1471-4159.2003.01826.x. [DOI] [PubMed] [Google Scholar]
- 34.Wright LS, Prowse KR, Wallace K, Linskens MH, Svendsen CN. Human progenitor cells isolated from the developing cortex undergo decreased neurogenesis and eventual senescence following expansion in vitro. Exp. Cell Res. 2006;312:2107–2120. doi: 10.1016/j.yexcr.2006.03.012. [DOI] [PubMed] [Google Scholar]
- 35.Yan YP, Sailor KA, Lang BT, Park SW, Vemuganti R, Dempsey RJ. Monocyte chemoattractant protein-1 plays a critical role in neuroblast migration after focal cerebral ischemia. J. Cereb. Blood Flow Metab. 2007;27:1213–1224. doi: 10.1038/sj.jcbfm.9600432. [DOI] [PubMed] [Google Scholar]
- 36.Yan YP, Sailor KA, Vemuganti R, Dempsey RJ. Insulin-like growth factor-1 is an endogenous mediator of focal ischemia-induced neural progenitor proliferation. Eur. J. Neurosci. 2006;24:45–54. doi: 10.1111/j.1460-9568.2006.04872.x. [DOI] [PubMed] [Google Scholar]